Abstract
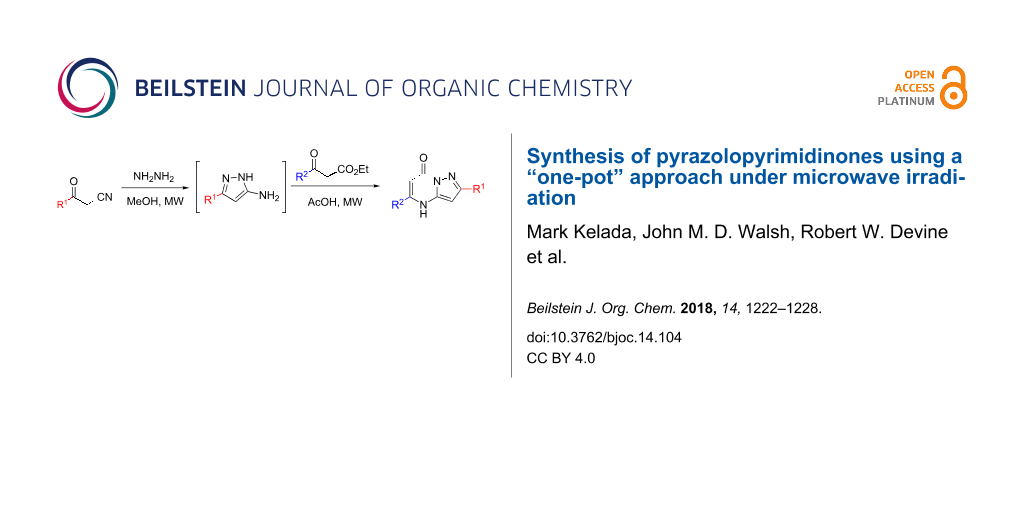
A simple one-pot method for the microwave-assisted synthesis of substituted pyrazolo[1,5-a]pyrimidinones, a core scaffold in many bioactive and pharmaceutically relevant compounds, has been established. A variety of substituents was tolerated at the 2 and 5 positions, including functionalized aryls, heterocycles, and alkyl groups.
Graphical Abstract
Introduction
The pyrazolo[1,5-a]pyrimidinone is a fused nitrogen-containing heterocyclic system and is of interest due to its role as a basic core scaffold in many bioactive and pharmaceutically relevant compounds, as well as its structural similarity to purine [1-4]. Pyrazolo[1,5-a]pyrimidinone derivatives have found use in the battle against several illnesses including cancer [5], viral infections [6-8], obesity [9], and cystic fibrosis [10] (Figure 1). It is these pharmacological properties, coupled with the pharmaceutical and fine chemical industries interest in synthetic processes that utilize cleaner and more efficient technology, which has stimulated the search for improved synthetic procedures for their generation.
Figure 1: Bioactive pyrazolo[1,5-a]pyrimidinones.
Figure 1: Bioactive pyrazolo[1,5-a]pyrimidinones.
Consequently, strategies for the synthesis of compounds of value, such as the pyrazolo[1,5-a]pyrimidinones, that employ one-pot syntheses and/or processes have been encouraged and have become a major focus for many synthetic chemists [11]. As such, a convenient and general method of preparing the scaffold would be of significant interest to the pharmaceutical industry. Herein, we report such a strategy for the simple synthesis of functionalized pyrazolo[1,5-a]pyrimidinones that employs a one-pot microwave-assisted approach.
Findings
A number of synthetic approaches have been developed for the synthesis of privileged nitrogen-fused bicyclic systems such as the pyrazolo[3,4-b]pyridines, pyrazolo[1,5-a]pyrimidines and pyrazolo[1,5-a]pyrimidinones [12-18]. The majority of methods used in the generation of the pyrazolo[1,5-a]pyrimidinones employ a two-step process, which first requires the synthesis and isolation of the intermediate 5-aminopyrazoles followed by their reaction with an appropriate β-dicarbonyl compound [1-4,19-29]. A very recent and excellent review by Aggarwal et al. covers the use and versatility of 5-aminopyrazoles in the synthesis of a range of pyrazoloazines [30].
As part of a study into the development of novel biologically active compounds based on the pyrazolopyrimidinone scaffold, we sought to develop a simple one-pot synthesis of the nitrogen-fused bicyclic system. In order to establish reaction conditions for this one-pot synthesis, we began by seeking general reaction conditions for the microwave-assisted generation of the intermediate 5-aminopyrazoles. Once established, this methodology could be expanded to generate a one-pot synthesis of pyrazolo[1,5-a]pyrimidinones.
Typical methods for preparing 5-aminopyrazoles require conventional heating of the appropriate β-ketonitrile in the presence of a hydrazine [1-4,19-29], with Rao et al. [31] and Bagley et al. [32] reporting microwave-assisted protocols, the former requiring an acid catalyst. In order to conduct a solvent screen we chose β-ketonitrile 1a as a model substrate and reacted it with hydrazine, employing a constant temperature of 150 °C and a reaction time of 5 min. Results from the solvent screen can be found in Table 1, with high isolated yields of the product 5-aminopyrazole 2a in all cases. As expected, a significant improvement in reaction time was observed under microwave conditions in comparison with conventional heating (Table 1, entries 7, 8 and 9). Methanol was subsequently selected as the solvent of choice, due to the higher yield generated and its lower cost when compared with acetonitrile. Temperature and time were then varied with the best combination of temperature and time found to be 150 °C for 5 min (Table 2, entry 5).
Table 1: Solvent screen for the synthesis of 5-aminopyrazole 2a.a
|
|
|||
| Entry | Heating method | Solvent | Yieldb % |
| 1 | MW | DCM | 82 |
| 2 | MW | toluene | 83 |
| 3 | MW | Pet ether | 88 |
| 4 | MW | EtOAc | 92 |
| 5 | MW | MeCN | 96 |
| 6 | MW | petroleum ether/MeOH 9:1 | 99 |
| 7 | MW | MeOH | 99 |
| 8c | convent. | MeOH | 60 |
| 9d | convent. | MeOH | 30 |
aReaction conditions: ketonitrile (0.9 mmol, 1.0 equiv), hydrazine (1.2 mmol, 1.3 equiv), solvent (1 mL) were heated under microwave conditions (100 W, 150 °C) for 5 min. bIsolated yield. cReaction conditions: ketonitrile (0.9 mmol, 1.0 equiv), hydrazine (1.2 mmol, 1.3 equiv), solvent (1 mL) were heated under conventional heating conditions at reflux for 17 h. dReaction conditions: ketonitrile (0.9 mmol, 1.0 equiv), hydrazine (1.2 mmol, 1.3 equiv), solvent (1 mL) were heated under conventional heating conditions at reflux for 5 min.
Table 2: Temperature and time variations for the synthesis of 5-aminopyrazole 2a.a
| Entry | T (°C) | Time (min) | Yieldb % |
|---|---|---|---|
| 1 | 120 | 40 | 84 |
| 2 | 130 | 30 | 96 |
| 3 | 130 | 5 | 53 |
| 4 | 140 | 20 | 98 |
| 5 | 150 | 5 | 99 |
aReaction conditions: ketonitrile (0.9 mmol, 1.0 equiv), hydrazine (1.2 mmol, 1.3 equiv), MeOH (1 mL) were heated under microwave conditions (100 W). bIsolated yield.
A substrate scope study showed that a variety of substituents is tolerated. Aromatic groups with electron-withdrawing and electron-donating substituents at the ortho, meta and para positions were successfully explored (Scheme 1). The heterocyclic furan and thiophene substituents allowed generation of the desired 5-aminopyrazoles in 75% and 81% yields, respectively (Scheme 1). Alkyl groups appeared to be less well tolerated, where the corresponding alkyl-substituted 5-aminopyrazoles were isolated in lower yields.
Scheme 1: Synthesis of 5-aminopyrazoles. Reaction conditions: ketonitrile (2.0 mmol, 1.0 equiv), hydrazine (2.6 mmol, 1.3 equiv), MeOH (1 mL) were heated under microwave conditions (100 W, 150 °C) for 5 min. Isolated yields.
Scheme 1: Synthesis of 5-aminopyrazoles. Reaction conditions: ketonitrile (2.0 mmol, 1.0 equiv), hydrazine (2...
With a convenient and general microwave-assisted method for the synthesis of the 5-aminopyrazoles in hand, we next focused our attention on its application to the one-pot synthesis of pyrazolo[1,5-a]pyrimidinones.
The synthesis of pyrazolo[1,5-a]pyrimidinone 3a was chosen as the model reaction (Scheme 2). Initial reaction conditions were chosen to match the already developed microwave-assisted synthesis of the 5-aminopyrazoles. A solution of the β-ketonitrile in methanol was treated with hydrazine and heated to 150 °C under microwave irradiation for 5 min. The β-ketoester and acetic acid were then simply added to the pot and the reaction heated at the same temperature, 150 °C, under microwave irradiation for a further 2 h. The target pyrazolo[1,5-a]pyrimidinone was subsequently isolated in an overall yield of 52%. Once more, a significant improvement was observed using microwave conditions in comparison with conventional heating.
Scheme 2: One-pot synthesis of pyrazolo[1,5-a]pyrimidinones. aReaction conditions: ketonitrile (0.9 mmol, 1.0 equiv), hydrazine (1.2 mmol, 1.3 equiv), MeOH (1 mL) were heated under microwave conditions (100 W, 150 °C) for 5 min followed by the addition of AcOH (0.5 mmol, 0.6 equiv) and ketoester (0.9 mmol, 1.0 equiv) and heated under microwave conditions (100 W, 150 °C) for a further 2 h. bIsolated yield. cReactions conditions: isolated aminopyrazole (0.9 mmol, 1.0 equiv), AcOH (0.5 mmol, 0.6 equiv), ketoester (0.9 mmol, 1.0 equiv) in methanol (2 mL) were heated using conventional heating conditions at reflux for 18 h. dReactions conditions: isolated aminopyrazole (0.9 mmol, 1.0 equiv), AcOH (0.5 mmol, 0.6 equiv), ketoester (0.9 mmol, 1.0 equiv) in methanol (2 mL) were heated using conventional heating conditions at reflux for 2 h.
Scheme 2: One-pot synthesis of pyrazolo[1,5-a]pyrimidinones. aReaction conditions: ketonitrile (0.9 mmol, 1.0...
When a mixture of previously isolated 5-aminopyrazole 2a, β-ketoester, and acetic acid were heated under conventional refluxing conditions for 18 h, the product pyrazolo[1,5-a]pyrimidinone 3a could only be isolated in a 25% yield. As expected, heating the reaction mixture for 2 h at reflux gave a lower isolated yield of 11%. A one-pot procedure under conventional refluxing conditions was also carried out in direct comparison with the microwave method, i.e., a solution of the β-ketonitrile in methanol was treated with hydrazine and refluxed for 5 min. The β-ketoester and acetic acid were then added to the pot and the reaction refluxed for a further 2 h. A complex mixture resulted consisting mostly of starting β-ketonitrile.
The superior performance of the microwave reaction, in terms of yield and reaction time, could result from the higher temperature and pressure achieved. The substrate scope for the one-pot reaction was then explored, with variations of both R1 and R2 groups at the pyrazolo[1,5-a]pyrimidinone core (Scheme 2).
Pyrazolo[1,5-a]pyrimidinones containing aromatic groups with electron-withdrawing and electron-donating substituents at the ortho, meta and para positions were generated, including the multi-substituted 3n, as were those with heterocyclic furyl and thienyl substituents (Scheme 2). Alkyl-substituted pyrazolo[1,5-a]pyrimidinones were also synthesized using this one-pot method (Scheme 2).
The structures of the pyrazolo[1,5-a]pyrimidinones were characterized using 1H and 13C NMR spectral data, HRMS, and IR spectroscopy. In addition, an X-ray crystal structure was obtained for pyrazolo[1,5-a]pyrimidinone 3m and is shown in Figure 2.
![[1860-5397-14-104-2]](/bjoc/content/figures/1860-5397-14-104-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: X-ray crystal structure of pyrazolo[1,5-a]pyrimidinone 3m with ellipsoids at 50% probability.
Figure 2: X-ray crystal structure of pyrazolo[1,5-a]pyrimidinone 3m with ellipsoids at 50% probability.
Conclusion
In conclusion, we have developed a new facile method for the one-pot microwave-assisted synthesis of substituted pyrazolo[1,5-a]pyrimidinones, a core scaffold in many bioactive and pharmaceutically relevant compounds. A variety of functional groups was tolerated at the 2 and 5 positions, including functionalized aryls, heterocycles, and alkyl groups. Furthermore, an efficient and general microwave-assisted synthesis of versatile 5-aminopyrazoles is reported.
Supporting Information
CCDC-1588686 contains the crystallographic data for 3m. The data can be obtained from The Cambridge Crystallographic Data Centre (CCDC) via http://www.ccdc.cam.ac.uk/data_request/cif.
| Supporting Information File 1: Experimental section, NMR spectra of all synthesized compounds and crystallographic data of compound 3m. | ||
| Format: PDF | Size: 3.9 MB | Download |
References
-
Qi, J.; Zhang, F.; Mi, Y.; Fu, Y.; Xu, W.; Zhang, D.; Wu, Y.; Du, X.; Jia, Q.; Wang, K.; Zhang, H. Eur. J. Med. Chem. 2011, 46, 934–943. doi:10.1016/j.ejmech.2011.01.010
Return to citation in text: [1] [2] [3] -
Gehling, V. S.; Bellon, S. F.; Harmange, J.-C.; LeBlanc, Y.; Poy, F.; Odate, S.; Buker, S.; Lan, F.; Arora, S.; Williamson, K. E.; Sandy, P.; Cummings, R. T.; Bailey, C. M.; Bergeron, L.; Mao, W.; Gustafson, A.; Liu, Y.; VanderPorten, E.; Audia, J. E.; Trojer, P.; Albrecht, B. K. Bioorg. Med. Chem. Lett. 2016, 26, 4350–4354. doi:10.1016/j.bmcl.2016.07.026
Return to citation in text: [1] [2] [3] -
Liang, J.; Zhang, B.; Labadie, S.; Ortwine, D. F.; Vinogradova, M.; Kiefer, J. R.; Gehling, V. S.; Harmange, J.-C.; Cummings, R.; Lai, T.; Liao, J.; Zheng, X.; Liu, Y.; Gustafson, A.; Van der Porten, E.; Mao, W.; Liederer, B. M.; Deshmukh, G.; Classon, M.; Trojer, P.; Dragovich, P. S.; Murray, L. Bioorg. Med. Chem. Lett. 2016, 26, 4036–4041. doi:10.1016/j.bmcl.2016.06.078
Return to citation in text: [1] [2] [3] -
Senga, K.; Novinson, T.; Wilson, H. R.; Robins, R. K. J. Med. Chem. 1981, 24, 610–613. doi:10.1021/jm00137a023
Return to citation in text: [1] [2] [3] -
Lin, H.; Moore, M. L.; Qu, J.; Rivero, R. A.; Tedesco, R.; Yu, H.; Luengo, J. I. Pyrazolopyrimidine derivative as PI3 Kinase inhibitors. WO Patent WO2013028263, Feb 28, 2013.
Return to citation in text: [1] -
Naidu, B. N.; Patel, M.; D’Andrea, S.; Zheng, Z. B.; Connolly, T. P.; Langley, D. R.; Peese, K.; Wang, Z.; Walker, M. A.; Kadow, J. F. Inhibitors of human immunodeficiency virus replication. WO Patent WO2014028384, Feb 20, 2014.
Return to citation in text: [1] -
Babaoglu, K.; Boojamra, C. G.; Eisenberg, E. J.; Hui, H. C.; Mackman, R. L.; Parrish, J. P.; Sangi, M.; Saunders, O. L.; Siegel, D.; Sperandio, D.; Yang, H. Pyrazolo[1,5-a]pyrimidines as antiviral agents. WO Patent WO2011/163518, Dec 29, 2011.
Return to citation in text: [1] -
Gu, B.; Block, T.; Cuconati, A. Small molecule inhibitors against west nile virus replication. WO Patent WO2007005541, Jan 11, 2007.
Return to citation in text: [1] -
Griffith, D. A. Pyrazolo[1,5-a]pyrimidin-7-one compounds and uses thereof. WO Patent WO2005103052, Nov 3, 2005.
Return to citation in text: [1] -
Binch, H.; Grootenhuis, P. D. J.; Pierce, A.; Fanning, L. T. D. Modulators of cystic fibrosis transmembrane conductance regulator. WO Patent WO2009076593, June 18, 2009.
Return to citation in text: [1] -
Volla, C. M. R.; Atodiresei, I.; Rueping, M. Chem. Rev. 2014, 114, 2390–2431. doi:10.1021/cr400215u
and references cited therein.
Return to citation in text: [1] -
El-borai, M. A.; Rizk, H. F.; Abd-Aal, M. F.; El-Deeb, I. Y. Eur. J. Med. Chem. 2012, 48, 92–96. doi:10.1016/j.ejmech.2011.11.038
Return to citation in text: [1] -
Quiroga, J.; Portilla, J.; Abonía, R.; Insuasty, B.; Nogueras, M.; Cobo, J. Tetrahedron Lett. 2008, 49, 6254–6256. doi:10.1016/j.tetlet.2008.08.044
Return to citation in text: [1] -
Quiroga, J.; Portilla, J.; Abonía, R.; Insuasty, B.; Nogueras, M.; Cobo, J. Tetrahedron Lett. 2007, 48, 6352–6355. doi:10.1016/j.tetlet.2007.07.041
Return to citation in text: [1] -
Hédou, D.; Deau, E.; Dubouilh-Benard, C.; Sanselme, M.; Martinet, A.; Chosson, E.; Levacher, V.; Besson, T. Eur. J. Org. Chem. 2013, 7533–7545. doi:10.1002/ejoc.201301014
Return to citation in text: [1] -
Loidreau, Y.; Dubouilh-Benard, C.; Marchand, P.; Nourrisson, M.-R.; Duflos, M.; Buquet, C.; Corbière, C.; Besson, T. J. Heterocycl. Chem. 2013, 50, 1187–1197. doi:10.1002/jhet.1716
Return to citation in text: [1] -
Rimland, J.; Dunne, A.; Hunjan, S. S.; Sasse, R.; Uings, I.; Montanari, D.; Caivano, M.; Shah, P.; Standing, D.; Gray, D.; Brown, D.; Cairns, W.; Trump, R.; Smith, P. W.; Bertheleme, N.; D’Alessandro, P.; Gul, S.; Vimal, M.; Smith, D. N.; Watson, S. P. Bioorg. Med. Chem. Lett. 2010, 20, 2340–2343. doi:10.1016/j.bmcl.2010.01.133
Return to citation in text: [1] -
Besson, T.; Brain, C. T. Heterocyclic Chemistry Using Microwave-Assisted Approaches. In Microwave Assisted Organic Synthesis; Tierney, J. P.; Lidström, P., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2005. doi:10.1002/9781444305548.ch3
Return to citation in text: [1] -
Wustrow, D. J.; Capiris, T.; Rubin, R.; Knobelsdorf, J. A.; Akunne, H.; Duff Davis, M.; MacKenzie, R.; Pugsley, T. A.; Zoski, K. T.; Heffner, T. G.; Wise, L. D. Bioorg. Med. Chem. Lett. 1998, 8, 2067–2070. doi:10.1016/S0960-894X(98)00372-2
Return to citation in text: [1] [2] -
Nam, N. L.; Grandberg, I. I.; Sorokin, V. I. Chem. Heterocycl. Compd. 2003, 39, 1210–1212. doi:10.1023/B:COHC.0000008268.94940.5c
Return to citation in text: [1] [2] -
Gavrin, L. K.; Lee, A.; Provencher, B. A.; Massefski, W. W.; Huhn, S. D.; Ciszewski, G. M.; Cole, D. C.; McKew, J. C. J. Org. Chem. 2007, 72, 1043–1046. doi:10.1021/jo062120g
Return to citation in text: [1] [2] -
Brigance, R. P.; Meng, W.; Fura, A.; Harrity, T.; Wang, A.; Zahler, R.; Kirby, M. S.; Hamann, L. G. Bioorg. Med. Chem. Lett. 2010, 20, 4395–4398. doi:10.1016/j.bmcl.2010.06.063
Return to citation in text: [1] [2] -
Portilla, J.; Quiroga, J.; Nogueras, M.; Cobo, J. Tetrahedron 2012, 68, 988–994. doi:10.1016/j.tet.2011.12.001
Return to citation in text: [1] [2] -
Hwang, J. Y.; Windisch, M. P.; Jo, S.; Kim, K.; Kong, S.; Kim, H. C.; Kim, S.; Kim, H.; Lee, M. E.; Kim, Y.; Choi, J.; Park, D.-S.; Park, E.; Kwon, J.; Nam, J.; Ahn, S.; Cechetto, J.; Kim, J.; Liuzzi, M.; No, Z.; Lee, J. Bioorg. Med. Chem. Lett. 2012, 22, 7297–7301. doi:10.1016/j.bmcl.2012.10.123
Return to citation in text: [1] [2] -
Soares de Melo, C.; Feng, T.-S.; van der Westhuyzen, R.; Gessner, R. K.; Street, L. J.; Morgans, G. L.; Warner, D. F.; Moosa, A.; Naran, K.; Lawrence, N.; Boshoff, H. I. M.; Barry, C. E., III; Harris, C. J.; Gordon, R.; Chibale, K. Bioorg. Med. Chem. 2015, 23, 7240–7250. doi:10.1016/j.bmc.2015.10.021
Return to citation in text: [1] [2] -
Azeredo, L. F. S. P.; Coutinho, J. P.; Jabor, V. A. P.; Feliciano, P. R.; Nonato, M. C.; Kaiser, C. R.; Menezes, C. M. S.; Hammes, A. S. O.; Caffarena, E. R.; Hoelz, L. V. B.; de Souza, N. B.; Pereira, G. A. N.; Cerávolo, I. P.; Krettli, A. U.; Boechat, N. Eur. J. Med. Chem. 2017, 126, 72–83. doi:10.1016/j.ejmech.2016.09.073
Return to citation in text: [1] [2] -
Albrecht, B. K.; Bellon, S. F.; Gehling, V. S.; Harmange, J. C.; Leblanc, Y.; Liang, J.; Magnuson, S.; Tsui, V.; Zhang, B. Therapeutic compounds and uses thereof. U.S. Patent US20150065522, March 5, 2015.
Return to citation in text: [1] [2] -
Karthikeyan, C.; Lee, C.; Moore, J.; Mittal, R.; Suswam, E. A.; Abbott, K. L.; Pondugula, S. R.; Manne, U.; Narayanan, N. K.; Trivedi, P.; Tiwari, A. K. Bioorg. Med. Chem. 2015, 23, 602–611. doi:10.1016/j.bmc.2014.11.043
Return to citation in text: [1] [2] -
Poreba, K.; Wietrzyk, J.; Opolski, A. Acta Pol. Pharm. 2006, 63, 189–194.
Return to citation in text: [1] [2] -
Aggarwal, R.; Kumar, S. Beilstein J. Org. Chem. 2018, 14, 203–242. doi:10.3762/bjoc.14.15
and references cited therein.
Return to citation in text: [1] -
Rao, B. R.; Venkateshwarlu, G.; Sunitha, P.; Kumar, K. S. Org. Chem.: Indian J. 2008, 4, 235–239.
Return to citation in text: [1] -
Bagley, M. C.; Davis, T.; Dix, M. C.; Widdowson, C. S.; Kipling, D. Org. Biomol. Chem. 2006, 4, 4158–4164. doi:10.1039/b611493h
Return to citation in text: [1]
| 1. | Qi, J.; Zhang, F.; Mi, Y.; Fu, Y.; Xu, W.; Zhang, D.; Wu, Y.; Du, X.; Jia, Q.; Wang, K.; Zhang, H. Eur. J. Med. Chem. 2011, 46, 934–943. doi:10.1016/j.ejmech.2011.01.010 |
| 2. | Gehling, V. S.; Bellon, S. F.; Harmange, J.-C.; LeBlanc, Y.; Poy, F.; Odate, S.; Buker, S.; Lan, F.; Arora, S.; Williamson, K. E.; Sandy, P.; Cummings, R. T.; Bailey, C. M.; Bergeron, L.; Mao, W.; Gustafson, A.; Liu, Y.; VanderPorten, E.; Audia, J. E.; Trojer, P.; Albrecht, B. K. Bioorg. Med. Chem. Lett. 2016, 26, 4350–4354. doi:10.1016/j.bmcl.2016.07.026 |
| 3. | Liang, J.; Zhang, B.; Labadie, S.; Ortwine, D. F.; Vinogradova, M.; Kiefer, J. R.; Gehling, V. S.; Harmange, J.-C.; Cummings, R.; Lai, T.; Liao, J.; Zheng, X.; Liu, Y.; Gustafson, A.; Van der Porten, E.; Mao, W.; Liederer, B. M.; Deshmukh, G.; Classon, M.; Trojer, P.; Dragovich, P. S.; Murray, L. Bioorg. Med. Chem. Lett. 2016, 26, 4036–4041. doi:10.1016/j.bmcl.2016.06.078 |
| 4. | Senga, K.; Novinson, T.; Wilson, H. R.; Robins, R. K. J. Med. Chem. 1981, 24, 610–613. doi:10.1021/jm00137a023 |
| 10. | Binch, H.; Grootenhuis, P. D. J.; Pierce, A.; Fanning, L. T. D. Modulators of cystic fibrosis transmembrane conductance regulator. WO Patent WO2009076593, June 18, 2009. |
| 9. | Griffith, D. A. Pyrazolo[1,5-a]pyrimidin-7-one compounds and uses thereof. WO Patent WO2005103052, Nov 3, 2005. |
| 6. | Naidu, B. N.; Patel, M.; D’Andrea, S.; Zheng, Z. B.; Connolly, T. P.; Langley, D. R.; Peese, K.; Wang, Z.; Walker, M. A.; Kadow, J. F. Inhibitors of human immunodeficiency virus replication. WO Patent WO2014028384, Feb 20, 2014. |
| 7. | Babaoglu, K.; Boojamra, C. G.; Eisenberg, E. J.; Hui, H. C.; Mackman, R. L.; Parrish, J. P.; Sangi, M.; Saunders, O. L.; Siegel, D.; Sperandio, D.; Yang, H. Pyrazolo[1,5-a]pyrimidines as antiviral agents. WO Patent WO2011/163518, Dec 29, 2011. |
| 8. | Gu, B.; Block, T.; Cuconati, A. Small molecule inhibitors against west nile virus replication. WO Patent WO2007005541, Jan 11, 2007. |
| 5. | Lin, H.; Moore, M. L.; Qu, J.; Rivero, R. A.; Tedesco, R.; Yu, H.; Luengo, J. I. Pyrazolopyrimidine derivative as PI3 Kinase inhibitors. WO Patent WO2013028263, Feb 28, 2013. |
| 30. |
Aggarwal, R.; Kumar, S. Beilstein J. Org. Chem. 2018, 14, 203–242. doi:10.3762/bjoc.14.15
and references cited therein. |
| 31. | Rao, B. R.; Venkateshwarlu, G.; Sunitha, P.; Kumar, K. S. Org. Chem.: Indian J. 2008, 4, 235–239. |
| 1. | Qi, J.; Zhang, F.; Mi, Y.; Fu, Y.; Xu, W.; Zhang, D.; Wu, Y.; Du, X.; Jia, Q.; Wang, K.; Zhang, H. Eur. J. Med. Chem. 2011, 46, 934–943. doi:10.1016/j.ejmech.2011.01.010 |
| 2. | Gehling, V. S.; Bellon, S. F.; Harmange, J.-C.; LeBlanc, Y.; Poy, F.; Odate, S.; Buker, S.; Lan, F.; Arora, S.; Williamson, K. E.; Sandy, P.; Cummings, R. T.; Bailey, C. M.; Bergeron, L.; Mao, W.; Gustafson, A.; Liu, Y.; VanderPorten, E.; Audia, J. E.; Trojer, P.; Albrecht, B. K. Bioorg. Med. Chem. Lett. 2016, 26, 4350–4354. doi:10.1016/j.bmcl.2016.07.026 |
| 3. | Liang, J.; Zhang, B.; Labadie, S.; Ortwine, D. F.; Vinogradova, M.; Kiefer, J. R.; Gehling, V. S.; Harmange, J.-C.; Cummings, R.; Lai, T.; Liao, J.; Zheng, X.; Liu, Y.; Gustafson, A.; Van der Porten, E.; Mao, W.; Liederer, B. M.; Deshmukh, G.; Classon, M.; Trojer, P.; Dragovich, P. S.; Murray, L. Bioorg. Med. Chem. Lett. 2016, 26, 4036–4041. doi:10.1016/j.bmcl.2016.06.078 |
| 4. | Senga, K.; Novinson, T.; Wilson, H. R.; Robins, R. K. J. Med. Chem. 1981, 24, 610–613. doi:10.1021/jm00137a023 |
| 19. | Wustrow, D. J.; Capiris, T.; Rubin, R.; Knobelsdorf, J. A.; Akunne, H.; Duff Davis, M.; MacKenzie, R.; Pugsley, T. A.; Zoski, K. T.; Heffner, T. G.; Wise, L. D. Bioorg. Med. Chem. Lett. 1998, 8, 2067–2070. doi:10.1016/S0960-894X(98)00372-2 |
| 20. | Nam, N. L.; Grandberg, I. I.; Sorokin, V. I. Chem. Heterocycl. Compd. 2003, 39, 1210–1212. doi:10.1023/B:COHC.0000008268.94940.5c |
| 21. | Gavrin, L. K.; Lee, A.; Provencher, B. A.; Massefski, W. W.; Huhn, S. D.; Ciszewski, G. M.; Cole, D. C.; McKew, J. C. J. Org. Chem. 2007, 72, 1043–1046. doi:10.1021/jo062120g |
| 22. | Brigance, R. P.; Meng, W.; Fura, A.; Harrity, T.; Wang, A.; Zahler, R.; Kirby, M. S.; Hamann, L. G. Bioorg. Med. Chem. Lett. 2010, 20, 4395–4398. doi:10.1016/j.bmcl.2010.06.063 |
| 23. | Portilla, J.; Quiroga, J.; Nogueras, M.; Cobo, J. Tetrahedron 2012, 68, 988–994. doi:10.1016/j.tet.2011.12.001 |
| 24. | Hwang, J. Y.; Windisch, M. P.; Jo, S.; Kim, K.; Kong, S.; Kim, H. C.; Kim, S.; Kim, H.; Lee, M. E.; Kim, Y.; Choi, J.; Park, D.-S.; Park, E.; Kwon, J.; Nam, J.; Ahn, S.; Cechetto, J.; Kim, J.; Liuzzi, M.; No, Z.; Lee, J. Bioorg. Med. Chem. Lett. 2012, 22, 7297–7301. doi:10.1016/j.bmcl.2012.10.123 |
| 25. | Soares de Melo, C.; Feng, T.-S.; van der Westhuyzen, R.; Gessner, R. K.; Street, L. J.; Morgans, G. L.; Warner, D. F.; Moosa, A.; Naran, K.; Lawrence, N.; Boshoff, H. I. M.; Barry, C. E., III; Harris, C. J.; Gordon, R.; Chibale, K. Bioorg. Med. Chem. 2015, 23, 7240–7250. doi:10.1016/j.bmc.2015.10.021 |
| 26. | Azeredo, L. F. S. P.; Coutinho, J. P.; Jabor, V. A. P.; Feliciano, P. R.; Nonato, M. C.; Kaiser, C. R.; Menezes, C. M. S.; Hammes, A. S. O.; Caffarena, E. R.; Hoelz, L. V. B.; de Souza, N. B.; Pereira, G. A. N.; Cerávolo, I. P.; Krettli, A. U.; Boechat, N. Eur. J. Med. Chem. 2017, 126, 72–83. doi:10.1016/j.ejmech.2016.09.073 |
| 27. | Albrecht, B. K.; Bellon, S. F.; Gehling, V. S.; Harmange, J. C.; Leblanc, Y.; Liang, J.; Magnuson, S.; Tsui, V.; Zhang, B. Therapeutic compounds and uses thereof. U.S. Patent US20150065522, March 5, 2015. |
| 28. | Karthikeyan, C.; Lee, C.; Moore, J.; Mittal, R.; Suswam, E. A.; Abbott, K. L.; Pondugula, S. R.; Manne, U.; Narayanan, N. K.; Trivedi, P.; Tiwari, A. K. Bioorg. Med. Chem. 2015, 23, 602–611. doi:10.1016/j.bmc.2014.11.043 |
| 29. | Poreba, K.; Wietrzyk, J.; Opolski, A. Acta Pol. Pharm. 2006, 63, 189–194. |
| 32. | Bagley, M. C.; Davis, T.; Dix, M. C.; Widdowson, C. S.; Kipling, D. Org. Biomol. Chem. 2006, 4, 4158–4164. doi:10.1039/b611493h |
| 12. | El-borai, M. A.; Rizk, H. F.; Abd-Aal, M. F.; El-Deeb, I. Y. Eur. J. Med. Chem. 2012, 48, 92–96. doi:10.1016/j.ejmech.2011.11.038 |
| 13. | Quiroga, J.; Portilla, J.; Abonía, R.; Insuasty, B.; Nogueras, M.; Cobo, J. Tetrahedron Lett. 2008, 49, 6254–6256. doi:10.1016/j.tetlet.2008.08.044 |
| 14. | Quiroga, J.; Portilla, J.; Abonía, R.; Insuasty, B.; Nogueras, M.; Cobo, J. Tetrahedron Lett. 2007, 48, 6352–6355. doi:10.1016/j.tetlet.2007.07.041 |
| 15. | Hédou, D.; Deau, E.; Dubouilh-Benard, C.; Sanselme, M.; Martinet, A.; Chosson, E.; Levacher, V.; Besson, T. Eur. J. Org. Chem. 2013, 7533–7545. doi:10.1002/ejoc.201301014 |
| 16. | Loidreau, Y.; Dubouilh-Benard, C.; Marchand, P.; Nourrisson, M.-R.; Duflos, M.; Buquet, C.; Corbière, C.; Besson, T. J. Heterocycl. Chem. 2013, 50, 1187–1197. doi:10.1002/jhet.1716 |
| 17. | Rimland, J.; Dunne, A.; Hunjan, S. S.; Sasse, R.; Uings, I.; Montanari, D.; Caivano, M.; Shah, P.; Standing, D.; Gray, D.; Brown, D.; Cairns, W.; Trump, R.; Smith, P. W.; Bertheleme, N.; D’Alessandro, P.; Gul, S.; Vimal, M.; Smith, D. N.; Watson, S. P. Bioorg. Med. Chem. Lett. 2010, 20, 2340–2343. doi:10.1016/j.bmcl.2010.01.133 |
| 18. | Besson, T.; Brain, C. T. Heterocyclic Chemistry Using Microwave-Assisted Approaches. In Microwave Assisted Organic Synthesis; Tierney, J. P.; Lidström, P., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2005. doi:10.1002/9781444305548.ch3 |
| 11. |
Volla, C. M. R.; Atodiresei, I.; Rueping, M. Chem. Rev. 2014, 114, 2390–2431. doi:10.1021/cr400215u
and references cited therein. |
| 1. | Qi, J.; Zhang, F.; Mi, Y.; Fu, Y.; Xu, W.; Zhang, D.; Wu, Y.; Du, X.; Jia, Q.; Wang, K.; Zhang, H. Eur. J. Med. Chem. 2011, 46, 934–943. doi:10.1016/j.ejmech.2011.01.010 |
| 2. | Gehling, V. S.; Bellon, S. F.; Harmange, J.-C.; LeBlanc, Y.; Poy, F.; Odate, S.; Buker, S.; Lan, F.; Arora, S.; Williamson, K. E.; Sandy, P.; Cummings, R. T.; Bailey, C. M.; Bergeron, L.; Mao, W.; Gustafson, A.; Liu, Y.; VanderPorten, E.; Audia, J. E.; Trojer, P.; Albrecht, B. K. Bioorg. Med. Chem. Lett. 2016, 26, 4350–4354. doi:10.1016/j.bmcl.2016.07.026 |
| 3. | Liang, J.; Zhang, B.; Labadie, S.; Ortwine, D. F.; Vinogradova, M.; Kiefer, J. R.; Gehling, V. S.; Harmange, J.-C.; Cummings, R.; Lai, T.; Liao, J.; Zheng, X.; Liu, Y.; Gustafson, A.; Van der Porten, E.; Mao, W.; Liederer, B. M.; Deshmukh, G.; Classon, M.; Trojer, P.; Dragovich, P. S.; Murray, L. Bioorg. Med. Chem. Lett. 2016, 26, 4036–4041. doi:10.1016/j.bmcl.2016.06.078 |
| 4. | Senga, K.; Novinson, T.; Wilson, H. R.; Robins, R. K. J. Med. Chem. 1981, 24, 610–613. doi:10.1021/jm00137a023 |
| 19. | Wustrow, D. J.; Capiris, T.; Rubin, R.; Knobelsdorf, J. A.; Akunne, H.; Duff Davis, M.; MacKenzie, R.; Pugsley, T. A.; Zoski, K. T.; Heffner, T. G.; Wise, L. D. Bioorg. Med. Chem. Lett. 1998, 8, 2067–2070. doi:10.1016/S0960-894X(98)00372-2 |
| 20. | Nam, N. L.; Grandberg, I. I.; Sorokin, V. I. Chem. Heterocycl. Compd. 2003, 39, 1210–1212. doi:10.1023/B:COHC.0000008268.94940.5c |
| 21. | Gavrin, L. K.; Lee, A.; Provencher, B. A.; Massefski, W. W.; Huhn, S. D.; Ciszewski, G. M.; Cole, D. C.; McKew, J. C. J. Org. Chem. 2007, 72, 1043–1046. doi:10.1021/jo062120g |
| 22. | Brigance, R. P.; Meng, W.; Fura, A.; Harrity, T.; Wang, A.; Zahler, R.; Kirby, M. S.; Hamann, L. G. Bioorg. Med. Chem. Lett. 2010, 20, 4395–4398. doi:10.1016/j.bmcl.2010.06.063 |
| 23. | Portilla, J.; Quiroga, J.; Nogueras, M.; Cobo, J. Tetrahedron 2012, 68, 988–994. doi:10.1016/j.tet.2011.12.001 |
| 24. | Hwang, J. Y.; Windisch, M. P.; Jo, S.; Kim, K.; Kong, S.; Kim, H. C.; Kim, S.; Kim, H.; Lee, M. E.; Kim, Y.; Choi, J.; Park, D.-S.; Park, E.; Kwon, J.; Nam, J.; Ahn, S.; Cechetto, J.; Kim, J.; Liuzzi, M.; No, Z.; Lee, J. Bioorg. Med. Chem. Lett. 2012, 22, 7297–7301. doi:10.1016/j.bmcl.2012.10.123 |
| 25. | Soares de Melo, C.; Feng, T.-S.; van der Westhuyzen, R.; Gessner, R. K.; Street, L. J.; Morgans, G. L.; Warner, D. F.; Moosa, A.; Naran, K.; Lawrence, N.; Boshoff, H. I. M.; Barry, C. E., III; Harris, C. J.; Gordon, R.; Chibale, K. Bioorg. Med. Chem. 2015, 23, 7240–7250. doi:10.1016/j.bmc.2015.10.021 |
| 26. | Azeredo, L. F. S. P.; Coutinho, J. P.; Jabor, V. A. P.; Feliciano, P. R.; Nonato, M. C.; Kaiser, C. R.; Menezes, C. M. S.; Hammes, A. S. O.; Caffarena, E. R.; Hoelz, L. V. B.; de Souza, N. B.; Pereira, G. A. N.; Cerávolo, I. P.; Krettli, A. U.; Boechat, N. Eur. J. Med. Chem. 2017, 126, 72–83. doi:10.1016/j.ejmech.2016.09.073 |
| 27. | Albrecht, B. K.; Bellon, S. F.; Gehling, V. S.; Harmange, J. C.; Leblanc, Y.; Liang, J.; Magnuson, S.; Tsui, V.; Zhang, B. Therapeutic compounds and uses thereof. U.S. Patent US20150065522, March 5, 2015. |
| 28. | Karthikeyan, C.; Lee, C.; Moore, J.; Mittal, R.; Suswam, E. A.; Abbott, K. L.; Pondugula, S. R.; Manne, U.; Narayanan, N. K.; Trivedi, P.; Tiwari, A. K. Bioorg. Med. Chem. 2015, 23, 602–611. doi:10.1016/j.bmc.2014.11.043 |
| 29. | Poreba, K.; Wietrzyk, J.; Opolski, A. Acta Pol. Pharm. 2006, 63, 189–194. |
© 2018 Kelada et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)