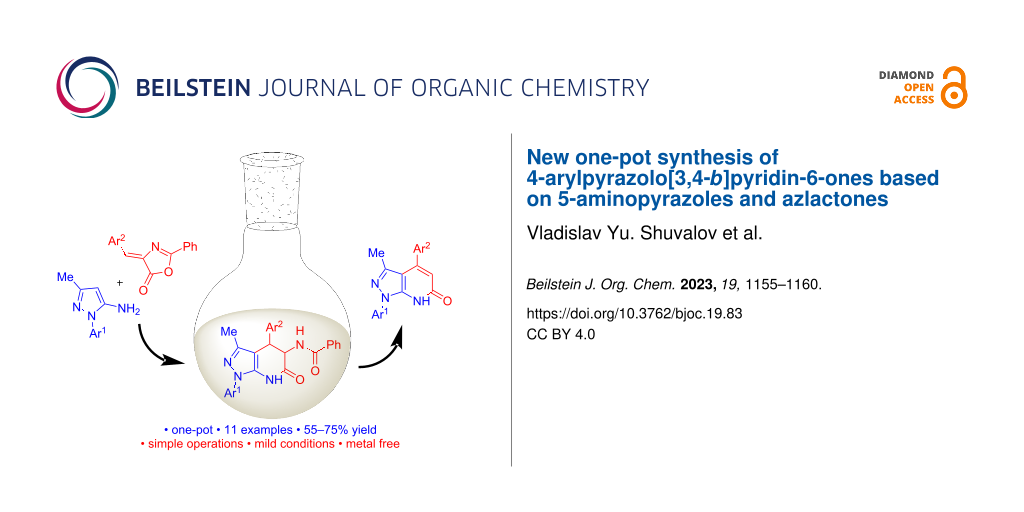
Abstract
An effective one-pot strategy was developed for the synthesis of 4-arylpyrazolo[3,4-b]pyridin-6-ones from pyrazolo[3,4-b]pyridin-6-ones, obtained by reacting 5-aminopyrazoles with 4-arylidene-2-phenyloxazol-5(4H)-ones (azlactones) under solvent-free conditions, through subsequent elimination of a benzamide molecule in a superbasic medium (t-BuOK/DMSO). The fluorescent properties of the synthesized compounds were studied. 4-Arylpyrazolo[3,4-b]pyridin-6-ones luminesce in the region of 409–440 nm with a quantum yield of 0.09–0.23 when irradiated with UV light.
Graphical Abstract
Introduction
The pyrazolo[3,4-b]pyridine scaffold is present in many biologically active compounds [1-12]. Among them, 4-aryl-substituted derivatives should be distinguished, exhibiting antiviral [13] and anti-inflammatory properties [14], being modulators of estrogen-related receptor alpha [15], JAK1 kinase inhibitor [16], GSK3 [17] and GyrB [8] inhibitors (Figure 1).
Figure 1: Biologically active 4-arylpyrazolo[3,4-b]pyridin-6-ones.
Figure 1: Biologically active 4-arylpyrazolo[3,4-b]pyridin-6-ones.
Despite the high demand, their synthesis methods are few (Scheme 1). To obtain 4-arylpyrazolo[3,4-b]pyridin-6-ones, the only known one-step method is most often used, including the acid-catalyzed condensation of aminopyrazoles with ketoesters [1,16,18] (method A). Its significant disadvantage is the low yields of the target products (11–60%). Yields are also low in two-stage synthesis methods. The first of them is based on the three-component condensation of aminopyrazoles, Meldrum's acid, and aromatic aldehydes, followed by the oxidation of the intermediate with DDQ [13,16,19] (method B). The second one includes the reaction of an aromatic aldehyde with thioglycolic acid and aminopyrazole, followed by the extrusion of sulfur from the resulting thiazepine [20] (method C). The three-stage synthesis of 4-arylpyrazolo[3,4-b]pyridin-6-ones, involving the preparation of 3-aryl-N-(1H-pyrazol-5-yl)propiolamides (method D), also leads to the formation of the target products with low yields [21]. Therefore, the development of a new effective method for the preparation of 4-arylpyrazolo[3,4-b]pyridin-6-ones is an urgent task.
Scheme 1: Methods for the synthesis of 4-arylpyrazolo[3,4-b]pyridin-6-ones.
Scheme 1: Methods for the synthesis of 4-arylpyrazolo[3,4-b]pyridin-6-ones.
Results and Discussion
One of the rational approaches to the synthesis of fused pyridine derivatives is based on the domino reaction of enamines with azlactones [22-30]. We have previously reported a plausible mechanism of such reactions [22,25]. 1H-Pyrazol-5-amines also enter into similar transformations with azlactones in various solvents. The yields of tetrahydro-1H-pyrazolo[3,4-b]pyridones 3 obtained by this method vary widely [31-33]. Solvent-free reactions are convenient from both economic and environmental points of view. We obtained tetrahydro-1H-pyrazolo[3,4-b]pyridinone 3a by heating 5-aminopyrazole 1 with azlactone 2a in the absence of solvent at 150 °C in 62% yield (Table 1). For compound 3a, the possibility of benzamide elimination was studied. The benzamide fragment is a poor leaving group; however, in a superbasic medium, we were able to eliminate this group in compound 3a. In order to select optimal synthesis conditions, we heated compound 3a in DMSO at temperatures from 90 to 150 °C for 1.5, 3.5 and 6 h in the presence of KOH or t-BuOK (Table 1).
Table 1: Optimization of reaction conditionsa.
|
|
|||
| entry | conditions (I) | conditions (II) | yield of 4a (%)b |
| 1 | 150 °C, 40 min, (62%)b | KOH (1 equiv), DMSO, 90 °C, 6 h | traces |
| 2 | KOH (1 equiv), DMSO, 150 °C, 6 h | 58с | |
| 3 | KOH (1.5 equiv), DMSO, 150 °C, 3.5 h | 63 | |
| 4 | t-BuOK (1.5 equiv), DMSO, 150 °C, 1.5 h | 81 | |
| 5d | 150 °C, 40 min then t-BuOK (1.5 equiv), DMSO, 150 °C, 1.5 h | 73 | |
| 6d | DMSO, 150 °C, 2.5 h then t-BuOK (1.5 equiv), 150 °C, 1.5 h | 60 | |
aReaction conditions: 1 (2 mmol), 2a (2 mmol). bIsolated yield after column chromatography. сCompound 3а was additionally isolated in 6% yield. dOne-pot method.
The best yield of 4-phenylpyrazolo[3,4-b]pyridin-6-one 4а (81%) was achieved at 150 °C in DMSO containing 1.5 equiv of t-BuOK for 1.5 h. Obviously, the preparation of 4-phenylpyrazolo[3,4-b]pyridin-6-one 4а could be carried out as one-pot synthesis, without isolation of the intermediate dihydro derivative 3а. In this case, the solvent (DMSO) could be added at the stage of obtaining dihydro derivative 3a or introduced into the reaction together with t-BuOK. We have explored both variants. When intermediate 3a was obtained under solvent-free conditions followed by the addition of t-BuOK in DMSO, the yield of pyrazolo[3,4-b]pyridin-6-one 4a was higher (73%, Table 1, entry 5) than when performing the reaction in a solvent (60%, Table 1, entry 6). Therefore, this procedure was used for the synthesis of compounds 4b–i, 9a, 10a. The yields of pyrazolo[3,4-b]pyridin-6-ones 4a–i, 9a, 10a obtained by this method are in the range of 55–75% (Scheme 2).
Scheme 2: One-pot synthesis of 4-arylpyrazolo[3,4-b]pyridin-6-ones 4a–i, 9a, and 10a.
Scheme 2: One-pot synthesis of 4-arylpyrazolo[3,4-b]pyridin-6-ones 4a–i, 9a, and 10a.
It should be noted that for compounds containing an electron-donating substituent in the C-4 position, such as 4-methoxyphenyl- (4c), 3,4-dimethoxyphenyl- (4d), 3,4,5-trimethoxyphenyl- (4e), 2-furyl- (4h) and 2-thienyl- (4i), the product yields are reduced to 55–60% (Scheme 2).
All the compounds obtained are colorless crystalline substances. When dissolved, they produce colorless solutions exhibiting distinct fluorescent properties with blue emission when exposed to UV light. We recorded absorption and fluorescence spectra of ethanolic solutions of compounds 4a–i, 9a, and 10a. The emission and absorption spectra of all the compounds differ slightly from each other. Their spectral parameters are presented in Table 2.
Table 2: Data of absorption and fluorescence spectra of compounds 4a–i, 9a, and 10a.a
| Compound | UV–vis | Photoluminescence | ||||
| maxλabs, nm |
ε, 103,
M–1·cm–1 (λ, nm) |
λex, nm | maxλem, nm |
Stokes shift,
nm; eV |
Quantum yield Φflb | |
| 4a | 260; 302 |
30.3 ± 0.7
(260) |
300; 320 | 419 | 117; 1.15 | 0.22 ± 0.01 |
| 4b | 260; 302 |
38.3 ± 0.7
(260) |
300; 320 | 428 | 126; 1.21 | 0.23 ± 0.01 |
| 4c | 262; 302 |
22.2 ± 0.8
(262) |
300; 320 | 409 | 107; 1.07 | 0.16 ± 0.01 |
| 4d | 260; 301 |
35.1 ± 0.9
(260) |
300; 320 | 414 | 113; 1.12 | 0.15 ± 0.01 |
| 4e | 262; 301 |
22.7 ± 0.9
(262) |
300; 320 | 416 | 115; 1.14 | 0.18 ± 0.01 |
| 4f | 260; 302 |
27.6 ± 0.8
(260) |
300; 320 | 415 | 113; 1.12 | 0.20 ± 0.01 |
| 4g | 261; 300 |
41.5 ± 0.9
(261) |
300; 320 | 411 | 111; 1.12 | 0.20 ± 0.01 |
| 4h | 265; 305 |
32.4 ± 1.0
(265) |
300; 310 | 421 | 116; 1.12 | 0.23 ± 0.01 |
| 4i | 263; 301 |
26.2 ± 0.8
(263) |
300; 310 | 431 | 130; 1.24 | 0.09 ± 0.00 |
| 9a | 259; 303 |
40.0 ± 0.9
(261) |
305 | 433 | 130; 1.23 | 0.19 ± 0.01 |
| 10a | 261; 288 |
34.9 ± 0.5
(259) |
290 | 440 | 152; 1.49 | 0.11 ± 0.01 |
aIn EtOH solution, c = 1.0·10−5 mol·L−1. bQuantum yield determined relative to quinine sulfate standard in 0.5 M H2SO4 (Фf = 0.546).
In the UV spectra of ethanolic solutions of compounds 4a–i, 9a, and 10a, a band with a maximum at 260–265 nm is observed, which has a shoulder at 300–305 nm. These signals seem to correspond to π–π* and n–π* transitions. In the luminescence spectra of compounds 4a–i, 9a, and 10a, there is one broadened band with an emission maximum at 409–440 nm (Figure 2). Their diluted alcohol solutions luminesce with a quantum yield of 0.09–0.23. Pyrazolo[3,4-b]pyridinones 4a–i, 9a, and 10a are characterized by an abnormally high Stokes shift (107–152 nm, 1.07–1.49 eV, Table 2). Such luminophores, which are colorless in daylight but become colored when irradiated with UV light, are used in forensics, in protection against forgery of banknotes, securities, and other important documents [34].
![[1860-5397-19-83-2]](/bjoc/content/figures/1860-5397-19-83-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: Normalized absorption and fluorescence spectra of solutions of compounds 4a–i, 9a, and 10a in EtOH.
Figure 2: Normalized absorption and fluorescence spectra of solutions of compounds 4a–i, 9a, and 10a in EtOH.
Conclusion
In summary, we developed a simple one-pot synthesis of 4-arylpyrazolo[3,4-b]pyridin-6-ones, based on the solvent-free reaction of the available starting compounds 5-aminopyrazoles 1, 5, 6 and azlactones 2a–i, followed by heating the resulting intermediate in DMSO in the presence of t-BuOK. Photophysical properties of the obtained compounds were studied.
Supporting Information
| Supporting Information File 1: Experimental procedures, characterization data, and 1H and 13C NMR spectra for all new compounds. | ||
| Format: PDF | Size: 678.4 KB | Download |
References
-
Cross, J. B.; Zhang, J.; Yang, Q.; Mesleh, M. F.; Romero, J. A. C.; Wang, B.; Bevan, D.; Poutsiaka, K. M.; Epie, F.; Moy, T.; Daniel, A.; Shotwell, J.; Chamberlain, B.; Carter, N.; Andersen, O.; Barker, J.; Ryan, M. D.; Metcalf, C. A., III; Silverman, J.; Nguyen, K.; Lippa, B.; Dolle, R. E. ACS Med. Chem. Lett. 2016, 7, 374–378. doi:10.1021/acsmedchemlett.5b00368
Return to citation in text: [1] [2] -
Luo, D.; Guo, Z.; Zhao, X.; Wu, L.; Liu, X.; Zhang, Y.; Zhang, Y.; Deng, Z.; Qu, X.; Cui, S.; Wan, S. Eur. J. Med. Chem. 2022, 227, 113923. doi:10.1016/j.ejmech.2021.113923
Return to citation in text: [1] -
Tucker, T. J.; Sisko, J. T.; Tynebor, R. M.; Williams, T. M.; Felock, P. J.; Flynn, J. A.; Lai, M.-T.; Liang, Y.; McGaughey, G.; Liu, M.; Miller, M.; Moyer, G.; Munshi, V.; Perlow-Poehnelt, R.; Prasad, S.; Reid, J. C.; Sanchez, R.; Torrent, M.; Vacca, J. P.; Wan, B.-L.; Yan, Y. J. Med. Chem. 2008, 51, 6503–6511. doi:10.1021/jm800856c
Return to citation in text: [1] -
Hamblin, J. N.; Angell, T. D. R.; Ballantine, S. P.; Cook, C. M.; Cooper, A. W. J.; Dawson, J.; Delves, C. J.; Jones, P. S.; Lindvall, M.; Lucas, F. S.; Mitchell, C. J.; Neu, M. Y.; Ranshaw, L. E.; Solanke, Y. E.; Somers, D. O.; Wiseman, J. O. Bioorg. Med. Chem. Lett. 2008, 18, 4237–4241. doi:10.1016/j.bmcl.2008.05.052
Return to citation in text: [1] -
Barghash, R. F.; Eldehna, W. M.; Kovalová, M.; Vojáčková, V.; Kryštof, V.; Abdel-Aziz, H. A. Eur. J. Med. Chem. 2022, 227, 113952. doi:10.1016/j.ejmech.2021.113952
Return to citation in text: [1] -
Ribeiro, J. L. S.; Soares, J. C. A. V.; Portapilla, G. B.; Providello, M. V.; Lima, C. H. S.; Muri, E. M. F.; de Albuquerque, S.; Dias, L. R. S. Bioorg. Med. Chem. 2021, 29, 115855. doi:10.1016/j.bmc.2020.115855
Return to citation in text: [1] -
Sharma, P. K.; Singh, K.; Kumar, S.; Kumar, P.; Dhawan, S. N.; Lal, S.; Ulbrich, H.; Dannhardt, G. Med. Chem. Res. 2011, 20, 239–244. doi:10.1007/s00044-010-9312-7
Return to citation in text: [1] -
Mesleh, M. F.; Cross, J. B.; Zhang, J.; Kahmann, J.; Andersen, O. A.; Barker, J.; Cheng, R. K.; Felicetti, B.; Wood, M.; Hadfield, A. T.; Scheich, C.; Moy, T. I.; Yang, Q.; Shotwell, J.; Nguyen, K.; Lippa, B.; Dolle, R.; Ryan, M. D. Bioorg. Med. Chem. Lett. 2016, 26, 1314–1318. doi:10.1016/j.bmcl.2016.01.009
Return to citation in text: [1] [2] -
Lu, Y.; Mao, F.; Li, X.; Zheng, X.; Wang, M.; Xu, Q.; Zhu, J.; Li, J. J. Med. Chem. 2017, 60, 5099–5119. doi:10.1021/acs.jmedchem.7b00468
Return to citation in text: [1] -
Wager, T. T. Pyrazolo[3,4-c]pyridines as gsk-3 inhibitors. PCT Pat. Appl. WO2005000303А1, Jan 6, 2005.
Return to citation in text: [1] -
Behnke, D.; Cotesta, S.; Hintermann, S.; Fendt, M.; Gee, C. E.; Jacobson, L. H.; Laue, G.; Meyer, A.; Wagner, T.; Badiger, S.; Chaudhari, V.; Chebrolu, M.; Pandit, C.; Hoyer, D.; Betschart, C. Bioorg. Med. Chem. Lett. 2015, 25, 5555–5560. doi:10.1016/j.bmcl.2015.10.055
Return to citation in text: [1] -
Choi, P. J.; Lu, G.-L.; Sutherland, H. S.; Giddens, A. C.; Franzblau, S. G.; Cooper, C. B.; Denny, W. A.; Palmer, B. D. Tetrahedron Lett. 2022, 90, 153611. doi:10.1016/j.tetlet.2021.153611
Return to citation in text: [1] -
Plemper, R. K.; Lee, E.; Vernachio, J.; Bourque, E. Bicyclic fused pyrazole derivatives for the treatment of rsv. PCT Pat. Appl. WO2017196982А1, Nov 16, 2017.
Return to citation in text: [1] [2] -
Uchikawa, O.; Mitsui, K.; Asakawa, A.; Morimoto, S.; Yamamoto, M.; Kimura, H.; Moriya, T.; Mizuno, M. Condensed pyrazole derivatives, process for producing the same and use thereof. U.S. Patent US2003187014A1, Oct 2, 2003.
Return to citation in text: [1] -
Lemmers, J. G. H.; Deretey, E.; Klomp, J. P. G.; Cals, J. M. G. B.; Oubrie, A. Estrogen-related receptor alpha (ERRα) modulators. PCT Pat. Appl. WO2021001453A1, Jan 7, 2021.
Return to citation in text: [1] -
Hansen, B. B.; Jepsen, T. H.; Larsen, M.; Sindet, R.; Vifian, T.; Burhardt, M. N.; Larsen, J.; Seitzberg, J. G.; Carnerup, M. A.; Jerre, A.; Mølck, C.; Lovato, P.; Rai, S.; Nasipireddy, V. R.; Ritzén, A. J. Med. Chem. 2020, 63, 7008–7032. doi:10.1021/acs.jmedchem.0c00359
Return to citation in text: [1] [2] [3] -
Wager, T. GSK-3 inhibitors. U.S. Patent US2005026946A1, Feb 3, 2005.
Return to citation in text: [1] -
Ratajczyk, J. D.; Swett, L. R. J. Heterocycl. Chem. 1975, 12, 517–522. doi:10.1002/jhet.5570120315
Return to citation in text: [1] -
Quiroga, J.; Hormaza, A.; Insuasty, B.; Márquez, M. J. Heterocycl. Chem. 1998, 35, 409–412. doi:10.1002/jhet.5570350225
Return to citation in text: [1] -
Swett, L. R.; Ratajczyk, J. D.; Nordeen, C. W.; Aynilian, G. H. J. Heterocycl. Chem. 1975, 12, 1137–1142. doi:10.1002/jhet.5570120611
Return to citation in text: [1] -
Minami, S.; Tomita, M.; Kawaguchi, K. Chem. Pharm. Bull. 1972, 20, 1716–1728. doi:10.1248/cpb.20.1716
Return to citation in text: [1] -
Shuvalov, V. Y.; Samsonenko, A. L.; Rozhkova, Y. S.; Morozov, V. V.; Shklyaev, Y. V.; Fisyuk, A. S. ChemistrySelect 2021, 6, 11265–11269. doi:10.1002/slct.202103028
Return to citation in text: [1] [2] -
Shuvalov, V. Yu.; Chernenko, S. A.; Shatsauskas, A. L.; Samsonenko, A. L.; Dmitriev, M. V.; Fisyuk, A. S. Chem. Heterocycl. Compd. 2021, 57, 764–771. doi:10.1007/s10593-021-02980-w
Return to citation in text: [1] -
Shuvalov, V. Y.; Rozhkova, Y. S.; Plekhanova, I. V.; Kostyuchenko, A. S.; Shklyaev, Y. V.; Fisyuk, A. S. Chem. Heterocycl. Compd. 2022, 58, 7–14. doi:10.1007/s10593-022-03050-5
Return to citation in text: [1] -
Shuvalov, V. Yu.; Fisyuk, A. S. Synthesis 2023, 55, 1267–1273. doi:10.1055/a-1993-3714
Return to citation in text: [1] [2] -
Cunha, S.; dos Santos Filho, R. F.; Saraiva, K. H.; Azevedo-Santos, A. V.; Menezes, D. Tetrahedron Lett. 2013, 54, 3366–3370. doi:10.1016/j.tetlet.2013.04.055
Return to citation in text: [1] -
Chen, X.; Zhu, D.; Wang, X.; Yan, S.; Lin, J. Tetrahedron 2013, 69, 9224–9236. doi:10.1016/j.tet.2013.08.052
Return to citation in text: [1] -
Vanden Eynde, J. J.; Labuche, N.; Van Haverbeke, Y. Synth. Commun. 1997, 27, 3683–3690. doi:10.1080/00397919708007288
Return to citation in text: [1] -
Worayuthakarn, R.; Nealmongkol, P.; Ruchirawat, S.; Thasana, N. Tetrahedron 2012, 68, 2864–2875. doi:10.1016/j.tet.2012.01.094
Return to citation in text: [1] -
Liu, X.-Q.; Liu, Y.-Q.; Shao, X.-S.; Xu, Z.-P.; Xu, X.-Y.; Li, Z. Chin. Chem. Lett. 2016, 27, 7–10. doi:10.1016/j.cclet.2015.10.002
Return to citation in text: [1] -
Shi, F.; Zhang, J.; Tu, S.; Jia, R.; Zhang, Y.; Jiang, B.; Jiang, H. J. Heterocycl. Chem. 2007, 44, 1013–1017. doi:10.1002/jhet.5570440506
Return to citation in text: [1] -
Kim, H. S.; Hammill, J. T.; Scott, D. C.; Chen, Y.; Min, J.; Rector, J.; Singh, B.; Schulman, B. A.; Guy, R. K. J. Med. Chem. 2019, 62, 8429–8442. doi:10.1021/acs.jmedchem.9b00410
Return to citation in text: [1] -
Kim, H. S.; Hammill, J. T.; Scott, D. C.; Chen, Y.; Rice, A. L.; Pistel, W.; Singh, B.; Schulman, B. A.; Guy, R. K. J. Med. Chem. 2021, 64, 5850–5862. doi:10.1021/acs.jmedchem.1c00035
Return to citation in text: [1] -
Ulyankin, E. B.; Bogza, Y. P.; Kostyuchenko, A. S.; Chernenko, S. A.; Samsonenko, A. L.; Shatsauskas, A. L.; Yurpalov, V. L.; Fisyuk, A. S. Synlett 2021, 32, 790–794. doi:10.1055/a-1392-2209
Return to citation in text: [1]
| 1. | Cross, J. B.; Zhang, J.; Yang, Q.; Mesleh, M. F.; Romero, J. A. C.; Wang, B.; Bevan, D.; Poutsiaka, K. M.; Epie, F.; Moy, T.; Daniel, A.; Shotwell, J.; Chamberlain, B.; Carter, N.; Andersen, O.; Barker, J.; Ryan, M. D.; Metcalf, C. A., III; Silverman, J.; Nguyen, K.; Lippa, B.; Dolle, R. E. ACS Med. Chem. Lett. 2016, 7, 374–378. doi:10.1021/acsmedchemlett.5b00368 |
| 2. | Luo, D.; Guo, Z.; Zhao, X.; Wu, L.; Liu, X.; Zhang, Y.; Zhang, Y.; Deng, Z.; Qu, X.; Cui, S.; Wan, S. Eur. J. Med. Chem. 2022, 227, 113923. doi:10.1016/j.ejmech.2021.113923 |
| 3. | Tucker, T. J.; Sisko, J. T.; Tynebor, R. M.; Williams, T. M.; Felock, P. J.; Flynn, J. A.; Lai, M.-T.; Liang, Y.; McGaughey, G.; Liu, M.; Miller, M.; Moyer, G.; Munshi, V.; Perlow-Poehnelt, R.; Prasad, S.; Reid, J. C.; Sanchez, R.; Torrent, M.; Vacca, J. P.; Wan, B.-L.; Yan, Y. J. Med. Chem. 2008, 51, 6503–6511. doi:10.1021/jm800856c |
| 4. | Hamblin, J. N.; Angell, T. D. R.; Ballantine, S. P.; Cook, C. M.; Cooper, A. W. J.; Dawson, J.; Delves, C. J.; Jones, P. S.; Lindvall, M.; Lucas, F. S.; Mitchell, C. J.; Neu, M. Y.; Ranshaw, L. E.; Solanke, Y. E.; Somers, D. O.; Wiseman, J. O. Bioorg. Med. Chem. Lett. 2008, 18, 4237–4241. doi:10.1016/j.bmcl.2008.05.052 |
| 5. | Barghash, R. F.; Eldehna, W. M.; Kovalová, M.; Vojáčková, V.; Kryštof, V.; Abdel-Aziz, H. A. Eur. J. Med. Chem. 2022, 227, 113952. doi:10.1016/j.ejmech.2021.113952 |
| 6. | Ribeiro, J. L. S.; Soares, J. C. A. V.; Portapilla, G. B.; Providello, M. V.; Lima, C. H. S.; Muri, E. M. F.; de Albuquerque, S.; Dias, L. R. S. Bioorg. Med. Chem. 2021, 29, 115855. doi:10.1016/j.bmc.2020.115855 |
| 7. | Sharma, P. K.; Singh, K.; Kumar, S.; Kumar, P.; Dhawan, S. N.; Lal, S.; Ulbrich, H.; Dannhardt, G. Med. Chem. Res. 2011, 20, 239–244. doi:10.1007/s00044-010-9312-7 |
| 8. | Mesleh, M. F.; Cross, J. B.; Zhang, J.; Kahmann, J.; Andersen, O. A.; Barker, J.; Cheng, R. K.; Felicetti, B.; Wood, M.; Hadfield, A. T.; Scheich, C.; Moy, T. I.; Yang, Q.; Shotwell, J.; Nguyen, K.; Lippa, B.; Dolle, R.; Ryan, M. D. Bioorg. Med. Chem. Lett. 2016, 26, 1314–1318. doi:10.1016/j.bmcl.2016.01.009 |
| 9. | Lu, Y.; Mao, F.; Li, X.; Zheng, X.; Wang, M.; Xu, Q.; Zhu, J.; Li, J. J. Med. Chem. 2017, 60, 5099–5119. doi:10.1021/acs.jmedchem.7b00468 |
| 10. | Wager, T. T. Pyrazolo[3,4-c]pyridines as gsk-3 inhibitors. PCT Pat. Appl. WO2005000303А1, Jan 6, 2005. |
| 11. | Behnke, D.; Cotesta, S.; Hintermann, S.; Fendt, M.; Gee, C. E.; Jacobson, L. H.; Laue, G.; Meyer, A.; Wagner, T.; Badiger, S.; Chaudhari, V.; Chebrolu, M.; Pandit, C.; Hoyer, D.; Betschart, C. Bioorg. Med. Chem. Lett. 2015, 25, 5555–5560. doi:10.1016/j.bmcl.2015.10.055 |
| 12. | Choi, P. J.; Lu, G.-L.; Sutherland, H. S.; Giddens, A. C.; Franzblau, S. G.; Cooper, C. B.; Denny, W. A.; Palmer, B. D. Tetrahedron Lett. 2022, 90, 153611. doi:10.1016/j.tetlet.2021.153611 |
| 16. | Hansen, B. B.; Jepsen, T. H.; Larsen, M.; Sindet, R.; Vifian, T.; Burhardt, M. N.; Larsen, J.; Seitzberg, J. G.; Carnerup, M. A.; Jerre, A.; Mølck, C.; Lovato, P.; Rai, S.; Nasipireddy, V. R.; Ritzén, A. J. Med. Chem. 2020, 63, 7008–7032. doi:10.1021/acs.jmedchem.0c00359 |
| 34. | Ulyankin, E. B.; Bogza, Y. P.; Kostyuchenko, A. S.; Chernenko, S. A.; Samsonenko, A. L.; Shatsauskas, A. L.; Yurpalov, V. L.; Fisyuk, A. S. Synlett 2021, 32, 790–794. doi:10.1055/a-1392-2209 |
| 15. | Lemmers, J. G. H.; Deretey, E.; Klomp, J. P. G.; Cals, J. M. G. B.; Oubrie, A. Estrogen-related receptor alpha (ERRα) modulators. PCT Pat. Appl. WO2021001453A1, Jan 7, 2021. |
| 14. | Uchikawa, O.; Mitsui, K.; Asakawa, A.; Morimoto, S.; Yamamoto, M.; Kimura, H.; Moriya, T.; Mizuno, M. Condensed pyrazole derivatives, process for producing the same and use thereof. U.S. Patent US2003187014A1, Oct 2, 2003. |
| 22. | Shuvalov, V. Y.; Samsonenko, A. L.; Rozhkova, Y. S.; Morozov, V. V.; Shklyaev, Y. V.; Fisyuk, A. S. ChemistrySelect 2021, 6, 11265–11269. doi:10.1002/slct.202103028 |
| 25. | Shuvalov, V. Yu.; Fisyuk, A. S. Synthesis 2023, 55, 1267–1273. doi:10.1055/a-1993-3714 |
| 13. | Plemper, R. K.; Lee, E.; Vernachio, J.; Bourque, E. Bicyclic fused pyrazole derivatives for the treatment of rsv. PCT Pat. Appl. WO2017196982А1, Nov 16, 2017. |
| 31. | Shi, F.; Zhang, J.; Tu, S.; Jia, R.; Zhang, Y.; Jiang, B.; Jiang, H. J. Heterocycl. Chem. 2007, 44, 1013–1017. doi:10.1002/jhet.5570440506 |
| 32. | Kim, H. S.; Hammill, J. T.; Scott, D. C.; Chen, Y.; Min, J.; Rector, J.; Singh, B.; Schulman, B. A.; Guy, R. K. J. Med. Chem. 2019, 62, 8429–8442. doi:10.1021/acs.jmedchem.9b00410 |
| 33. | Kim, H. S.; Hammill, J. T.; Scott, D. C.; Chen, Y.; Rice, A. L.; Pistel, W.; Singh, B.; Schulman, B. A.; Guy, R. K. J. Med. Chem. 2021, 64, 5850–5862. doi:10.1021/acs.jmedchem.1c00035 |
| 13. | Plemper, R. K.; Lee, E.; Vernachio, J.; Bourque, E. Bicyclic fused pyrazole derivatives for the treatment of rsv. PCT Pat. Appl. WO2017196982А1, Nov 16, 2017. |
| 16. | Hansen, B. B.; Jepsen, T. H.; Larsen, M.; Sindet, R.; Vifian, T.; Burhardt, M. N.; Larsen, J.; Seitzberg, J. G.; Carnerup, M. A.; Jerre, A.; Mølck, C.; Lovato, P.; Rai, S.; Nasipireddy, V. R.; Ritzén, A. J. Med. Chem. 2020, 63, 7008–7032. doi:10.1021/acs.jmedchem.0c00359 |
| 19. | Quiroga, J.; Hormaza, A.; Insuasty, B.; Márquez, M. J. Heterocycl. Chem. 1998, 35, 409–412. doi:10.1002/jhet.5570350225 |
| 21. | Minami, S.; Tomita, M.; Kawaguchi, K. Chem. Pharm. Bull. 1972, 20, 1716–1728. doi:10.1248/cpb.20.1716 |
| 1. | Cross, J. B.; Zhang, J.; Yang, Q.; Mesleh, M. F.; Romero, J. A. C.; Wang, B.; Bevan, D.; Poutsiaka, K. M.; Epie, F.; Moy, T.; Daniel, A.; Shotwell, J.; Chamberlain, B.; Carter, N.; Andersen, O.; Barker, J.; Ryan, M. D.; Metcalf, C. A., III; Silverman, J.; Nguyen, K.; Lippa, B.; Dolle, R. E. ACS Med. Chem. Lett. 2016, 7, 374–378. doi:10.1021/acsmedchemlett.5b00368 |
| 16. | Hansen, B. B.; Jepsen, T. H.; Larsen, M.; Sindet, R.; Vifian, T.; Burhardt, M. N.; Larsen, J.; Seitzberg, J. G.; Carnerup, M. A.; Jerre, A.; Mølck, C.; Lovato, P.; Rai, S.; Nasipireddy, V. R.; Ritzén, A. J. Med. Chem. 2020, 63, 7008–7032. doi:10.1021/acs.jmedchem.0c00359 |
| 18. | Ratajczyk, J. D.; Swett, L. R. J. Heterocycl. Chem. 1975, 12, 517–522. doi:10.1002/jhet.5570120315 |
| 22. | Shuvalov, V. Y.; Samsonenko, A. L.; Rozhkova, Y. S.; Morozov, V. V.; Shklyaev, Y. V.; Fisyuk, A. S. ChemistrySelect 2021, 6, 11265–11269. doi:10.1002/slct.202103028 |
| 23. | Shuvalov, V. Yu.; Chernenko, S. A.; Shatsauskas, A. L.; Samsonenko, A. L.; Dmitriev, M. V.; Fisyuk, A. S. Chem. Heterocycl. Compd. 2021, 57, 764–771. doi:10.1007/s10593-021-02980-w |
| 24. | Shuvalov, V. Y.; Rozhkova, Y. S.; Plekhanova, I. V.; Kostyuchenko, A. S.; Shklyaev, Y. V.; Fisyuk, A. S. Chem. Heterocycl. Compd. 2022, 58, 7–14. doi:10.1007/s10593-022-03050-5 |
| 25. | Shuvalov, V. Yu.; Fisyuk, A. S. Synthesis 2023, 55, 1267–1273. doi:10.1055/a-1993-3714 |
| 26. | Cunha, S.; dos Santos Filho, R. F.; Saraiva, K. H.; Azevedo-Santos, A. V.; Menezes, D. Tetrahedron Lett. 2013, 54, 3366–3370. doi:10.1016/j.tetlet.2013.04.055 |
| 27. | Chen, X.; Zhu, D.; Wang, X.; Yan, S.; Lin, J. Tetrahedron 2013, 69, 9224–9236. doi:10.1016/j.tet.2013.08.052 |
| 28. | Vanden Eynde, J. J.; Labuche, N.; Van Haverbeke, Y. Synth. Commun. 1997, 27, 3683–3690. doi:10.1080/00397919708007288 |
| 29. | Worayuthakarn, R.; Nealmongkol, P.; Ruchirawat, S.; Thasana, N. Tetrahedron 2012, 68, 2864–2875. doi:10.1016/j.tet.2012.01.094 |
| 30. | Liu, X.-Q.; Liu, Y.-Q.; Shao, X.-S.; Xu, Z.-P.; Xu, X.-Y.; Li, Z. Chin. Chem. Lett. 2016, 27, 7–10. doi:10.1016/j.cclet.2015.10.002 |
| 8. | Mesleh, M. F.; Cross, J. B.; Zhang, J.; Kahmann, J.; Andersen, O. A.; Barker, J.; Cheng, R. K.; Felicetti, B.; Wood, M.; Hadfield, A. T.; Scheich, C.; Moy, T. I.; Yang, Q.; Shotwell, J.; Nguyen, K.; Lippa, B.; Dolle, R.; Ryan, M. D. Bioorg. Med. Chem. Lett. 2016, 26, 1314–1318. doi:10.1016/j.bmcl.2016.01.009 |
| 20. | Swett, L. R.; Ratajczyk, J. D.; Nordeen, C. W.; Aynilian, G. H. J. Heterocycl. Chem. 1975, 12, 1137–1142. doi:10.1002/jhet.5570120611 |
© 2023 Shuvalov et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.