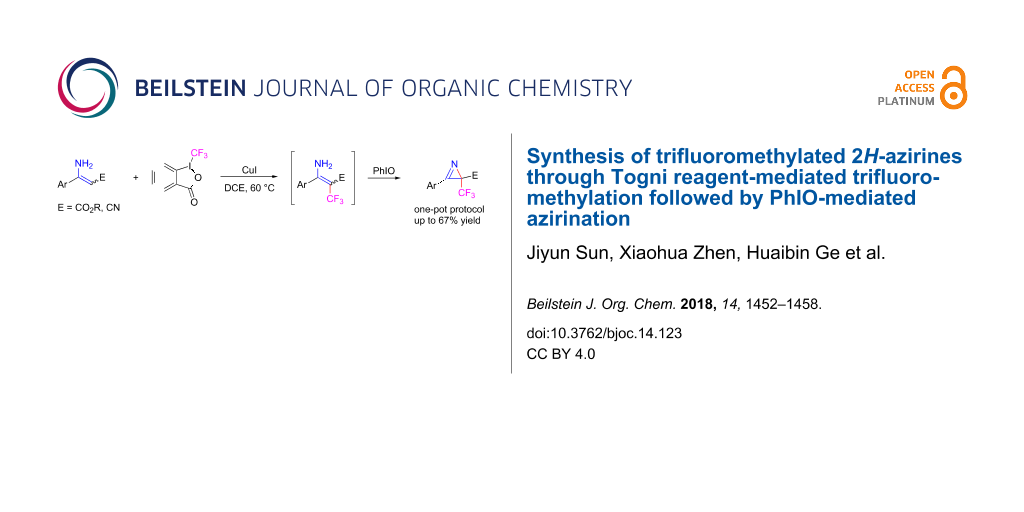
Abstract
The reaction of enamine compounds with the Togni reagent in the presence of CuI afforded β-trifluoromethylated enamine intermediates, which were converted directly to biologically interesting trifluoromethylated 2H-azirines by an iodosobenzene (PhIO)-mediated intramolecular azirination in a one-pot process.
Graphical Abstract
Introduction
The trifluoromethyl group is a striking structural motif, which can be widely found in the fields of pharmaceutical and agrochemical sciences. The introduction of this functional group in drug molecules can enhance their chemical and metabolic stability, improve their lipophilicity and bioavailability, and increase protein-binding affinity [1-6]. In this regard, the CF3 group has been introduced into many pharmaceutical agents [7-16]. For example, fluoxetine hydrochloride (Figure 1, A) [4,9,10] (Prozac®, an antidepressant and a selective serotonin reuptake inhibitor for the treatment of major depressive disorders, obsessive–compulsive disorders, etc.), teriflunomide (Figure 1, B) [11-13] (Aubagio®, the active metabolite of leflunomide for the treatment of multiple sclerosis), and pleconaril (Figure 1, C) [14-16] (an antiviral drug), all possess this privileged substituent. Although many useful synthetic methods [17-21] have been established for introducing the CF3 group into various organic molecules, the further development of novel routes for the selective trifluoromethylation is of continuing interest for synthetic and medicinal chemists.
Figure 1: Representative pharmaceutical agents bearing the CF3 group.
Figure 1: Representative pharmaceutical agents bearing the CF3 group.
Togni reagents, including 1-(trifluoromethyl)-1,2-benziodoxol-3(1H)-one (1) and trifluoromethyl-1,3-dihydro-3,3-dimethyl-1,2-benziodoxole (1’, Figure 2), are effective and efficient hypervalent iodine reagents for trifluoromethylation reactions of a variety of substrates [22,23]. These reagents have found wide applications in the area of organofluorine chemistry, synthetic method development as well as medicinal chemistry [24-40]. For example, the Togni reagents have been successfully applied to introduce the CF3 group into pharmaceutical agents such as the fluoxetine derivative D (Figure 1), the mefloquine derivative E [41] and compound F [42] – a potential anti-HIV drug bearing a NCF3 moiety.
Figure 2: The structures of the Togni reagents 1-(trifluoromethyl)-1,2-benziodoxol-3(1H)-one (1) and trifluoromethyl-1,3-dihydro-3,3-dimethyl-1,2-benziodoxole (1’).
Figure 2: The structures of the Togni reagents 1-(trifluoromethyl)-1,2-benziodoxol-3(1H)-one (1) and trifluor...
2H-Azirines are a class of highly strained and reactive molecules containing a C–N double bond. The exclusive framework can be found in some natural products [43-47], which were shown to possess antibiotic activities [43,44]. Furthermore, compounds with this structural motif are also useful building blocks for the synthesis of functionalized amino derivatives and N-containing heterocyclic derivatives [48-51]. Thus, this class of compounds has gained considerable attention from synthetic chemists and many useful synthetic approaches [52-55] have been developed for accessing this exclusive class of heterocycles. In our previous works, we have realized the application of hypervalent iodine reagents for the construction of the 2H-azirine skeleton starting from enamines 2 via intramolecular oxidative cyclization (Scheme 1) [56,57]. When the R2 substituent is alkyl or aryl, the corresponding substrates 2 were converted to a series of alkylated or arylated 2H-azirines 3 in the presence of phenyliodine diacetate (PIDA) in 1,2-dichloroethane (DCE) [56]. Alternatively, the treatment of β-unsubstituted enamine substrates (2, R2 = H) with PhIO in 2,2,2-trifluoroethanol (TFE) afforded 2-trifluoroethoxy-2H-azirines 4 [57]. The latter process involves an intermolecular oxidative trifluoroethoxylation and the subsequent oxidative intramolecular azirination. In continuation of our interest in the construction of the 2H-azirine skeleton bearing versatile substituents, we herein report that the biologically interesting CF3 group can be incorporated into the privileged 2H-azirine framework through the Togni reagent 1-mediated trifluoromethylation followed by PhIO-mediated azirination in a one-pot process.
Scheme 1: Our previous hypervalent iodine-mediated synthesis of 2H-azirine compounds.
Scheme 1: Our previous hypervalent iodine-mediated synthesis of 2H-azirine compounds.
Results and Discussion
It is well documented that Togni reagents can realize the direct trifluoromethylation of alkenes [58-60] and electron-rich enamides [61]. Inspired by this, we envisaged that Togni reagent 1 could also enable the introduction of a CF3 group to the β-position of enamine substrates, and the so-obtained trifluoromethylated enamines could undergo a hypervalent iodine-mediated intramolecular azirination to give the corresponding trifluoromethylated 2H-azirines [56,57]. To test this conversion, the readily available enamine 5a was used as a model substrate. The treatment of 5a with Togni reagent 1 in the presence of CuI in N,N-dimethylformamide (DMF) [62] at room temperature for two hours afforded the β-trifluoromethylated enamine 6a in a 23% yield. Subjecting enamine 6a to PhIO in 1,2-dichloroethane (DCE) for 12 hours at room temperature led to the formation of the desired β-trifluoromethylated 2H-azirine 7a in a yield of 60% (Scheme 2).
Scheme 2: Study on the presumed Togni reagent 1-mediated trifluoromethylation followed by PhIO-mediated azirination.
Scheme 2: Study on the presumed Togni reagent 1-mediated trifluoromethylation followed by PhIO-mediated aziri...
In order to make the synthesis of β-trifluoromethylated 2H-azirine more concise and convenient, we were keen to probe whether the two-step synthesis could be combined into a one-pot process. For this purpose, we first carried out the reaction of Togni reagent 1 and 5a in the presence of CuI in DMF at room temperature, followed by an addition of PhIO. However, only trace amounts of the expected product 7a were obtained (Table 1, entry 1). We next screened various solvents to increase the reaction outcome (Table 1, entries 1–4). Judging by the yield of the desired product, it was concluded that DCE was the best solvent (Table 1, entry 3). By increasing the reaction temperature from rt to 60 °C, the yields significantly increased to 55% (Table 1, entries 3, 5 and 6). However, an attempt to improve the product yield by operating the reaction at a higher temperature was unsuccessful (Table 1, entry 7). Replacing the catalyst CuI with other commonly used copper catalysts including CuCl, CuBr and CuOAc led to a decreased yield in each case (Table 1, entries 8–10). In addition the other commonly employed hypervalent iodine(III) reagents, namely, PIDA and phenyliodine bis(trifluoroacetate) (PIFA) were tested, but the results indicated that they were ineffective to further improve the yields (Table 1, entries 11 and 12).
Table 1: Optimization of reaction conditions.a
|
|
|||||
| Entry | Catalyst | Oxidantb | Solvent | Temp. (°C) | Yieldc (%) |
| 1 | CuI | PhIO | DMF | rt | trace |
| 2 | CuI | PhIO | CH3CN | rt | 13 |
| 3 | CuI | PhIO | DCE | rt | 24 |
| 4 | CuI | PhIO | toluene | rt | 12 |
| 5 | CuI | PhIO | DCE | 40 | 35 |
| 6 | CuI | PhIO | DCE | 60 | 55 |
| 7 | CuI | PhIO | DCE | reflux | 48 |
| 8 | CuCl | PhIO | DCE | 60 | 49 |
| 9 | CuBr | PhIO | DCE | 60 | 50 |
| 10 | CuOAc | PhIO | DCE | 60 | 38 |
| 11 | CuI | PIDA | DCE | 60 | 46 |
| 12 | CuI | PIFA | DCE | 60 | 30 |
aReaction conditions: Togni reagent 1 (1.2 mmol), 5a (1.0 mmol), catalyst (0.2 mmol), oxidant (1.5 mmol) in solvent (10 mL) unless otherwise stated. bThe oxidant was added to the reaction mixture after the substrate 5a was completely consumed (TLC analysis). cIsolated yield.
With the optimized conditions in hand, we next explored the substrate scope for this newly established one-pot oxidative trifluoromethylation and azirination reaction. As shown in Scheme 3, a variety of substrates bearing halogen substituents at the ortho, meta and para-positions of the phenyl ring in the substrates were converted to the expected 2H-azirines 7b–e in 45–65% one-pot yield. Notably, the substrate having a trifluoromethyl group at the meta-position in the phenyl ring also afforded the desired 2H-azirine product 7f bearing two CF3 substituents in a satisfactory one-pot yield. Various enamine substrates with electron-donating groups (p-Me, o-Me and 3,4-di-OMe) in the aryl ring, also reacted efficiently under the conditions of the one-pot process to afford the corresponding products 7g–i in a yield of 40–67%. Furthermore, when replacing the methoxycarbonyl group in 5a with a cyano or N-methyl-N-phenylformyl group, the corresponding substrates 5j and 5k were converted to the desired products 7j and 7k in a yield of 49% and 57%, respectively. The methoxy group in the ester moiety could also be replaced by the n-butoxy group, with the desired product 7l being isolated in a yield of 62%. In addition, this method was also applicable to substrates bearing naphthyl or thienyl groups at R substitution to give the desired products 7m and 7n in a yield of 43% and 45%, respectively. However, the method was not applicable to the substrate bearing an alkyl group, as the reaction of 5o, even at lower temperatures (−20 °C, 0 °C, 20 °C and 40 °C) gave a complex mixture after adding PhIO.
Scheme 3: Togni reagent/PhIO-mediated one-pot synthesis of β-trifluoromethyl 2H-azirines. Reaction conditions: 1 (1.2 mmol), 5 (1.0 mmol), CuI (0.2 mmol), PhIO (1.5 mmol) in DCE (10 mL) unless otherwise stated. PhIO was added to the reaction mixture after the substrate 5 was completely consumed (TLC analysis). Yields refer to isolated yields.
Scheme 3: Togni reagent/PhIO-mediated one-pot synthesis of β-trifluoromethyl 2H-azirines. Reaction conditions...
To gain further insights into the reaction mechanism, 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO), a well-known radical scavenger, was introduced to the model reaction (Scheme 4) following the method previously reported in the literature [63]. It was found that the trifluoromethylation was hampered and the TEMPO-CF3 adduct 8 was formed as a major product based on the analysis of its 19F NMR (δ −55.67).
The above results from the experiment provided supportive evidence that the CF3 radical was likely involved as a reactive species in the reaction process. Based on this and previous reports [62-68], a possible reaction pathway has been proposed and is outlined in Scheme 5. Initially, CuI catalytically activates the Togni reagent 1, leading to the formation of the CF3-containing radical intermediate 9. Decomposition of the intermediate 9 produces (2-iodobenzoyloxy)copper(II) iodide (10) [65,66] with the simultaneous release of a CF3 radical. Then, the reaction of enamine 5a with the CF3 radical affords the carbon-centered radical 11. Next, the reaction of 10 and 11, possibly through an electron-transfer process, along with the conversion of intermediate 10 to 2-iodobenzoic acid enables the conversion of intermediate 11 to 6a (possibly tautomerized from its imine isomer) [69]. Finally, the β-trifluoromethylated enamine 6a undergoes intramolecular azirination affording the corresponding β-trifluoromethylated 2H-azirine via a known pathway [56,57].
![[1860-5397-14-123-i5]](/bjoc/content/inline/1860-5397-14-123-i5.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 5: Proposed mechanism for the Togni reagent-mediated trifluoromethylation of enamines.
Scheme 5: Proposed mechanism for the Togni reagent-mediated trifluoromethylation of enamines.
Conclusion
In summary, we have reported an efficient hypervalent iodine-mediated trifluoromethylation and azirination process. In this transformation, the introduction of the CF3 group to the β-position of enamines followed by the intramolecular azirination was realized in a one-pot process, providing a general and straightforward access to biologically interesting trifluoromethylated 2H-azirine compounds. This method features mild reaction conditions, a simple operation, and metal-free characteristics. The presence of both, the biologically interesting CF3 group and the 2H-azirine skeleton in the products obtained might making them interesting for further applications in biological studies.
Supporting Information
| Supporting Information File 1: Synthetic details and characterization data. | ||
| Format: PDF | Size: 2.5 MB | Download |
Acknowledgements
We acknowledge the National Natural Science Foundation of China (No. 21472136) and Tianjin Research Program of Application Foundation and Advanced Technology (15JCZDJC32900) for their financial support. We also thank Ms Jeanette Mar [School of Health Science Platform, Tianjin University] for editing the English version in this paper.
References
-
Hiyama, T. In Organofluorine Compounds: Chemistry and Applications; Yamamoto, H., Ed.; Springer: Berlin, 2000. doi:10.1007/978-3-662-04164-2
Return to citation in text: [1] -
Ojima, I., Ed. Fluorine in Medicinal Chemistry and Chemical Biology; Wiley-Blackwell: Chichester, U.K., 2009. doi:10.1002/9781444312096
Return to citation in text: [1] -
Kirsch, P. Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications; Wiley-VCH: Weinheim, Germany, 2013. doi:10.1002/9783527651351
Return to citation in text: [1] -
Müller, K.; Faeh, C.; Diederich, F. Science 2007, 317, 1881–1886. doi:10.1126/science.1131943
Return to citation in text: [1] [2] -
Hagmann, W. K. J. Med. Chem. 2008, 51, 4359–4369. doi:10.1021/jm800219f
Return to citation in text: [1] -
Meanwell, N. A. J. Med. Chem. 2011, 54, 2529–2591. doi:10.1021/jm1013693
Return to citation in text: [1] -
Tomashenko, O. A.; Grushin, V. V. Chem. Rev. 2011, 111, 4475–4521. doi:10.1021/cr1004293
Return to citation in text: [1] -
Roy, S.; Gregg, B. T.; Gribble, G. W.; Le, V.-D.; Roy, S. Tetrahedron 2011, 67, 2161–2195. doi:10.1016/j.tet.2011.01.002
Return to citation in text: [1] -
Wong, D. T.; Bymaster, F. P.; Engleman, E. A. Life Sci. 1995, 57, 411–441. doi:10.1016/0024-3205(95)00209-O
Return to citation in text: [1] [2] -
Otton, S. V.; Wu, D.; Joffe, R. T.; Cheung, S. W.; Sellers, E. M. Clin. Pharmacol. Ther. (Hoboken, NJ, U. S.) 1993, 53, 401–409. doi:10.1038/clpt.1993.43
Return to citation in text: [1] [2] -
Bar-Or, A.; Pachner, A.; Menguy-Vacheron, F.; Kaplan, J.; Wiendl, H. Drugs 2014, 74, 659–674. doi:10.1007/s40265-014-0212-x
Return to citation in text: [1] [2] -
Claussen, M. C.; Korn, T. Clin. Immunol. 2012, 142, 49–56. doi:10.1016/j.clim.2011.02.011
Return to citation in text: [1] [2] -
Zeyda, M.; Poglitsch, M.; Geyeregger, R.; Smolen, J. S.; Zlabinger, G. J.; Hörl, W. H.; Waldhäusl, W.; Stulnig, T. M.; Säemann, M. D. Arthritis Rheumatol. 2005, 52, 2730–2739. doi:10.1002/art.21255
Return to citation in text: [1] [2] -
Pevear, D. C.; Tull, T. M.; Seipel, M. E.; Groarke, J. M. Antimicrob. Agents Chemother. 1999, 43, 2109–2115.
Return to citation in text: [1] [2] -
Rotbart, H. A.; Webster, A. D. Clin. Infect. Dis. 2001, 32, 228–235. doi:10.1086/318452
Return to citation in text: [1] [2] -
Romero, J. R. Expert Opin. Invest. Drugs 2001, 10, 369–379. doi:10.1517/13543784.10.2.369
Return to citation in text: [1] [2] -
Furuya, T.; Kamlet, A. S.; Ritter, T. Nature 2011, 473, 470–477. doi:10.1038/nature10108
Return to citation in text: [1] -
Zhang, C. ARKIVOC 2014, No. i, 453–469. doi:10.3998/ark.5550190.p008.656
Return to citation in text: [1] -
Studer, A. Angew. Chem., Int. Ed. 2012, 51, 8950–8958. doi:10.1002/anie.201202624
Return to citation in text: [1] -
Liang, T.; Neumann, C. N.; Ritter, T. Angew. Chem., Int. Ed. 2013, 52, 8214–8264. doi:10.1002/anie.201206566
Return to citation in text: [1] -
Zhu, W.; Wang, J.; Wang, S.; Gu, Z.; Aceña, J. L.; Izawa, K.; Liu, H.; Soloshonok, V. A. J. Fluorine Chem. 2014, 167, 37–54. doi:10.1016/j.jfluchem.2014.06.026
Return to citation in text: [1] -
Eisenberger, P.; Gischig, S.; Togni, A. Chem. – Eur. J. 2006, 12, 2579–2586. doi:10.1002/chem.200501052
Return to citation in text: [1] -
Charpentier, J.; Früh, N.; Togni, A. Chem. Rev. 2015, 115, 650–682. doi:10.1021/cr500223h
Return to citation in text: [1] -
Shibata, N.; Matsnev, A.; Cahard, D. Beilstein J. Org. Chem. 2010, 6, No. 65. doi:10.3762/bjoc.6.65
Return to citation in text: [1] -
Macé, Y.; Magnier, E. Eur. J. Org. Chem. 2012, 2479–2494. doi:10.1002/ejoc.201101535
Return to citation in text: [1] -
Barata-Vallejo, S.; Lantaño, B.; Postigo, A. Chem. – Eur. J. 2014, 20, 16806–16829. doi:10.1002/chem.201404005
Return to citation in text: [1] -
Alonso, C.; Martinez de Marigorta, E.; Rubiales, G.; Palacios, F. Chem. Rev. 2015, 115, 1847–1935. doi:10.1021/cr500368h
Return to citation in text: [1] -
Wang, S.-M.; Han, J.-B.; Zhang, C.-P.; Qin, H.-L.; Xiao, J.-C. Tetrahedron 2015, 71, 7949–7976. doi:10.1016/j.tet.2015.06.056
Return to citation in text: [1] -
Koller, R.; Huchet, Q.; Battaglia, P.; Welch, J. M.; Togni, A. Chem. Commun. 2009, 5993–5995. doi:10.1039/b913962a
Return to citation in text: [1] -
Santschi, N.; Geissbühler, P.; Togni, A. J. Fluorine Chem. 2012, 135, 83–86. doi:10.1016/j.jfluchem.2011.08.014
Return to citation in text: [1] -
Eisenberger, P.; Kieltsch, I.; Armanino, N.; Togni, A. Chem. Commun. 2008, 1575–1577. doi:10.1039/b801424h
Return to citation in text: [1] -
Niedermann, K.; Früh, N.; Vinogradova, E.; Wiehn, M. S.; Moreno, A.; Togni, A. Angew. Chem., Int. Ed. 2011, 50, 1059–1063. doi:10.1002/anie.201006021
Return to citation in text: [1] -
Wiehn, M. S.; Vinogradova, E. V.; Togni, A. J. Fluorine Chem. 2010, 131, 951–957. doi:10.1016/j.jfluchem.2010.06.020
Return to citation in text: [1] -
Kieltsch, I. Ph.D. Thesis, 1799, Swiss Federal Institute of Technology, Zürich, 2008.
Return to citation in text: [1] -
Mejía, E.; Togni, A. ACS Catal. 2012, 2, 521–527. doi:10.1021/cs300089y
Return to citation in text: [1] -
Xie, J.; Yuan, X.; Abdukader, A.; Zhu, C.; Ma, J. Org. Lett. 2014, 16, 1768–1771. doi:10.1021/ol500469a
Return to citation in text: [1] -
Schmidt, B. M.; Seki, S.; Topolinski, B.; Ohkubo, K.; Fukuzumi, S.; Sakurai, H.; Lentz, D. Angew. Chem., Int. Ed. 2012, 51, 11385–11388. doi:10.1002/anie.201205757
Return to citation in text: [1] -
He, Z.; Luo, T.; Hu, M.; Cao, Y.; Hu, J. Angew. Chem., Int. Ed. 2012, 51, 3944–3947. doi:10.1002/anie.201200140
Return to citation in text: [1] -
Capone, S.; Kieltsch, I.; Flögel, O.; Lelais, G.; Togni, A.; Seebach, D. Helv. Chim. Acta 2008, 91, 2035–2056. doi:10.1002/hlca.200890217
Return to citation in text: [1] -
Seebach, D.; Widmer, H.; Capone, S.; Ernst, R.; Bremi, T.; Kieltsch, I.; Togni, A.; Monna, D.; Langenegger, D.; Hoyer, D. Helv. Chim. Acta 2009, 92, 2577–2586. doi:10.1002/hlca.200900279
Return to citation in text: [1] -
Matoušek, V.; Pietrasiak, E.; Sigrist, L.; Czarniecki, B.; Togni, A. Eur. J. Org. Chem. 2014, 3087–3092. doi:10.1002/ejoc.201402225
Return to citation in text: [1] -
Babaoglu, K.; Brizgys, G.; Cha, J.; Chen, X.; Guo, H.; Halcomb, R. L.; Han, X.; Huang, R.; Liu, H.; McFadden, R.; Mitchell, M. L.; Qi, Y.; Roethle, P. A.; Xu, L.; Yang, H. Benzothiazol-6-ylacetic acid derivatives as anti-HIV agents and their preparation and use for treating an HIV infection. WO Patent WO2013/159064, Nov 29, 2013.
Return to citation in text: [1] -
Stapley, E. O.; Hendlin, D.; Jackson, M.; Miller, A. K.; Hernandez, S.; Mata, J. M. J. Antibiot. 1971, 24, 42–47. doi:10.7164/antibiotics.24.42
Return to citation in text: [1] [2] -
Miller, T. W.; Tristram, E. W.; Wolf, F. J. J. Antibiot. 1971, 24, 48–50. doi:10.7164/antibiotics.24.48
Return to citation in text: [1] [2] -
Molinski, T. F.; Ireland, C. M. J. Org. Chem. 1988, 53, 2103–2105. doi:10.1021/jo00244a049
Return to citation in text: [1] -
Salomon, C. E.; Williams, D. H.; Faulkner, D. J. J. Nat. Prod. 1995, 58, 1463–1466. doi:10.1021/np50123a021
Return to citation in text: [1] -
Skepper, C. K.; Molinski, T. F. J. Org. Chem. 2008, 73, 2592–2597. doi:10.1021/jo702435s
Return to citation in text: [1] -
Yoshimura, A.; Zhdankin, V. V. Chem. Rev. 2016, 116, 3328–3435. doi:10.1021/acs.chemrev.5b00547
Return to citation in text: [1] -
Reddy, C. R.; Prajapti, S. K.; Warudikar, K.; Ranjan, R.; Rao, B. B. Org. Biomol. Chem. 2017, 15, 3130–3151. doi:10.1039/C7OB00405B
Return to citation in text: [1] -
Zheng, Z.; Zhang-Negrerie, D.; Du, Y.; Zhao, K. Sci. China: Chem. 2014, 57, 189–214. doi:10.1007/s11426-013-5043-1
Return to citation in text: [1] -
Zheng, Y.; Yang, C.; Zhang-Negrerie, D.; Du, Y.; Zhao, K. Tetrahedron Lett. 2013, 54, 6157–6160. doi:10.1016/j.tetlet.2013.08.079
Return to citation in text: [1] -
Palacios, F.; Ochoa de Retana, A. M.; Martinez de Marigorta, E.; de los Santos, J. M. Org. Prep. Proced. Int. 2002, 34, 219–269. doi:10.1080/00304940209356770
Return to citation in text: [1] -
Palacios, F.; Ochoa de Retana, A. M.; Martinez de Marigorta, E.; de los Santos, J. M. Eur. J. Org. Chem. 2001, 2401–2414. doi:10.1002/1099-0690(200107)2001:13<2401::AID-EJOC2401>3.0.CO;2-U
Return to citation in text: [1] -
Pinho e Melo, T. M. V. D.; d'A Rocha Gonsalves, A. M. Curr. Org. Chem. 2004, 1, 275–292. doi:10.2174/1570179043366729
Return to citation in text: [1] -
Katritzky, A. R.; Wang, M.; Wilkerson, C. R.; Yang, H. J. Org. Chem. 2003, 68, 9105–9108. doi:10.1021/jo034472i
Return to citation in text: [1] -
Li, X.; Du, Y.; Liang, Z.; Li, X.; Pan, Y.; Zhao, K. Org. Lett. 2009, 11, 2643–2646. doi:10.1021/ol9006663
Return to citation in text: [1] [2] [3] [4] -
Sun, X.; Lyu, Y.; Zhang-Negrerie, D.; Du, Y.; Zhao, K. Org. Lett. 2013, 15, 6222–6225. doi:10.1021/ol4030716
Return to citation in text: [1] [2] [3] [4] -
Wang, X.-P.; Lin, J.-H.; Zhang, C.-P.; Xiao, J.-C.; Zheng, X. Beilstein J. Org. Chem. 2013, 9, 2635–2640. doi:10.3762/bjoc.9.299
Return to citation in text: [1] -
Janson, P. G.; Ghoneim, I.; Ilchenko, N. O.; Szabó, K. J. Org. Lett. 2012, 14, 2882–2885. doi:10.1021/ol3011419
Return to citation in text: [1] -
Egami, H.; Shimizu, R.; Sodeoka, M. Tetrahedron Lett. 2012, 53, 5503–5506. doi:10.1016/j.tetlet.2012.07.134
Return to citation in text: [1] -
Feng, C.; Loh, T.-P. Chem. Sci. 2012, 3, 3458–3462. doi:10.1039/c2sc21164e
Return to citation in text: [1] -
Fang, Z.; Ning, Y.; Mi, P.; Liao, P.; Bi, X. Org. Lett. 2014, 16, 1522–1525. doi:10.1021/ol5004498
Return to citation in text: [1] [2] -
Wang, X.; Ye, Y.; Zhang, S.; Feng, J.; Xu, Y.; Zhang, Y.; Wang, J. J. Am. Chem. Soc. 2011, 133, 16410–16413. doi:10.1021/ja207775a
Return to citation in text: [1] [2] -
Li, Z.; Li, C.-J. J. Am. Chem. Soc. 2006, 128, 56–57. doi:10.1021/ja056541b
Return to citation in text: [1] -
Lee, J. M.; Park, E. J.; Cho, S. H.; Chang, S. J. Am. Chem. Soc. 2008, 130, 7824–7825. doi:10.1021/ja8031218
Return to citation in text: [1] [2] -
Cai, S.; Chen, C.; Sun, Z.; Xi, C. Chem. Commun. 2013, 49, 4552–4554. doi:10.1039/c3cc41331d
Return to citation in text: [1] [2] -
Zhou, W.; Zhang, L.; Jiao, N. Angew. Chem., Int. Ed. 2009, 48, 7094–7097. doi:10.1002/anie.200903838
Return to citation in text: [1] -
Qin, C.; Jiao, N. J. Am. Chem. Soc. 2010, 132, 15893–15895. doi:10.1021/ja1070202
Return to citation in text: [1] -
Sun, J.; Zhang-Negrerie, D.; Du, Y. Adv. Synth. Catal. 2016, 358, 2035–2040. doi:10.1002/adsc.201501099
Return to citation in text: [1]
| 1. | Hiyama, T. In Organofluorine Compounds: Chemistry and Applications; Yamamoto, H., Ed.; Springer: Berlin, 2000. doi:10.1007/978-3-662-04164-2 |
| 2. | Ojima, I., Ed. Fluorine in Medicinal Chemistry and Chemical Biology; Wiley-Blackwell: Chichester, U.K., 2009. doi:10.1002/9781444312096 |
| 3. | Kirsch, P. Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications; Wiley-VCH: Weinheim, Germany, 2013. doi:10.1002/9783527651351 |
| 4. | Müller, K.; Faeh, C.; Diederich, F. Science 2007, 317, 1881–1886. doi:10.1126/science.1131943 |
| 5. | Hagmann, W. K. J. Med. Chem. 2008, 51, 4359–4369. doi:10.1021/jm800219f |
| 6. | Meanwell, N. A. J. Med. Chem. 2011, 54, 2529–2591. doi:10.1021/jm1013693 |
| 14. | Pevear, D. C.; Tull, T. M.; Seipel, M. E.; Groarke, J. M. Antimicrob. Agents Chemother. 1999, 43, 2109–2115. |
| 15. | Rotbart, H. A.; Webster, A. D. Clin. Infect. Dis. 2001, 32, 228–235. doi:10.1086/318452 |
| 16. | Romero, J. R. Expert Opin. Invest. Drugs 2001, 10, 369–379. doi:10.1517/13543784.10.2.369 |
| 56. | Li, X.; Du, Y.; Liang, Z.; Li, X.; Pan, Y.; Zhao, K. Org. Lett. 2009, 11, 2643–2646. doi:10.1021/ol9006663 |
| 57. | Sun, X.; Lyu, Y.; Zhang-Negrerie, D.; Du, Y.; Zhao, K. Org. Lett. 2013, 15, 6222–6225. doi:10.1021/ol4030716 |
| 11. | Bar-Or, A.; Pachner, A.; Menguy-Vacheron, F.; Kaplan, J.; Wiendl, H. Drugs 2014, 74, 659–674. doi:10.1007/s40265-014-0212-x |
| 12. | Claussen, M. C.; Korn, T. Clin. Immunol. 2012, 142, 49–56. doi:10.1016/j.clim.2011.02.011 |
| 13. | Zeyda, M.; Poglitsch, M.; Geyeregger, R.; Smolen, J. S.; Zlabinger, G. J.; Hörl, W. H.; Waldhäusl, W.; Stulnig, T. M.; Säemann, M. D. Arthritis Rheumatol. 2005, 52, 2730–2739. doi:10.1002/art.21255 |
| 56. | Li, X.; Du, Y.; Liang, Z.; Li, X.; Pan, Y.; Zhao, K. Org. Lett. 2009, 11, 2643–2646. doi:10.1021/ol9006663 |
| 4. | Müller, K.; Faeh, C.; Diederich, F. Science 2007, 317, 1881–1886. doi:10.1126/science.1131943 |
| 9. | Wong, D. T.; Bymaster, F. P.; Engleman, E. A. Life Sci. 1995, 57, 411–441. doi:10.1016/0024-3205(95)00209-O |
| 10. | Otton, S. V.; Wu, D.; Joffe, R. T.; Cheung, S. W.; Sellers, E. M. Clin. Pharmacol. Ther. (Hoboken, NJ, U. S.) 1993, 53, 401–409. doi:10.1038/clpt.1993.43 |
| 48. | Yoshimura, A.; Zhdankin, V. V. Chem. Rev. 2016, 116, 3328–3435. doi:10.1021/acs.chemrev.5b00547 |
| 49. | Reddy, C. R.; Prajapti, S. K.; Warudikar, K.; Ranjan, R.; Rao, B. B. Org. Biomol. Chem. 2017, 15, 3130–3151. doi:10.1039/C7OB00405B |
| 50. | Zheng, Z.; Zhang-Negrerie, D.; Du, Y.; Zhao, K. Sci. China: Chem. 2014, 57, 189–214. doi:10.1007/s11426-013-5043-1 |
| 51. | Zheng, Y.; Yang, C.; Zhang-Negrerie, D.; Du, Y.; Zhao, K. Tetrahedron Lett. 2013, 54, 6157–6160. doi:10.1016/j.tetlet.2013.08.079 |
| 7. | Tomashenko, O. A.; Grushin, V. V. Chem. Rev. 2011, 111, 4475–4521. doi:10.1021/cr1004293 |
| 8. | Roy, S.; Gregg, B. T.; Gribble, G. W.; Le, V.-D.; Roy, S. Tetrahedron 2011, 67, 2161–2195. doi:10.1016/j.tet.2011.01.002 |
| 9. | Wong, D. T.; Bymaster, F. P.; Engleman, E. A. Life Sci. 1995, 57, 411–441. doi:10.1016/0024-3205(95)00209-O |
| 10. | Otton, S. V.; Wu, D.; Joffe, R. T.; Cheung, S. W.; Sellers, E. M. Clin. Pharmacol. Ther. (Hoboken, NJ, U. S.) 1993, 53, 401–409. doi:10.1038/clpt.1993.43 |
| 11. | Bar-Or, A.; Pachner, A.; Menguy-Vacheron, F.; Kaplan, J.; Wiendl, H. Drugs 2014, 74, 659–674. doi:10.1007/s40265-014-0212-x |
| 12. | Claussen, M. C.; Korn, T. Clin. Immunol. 2012, 142, 49–56. doi:10.1016/j.clim.2011.02.011 |
| 13. | Zeyda, M.; Poglitsch, M.; Geyeregger, R.; Smolen, J. S.; Zlabinger, G. J.; Hörl, W. H.; Waldhäusl, W.; Stulnig, T. M.; Säemann, M. D. Arthritis Rheumatol. 2005, 52, 2730–2739. doi:10.1002/art.21255 |
| 14. | Pevear, D. C.; Tull, T. M.; Seipel, M. E.; Groarke, J. M. Antimicrob. Agents Chemother. 1999, 43, 2109–2115. |
| 15. | Rotbart, H. A.; Webster, A. D. Clin. Infect. Dis. 2001, 32, 228–235. doi:10.1086/318452 |
| 16. | Romero, J. R. Expert Opin. Invest. Drugs 2001, 10, 369–379. doi:10.1517/13543784.10.2.369 |
| 52. | Palacios, F.; Ochoa de Retana, A. M.; Martinez de Marigorta, E.; de los Santos, J. M. Org. Prep. Proced. Int. 2002, 34, 219–269. doi:10.1080/00304940209356770 |
| 53. | Palacios, F.; Ochoa de Retana, A. M.; Martinez de Marigorta, E.; de los Santos, J. M. Eur. J. Org. Chem. 2001, 2401–2414. doi:10.1002/1099-0690(200107)2001:13<2401::AID-EJOC2401>3.0.CO;2-U |
| 54. | Pinho e Melo, T. M. V. D.; d'A Rocha Gonsalves, A. M. Curr. Org. Chem. 2004, 1, 275–292. doi:10.2174/1570179043366729 |
| 55. | Katritzky, A. R.; Wang, M.; Wilkerson, C. R.; Yang, H. J. Org. Chem. 2003, 68, 9105–9108. doi:10.1021/jo034472i |
| 41. | Matoušek, V.; Pietrasiak, E.; Sigrist, L.; Czarniecki, B.; Togni, A. Eur. J. Org. Chem. 2014, 3087–3092. doi:10.1002/ejoc.201402225 |
| 43. | Stapley, E. O.; Hendlin, D.; Jackson, M.; Miller, A. K.; Hernandez, S.; Mata, J. M. J. Antibiot. 1971, 24, 42–47. doi:10.7164/antibiotics.24.42 |
| 44. | Miller, T. W.; Tristram, E. W.; Wolf, F. J. J. Antibiot. 1971, 24, 48–50. doi:10.7164/antibiotics.24.48 |
| 45. | Molinski, T. F.; Ireland, C. M. J. Org. Chem. 1988, 53, 2103–2105. doi:10.1021/jo00244a049 |
| 46. | Salomon, C. E.; Williams, D. H.; Faulkner, D. J. J. Nat. Prod. 1995, 58, 1463–1466. doi:10.1021/np50123a021 |
| 47. | Skepper, C. K.; Molinski, T. F. J. Org. Chem. 2008, 73, 2592–2597. doi:10.1021/jo702435s |
| 24. | Shibata, N.; Matsnev, A.; Cahard, D. Beilstein J. Org. Chem. 2010, 6, No. 65. doi:10.3762/bjoc.6.65 |
| 25. | Macé, Y.; Magnier, E. Eur. J. Org. Chem. 2012, 2479–2494. doi:10.1002/ejoc.201101535 |
| 26. | Barata-Vallejo, S.; Lantaño, B.; Postigo, A. Chem. – Eur. J. 2014, 20, 16806–16829. doi:10.1002/chem.201404005 |
| 27. | Alonso, C.; Martinez de Marigorta, E.; Rubiales, G.; Palacios, F. Chem. Rev. 2015, 115, 1847–1935. doi:10.1021/cr500368h |
| 28. | Wang, S.-M.; Han, J.-B.; Zhang, C.-P.; Qin, H.-L.; Xiao, J.-C. Tetrahedron 2015, 71, 7949–7976. doi:10.1016/j.tet.2015.06.056 |
| 29. | Koller, R.; Huchet, Q.; Battaglia, P.; Welch, J. M.; Togni, A. Chem. Commun. 2009, 5993–5995. doi:10.1039/b913962a |
| 30. | Santschi, N.; Geissbühler, P.; Togni, A. J. Fluorine Chem. 2012, 135, 83–86. doi:10.1016/j.jfluchem.2011.08.014 |
| 31. | Eisenberger, P.; Kieltsch, I.; Armanino, N.; Togni, A. Chem. Commun. 2008, 1575–1577. doi:10.1039/b801424h |
| 32. | Niedermann, K.; Früh, N.; Vinogradova, E.; Wiehn, M. S.; Moreno, A.; Togni, A. Angew. Chem., Int. Ed. 2011, 50, 1059–1063. doi:10.1002/anie.201006021 |
| 33. | Wiehn, M. S.; Vinogradova, E. V.; Togni, A. J. Fluorine Chem. 2010, 131, 951–957. doi:10.1016/j.jfluchem.2010.06.020 |
| 34. | Kieltsch, I. Ph.D. Thesis, 1799, Swiss Federal Institute of Technology, Zürich, 2008. |
| 35. | Mejía, E.; Togni, A. ACS Catal. 2012, 2, 521–527. doi:10.1021/cs300089y |
| 36. | Xie, J.; Yuan, X.; Abdukader, A.; Zhu, C.; Ma, J. Org. Lett. 2014, 16, 1768–1771. doi:10.1021/ol500469a |
| 37. | Schmidt, B. M.; Seki, S.; Topolinski, B.; Ohkubo, K.; Fukuzumi, S.; Sakurai, H.; Lentz, D. Angew. Chem., Int. Ed. 2012, 51, 11385–11388. doi:10.1002/anie.201205757 |
| 38. | He, Z.; Luo, T.; Hu, M.; Cao, Y.; Hu, J. Angew. Chem., Int. Ed. 2012, 51, 3944–3947. doi:10.1002/anie.201200140 |
| 39. | Capone, S.; Kieltsch, I.; Flögel, O.; Lelais, G.; Togni, A.; Seebach, D. Helv. Chim. Acta 2008, 91, 2035–2056. doi:10.1002/hlca.200890217 |
| 40. | Seebach, D.; Widmer, H.; Capone, S.; Ernst, R.; Bremi, T.; Kieltsch, I.; Togni, A.; Monna, D.; Langenegger, D.; Hoyer, D. Helv. Chim. Acta 2009, 92, 2577–2586. doi:10.1002/hlca.200900279 |
| 43. | Stapley, E. O.; Hendlin, D.; Jackson, M.; Miller, A. K.; Hernandez, S.; Mata, J. M. J. Antibiot. 1971, 24, 42–47. doi:10.7164/antibiotics.24.42 |
| 44. | Miller, T. W.; Tristram, E. W.; Wolf, F. J. J. Antibiot. 1971, 24, 48–50. doi:10.7164/antibiotics.24.48 |
| 22. | Eisenberger, P.; Gischig, S.; Togni, A. Chem. – Eur. J. 2006, 12, 2579–2586. doi:10.1002/chem.200501052 |
| 23. | Charpentier, J.; Früh, N.; Togni, A. Chem. Rev. 2015, 115, 650–682. doi:10.1021/cr500223h |
| 17. | Furuya, T.; Kamlet, A. S.; Ritter, T. Nature 2011, 473, 470–477. doi:10.1038/nature10108 |
| 18. | Zhang, C. ARKIVOC 2014, No. i, 453–469. doi:10.3998/ark.5550190.p008.656 |
| 19. | Studer, A. Angew. Chem., Int. Ed. 2012, 51, 8950–8958. doi:10.1002/anie.201202624 |
| 20. | Liang, T.; Neumann, C. N.; Ritter, T. Angew. Chem., Int. Ed. 2013, 52, 8214–8264. doi:10.1002/anie.201206566 |
| 21. | Zhu, W.; Wang, J.; Wang, S.; Gu, Z.; Aceña, J. L.; Izawa, K.; Liu, H.; Soloshonok, V. A. J. Fluorine Chem. 2014, 167, 37–54. doi:10.1016/j.jfluchem.2014.06.026 |
| 42. | Babaoglu, K.; Brizgys, G.; Cha, J.; Chen, X.; Guo, H.; Halcomb, R. L.; Han, X.; Huang, R.; Liu, H.; McFadden, R.; Mitchell, M. L.; Qi, Y.; Roethle, P. A.; Xu, L.; Yang, H. Benzothiazol-6-ylacetic acid derivatives as anti-HIV agents and their preparation and use for treating an HIV infection. WO Patent WO2013/159064, Nov 29, 2013. |
| 57. | Sun, X.; Lyu, Y.; Zhang-Negrerie, D.; Du, Y.; Zhao, K. Org. Lett. 2013, 15, 6222–6225. doi:10.1021/ol4030716 |
| 58. | Wang, X.-P.; Lin, J.-H.; Zhang, C.-P.; Xiao, J.-C.; Zheng, X. Beilstein J. Org. Chem. 2013, 9, 2635–2640. doi:10.3762/bjoc.9.299 |
| 59. | Janson, P. G.; Ghoneim, I.; Ilchenko, N. O.; Szabó, K. J. Org. Lett. 2012, 14, 2882–2885. doi:10.1021/ol3011419 |
| 60. | Egami, H.; Shimizu, R.; Sodeoka, M. Tetrahedron Lett. 2012, 53, 5503–5506. doi:10.1016/j.tetlet.2012.07.134 |
| 56. | Li, X.; Du, Y.; Liang, Z.; Li, X.; Pan, Y.; Zhao, K. Org. Lett. 2009, 11, 2643–2646. doi:10.1021/ol9006663 |
| 57. | Sun, X.; Lyu, Y.; Zhang-Negrerie, D.; Du, Y.; Zhao, K. Org. Lett. 2013, 15, 6222–6225. doi:10.1021/ol4030716 |
| 65. | Lee, J. M.; Park, E. J.; Cho, S. H.; Chang, S. J. Am. Chem. Soc. 2008, 130, 7824–7825. doi:10.1021/ja8031218 |
| 66. | Cai, S.; Chen, C.; Sun, Z.; Xi, C. Chem. Commun. 2013, 49, 4552–4554. doi:10.1039/c3cc41331d |
| 69. | Sun, J.; Zhang-Negrerie, D.; Du, Y. Adv. Synth. Catal. 2016, 358, 2035–2040. doi:10.1002/adsc.201501099 |
| 63. | Wang, X.; Ye, Y.; Zhang, S.; Feng, J.; Xu, Y.; Zhang, Y.; Wang, J. J. Am. Chem. Soc. 2011, 133, 16410–16413. doi:10.1021/ja207775a |
| 62. | Fang, Z.; Ning, Y.; Mi, P.; Liao, P.; Bi, X. Org. Lett. 2014, 16, 1522–1525. doi:10.1021/ol5004498 |
| 63. | Wang, X.; Ye, Y.; Zhang, S.; Feng, J.; Xu, Y.; Zhang, Y.; Wang, J. J. Am. Chem. Soc. 2011, 133, 16410–16413. doi:10.1021/ja207775a |
| 64. | Li, Z.; Li, C.-J. J. Am. Chem. Soc. 2006, 128, 56–57. doi:10.1021/ja056541b |
| 65. | Lee, J. M.; Park, E. J.; Cho, S. H.; Chang, S. J. Am. Chem. Soc. 2008, 130, 7824–7825. doi:10.1021/ja8031218 |
| 66. | Cai, S.; Chen, C.; Sun, Z.; Xi, C. Chem. Commun. 2013, 49, 4552–4554. doi:10.1039/c3cc41331d |
| 67. | Zhou, W.; Zhang, L.; Jiao, N. Angew. Chem., Int. Ed. 2009, 48, 7094–7097. doi:10.1002/anie.200903838 |
| 68. | Qin, C.; Jiao, N. J. Am. Chem. Soc. 2010, 132, 15893–15895. doi:10.1021/ja1070202 |
| 56. | Li, X.; Du, Y.; Liang, Z.; Li, X.; Pan, Y.; Zhao, K. Org. Lett. 2009, 11, 2643–2646. doi:10.1021/ol9006663 |
| 57. | Sun, X.; Lyu, Y.; Zhang-Negrerie, D.; Du, Y.; Zhao, K. Org. Lett. 2013, 15, 6222–6225. doi:10.1021/ol4030716 |
| 62. | Fang, Z.; Ning, Y.; Mi, P.; Liao, P.; Bi, X. Org. Lett. 2014, 16, 1522–1525. doi:10.1021/ol5004498 |
© 2018 Sun et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)