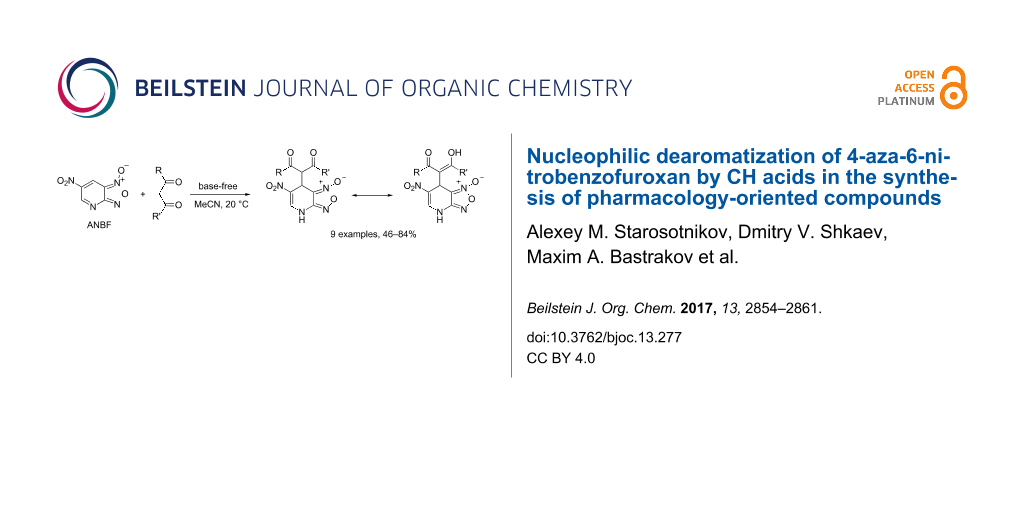
Abstract
4-Aza-6-nitrobenzofuroxan (ANBF) reacts with 1,3-dicarbonyl compounds and other CH acids to give carbon-bonded 1,4-adducts – 1,4-dihydropyridines fused with furoxan ring. In the case of most acidic β-diketones, which exist mainly in the enol form in polar solvents, the reactions proceed in the absence of any added base emphasizing the highly electrophilic character of ANBF. The resulting compounds combine in one molecule NO-donor furoxan ring along with a pharmacologically important 1,4-dihydropyridine fragment and therefore can be considered as prospective platforms for the design of pharmacology-oriented heterocyclic systems.
Graphical Abstract
Introduction
The reactions of condensed furoxans with CH acidic compounds have been extensively studied. There are two main possibilities for these reactions to proceed depending on the structure of the substrate and the nucleophile. The first, Beirut reaction [1-10], allowing to transform the furoxan ring to pyrazine or imidazole N-oxides as well as N-hydroxyimidazole occurs under the action of α-unsubstituted aldehydes, ketones, 1,3-dicarbonyl compounds, etc. on furoxans annelated with benzene or heterocyclic ring in the presence of base (Scheme 1).
Mononitrobenzofuroxans as well as pyridofuroxan (4-azabenzofuroxan) react as mentioned above [11-14]. At the same time in the case of highly electrophilic 4,6-dinitrobenzofuroxan (DNBF) another reaction pathway was observed. DNBF react with β-diketones and even monoketones in DMSO solution to give carbon-bonded σ-adducts in the absence of any added base [15] (Scheme 2). However, DNBF is a typical superelectrophile [16], it reacts very easily with water or methanol without base to give the σ-adducts.
Scheme 2: Reactivity of 4,6-dinitrobenzofuroxan.
Scheme 2: Reactivity of 4,6-dinitrobenzofuroxan.
Earlier it was found that an aza analog of DNBF – 4-aza-6-nitrobenzofuroxan (1, ANBF) ranks among the most electrophilic heteroaromatics known to date [17,18]. Compound 1 gives a remarkably stable hydrate in aqueous solution and forms Diels–Alder cycloadduts 2 with dienes (Scheme 3). In addition, ANBF readily forms σ-adduct 3 with N-methylindole which was isolated as sodium salt [18] (Scheme 3).
In this work we examined the reactivity of ANBF toward CH-acidic compounds containing a variety of functional groups. This allows to synthesize highly functionalized heteroaromatics combining several pharmacophoric moieties in the same molecule, interesting from the standpoint of design of novel pharmaceuticals. The work continues our ongoing project on the synthesis of complex hybrid molecules – furoxan-containing potential NO donors [19-24].
Results and Discussion
The only method for the synthesis of 1 described so far deals with the reaction of commercially available 2-chloro-3,5-dinitropyridine (4) with NaN3 followed by thermolysis of the intermediate azide [25,26]. We developed an alternative safe and efficient method for the synthesis of 1 starting from chloride 4 which was treated with methanolic ammonia solution to give 2-amino-3,5-dinitropyridine (5). Oxidative cyclization of 5 under the action of PhI(OAc)2 gave ANBF in 87% overall yield (Scheme 4).
The 1H NMR spectra of compound 1 in dry deuterated DMSO and acetone coincided with those described earlier [17,26,27]. Unlike for DNBF [15], no evidence of formation of any adduct was obtained on addition of an equimolar amount of acetone to a solution of ANBF in DMSO-d6. Moreover, reactions of 1 with acetone or acetophenone in the presence of 1 equiv of Et3N led to fast consumption of the starting material and resinification. Apparently, it was caused by interaction of Et3N with ANBF because the blank experiment (1 + Et3N with no ketone added) also resulted in decomposition of ANBF. Therefore we studied reactions of 1 with more acidic 1,3-diketones, ketoesters and related compounds. The addition of equimolar quantity of certain CH-acid to the solution of ANBF in dry MeCN or DMF resulted in rapid formation of the adducts 6–14; TLC analysis showed full consumption of starting materials after 15–20 min stirring at room temperature (Scheme 5, Table 1).
Scheme 5: Reactions of ANBF with β-dicarbonyl compounds.
Scheme 5: Reactions of ANBF with β-dicarbonyl compounds.
Table 1: Reactions of ANBF with β-dicarbonyl compounds.
| Entry | CH acid | Products | Isolated yield, % |
| 1 |
|
|
74 |
| 2 |
|
|
84 |
| 3 |
|
|
46 |
| 4 |
|
|
78 |
| 5 |
|
|
50 |
| 6 |
|
|
83 |
| 7 |
|
|
70 |
| 8 |
|
|
78a |
| 9 |
|
|
–b |
aReaction was carried out in the presence of 1 equiv of Et3N. bNot isolated due to the low stability of the product.
The reaction with 2,4-pentanedione gave a mixture of diketo-adduct 6a and a pair of enol tautomers 6b, NMR spectra in DMSO exhibited two sets of signals with relative intensities about 3:5 at 27 °C. The chirality of carbon atom C7 in compound 6a makes two methyl groups nonequivalent (δ 2.14 and 2.36 ppm, Table 2). In addition, two doublets at 4.71 and 5.15 ppm (J = 2.4 Hz) corresponding to H1’ and H7 atoms were observed. Another set of signals was referred to tautomers 6b. Due to fast interconversion of these tautomers the signals of their methyl groups represent broad singlets at 2.06 and 2.40 ppm.
Table 2: Selected NMR parameters of the ANBF-CH-acid adducts (in DMSO-d6)a.
| Adduct | Chemical shifts and coupling constants, δ (J)b | ||||||
| H(5) | H(7) | H(1’) | NH | OH | CH3 | CH2 | |
| 6a | 8.41 | 5.15 (2.4) | 4.71 (2.4) | 11.72 | 2.14; 2.36 | ||
| 6b | 8.34 | 5.44 | 11.72 | 14.79 | 2.06; 2.40 | ||
| 7a | 8.44 | 5.59 (2.6) | 5.04 (2.6) | 11.78 | 2.17 | ||
| 7b | 8.40 | 5.52 (2.9) | 5.34 (2.9) | 2.42 | |||
| 8 | 8.26 | 5.54 | 11.47 | 1.77; 2.33 | |||
| 9 | 8.26 | 5.52 | 11.42 | 0.95 | 2.23 | ||
| 13 | 8.46 | 5.05 (3.0) | 4.10 (2.9) | 12.03 | 1.10-1.22 | 3.99–4.17 | |
| 14 | 8.35 | 5.38 | 11.70 | 2.34 | |||
aFull spectroscopic data can be found in Supporting Information File 1. bChemical shifts in ppm from Me4Si, J values in Hz.
The reaction of ANBF with 1-phenyl-1,3-butanedione resulted in the formation of two diastereomers 7a and 7b in a ratio of 6:5 which in DMSO solution exist in diketonic form (Table 1, entry 2). This was confirmed by spectral data: two pairs of dublets (5.04 and 5.59 ppm, J = 2.6 Hz, for the major diastereomer and 5.34 and 5.52 ppm, J = 2.9 Hz, for the minor diastereomer) indicate the coupling of H7 and H1’ in both isomers and the absence of the enolic forms (Table 2). However, we were unable to attribute each set of signal to specific diastereomer 7a or 7b because the coupling constant values for H7 and H1’ were very close.
1,3-Cyclohexanedione and dimedone gave adducts 8 and 9, respectively (Table 1, entries 3 and 4) which exist predominantly in the enolic form. In favor of this assertion, in proton NMR spectra of 8 and 9 broad singlets at 2.33 and 2.23 ppm were observed (Table 2), corresponding to methylene groups. At the same time signals of diketonic form were found as traces.
Meldrum’s acid and 1,3-dimethylbarbituric acid react with ANBF similarly in the absence of base to give compounds 10 and 11, respectively (Table 1, entries 5 and 6). 1H NMR spectra of these adducts in DMSO-d6 contain double sets of signals that may be attributed to dioxo- and enolic forms. However, analysis of their 1H and 13C NMR spectra was quite complicated due to the broadening of the signals as a result of interconversion of tautomers. On the contrary, 1H NMR spectra in acetone-d6 are much simpler: in each case the single set of signals corresponds to the dioxo form, although the spectrum of 10 contains an additional “enolic” set of signals as traces.
Addition of ethyl acetoacetate to the solution of ANBF in acetonitrile resulted in the formation of the corresponding adduct 12 which was isolated in 70% yield (Table 1, entry 7). In this case the product in DMSO solution exists as a mixture of enolic form 12a and two diastereomeric dicarbonyl compounds 12b and 12c in a ratio of 2:1:1. The signals of each form are sometimes overlapping making it difficult to attribute any certain proton. The structure of 12 was confirmed by an X-ray diffraction experiment (Figure 1) [28]. Compound 12 crystallizes as a solvate with one DMF molecule that is connected to the hydrogen atom of the amino fragment by an intermolecular H-bond. In the crystal the position of hydrogen atom H3 (Figure 1), as well as bond lengths (C14–O1 1.2352(17), C12–O3 1.3386(18), C11–C12 1.3687(19), C11–C14 1.450(2) Å) clearly indicate the enolic form of the compound.
![[1860-5397-13-277-1]](/bjoc/content/figures/1860-5397-13-277-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: General view of molecule 12 in crystal. Anisotropic displacement parameters for non-hydrogen atoms are drawn at 50% probability; a DMF molecule is omitted for clarity.
Figure 1: General view of molecule 12 in crystal. Anisotropic displacement parameters for non-hydrogen atoms ...
The reaction of 1 with less acidic diethyl malonate required the addition of 1 equivalent of Et3N. In this case product 13 was isolated in 78% yield (Table 1, entry 8). Again, due to the chirality of C7, the two ethoxy groups are non-equivalent. It causes splitting of the signals of CH3, CH2 and C=O groups in 1H and 13C NMR spectra of 13.
Like all other diketones studied, 1,1,1-trifluoro-2,4-pentanedione gave the corresponding adduct with ANBF (Table 1, entry 9). However, all attempts to isolate compound 14 were unsuccessful due to its instability. Nevertheless, the 1H NMR spectrum of crude 14 contains one set of signals which was attributed to the enolic form indicated in Table 2. In particular, the sharp singlet at 5.38 ppm corresponds to the proton H7 of the dihydropyridine ring while the singlet at 2.34 ppm belongs to the methyl group. This is consistent with known data on fluorinated diketones: stability of the enol tautomer increases with the degree of fluorination [29,30]. Apparently this could explain the difference in ratios of tautomers in addition products of structurally similar linear 1,3-diketones (Table 1, entries 1, 2 and 9).
Another class of CH acids was studies in reactions with ANBF. In particular, 2,4-dinitrotoluene in the presence of Et3N was found to be unreactive, while 2,4,6-trinitrotoluene (TNT) gave the expected 1,4-adduct 15 in 74% yield (Scheme 6).
Scheme 6: Reaction of ANBF with 2,4,6-trinitrotoluene.
Scheme 6: Reaction of ANBF with 2,4,6-trinitrotoluene.
Due to the chirality of carbon atom C7, the protons HA and HB of the methylene group are diastereotopic and therefore have different chemical shifts (3.34 and 3.74 ppm). The geminal protons and H7 represent a well-resolved AMX system, Figure 2.
![[1860-5397-13-277-2]](/bjoc/content/figures/1860-5397-13-277-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: Partial 1H NMR spectrum of compound 15 in DMSO-d6.
Figure 2: Partial 1H NMR spectrum of compound 15 in DMSO-d6.
The structure of compound 15 was additionally studied using X-ray analysis [28] (Figure 3). Compound 15 crystallizes as a solvate with one DMSO molecule connected to the hydrogen atom of the amino fragment by an intermolecular H-bond. Bond lengths and angles in the dearomatized 4-aza-6-nitrobenzofuroxan ring are very similar to those observed in the crystal of 12·DMF. Due to significant steric effects, the single bond C4–C11 is significantly elongated (1.567(2) Å), and two nitro groups in ortho-positions of the connected picryl fragment are rotated out of the plane of the benzene ring.
![[1860-5397-13-277-3]](/bjoc/content/figures/1860-5397-13-277-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: General view of molecule 15 in crystal. Anisotropic displacement parameters for non-hydrogen atoms are drawn at 50% probability; a DMSO molecule and the minor component of the disordered nitro group (N18 O19” O20”) is omitted for clarity.
Figure 3: General view of molecule 15 in crystal. Anisotropic displacement parameters for non-hydrogen atoms ...
Thus, reactions of ANBF with strong CH acids (pKa(H2O) 4–11) proceed without added base while in case of diethyl malonate (pKa(H2O) 13.3) and TNT (pKa(H2O) 13.6) the addition of one equivalent of Et3N is required. In the latter case the formation of the adducts proceeds through the attack of the anion on C7. All other CH acids used exist mainly in enol form in polar solvents (DMSO, MeCN) and therefore, the mechanism depicted on Scheme 7 seems reasonable.
Scheme 7: Plausible mechanism of adducts formation.
Scheme 7: Plausible mechanism of adducts formation.
Adducts of 1,3-dicarbonyl compounds to ANBF are generally enolic. At the same time ratios of enolic and diketonic forms depend on the nature of certain CH acid. In case of most acidic diketones (Table 1, entries 3, 4 and 9) the enol form of the adduct is predominant and the ketonic form was detected by proton NMR as traces. In other cases the products contained comparable quantities of both tautomers, however, enol forms were prevailing. Surprisingly, the benzoylacetone adduct (Table 1, entry 2) represent a mixture of diketonic diastereomers and no enol form was detected.
All compounds synthesized contain two pharmacologically important structural fragments in one molecule, namely dihydropyridine and furoxan rings. The formers are well known L-type calcium channel blockers, used in the treatment of hypertension. Dihydropyridine derivatives are relatively vascular selective in their mechanism of action in lowering blood pressure [31,32]. Dimeric dihydropyridines are used as the precursors for HIV-1 protease inhibitors [33,34]. The furoxan system is used in the design of new NO donors [35-39] and its chemistry is being extensively studied (see for example [40,41]). Additional functionality, such as 1,3-dicarbonyl moiety and nitro group, would provide a number of transformations, heterocyclizations, etc. useful for diversification of synthesized derivatives.
Conclusion
In conclusion, reactions of 4-aza-6-nitrobenzofuroxan with CH acids gave carbon-bonded 1,4-adducts – 1,4-dihydropyridines fused with furoxan ring. In the case of the most acidic 1,3-dicarbonyl compounds the reactions proceed in the absence of a base. The resulting compounds combine in one molecule the NO-donor furoxan ring along with the pharmacologically important 1,4-dihydropyridine fragment and therefore can be considered as prospective platforms for the design of pharmacology-oriented heterocyclic systems.
Supporting Information
| Supporting Information File 1: Experimental section, NMR spectra, HRMS and X-ray analysis data. | ||
| Format: PDF | Size: 3.3 MB | Download |
References
-
Haddadin, M. J.; Issidoridis, C. H. Heterocycles 1976, 4, 767–816. doi:10.3987/R-1976-04-0767
Return to citation in text: [1] -
Gasco, A.; Boulton, A. J. In Advances in Heterocyclic Chemistry; Katritzky, A. R.; Boulton, A. J., Eds.; Academic Press: New York, 1981; Vol. 29, pp 251–340.
Return to citation in text: [1] -
Tinsley, J. M. Beirut reaction. In Name Reactions in Heterocyclic Chemistry; Li, J. J.; Corey, E. J., Eds.; John Wiley & Sons: Hoboken, NJ, 2005; pp 504–509.
Return to citation in text: [1] -
Issidoridis, C. H.; Haddadin, M. J. J. Org. Chem. 1966, 31, 4067–4068. doi:10.1021/jo01350a043
Return to citation in text: [1] -
Haddadin, M. J.; Issidoridis, C. H. Tetrahedron Lett. 1965, 6, 3253–3256. doi:10.1016/S0040-4039(01)89222-4
Return to citation in text: [1] -
Atfah, A.; Hill, J. J. Chem. Soc., Perkin Trans. 1 1989, 221–224. doi:10.1039/p19890000221
Return to citation in text: [1] -
Haddadin, M. J.; Issidorides, C. H. Heterocycles 1993, 35, 1503–1525. doi:10.3987/REV-92-SR(T)8
Return to citation in text: [1] -
El-Abadelah, M. M.; Nazer, M. Z.; El-Abadla, N. S.; Meier, H. Heterocycles 1995, 41, 2203–2219. doi:10.3987/COM-95-7128
Return to citation in text: [1] -
Takabatake, T.; Miyazawa, T.; Kojo, M.; Hasegawa, M. Heterocycles 2000, 53, 2151–2162. doi:10.3987/COM-00-8957
Return to citation in text: [1] -
Türker, L.; Dura, E. J. Mol. Struct.: THEOCHEM 2002, 593, 143–147. doi:10.1016/S0166-1280(02)00311-1
Return to citation in text: [1] -
Monge, A.; Palop, J. A.; Lopez de Ceráin, A.; Senador, V.; Martinez-Crespo, F. J.; Sainz, Y.; Narro, S.; Garcia, E.; de Miguel, C.; González, M.; Hamilton, E.; Barker, A. J.; Clarke, E. D.; Greenhow, D. T. J. Med. Chem. 1995, 38, 1786–1792. doi:10.1021/jm00010a023
Return to citation in text: [1] -
Panasyuk, P. M.; Mel’nikova, S. F.; Tselinskii, I. V. Russ. J. Org. Chem. 2001, 37, 891. doi:10.1023/A:1012486204080
Return to citation in text: [1] -
Takabatake, T.; Miyazawa, T.; Hasegawa, M. Heterocycles 1997, 45, 107–118. doi:10.3987/COM-96-7640
Return to citation in text: [1] -
Binder, D.; Noe, C. R.; Nußbaumer, J.; Prager, B. C. Monatsh. Chem. 1980, 111, 407–411. doi:10.1007/BF00903236
Return to citation in text: [1] -
Terrier, F.; Simonnin, M. P.; Pouet, M. J.; Strauss, M. J. J. Org. Chem. 1981, 46, 3537–3543. doi:10.1021/jo00330a033
Return to citation in text: [1] [2] -
Terrier, F.; Millot, F.; Norris, W. P. J. Am. Chem. Soc. 1976, 98, 5883–5890. doi:10.1021/ja00435a022
Return to citation in text: [1] -
Terrier, F.; Sebban, M.; Goumont, R.; Hallé, J. C.; Moutiers, G.; Cangelosi, I.; Buncel, E. J. Org. Chem. 2000, 65, 7391–7398. doi:10.1021/jo0005114
Return to citation in text: [1] [2] -
Terrier, F.; Lakhdar, S.; Boubaker, T.; Goumont, R. J. Org. Chem. 2005, 70, 6242–6253. doi:10.1021/jo0505526
Return to citation in text: [1] [2] -
Ananikov, V. P.; Khokhlova, E. A.; Egorov, M. P.; Sakharov, A. M.; Zlotin, S. G.; Kucherov, A. V.; Kustov, L. M.; Gening, M. L.; Nifantiev, N. E. Mendeleev Commun. 2015, 25, 75–82. doi:10.1016/j.mencom.2015.03.001
Return to citation in text: [1] -
Zlotin, S. G.; Churakov, A. M.; Luk'yanov, O. A.; Makhova, N. N.; Sukhorukov, A. Yu.; Tartakovsky, V. A. Mendeleev Commun. 2015, 25, 399–409. doi:10.1016/j.mencom.2015.11.001
Return to citation in text: [1] -
Bastrakov, M. A.; Starosotnikov, A. M.; Kachala, V. V.; Dalinger, I. L.; Shevelev, S. A. Chem. Heterocycl. Compd. 2015, 51, 496–499. doi:10.1007/s10593-015-1726-1
Return to citation in text: [1] -
Starosotnikov, A. M.; Bastrakov, M. A.; Pavlov, A. A.; Fedyanin, I. V.; Dalinger, I. L.; Shevelev, S. A. Mendeleev Commun. 2016, 26, 217–219. doi:10.1016/j.mencom.2016.04.013
Return to citation in text: [1] -
Bastrakov, M. A.; Starosotnikov, A. M.; Pavlov, A. A.; Dalinger, I. L.; Shevelev, S. A. Chem. Heterocycl. Compd. 2016, 52, 690–693. doi:10.1007/s10593-016-1950-3
Return to citation in text: [1] -
Bastrakov, M. A.; Kucherova, A. Yu.; Fedorenko, A. K.; Starosotnikov, A. M.; Fedyanin, I. V.; Dalinger, I. L.; Shevelev, S. A. ARKIVOC 2017, No. iii, 181–190. doi:10.24820/ark.5550190.p010.185
Return to citation in text: [1] -
Ayyangar, N. R.; Madan Kumar, S.; Srinivasan, K. V. Synthesis 1987, 616–618. doi:10.1055/s-1987-28023
Return to citation in text: [1] -
Lowe-Ma, C. K.; Nissan, R. A.; Wilson, W. S. J. Org. Chem. 1990, 55, 3755–3761. doi:10.1021/jo00299a014
Return to citation in text: [1] [2] -
Cmoch, P.; Kamieński, B.; Kamieńska-Trela, K.; Stefaniak, L.; Webb, G. A. J. Phys. Org. Chem. 2000, 13, 480–488. doi:10.1002/1099-1395(200008)13:8<480::AID-POC268>3.0.CO;2-Z
Return to citation in text: [1] -
Crystallographic data for 12·DMF and 15·DMSO crystals have been deposited at the Cambridge Crystallographic Data Centre (CCDC) with the reference numbers 1574256 and 1574257, respectively. These data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures. Details on the X-ray experiments and additional crystallographic information are provided in Supporting Information File 1.
Return to citation in text: [1] [2] -
Wallen, S. L.; Yonker, C. R.; Phelps, C. L.; Wai, C. M. J. Chem. Soc., Faraday Trans. 1 1997, 93, 2391–2394. doi:10.1039/a701851g
Return to citation in text: [1] -
Pashkevich, K. I.; Saloutin, V. I.; Postovskii, I. Y. Russ. Chem. Rev. 1981, 50, 180–195. doi:10.1070/RC1981v050n02ABEH002555
Return to citation in text: [1] -
Siddiqui, M. A. A.; Polsker, G. L. Drugs 2004, 64, 1135–1148. doi:10.2165/00003495-200464100-00009
Return to citation in text: [1] -
Peterson, B. Z.; Tanada, T. N.; Catterall, W. A. J. Biol. Chem. 1996, 271, 5293–5296. doi:10.1074/jbc.271.10.5293
Return to citation in text: [1] -
Coburger, C.; Wollmann, J.; Baumert, C.; Krug, M.; Molnár, J.; Lage, H.; Hilgeroth, A. J. Med. Chem. 2008, 51, 5871–5874. doi:10.1021/jm800480y
Return to citation in text: [1] -
Richter, M.; Molnár, J.; Hilgeroth, A. J. Med. Chem. 2006, 49, 2838–2840. doi:10.1021/jm058046w
Return to citation in text: [1] -
Feelisch, M.; Schönafingeri, K.; Noack, H. Biochem. Pharmacol. 1992, 44, 1149–1157. doi:10.1016/0006-2952(92)90379-W
Return to citation in text: [1] -
Sorba, G.; Medana, C.; Fruttero, R.; Cena, C.; Di Stilo, A.; Galli, U.; Gasco, A. J. Med. Chem. 1997, 40, 463–469. doi:10.1021/jm960379t
Return to citation in text: [1] -
Medana, C.; Ermondi, G.; Fruttero, R.; Di Stilo, A.; Ferretti, C.; Gasco, A. J. Med. Chem. 1994, 37, 4412–4416. doi:10.1021/jm00051a020
Return to citation in text: [1] -
Ferioli, R.; Folco, G. C.; Ferretti, C.; Gasco, A. M.; Medana, C.; Fruttero, R.; Civelli, M.; Gasco, A. Br. J. Pharmacol. 1995, 114, 816–820. doi:10.1111/j.1476-5381.1995.tb13277.x
Return to citation in text: [1] -
Cena, C.; Bertinaria, M.; Boschi, D.; Giorgis, M.; Gasco, A. ARKIVOC 2006, No. vii, 301–309. doi:10.3998/ark.5550190.0007.722
Return to citation in text: [1] -
Fershtat, L. L.; Larin, A. A.; Epishina, M. A.; Ovchinnikov, I. V.; Kulikov, A. S.; Ananyev, I. V.; Makhova, N. N. RSC Adv. 2016, 6, 31526–31539. doi:10.1039/C6RA05110C
Return to citation in text: [1] -
Fershtat, L. L.; Epishina, M. A.; Ovchinnikov, I. V.; Kachala, V. V.; Makhova, N. N. Chem. Heterocycl. Compd. 2015, 51, 754–759. doi:10.1007/s10593-015-1771-9
Return to citation in text: [1]
| 40. | Fershtat, L. L.; Larin, A. A.; Epishina, M. A.; Ovchinnikov, I. V.; Kulikov, A. S.; Ananyev, I. V.; Makhova, N. N. RSC Adv. 2016, 6, 31526–31539. doi:10.1039/C6RA05110C |
| 41. | Fershtat, L. L.; Epishina, M. A.; Ovchinnikov, I. V.; Kachala, V. V.; Makhova, N. N. Chem. Heterocycl. Compd. 2015, 51, 754–759. doi:10.1007/s10593-015-1771-9 |
| 1. | Haddadin, M. J.; Issidoridis, C. H. Heterocycles 1976, 4, 767–816. doi:10.3987/R-1976-04-0767 |
| 2. | Gasco, A.; Boulton, A. J. In Advances in Heterocyclic Chemistry; Katritzky, A. R.; Boulton, A. J., Eds.; Academic Press: New York, 1981; Vol. 29, pp 251–340. |
| 3. | Tinsley, J. M. Beirut reaction. In Name Reactions in Heterocyclic Chemistry; Li, J. J.; Corey, E. J., Eds.; John Wiley & Sons: Hoboken, NJ, 2005; pp 504–509. |
| 4. | Issidoridis, C. H.; Haddadin, M. J. J. Org. Chem. 1966, 31, 4067–4068. doi:10.1021/jo01350a043 |
| 5. | Haddadin, M. J.; Issidoridis, C. H. Tetrahedron Lett. 1965, 6, 3253–3256. doi:10.1016/S0040-4039(01)89222-4 |
| 6. | Atfah, A.; Hill, J. J. Chem. Soc., Perkin Trans. 1 1989, 221–224. doi:10.1039/p19890000221 |
| 7. | Haddadin, M. J.; Issidorides, C. H. Heterocycles 1993, 35, 1503–1525. doi:10.3987/REV-92-SR(T)8 |
| 8. | El-Abadelah, M. M.; Nazer, M. Z.; El-Abadla, N. S.; Meier, H. Heterocycles 1995, 41, 2203–2219. doi:10.3987/COM-95-7128 |
| 9. | Takabatake, T.; Miyazawa, T.; Kojo, M.; Hasegawa, M. Heterocycles 2000, 53, 2151–2162. doi:10.3987/COM-00-8957 |
| 10. | Türker, L.; Dura, E. J. Mol. Struct.: THEOCHEM 2002, 593, 143–147. doi:10.1016/S0166-1280(02)00311-1 |
| 17. | Terrier, F.; Sebban, M.; Goumont, R.; Hallé, J. C.; Moutiers, G.; Cangelosi, I.; Buncel, E. J. Org. Chem. 2000, 65, 7391–7398. doi:10.1021/jo0005114 |
| 18. | Terrier, F.; Lakhdar, S.; Boubaker, T.; Goumont, R. J. Org. Chem. 2005, 70, 6242–6253. doi:10.1021/jo0505526 |
| 33. | Coburger, C.; Wollmann, J.; Baumert, C.; Krug, M.; Molnár, J.; Lage, H.; Hilgeroth, A. J. Med. Chem. 2008, 51, 5871–5874. doi:10.1021/jm800480y |
| 34. | Richter, M.; Molnár, J.; Hilgeroth, A. J. Med. Chem. 2006, 49, 2838–2840. doi:10.1021/jm058046w |
| 16. | Terrier, F.; Millot, F.; Norris, W. P. J. Am. Chem. Soc. 1976, 98, 5883–5890. doi:10.1021/ja00435a022 |
| 35. | Feelisch, M.; Schönafingeri, K.; Noack, H. Biochem. Pharmacol. 1992, 44, 1149–1157. doi:10.1016/0006-2952(92)90379-W |
| 36. | Sorba, G.; Medana, C.; Fruttero, R.; Cena, C.; Di Stilo, A.; Galli, U.; Gasco, A. J. Med. Chem. 1997, 40, 463–469. doi:10.1021/jm960379t |
| 37. | Medana, C.; Ermondi, G.; Fruttero, R.; Di Stilo, A.; Ferretti, C.; Gasco, A. J. Med. Chem. 1994, 37, 4412–4416. doi:10.1021/jm00051a020 |
| 38. | Ferioli, R.; Folco, G. C.; Ferretti, C.; Gasco, A. M.; Medana, C.; Fruttero, R.; Civelli, M.; Gasco, A. Br. J. Pharmacol. 1995, 114, 816–820. doi:10.1111/j.1476-5381.1995.tb13277.x |
| 39. | Cena, C.; Bertinaria, M.; Boschi, D.; Giorgis, M.; Gasco, A. ARKIVOC 2006, No. vii, 301–309. doi:10.3998/ark.5550190.0007.722 |
| 15. | Terrier, F.; Simonnin, M. P.; Pouet, M. J.; Strauss, M. J. J. Org. Chem. 1981, 46, 3537–3543. doi:10.1021/jo00330a033 |
| 28. | Crystallographic data for 12·DMF and 15·DMSO crystals have been deposited at the Cambridge Crystallographic Data Centre (CCDC) with the reference numbers 1574256 and 1574257, respectively. These data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures. Details on the X-ray experiments and additional crystallographic information are provided in Supporting Information File 1. |
| 11. | Monge, A.; Palop, J. A.; Lopez de Ceráin, A.; Senador, V.; Martinez-Crespo, F. J.; Sainz, Y.; Narro, S.; Garcia, E.; de Miguel, C.; González, M.; Hamilton, E.; Barker, A. J.; Clarke, E. D.; Greenhow, D. T. J. Med. Chem. 1995, 38, 1786–1792. doi:10.1021/jm00010a023 |
| 12. | Panasyuk, P. M.; Mel’nikova, S. F.; Tselinskii, I. V. Russ. J. Org. Chem. 2001, 37, 891. doi:10.1023/A:1012486204080 |
| 13. | Takabatake, T.; Miyazawa, T.; Hasegawa, M. Heterocycles 1997, 45, 107–118. doi:10.3987/COM-96-7640 |
| 14. | Binder, D.; Noe, C. R.; Nußbaumer, J.; Prager, B. C. Monatsh. Chem. 1980, 111, 407–411. doi:10.1007/BF00903236 |
| 31. | Siddiqui, M. A. A.; Polsker, G. L. Drugs 2004, 64, 1135–1148. doi:10.2165/00003495-200464100-00009 |
| 32. | Peterson, B. Z.; Tanada, T. N.; Catterall, W. A. J. Biol. Chem. 1996, 271, 5293–5296. doi:10.1074/jbc.271.10.5293 |
| 17. | Terrier, F.; Sebban, M.; Goumont, R.; Hallé, J. C.; Moutiers, G.; Cangelosi, I.; Buncel, E. J. Org. Chem. 2000, 65, 7391–7398. doi:10.1021/jo0005114 |
| 26. | Lowe-Ma, C. K.; Nissan, R. A.; Wilson, W. S. J. Org. Chem. 1990, 55, 3755–3761. doi:10.1021/jo00299a014 |
| 27. | Cmoch, P.; Kamieński, B.; Kamieńska-Trela, K.; Stefaniak, L.; Webb, G. A. J. Phys. Org. Chem. 2000, 13, 480–488. doi:10.1002/1099-1395(200008)13:8<480::AID-POC268>3.0.CO;2-Z |
| 28. | Crystallographic data for 12·DMF and 15·DMSO crystals have been deposited at the Cambridge Crystallographic Data Centre (CCDC) with the reference numbers 1574256 and 1574257, respectively. These data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures. Details on the X-ray experiments and additional crystallographic information are provided in Supporting Information File 1. |
| 25. | Ayyangar, N. R.; Madan Kumar, S.; Srinivasan, K. V. Synthesis 1987, 616–618. doi:10.1055/s-1987-28023 |
| 26. | Lowe-Ma, C. K.; Nissan, R. A.; Wilson, W. S. J. Org. Chem. 1990, 55, 3755–3761. doi:10.1021/jo00299a014 |
| 29. | Wallen, S. L.; Yonker, C. R.; Phelps, C. L.; Wai, C. M. J. Chem. Soc., Faraday Trans. 1 1997, 93, 2391–2394. doi:10.1039/a701851g |
| 30. | Pashkevich, K. I.; Saloutin, V. I.; Postovskii, I. Y. Russ. Chem. Rev. 1981, 50, 180–195. doi:10.1070/RC1981v050n02ABEH002555 |
| 19. | Ananikov, V. P.; Khokhlova, E. A.; Egorov, M. P.; Sakharov, A. M.; Zlotin, S. G.; Kucherov, A. V.; Kustov, L. M.; Gening, M. L.; Nifantiev, N. E. Mendeleev Commun. 2015, 25, 75–82. doi:10.1016/j.mencom.2015.03.001 |
| 20. | Zlotin, S. G.; Churakov, A. M.; Luk'yanov, O. A.; Makhova, N. N.; Sukhorukov, A. Yu.; Tartakovsky, V. A. Mendeleev Commun. 2015, 25, 399–409. doi:10.1016/j.mencom.2015.11.001 |
| 21. | Bastrakov, M. A.; Starosotnikov, A. M.; Kachala, V. V.; Dalinger, I. L.; Shevelev, S. A. Chem. Heterocycl. Compd. 2015, 51, 496–499. doi:10.1007/s10593-015-1726-1 |
| 22. | Starosotnikov, A. M.; Bastrakov, M. A.; Pavlov, A. A.; Fedyanin, I. V.; Dalinger, I. L.; Shevelev, S. A. Mendeleev Commun. 2016, 26, 217–219. doi:10.1016/j.mencom.2016.04.013 |
| 23. | Bastrakov, M. A.; Starosotnikov, A. M.; Pavlov, A. A.; Dalinger, I. L.; Shevelev, S. A. Chem. Heterocycl. Compd. 2016, 52, 690–693. doi:10.1007/s10593-016-1950-3 |
| 24. | Bastrakov, M. A.; Kucherova, A. Yu.; Fedorenko, A. K.; Starosotnikov, A. M.; Fedyanin, I. V.; Dalinger, I. L.; Shevelev, S. A. ARKIVOC 2017, No. iii, 181–190. doi:10.24820/ark.5550190.p010.185 |
| 18. | Terrier, F.; Lakhdar, S.; Boubaker, T.; Goumont, R. J. Org. Chem. 2005, 70, 6242–6253. doi:10.1021/jo0505526 |
| 15. | Terrier, F.; Simonnin, M. P.; Pouet, M. J.; Strauss, M. J. J. Org. Chem. 1981, 46, 3537–3543. doi:10.1021/jo00330a033 |
© 2017 Starosotnikov et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)