Abstract
Several 3,5-disubstituted isoxazoles were obtained in good yields by regiospecific 1,3-dipolar cycloaddition reactions between aromatic nitrile oxides, generated in situ from the corresponding hydroxyimidoyl chlorides, with non-symmetrical activated alkynes in the presence of catalytic amounts of copper(I) iodide. Effects of 3,5-disubstituted isoxazoles on nitric oxide and reactive oxygen species generation in Arabidopsis tissues was studied using specific diaminofluoresceine dyes as fluorescence indicators.
Graphical Abstract
Introduction
Isoxazoles are an interesting class of N-heterocyclic compounds intensely studied mainly due to their wide range of biological activity [1,2]. Isoxazole compounds show antiviral [3,4], antithrombotic [5-9], analgesic [9], COX-2 inhibitory [10,11], anti-inflamatory [9,11], antinociceptive [12] and anticancer [13] activities. Several isoxazole derivatives have GABAA antagonist [14] and T-type Ca2+ channel blocking activities [15]. Commercial drugs featuring an isoxazole moiety include the COX-2 inhibitor Valdecoxib and the β-lactam antibiotics Cloxacillin and Dicloxacillin. An isoxazole derivative, namely 3,5-difluorophenyl-[3-methyl-4-(methylsulfonyl)isoxazol-5-yl]methanone, was recently reported as an inducer of nitric oxide producing elicitor in plants [16,17]. Nitric oxide (NO), which has been demonstrated to be a major gasotransmitter in mammals, is also involved in the orchestration of various plant physiological responses, playing an important role in the regulation of interactions between plant and microorganisms and in plant defense mechanisms against stresses [18,19]. Consequently, there is interest in the biological evaluation of further isoxazole derivatives.
Many synthetic approaches towards the isoxazole core include the reactions of hydroxylamine with aryl-β-diketones [20], α,β-unsaturated carbonyl compounds [21], or α,β-unsaturated nitriles [22], and 1,3-dipolar cycloaddition reactions between alkenes or alkynes and nitrile oxides [23-25].
Nitrile oxides are known as reactive 1,3-dipoles involved in 1,3-dipolar cycloaddition reactions with various dipolarophiles generating five-membered heterocyclic compounds, such as isoxazoles, isoxazolines, oxadiazoles, oxadiazolines, dioxazolidines etc. [23-25]. Intermediate nitrile oxides are usually generated in situ by the oxidative dehydrogenation of aldoximes in the presence of various oxidants [26-29], or by the dehydrohalogenation of hydroxyiminoyl halides promoted by organic or inorganic bases [30-32]. A less used synthetic procedure involves the oxidative dehydration of primary nitro compounds with isocyanates in the presence of tertiary alkylamines [33].
Generally, the cycloaddition reactions of nitrile oxides to alkenes yield isoxazolines or a mixture of isoxalines and isoxazoles. Cycloaddition reactions of nitrile oxides to alkynes yield isoxazoles directly, without a catalyst, but the yields of isoxazole products are quite low because of side reactions and both regioisomers are generally obtained [23-25]. The one-pot 1,3-dipolar cycloaddition reaction of a nitrile oxide, generated in situ from the corresponding hydroxymoyl chloride, with an in situ brominated electron-deficient alkene led to the intermediate bromoisoxazoline from which, by loss of HBr, a 3,5-disubstituted isoxazole derivative is formed as major regioisomer [34]. Based on the copper(I)-catalyzed click reactions of organic azides with terminal acetylenes [35], different copper(I)-catalyzed synthetic procedures towards isoxazole derivatives were developed [36,37].
As part of our continued efforts to develop simple synthetic routes towards bioactive heterocyclic compounds [38-43], we report here the synthesis of several 3,5-isoxazole derivatives, bearing benzo[1,3]dioxole and thiophene scaffolds respectively, as well as their inductor effect on the generation of nitric oxide and reactive oxygen species in plant tissues. The benzo[1,3]dioxole framework is a constituent of some fragrances and flavors [44], and several bioactive compounds with a broad spectrum of applications [45-48]. The thiophene is a core system of a large number of bioactive molecules such as antineoplastic agents [49], non-steroidal anti-inflammatory drugs [50] or compounds with antibacterial activities against several Gram-positive strains [51]. Thus, 3,5-disubstituted isoxazole derivatives were obtained by regioselective 1,3-dipolar cycloaddition reactions of aromatic nitrile oxides to non-symmetrical activated alkyne derivatives in the presence of catalytic amounts of copper(I) iodide.
Results and Discussion
Synthesis of 3,5-disubstituted isoxazole derivatives
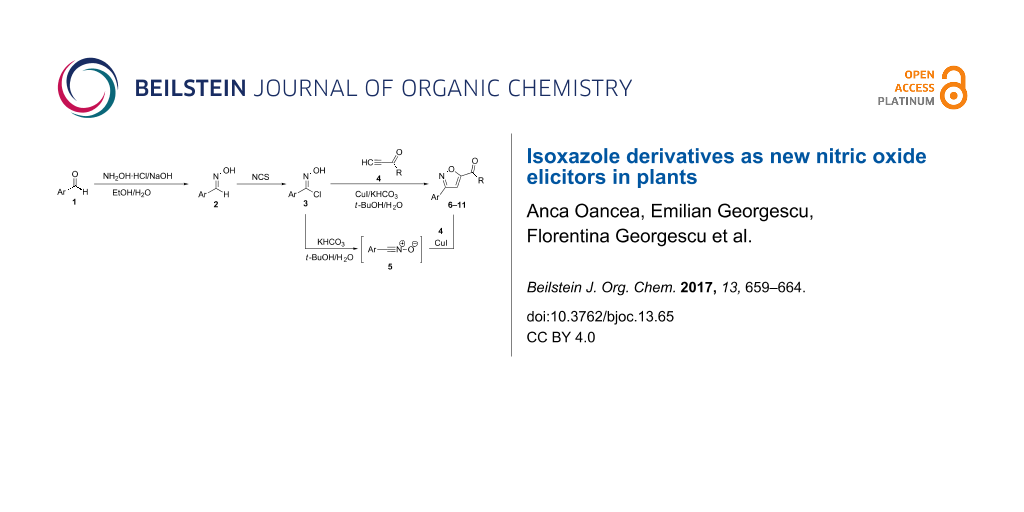
The regioselective cooper(I)-catalyzed 1,3-dipolar cycloaddition reactions of aromatic nitrile oxides, generated in situ from the corresponding crude imidoyl chlorides, and non-symmetrically activated alkynes led to 3,5-disubstituted isoxazole derivatives. For this, aromatic aldehydes 1 are first converted to the corresponding aldoximes 2, via reactions with hydroxylamine, and the crude reaction products are transformed to the corresponding imidoyl chlorides 3 which are directly used in the next step without purifications. Catalytic amounts of copper(I) iodide and a base, such as KHCO3, are added to an aqueous solution containing the crude imidoyl chlorides 3 and the non-symmetrical activated alkynes 4. The in situ generated aromatic nitrile oxides 5 undergo an addition to copper(I) acetylides formed in situ to give the 3,5-disubstituted isoxazoles 6–11 as single isomers, in moderate to good yields (Scheme 1).
Scheme 1: Synthetic route to 3,5-disubstituted isoxazoles.
Scheme 1: Synthetic route to 3,5-disubstituted isoxazoles.
All reactions between crude imidoyl chlorides 3 and the non-symmetrical activated alkynes 4 are carried out in aqueous solutions at room temperature. The final 3,5-disubstituted isoxazoles are easily separated by filtration, washed with water and recristallysed. The synthesized 3,5-disubstituted isoxazoles are presented in Table 1.
Table 1: Reported 3,5-disubstituted isoxazoles.
| Compound | Ar | R | mp (º) | Yield (%) |
|---|---|---|---|---|
| 6 |
|
Me |
159–160;
141–142 [51] |
81 |
| 7 | OEt | 100–101.5 | 70 | |
| 8 | Ph | 135–137 | 78 | |
| 9 |
|
Me | 113–115 | 73 |
| 10 | OEt | 78–80 | 67 | |
| 11 | Ph | 90–91 | 74 | |
3,5-Disubstituted isoxazoles 6, 7, 9 and 10 (Table 1) have been previously prepared by different synthetic procedures, but compounds 6 [52], 7 [49] and 10 [53] have been only partly characterized, while for compound 9 [54] no characterization data have been reported.
3,5-Disubstituted isoxazole structures were unambiguously assigned on the basis of chemical and spectral analysis (IR, 1H, 13C and 15N NMR spectra). NMR spectra clearly indicated the presence of only one regioisomer for all synthesized 3,5-disubstituted isoxazoles. Signals in 1H and 13C NMR spectra were fully assigned based on H,C-HSQC and H,C-HMBC experiments. The 3,5-disubstitution was also experimentally proven by NOE experiments which indicated through space interaction between the H-4 proton and the protons from both 3- and 5- substituents.
Biological activity
We investigated the inductor effects of 3,5-disubstituted isoxazoles 6–11 on NO and reactive oxygen species (ROS) production in plant tissues. Usually, NO and ROS, such as O2−, OH· and H2O2, together are required to induce the activation of various defense-related enzymes in plants [55]. Plant cells contain oxygen radical detoxifying enzymes and nonenzymatic antioxidants which have an essential role in protection of plant cells from oxidative damages at the sites of ROS and NO generation [56,57]. Measuring the ROS and NO levels in plant tissues is often difficult due to high reactivity and extremely short physiological half-life of these free radicals [58,59].
In this work, generation of both NO and ROS was proven by fluorescence microscopy using specific fluorescence indicators that help to exactly define the sites of generation. We used Arabidopsis thaliana wild type seeds, cultivated for six weeks in laboratory in Arasystem [60]. Arabidopsis thaliana was selected as model organisms for NO inductors because this is the flowering plant with the largest amount of knowledge on cellular and molecular biology and it has a relatively short life cycle. The Arabidopsis leaves were sprayed with fine suspensions of isoxazole inductors 6–11 at two different concentrations (10 μg/mL and 50 μg/mL, respectively) and collected after 24 h. Collected leaves were washed with distilled water and incubated with the specific fluorescence indicator for histochemical analysis of ROS and NO by fluorescence microscopy. Arabidopsis leaves untreated with inductor suspensions have been used as negative controls. As positive control, we used chitosan (β-1,4 linked glucosamine) with average molecular weight, a fungal elicitor with known effect as NO and ROS inductor on A. thaliana [61].
Intracellular ROS was visualized using 2’,7’-dichlorodihydrofluorescein diacetate (H2DCF DA) as fluorescence indicator. The method is based on the oxidation of the non-fluorescent probe of 2’,7’-dichlorodihydrofluorescein diacetate to the highly fluorescent 2’,7’-dichlorofluorescein diacetate [62,63].
Intracellular NO was visualized using 4-amino-5-methylamino-2’,7’-dichlorodihydrofluorescein diacetate (DAF-FM diacetate), a non-fluorescent compound, which reacts with NO to form a fluorescent benzotriazole and does not react with any ROS [64-67].
Fluorescence microscopy images of all 3,5-disubstituted isoxazoles-treated Arabidopsis leaves showed a pronounced presence of ROS at both concentrations of inductors (10 μg/mL and 50 μg/mL). Strong fluorescence densities were observed especially at higher concentration (50 μg/mL) of 3,5-disubstituted isoxazoles. Intensities of fluorescence revealed differences between tested compounds. These compounds can be listed in the increasing order of ROS generation efficacy as follows: 11 > 10 > 9 > 7 > 8 > 6 (Figure S2 in Supporting Information File 1).
Similarly, images of fluorescence microscopy revealed the presence of NO in all 3,5-disubstituted isoxazoles-treated Arabidopsis leaves, at both concentrations (10 μg/mL and 50 μg/mL), especially at higher concentration of compounds (50 μg/mL). The NO releasing capacity of newly synthesized 3,5-disubstituted isoxazoles followed the series: 9 > 11 > 10 > 8 > 7 = 6 (Figure S3 in Supporting Information File 1).
Fluorescence data indicate that the 3,5-disubstituted isoxazoles 6–11, particularly 9–11, are involved in NO and ROS production in Arabidopsis treated leaves.
Conclusion
Several 3,5-disubstituted isoxazoles were obtained by the convenient, regiospecific 1,3-dipolar cycloaddition reactions of aromatic nitrile oxides, generated in situ from the crude imidoyl chlorides, with non-symmetrical activated alkynes in the presence of catalytic amounts of copper(I) iodide. The effect of 3,5-disubstituted isoxazoles in generation of ROS and NO in plant tissues was investigated by fluorescent microscopy. The obtained data indicate that some of these compounds are chemical elicitors that induce NO and ROS generation in plant tissues and could activate various defense mechanisms in plants. Further research is in progress to assess the in planta mechanism of NO generation by these compounds.
Supporting Information
| Supporting Information File 1: Experimental procedures, characterization data, IR, 1H, 13C and 15N NMR data for all new compounds and fluorescence microscopy images of NO and ROS generation for all 3,5-disubstituted isoxazoles-treated Arabidopsis leaves. | ||
| Format: PDF | Size: 602.1 KB | Download |
Acknowledgments
This work was supported by the Ministry of National Education – Research Activity, UEFISCDI, Project PN-II-PT-PCCA-2013-4-0267 – SAFE-SEL, contract 186/2014. Access to research infrastructure developed in the “Petru Poni” Institute of Macromolecular Chemistry through the European Social Fund for Regional Development, Competitiveness Operational Programme Axis 1, Project InoMatPol (ID P_36_570, Contract 142/10.10.2016, cod MySMIS: 107464) is gratefully acknowledged.
References
-
Carlsen, L.; Döpp, D.; Döpp, H.; Duus, F.; Hartmann, H.; Lang-Fugmann, S.; Schulze, B.; Smalley, R. K.; Wakefield, B. J. In Houben-Weyl Methods in Organic Chemistry; Schaumann, E., Ed.; Georg Thieme Verlag: Stuttgart, 1992; Vol. E8a, pp 45–204.
Return to citation in text: [1] -
Banik, U.; Manna, K.; Ghosh, P. S.; Das, M. Int. J. Institutional Pharm. Life Sci. 2014, 4, 71–78.
Return to citation in text: [1] -
Lee, Y.-S.; Kim, B. H. Bioorg. Med. Chem. Lett. 2002, 12, 1395–1397. doi:10.1016/S0960-894X(02)00182-8
Return to citation in text: [1] -
Srivastava, S.; Bajpai, L. K.; Batra, S.; Bhaduri, A. P.; Maikhuri, J. P.; Gupta, G.; Dhar, J. D. Bioorg. Med. Chem. 1999, 7, 2607–2613. doi:10.1016/S0968-0896(99)00188-1
Return to citation in text: [1] -
Pruitt, J. R.; Pinto, D. J.; Estrella, M. J.; Bostrom, L. L.; Knabb, R. M.; Wong, P. C.; Wright, M. R.; Wexler, R. R. Bioorg. Med. Chem. Lett. 2000, 10, 685–689. doi:10.1016/S0960-894X(00)00097-4
Return to citation in text: [1] -
Nantermet, P. G.; Barrow, J. C.; Lundell, G. F.; Pellicore, J. M.; Rittle, K. E.; Young, M.; Freidinger, R. M.; Connolly, T. M.; Condra, C.; Karczewski, J.; Bednar, R. A.; Gaul, S. L.; Gould, R. J.; Prendergast, K.; Selnick, H. G. Bioorg. Med. Chem. Lett. 2002, 12, 319–323. doi:10.1016/S0960-894X(01)00745-4
Return to citation in text: [1] -
Batra, S.; Srinivasan, T.; Rastogi, S. K.; Kundu, B.; Patra, A.; Bhaduri, A. P.; Dixit, M. Bioorg. Med. Chem. Lett. 2002, 12, 1905–1908. doi:10.1016/S0960-894X(02)00333-5
Return to citation in text: [1] -
Batra, S.; Roy, A. K.; Patra, A.; Bhaduri, A. P.; Surin, W. R.; Raghavan, S. A. V.; Sharma, P.; Kapoor, K.; Dikshit, M. Bioorg. Med. Chem. 2004, 12, 2059–2077. doi:10.1016/j.bmc.2004.02.023
Return to citation in text: [1] -
Daidone, G.; Raffa, D.; Maggio, B.; Plescia, F.; Cutuli, V. M. C.; Mangano, N. G.; Caruso, A. Arch. Pharm. 1999, 332, 50–54. doi:10.1002/(SICI)1521-4184(19993)332:2<50::AID-ARDP50>3.0.CO;2-S
Return to citation in text: [1] [2] [3] -
Talley, J. J. Prog. Med. Chem. 1999, 36, 201–234. doi:10.1016/S0079-6468(08)70048-1
Return to citation in text: [1] -
Talley, J. J.; Brown, D. L.; Carter, J. S.; Graneto, M. J.; Koboldt, C. M.; Masferrer, J. L.; Perkins, W. E.; Rogers, R. S.; Shaffer, A. F.; Zhang, Y. Y.; Zweifel, B. S.; Seibert, K. J. Med. Chem. 2000, 43, 775–777. doi:10.1021/jm990577v
Return to citation in text: [1] [2] -
Giovannoni, M. P.; Vergelli, C.; Ghelardini, C.; Galeotti, N.; Bartolini, A.; Dal Piaz, V. J. Med. Chem. 2003, 46, 1055–1059. doi:10.1021/jm021057u
Return to citation in text: [1] -
Li, W.-T.; Hwang, D.-R.; Chen, C.-P.; Shen, C.-W.; Huang, C.-L.; Chen, T.-W.; Lin, C.-H.; Chang, Y.-L.; Chang, Y.-Y.; Lo, Y.-K.; Tseng, H.-Y.; Lin, C.-C.; Song, J.-S.; Chen, H.-C.; Chen, S.-J.; Wu, S.-H.; Chen, C.-T. J. Med. Chem. 2003, 46, 1706–1715. doi:10.1021/jm020471r
Return to citation in text: [1] -
Frølund, B.; Jørgensen, A. T.; Tagmose, L.; Stensbøl, T. B.; Vestergaard, H. T.; Engblom, C.; Kristiansen, U.; Sanchez, C.; Krogsgaard-Larsen, P.; Liljefors, T. J. Med. Chem. 2002, 45, 2454–2468. doi:10.1021/jm020027o
Return to citation in text: [1] -
Jung, H. K.; Doddareddy, M. R.; Cha, J. H.; Rhim, H.; Cho, Y. S.; Koh, H. Y.; Jung, B. Y.; Pae, A. N. Bioorg. Med. Chem. 2004, 12, 3965–3970. doi:10.1016/j.bmc.2004.06.011
Return to citation in text: [1] -
Monjil, M. S.; Shibata, Y.; Takemoto, D.; Kawakita, K. Nitric Oxide 2013, 29, 34–45. doi:10.1016/j.niox.2012.12.004
Return to citation in text: [1] -
Monjil, M. S.; Takemoto, D.; Kawakita, K. J. Gen. Plant Pathol. 2014, 80, 38–49. doi:10.1007/s10327-013-0493-z
Return to citation in text: [1] -
Wendehenne, D.; Pugin, A.; Klessig, D. F.; Durner, J. Trends Plant Sci. 2001, 6, 177–183. doi:10.1016/S1360-1385(01)01893-3
Return to citation in text: [1] -
Besson-Bard, A.; Pugin, A.; Wendehenne, D. Annu. Rev. Plant Biol. 2008, 59, 21–39. doi:10.1146/annurev.arplant.59.032607.092830
Return to citation in text: [1] -
Bandiera, T.; Grünanger, P.; Albini, F. M. J. Heterocycl. Chem. 1992, 29, 1423–1428. doi:10.1002/jhet.5570290609
Return to citation in text: [1] -
Cuadrado, P.; González-Nogal, A. M.; Valero, R. Tetrahedron 2002, 58, 4975–4980. doi:10.1016/S0040-4020(02)00386-1
Return to citation in text: [1] -
Vicentini, C. B.; Veronese, A. C.; Poli, T.; Guarneri, M.; Giori, P.; Ferretti, V. J. Heterocycl. Chem. 1990, 27, 1481–1484. doi:10.1002/jhet.5570270555
Return to citation in text: [1] -
Huisgen, R. In 1,3-Dipolar Cycloaddition Chemistry; Padwa, A., Ed.; Wiley: New York, 1984; Vol. 1, pp 1–176.
Return to citation in text: [1] [2] [3] -
Jaeger, V.; Colinas, P. A. Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products. In Chemistry of Heterocyclic Compounds; Padwa, A.; Pearson, W. H., Eds.; Wiley: Hoboken, 2002; Vol. 59, pp 363–461.
Return to citation in text: [1] [2] [3] -
Ajay Kumar, K.; Govindaraju, M.; Jayaroopa, P.; Vasanth Kumar, G. Int. J. Pharm., Chem. Biol. Sci. 2012, 3, 91–101.
Return to citation in text: [1] [2] [3] -
Just, G.; Dhal, K. Tetrahedron 1968, 24, 5251–5269. doi:10.1016/S0040-4020(01)96322-7
Return to citation in text: [1] -
Grundmann, C.; Dean, J. M. J. Org. Chem. 1965, 30, 2809–2812. doi:10.1021/jo01019a074
Return to citation in text: [1] -
Kim, J. N.; Ryu, E. K. Synth. Commun. 1990, 20, 1373–1377. doi:10.1080/00397919008052851
Return to citation in text: [1] -
Hassner, A.; Rai, K. M. L. Synthesis 1989, 57–59. doi:10.1055/s-1989-27152
Return to citation in text: [1] -
Grundmann, C.; Richter, R. J. Org. Chem. 1968, 33, 476–478. doi:10.1021/jo01265a120
Return to citation in text: [1] -
Liu, K.-C.; Shelton, B. R.; Howe, R. K. J. Org. Chem. 1980, 45, 3916–3918. doi:10.1021/jo01307a039
Return to citation in text: [1] -
Halling, K.; Torssell, K. B. G.; Hazell, R. G. Acta Chem. Scand. 1991, 45, 736–741. doi:10.3891/acta.chem.scand.45-0736
Return to citation in text: [1] -
Mukaiyama, T.; Hoshino, T. J. Am. Chem. Soc. 1960, 82, 5339–5342. doi:10.1021/ja01505a017
Return to citation in text: [1] -
Xu, J.; Hamme, A. T. Synlett 2008, 919–923. doi:10.1055/s-2008-1042906
Return to citation in text: [1] -
Tornøe, C. W.; Christiansen, C.; Meldal, M. J. Org. Chem. 2002, 67, 3057–3064. doi:10.1021/jo011148j
Return to citation in text: [1] -
Hansen, T. V.; Wu, P.; Fokin, V. V. J. Org. Chem. 2005, 70, 7761–7764. doi:10.1021/jo050163b
Return to citation in text: [1] -
Himo, T.; Lovell, T.; Hilgraf, R.; Rostovtsev, V. V.; Noodleman, L.; Sharpless, B. K.; Fokin, V. V. J. Am. Chem. Soc. 2005, 127, 210–216. doi:10.1021/ja0471525
Return to citation in text: [1] -
Georgescu, E.; Georgescu, F.; Popa, M. M.; Draghici, C.; Tarko, L.; Dumitrascu, F. ACS Comb. Sci. 2012, 14, 101–107. doi:10.1021/co2002125
Return to citation in text: [1] -
Georgescu, E.; Nicolescu, A.; Georgescu, F.; Teodorescu, F.; Marinescu, D.; Macsim, A.-M.; Deleanu, C. Beilstein J. Org. Chem. 2014, 10, 2377–2387. doi:10.3762/bjoc.10.248
Return to citation in text: [1] -
Popa, M. M.; Georgescu, E.; Caira, M. R.; Georgescu, F.; Draghici, C.; Stan, R.; Deleanu, C.; Dumitrascu, F. Beilstein J. Org. Chem. 2015, 11, 1079–1088. doi:10.3762/bjoc.11.121
Return to citation in text: [1] -
Georgescu, E.; Nicolescu, A.; Georgescu, F.; Shova, S.; Teodorescu, F.; Macsim, A.-M.; Deleanu, C. Synthesis 2015, 47, 1643–1655. doi:10.1055/s-0034-1380185
Return to citation in text: [1] -
Georgescu, E.; Nicolescu, A.; Georgescu, F.; Teodorescu, F.; Shova, S.; Marinoiu, A. T.; Dumitrascu, F.; Deleanu, C. Tetrahedron 2016, 72, 2507–2520. doi:10.1016/j.tet.2016.03.086
Return to citation in text: [1] -
Paraschivescu, C.C.; Matache, M.; Dobrotă, C.; Nicolescu, A.; Maxim, C.; Deleanu, C.; Fărcăşanu, I. C.; Hădade, N. D. J. Org. Chem. 2013, 78, 2670–2679. doi:10.1021/jo400023z
Return to citation in text: [1] -
Fahlbusch, K.-G.; Hammerschmidt, F.-J.; Panten, J.; Pickenhagen, W.; Schatkowski, D.; Bauer, K.; Garbe, D.; Surburg, H. "Flavors and Fragrances". Ullmann's Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, 2003.
Return to citation in text: [1] -
Gupta, O. P.; Nath, A.; Gupta, S. C.; Srivastava, T. N. Bull. Med. Ethnobot. Res. 1980, 1, 99–106.
Return to citation in text: [1] -
Rukachaisirikul, T.; Prabpai, S.; Champung, P.; Suksamrarn, A. Planta Med. 2002, 68, 853–855. doi:10.1055/s-2002-34410
Return to citation in text: [1] -
Deshpande, S. R.; Nagrale,, S. N.; Patil, M. V.; Chavan, P. S. Indian J. Pharm. Sci. 2015, 77, 24–33. doi:10.4103/0250-474X.151588
Return to citation in text: [1] -
Ren, J.; Yang, M.; Liu, H.; Cao, D.; Chen, D.; Li, L.; Tang, L.; He, J.; Chen, Y.-L.; Geng, M.; Xiong, B.; Shen, J. Org. Biomol. Chem. 2015, 13, 1531–1535. doi:10.1039/C4OB01865F
Return to citation in text: [1] -
Kamal, A.; Shaik, A. B.; Rao, B. B.; Khan, I.; Kumar, G. B.; Jain, N. Org. Biomol. Chem. 2015, 13, 10162–10178. doi:10.1039/C5OB01257K
Return to citation in text: [1] [2] -
Singh, P.; Sharma, P.; Bisetty, K.; Mahajan, M. P. ARKIVOC 2011, (x), 55–70. doi:10.3998/ark.5550190.0012.a05
Return to citation in text: [1] -
Pae, A. N.; Kim, H. Y.; Joo, H. J.; Kim, B. H.; Cho, Y. S.; Choi, K. I.; Choi, J. H.; Koh, H. Y. Bioorg. Med. Chem. Lett. 1999, 9, 2679–2684. doi:10.1016/S0960-894X(99)00473-4
Return to citation in text: [1] [2] -
Jiménez, R.; Peréz, L.; Tamariz, J.; Salgado, H. Heterocycles 1993, 35, 591–598. doi:10.3987/COM-92-S(T)70
Return to citation in text: [1] -
Bhosale, S.; Kurhade, S.; Prasad, U. V.; Palle, V. P.; Bhuniya, D. Tetrahedron Lett. 2009, 50, 3948–3951. doi:10.1016/j.tetlet.2009.04.073
Return to citation in text: [1] -
Inaba, T.; Haas, J.; Shiozaki, M.; Littman, N. M.; Yasue, K.; Andrews, S. W.; Sakai, A.; Fryer, A. M.; Matsuo, T.; Laird, E. R.; Suma, A.; Shinozaki, Y.; Hori, Y.; Imai, H.; Negoro, T. WO 2005058884 A2, June 30, 2005.
Return to citation in text: [1] -
Delledonne, M.; Xia, Y.; Dixon, R. A.; Lamb, C. Nature 1998, 394, 585–588. doi:10.1038/29087
Return to citation in text: [1] -
Kuźniak, E.; Skłodowska, M. Plant Sci. 2001, 160, 723–731. doi:10.1016/S0168-9452(00)00457-X
Return to citation in text: [1] -
Pnueli, L.; Liang, H.; Rozenberg, M.; Mittler, R. Plant J. 2003, 34, 187–203. doi:10.1046/j.1365-313X.2003.01715.x
Return to citation in text: [1] -
Bryan, N. S.; Grisham, M. B. Free Radical Biol. Med. 2007, 43, 645–657. doi:10.1016/j.freeradbiomed.2007.04.026
Return to citation in text: [1] -
Mur, L. A. J.; Mandon, J.; Persijn, S.; Cristescu, S. M.; Moshkov, I. E.; Novikova, G. V.; Hall, M. A.; Harren, F. J. M.; Hebelstrup, K. H.; Gupta, K. J. AoB Plants 2013, 5, No. pls052. doi:10.1093/aobpla/pls052
Return to citation in text: [1] -
Weigel, D.; Glazebrook, J. Arabidopsis: a laboratory manual, Cold Spring Harbor; Cold Spring Harbor Laboratory Press: New York, 2002; pp 143–170.
Return to citation in text: [1] -
Srivastava, N.; Gonugunta, V. K.; Puli, M. R.; Raghavendra, A. S. Planta 2009, 229, 757–765. doi:10.1007/s00425-008-0855-5
Return to citation in text: [1] -
Chen, X.; Zhong, Z.; Xu, Z.; Chen, L.; Wang, Y. Free Radical Res. 2010, 44, 587–604. doi:10.3109/10715761003709802
Return to citation in text: [1] -
Chen, Y.; Mo, H.-Z.; Hu, L.-B.; Li, Y.-Q.; Chen, J.; Yang, L.-F. PLoS One 2014, 9, e110901. doi:10.1371/journal.pone.0110901
Return to citation in text: [1] -
Kojima, H.; Nakatsubo, N.; Kikuchi, K.; Kawahara, S.; Kirino, Y.; Nagoshi, H.; Hirata, Y.; Nagano, T. Anal. Chem. 1998, 70, 2446–2453. doi:10.1021/ac9801723
Return to citation in text: [1] -
Kojima, H.; Sakurai, K.; Kikuchi, K.; Kawahara, S.; Kirino, Y.; Nagoshi, H.; Hirata, Y.; Nagano, T. Chem. Pharm. Bull. 1998, 46, 373–375. doi:10.1248/cpb.46.373
Return to citation in text: [1] -
Kojima, H.; Urano, Y.; Kikuchi, K.; Higuchi, T.; Hirata, Y.; Nagano, T. Angew. Chem., Int. Ed. Engl. 1999, 21, 3209–3212. doi:10.1002/(SICI)1521-3773(19991102)38:21<3209::AID-ANIE3209>3.0.CO;2-6
Return to citation in text: [1] -
Kolbert, Z.; Petô, A.; Lehotai, N.; Feigl, G.; Ördög, A.; Erdei, L. Acta Biol. (Szeged) 2012, 56, 37–41.
Return to citation in text: [1]
| 49. | Kamal, A.; Shaik, A. B.; Rao, B. B.; Khan, I.; Kumar, G. B.; Jain, N. Org. Biomol. Chem. 2015, 13, 10162–10178. doi:10.1039/C5OB01257K |
| 50. | Singh, P.; Sharma, P.; Bisetty, K.; Mahajan, M. P. ARKIVOC 2011, (x), 55–70. doi:10.3998/ark.5550190.0012.a05 |
| 51. | Pae, A. N.; Kim, H. Y.; Joo, H. J.; Kim, B. H.; Cho, Y. S.; Choi, K. I.; Choi, J. H.; Koh, H. Y. Bioorg. Med. Chem. Lett. 1999, 9, 2679–2684. doi:10.1016/S0960-894X(99)00473-4 |
| 1. | Carlsen, L.; Döpp, D.; Döpp, H.; Duus, F.; Hartmann, H.; Lang-Fugmann, S.; Schulze, B.; Smalley, R. K.; Wakefield, B. J. In Houben-Weyl Methods in Organic Chemistry; Schaumann, E., Ed.; Georg Thieme Verlag: Stuttgart, 1992; Vol. E8a, pp 45–204. |
| 2. | Banik, U.; Manna, K.; Ghosh, P. S.; Das, M. Int. J. Institutional Pharm. Life Sci. 2014, 4, 71–78. |
| 10. | Talley, J. J. Prog. Med. Chem. 1999, 36, 201–234. doi:10.1016/S0079-6468(08)70048-1 |
| 11. | Talley, J. J.; Brown, D. L.; Carter, J. S.; Graneto, M. J.; Koboldt, C. M.; Masferrer, J. L.; Perkins, W. E.; Rogers, R. S.; Shaffer, A. F.; Zhang, Y. Y.; Zweifel, B. S.; Seibert, K. J. Med. Chem. 2000, 43, 775–777. doi:10.1021/jm990577v |
| 22. | Vicentini, C. B.; Veronese, A. C.; Poli, T.; Guarneri, M.; Giori, P.; Ferretti, V. J. Heterocycl. Chem. 1990, 27, 1481–1484. doi:10.1002/jhet.5570270555 |
| 56. | Kuźniak, E.; Skłodowska, M. Plant Sci. 2001, 160, 723–731. doi:10.1016/S0168-9452(00)00457-X |
| 57. | Pnueli, L.; Liang, H.; Rozenberg, M.; Mittler, R. Plant J. 2003, 34, 187–203. doi:10.1046/j.1365-313X.2003.01715.x |
| 9. | Daidone, G.; Raffa, D.; Maggio, B.; Plescia, F.; Cutuli, V. M. C.; Mangano, N. G.; Caruso, A. Arch. Pharm. 1999, 332, 50–54. doi:10.1002/(SICI)1521-4184(19993)332:2<50::AID-ARDP50>3.0.CO;2-S |
| 23. | Huisgen, R. In 1,3-Dipolar Cycloaddition Chemistry; Padwa, A., Ed.; Wiley: New York, 1984; Vol. 1, pp 1–176. |
| 24. | Jaeger, V.; Colinas, P. A. Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products. In Chemistry of Heterocyclic Compounds; Padwa, A.; Pearson, W. H., Eds.; Wiley: Hoboken, 2002; Vol. 59, pp 363–461. |
| 25. | Ajay Kumar, K.; Govindaraju, M.; Jayaroopa, P.; Vasanth Kumar, G. Int. J. Pharm., Chem. Biol. Sci. 2012, 3, 91–101. |
| 58. | Bryan, N. S.; Grisham, M. B. Free Radical Biol. Med. 2007, 43, 645–657. doi:10.1016/j.freeradbiomed.2007.04.026 |
| 59. | Mur, L. A. J.; Mandon, J.; Persijn, S.; Cristescu, S. M.; Moshkov, I. E.; Novikova, G. V.; Hall, M. A.; Harren, F. J. M.; Hebelstrup, K. H.; Gupta, K. J. AoB Plants 2013, 5, No. pls052. doi:10.1093/aobpla/pls052 |
| 5. | Pruitt, J. R.; Pinto, D. J.; Estrella, M. J.; Bostrom, L. L.; Knabb, R. M.; Wong, P. C.; Wright, M. R.; Wexler, R. R. Bioorg. Med. Chem. Lett. 2000, 10, 685–689. doi:10.1016/S0960-894X(00)00097-4 |
| 6. | Nantermet, P. G.; Barrow, J. C.; Lundell, G. F.; Pellicore, J. M.; Rittle, K. E.; Young, M.; Freidinger, R. M.; Connolly, T. M.; Condra, C.; Karczewski, J.; Bednar, R. A.; Gaul, S. L.; Gould, R. J.; Prendergast, K.; Selnick, H. G. Bioorg. Med. Chem. Lett. 2002, 12, 319–323. doi:10.1016/S0960-894X(01)00745-4 |
| 7. | Batra, S.; Srinivasan, T.; Rastogi, S. K.; Kundu, B.; Patra, A.; Bhaduri, A. P.; Dixit, M. Bioorg. Med. Chem. Lett. 2002, 12, 1905–1908. doi:10.1016/S0960-894X(02)00333-5 |
| 8. | Batra, S.; Roy, A. K.; Patra, A.; Bhaduri, A. P.; Surin, W. R.; Raghavan, S. A. V.; Sharma, P.; Kapoor, K.; Dikshit, M. Bioorg. Med. Chem. 2004, 12, 2059–2077. doi:10.1016/j.bmc.2004.02.023 |
| 9. | Daidone, G.; Raffa, D.; Maggio, B.; Plescia, F.; Cutuli, V. M. C.; Mangano, N. G.; Caruso, A. Arch. Pharm. 1999, 332, 50–54. doi:10.1002/(SICI)1521-4184(19993)332:2<50::AID-ARDP50>3.0.CO;2-S |
| 20. | Bandiera, T.; Grünanger, P.; Albini, F. M. J. Heterocycl. Chem. 1992, 29, 1423–1428. doi:10.1002/jhet.5570290609 |
| 54. | Inaba, T.; Haas, J.; Shiozaki, M.; Littman, N. M.; Yasue, K.; Andrews, S. W.; Sakai, A.; Fryer, A. M.; Matsuo, T.; Laird, E. R.; Suma, A.; Shinozaki, Y.; Hori, Y.; Imai, H.; Negoro, T. WO 2005058884 A2, June 30, 2005. |
| 3. | Lee, Y.-S.; Kim, B. H. Bioorg. Med. Chem. Lett. 2002, 12, 1395–1397. doi:10.1016/S0960-894X(02)00182-8 |
| 4. | Srivastava, S.; Bajpai, L. K.; Batra, S.; Bhaduri, A. P.; Maikhuri, J. P.; Gupta, G.; Dhar, J. D. Bioorg. Med. Chem. 1999, 7, 2607–2613. doi:10.1016/S0968-0896(99)00188-1 |
| 21. | Cuadrado, P.; González-Nogal, A. M.; Valero, R. Tetrahedron 2002, 58, 4975–4980. doi:10.1016/S0040-4020(02)00386-1 |
| 55. | Delledonne, M.; Xia, Y.; Dixon, R. A.; Lamb, C. Nature 1998, 394, 585–588. doi:10.1038/29087 |
| 14. | Frølund, B.; Jørgensen, A. T.; Tagmose, L.; Stensbøl, T. B.; Vestergaard, H. T.; Engblom, C.; Kristiansen, U.; Sanchez, C.; Krogsgaard-Larsen, P.; Liljefors, T. J. Med. Chem. 2002, 45, 2454–2468. doi:10.1021/jm020027o |
| 16. | Monjil, M. S.; Shibata, Y.; Takemoto, D.; Kawakita, K. Nitric Oxide 2013, 29, 34–45. doi:10.1016/j.niox.2012.12.004 |
| 17. | Monjil, M. S.; Takemoto, D.; Kawakita, K. J. Gen. Plant Pathol. 2014, 80, 38–49. doi:10.1007/s10327-013-0493-z |
| 49. | Kamal, A.; Shaik, A. B.; Rao, B. B.; Khan, I.; Kumar, G. B.; Jain, N. Org. Biomol. Chem. 2015, 13, 10162–10178. doi:10.1039/C5OB01257K |
| 13. | Li, W.-T.; Hwang, D.-R.; Chen, C.-P.; Shen, C.-W.; Huang, C.-L.; Chen, T.-W.; Lin, C.-H.; Chang, Y.-L.; Chang, Y.-Y.; Lo, Y.-K.; Tseng, H.-Y.; Lin, C.-C.; Song, J.-S.; Chen, H.-C.; Chen, S.-J.; Wu, S.-H.; Chen, C.-T. J. Med. Chem. 2003, 46, 1706–1715. doi:10.1021/jm020471r |
| 18. | Wendehenne, D.; Pugin, A.; Klessig, D. F.; Durner, J. Trends Plant Sci. 2001, 6, 177–183. doi:10.1016/S1360-1385(01)01893-3 |
| 19. | Besson-Bard, A.; Pugin, A.; Wendehenne, D. Annu. Rev. Plant Biol. 2008, 59, 21–39. doi:10.1146/annurev.arplant.59.032607.092830 |
| 53. | Bhosale, S.; Kurhade, S.; Prasad, U. V.; Palle, V. P.; Bhuniya, D. Tetrahedron Lett. 2009, 50, 3948–3951. doi:10.1016/j.tetlet.2009.04.073 |
| 12. | Giovannoni, M. P.; Vergelli, C.; Ghelardini, C.; Galeotti, N.; Bartolini, A.; Dal Piaz, V. J. Med. Chem. 2003, 46, 1055–1059. doi:10.1021/jm021057u |
| 51. | Pae, A. N.; Kim, H. Y.; Joo, H. J.; Kim, B. H.; Cho, Y. S.; Choi, K. I.; Choi, J. H.; Koh, H. Y. Bioorg. Med. Chem. Lett. 1999, 9, 2679–2684. doi:10.1016/S0960-894X(99)00473-4 |
| 9. | Daidone, G.; Raffa, D.; Maggio, B.; Plescia, F.; Cutuli, V. M. C.; Mangano, N. G.; Caruso, A. Arch. Pharm. 1999, 332, 50–54. doi:10.1002/(SICI)1521-4184(19993)332:2<50::AID-ARDP50>3.0.CO;2-S |
| 11. | Talley, J. J.; Brown, D. L.; Carter, J. S.; Graneto, M. J.; Koboldt, C. M.; Masferrer, J. L.; Perkins, W. E.; Rogers, R. S.; Shaffer, A. F.; Zhang, Y. Y.; Zweifel, B. S.; Seibert, K. J. Med. Chem. 2000, 43, 775–777. doi:10.1021/jm990577v |
| 15. | Jung, H. K.; Doddareddy, M. R.; Cha, J. H.; Rhim, H.; Cho, Y. S.; Koh, H. Y.; Jung, B. Y.; Pae, A. N. Bioorg. Med. Chem. 2004, 12, 3965–3970. doi:10.1016/j.bmc.2004.06.011 |
| 52. | Jiménez, R.; Peréz, L.; Tamariz, J.; Salgado, H. Heterocycles 1993, 35, 591–598. doi:10.3987/COM-92-S(T)70 |
| 30. | Grundmann, C.; Richter, R. J. Org. Chem. 1968, 33, 476–478. doi:10.1021/jo01265a120 |
| 31. | Liu, K.-C.; Shelton, B. R.; Howe, R. K. J. Org. Chem. 1980, 45, 3916–3918. doi:10.1021/jo01307a039 |
| 32. | Halling, K.; Torssell, K. B. G.; Hazell, R. G. Acta Chem. Scand. 1991, 45, 736–741. doi:10.3891/acta.chem.scand.45-0736 |
| 23. | Huisgen, R. In 1,3-Dipolar Cycloaddition Chemistry; Padwa, A., Ed.; Wiley: New York, 1984; Vol. 1, pp 1–176. |
| 24. | Jaeger, V.; Colinas, P. A. Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products. In Chemistry of Heterocyclic Compounds; Padwa, A.; Pearson, W. H., Eds.; Wiley: Hoboken, 2002; Vol. 59, pp 363–461. |
| 25. | Ajay Kumar, K.; Govindaraju, M.; Jayaroopa, P.; Vasanth Kumar, G. Int. J. Pharm., Chem. Biol. Sci. 2012, 3, 91–101. |
| 60. | Weigel, D.; Glazebrook, J. Arabidopsis: a laboratory manual, Cold Spring Harbor; Cold Spring Harbor Laboratory Press: New York, 2002; pp 143–170. |
| 26. | Just, G.; Dhal, K. Tetrahedron 1968, 24, 5251–5269. doi:10.1016/S0040-4020(01)96322-7 |
| 27. | Grundmann, C.; Dean, J. M. J. Org. Chem. 1965, 30, 2809–2812. doi:10.1021/jo01019a074 |
| 28. | Kim, J. N.; Ryu, E. K. Synth. Commun. 1990, 20, 1373–1377. doi:10.1080/00397919008052851 |
| 29. | Hassner, A.; Rai, K. M. L. Synthesis 1989, 57–59. doi:10.1055/s-1989-27152 |
| 61. | Srivastava, N.; Gonugunta, V. K.; Puli, M. R.; Raghavendra, A. S. Planta 2009, 229, 757–765. doi:10.1007/s00425-008-0855-5 |
| 62. | Chen, X.; Zhong, Z.; Xu, Z.; Chen, L.; Wang, Y. Free Radical Res. 2010, 44, 587–604. doi:10.3109/10715761003709802 |
| 63. | Chen, Y.; Mo, H.-Z.; Hu, L.-B.; Li, Y.-Q.; Chen, J.; Yang, L.-F. PLoS One 2014, 9, e110901. doi:10.1371/journal.pone.0110901 |
| 44. | Fahlbusch, K.-G.; Hammerschmidt, F.-J.; Panten, J.; Pickenhagen, W.; Schatkowski, D.; Bauer, K.; Garbe, D.; Surburg, H. "Flavors and Fragrances". Ullmann's Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, 2003. |
| 45. | Gupta, O. P.; Nath, A.; Gupta, S. C.; Srivastava, T. N. Bull. Med. Ethnobot. Res. 1980, 1, 99–106. |
| 46. | Rukachaisirikul, T.; Prabpai, S.; Champung, P.; Suksamrarn, A. Planta Med. 2002, 68, 853–855. doi:10.1055/s-2002-34410 |
| 47. | Deshpande, S. R.; Nagrale,, S. N.; Patil, M. V.; Chavan, P. S. Indian J. Pharm. Sci. 2015, 77, 24–33. doi:10.4103/0250-474X.151588 |
| 48. | Ren, J.; Yang, M.; Liu, H.; Cao, D.; Chen, D.; Li, L.; Tang, L.; He, J.; Chen, Y.-L.; Geng, M.; Xiong, B.; Shen, J. Org. Biomol. Chem. 2015, 13, 1531–1535. doi:10.1039/C4OB01865F |
| 36. | Hansen, T. V.; Wu, P.; Fokin, V. V. J. Org. Chem. 2005, 70, 7761–7764. doi:10.1021/jo050163b |
| 37. | Himo, T.; Lovell, T.; Hilgraf, R.; Rostovtsev, V. V.; Noodleman, L.; Sharpless, B. K.; Fokin, V. V. J. Am. Chem. Soc. 2005, 127, 210–216. doi:10.1021/ja0471525 |
| 38. | Georgescu, E.; Georgescu, F.; Popa, M. M.; Draghici, C.; Tarko, L.; Dumitrascu, F. ACS Comb. Sci. 2012, 14, 101–107. doi:10.1021/co2002125 |
| 39. | Georgescu, E.; Nicolescu, A.; Georgescu, F.; Teodorescu, F.; Marinescu, D.; Macsim, A.-M.; Deleanu, C. Beilstein J. Org. Chem. 2014, 10, 2377–2387. doi:10.3762/bjoc.10.248 |
| 40. | Popa, M. M.; Georgescu, E.; Caira, M. R.; Georgescu, F.; Draghici, C.; Stan, R.; Deleanu, C.; Dumitrascu, F. Beilstein J. Org. Chem. 2015, 11, 1079–1088. doi:10.3762/bjoc.11.121 |
| 41. | Georgescu, E.; Nicolescu, A.; Georgescu, F.; Shova, S.; Teodorescu, F.; Macsim, A.-M.; Deleanu, C. Synthesis 2015, 47, 1643–1655. doi:10.1055/s-0034-1380185 |
| 42. | Georgescu, E.; Nicolescu, A.; Georgescu, F.; Teodorescu, F.; Shova, S.; Marinoiu, A. T.; Dumitrascu, F.; Deleanu, C. Tetrahedron 2016, 72, 2507–2520. doi:10.1016/j.tet.2016.03.086 |
| 43. | Paraschivescu, C.C.; Matache, M.; Dobrotă, C.; Nicolescu, A.; Maxim, C.; Deleanu, C.; Fărcăşanu, I. C.; Hădade, N. D. J. Org. Chem. 2013, 78, 2670–2679. doi:10.1021/jo400023z |
| 35. | Tornøe, C. W.; Christiansen, C.; Meldal, M. J. Org. Chem. 2002, 67, 3057–3064. doi:10.1021/jo011148j |
| 33. | Mukaiyama, T.; Hoshino, T. J. Am. Chem. Soc. 1960, 82, 5339–5342. doi:10.1021/ja01505a017 |
| 64. | Kojima, H.; Nakatsubo, N.; Kikuchi, K.; Kawahara, S.; Kirino, Y.; Nagoshi, H.; Hirata, Y.; Nagano, T. Anal. Chem. 1998, 70, 2446–2453. doi:10.1021/ac9801723 |
| 65. | Kojima, H.; Sakurai, K.; Kikuchi, K.; Kawahara, S.; Kirino, Y.; Nagoshi, H.; Hirata, Y.; Nagano, T. Chem. Pharm. Bull. 1998, 46, 373–375. doi:10.1248/cpb.46.373 |
| 66. | Kojima, H.; Urano, Y.; Kikuchi, K.; Higuchi, T.; Hirata, Y.; Nagano, T. Angew. Chem., Int. Ed. Engl. 1999, 21, 3209–3212. doi:10.1002/(SICI)1521-3773(19991102)38:21<3209::AID-ANIE3209>3.0.CO;2-6 |
| 67. | Kolbert, Z.; Petô, A.; Lehotai, N.; Feigl, G.; Ördög, A.; Erdei, L. Acta Biol. (Szeged) 2012, 56, 37–41. |
| 23. | Huisgen, R. In 1,3-Dipolar Cycloaddition Chemistry; Padwa, A., Ed.; Wiley: New York, 1984; Vol. 1, pp 1–176. |
| 24. | Jaeger, V.; Colinas, P. A. Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products. In Chemistry of Heterocyclic Compounds; Padwa, A.; Pearson, W. H., Eds.; Wiley: Hoboken, 2002; Vol. 59, pp 363–461. |
| 25. | Ajay Kumar, K.; Govindaraju, M.; Jayaroopa, P.; Vasanth Kumar, G. Int. J. Pharm., Chem. Biol. Sci. 2012, 3, 91–101. |
© 2017 Oancea et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)