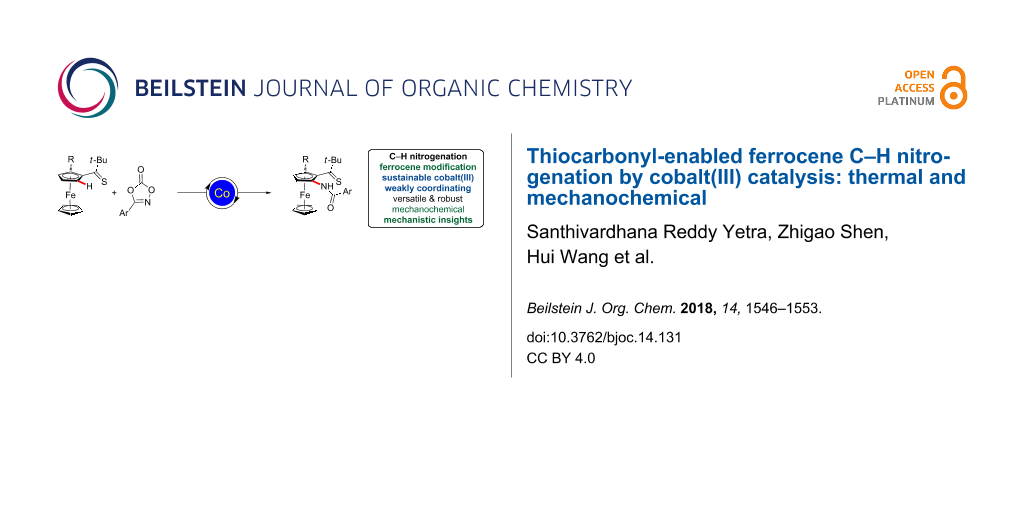
Abstract
Versatile C–H amidations of synthetically useful ferrocenes were accomplished by weakly-coordinating thiocarbonyl-assisted cobalt catalysis. Thus, carboxylates enabled ferrocene C–H nitrogenations with dioxazolones, featuring ample substrate scope and robust functional group tolerance. Mechanistic studies provided strong support for a facile organometallic C–H activation manifold.
Graphical Abstract
Introduction
C–H activation has surfaced as a transformative tool in molecular sciences [1-9]. While major advances have been accomplished with precious 4d transition metals, recent focus has shifted towards more sustainable base metals [10-17], with considerable progress by earth-abundant cobalt catalysts [18-22]. In this context, well-defined cyclopentadienyl-derived cobalt(III) complexes have proven instrumental for enabling a wealth of C–H transformations [23-41], prominently featuring transformative C–H nitrogenations [42,43] in an atom- and step-economical fashion [44-59]. Within our program on cobalt-catalyzed C–H activation [60-68], we have now devised C–H nitrogenations assisted by weakly-coordinating [69] thiocarbonyls [70,71], allowing the direct C–H activation on substituted ferrocenes [72-93] – key structural motifs of powerful transition metal catalyst ligands and organocatalysts (Figure 1) [94-97]. During the preparation of this article, the use of strongly-coordinating, difficult to remove directing groups has been reported [70,71]. In sharp contrast, notable features of our approach include (i) cobalt-catalyzed C–H amidations of thiocarbonylferrocenes by weak coordination, (ii) thermal and mechanochemical [98-100] cobalt-catalyzed ferrocene C–H nitrogenations, (iii) versatile access to synthetically useful aminoketones, and (iv) key mechanistic insights on facile C–H cobaltation.
Figure 1: Selected ferrocene-based ligands and organocatalysts.
Figure 1: Selected ferrocene-based ligands and organocatalysts.
Results and Discussion
We initiated our studies by probing various reaction conditions for the envisioned C–H amidation of ferrocene 1a (Table 1). Among a variety of ligands, N-heterocyclic carbenes and phosphines provided unsatisfactory results (Table 1, entries 1–3), while the product 3aa was formed when using amino acid derivatives, albeit as of yet in a racemic fashion (Table 1, entries 4–7). Yet, optimal catalytic performance was realized with 1-AdCO2H (Table 1, entries 8 and 9) [101-104], particularly when using DCE as the solvent (Table 1, entries 9–12). A control experiment verified the essential nature of the cobalt catalyst (Table 1, entry 13). In contrast to the thiocarbonyl-assisted C–H amidation, the corresponding ketone failed thus far to deliver the desired product, under otherwise identical reaction conditions.
Table 1: Thiocarbonyl-assisted C−H nitrogenation of ferrocene 1a.a
|
|
|||
| Entry | Solvent | Ligand | Yield (%) |
|---|---|---|---|
| 1 | DCE | – | – |
| 2 | DCE | IMes·HCl | – |
| 3 | DCE | PPh3 | – |
| 4 | DCE | Boc-Leu-OH | 40 |
| 5 | DCE | Boc-Val-OH | 55 |
| 6 | DCE | Boc-Pro-OH | 30 |
| 7 | DCE | Boc-Ala-OH | 62 |
| 8 | DCE | MesCO2H | 80 |
| 9 | DCE | 1-AdCO2H | 84 |
| 10 | 1,4-dioxane | 1-AdCO2H | 75 |
| 11 | toluene | 1-AdCO2H | 79 |
| 12 | GVL | 1-AdCO2H | 35 |
| 13 | DCE | 1-AdCO2H | –b |
aReaction conditions: 1a (0.13 mmol), 2a (0.15 mmol), ligand (30 mol %), [Co] (5.0 mol %), solvent (1.0 mL). bReaction performed in the absence of [Cp*Co(CH3CN)3][SbF6]2. Yields of isolated product.
With the optimized reaction conditions in hand, we explored the robustness of the cobalt-catalyzed ferrocene C–H amidation with a variety of 1,4,2-dioxazol-5-ones 2 (Scheme 1). Hence, the chemoselectivity of the cobalt catalyst was reflected by fully tolerating sensitive electrophilic functional groups, including amido, chloro, bromo and nitro substituents in the para-, meta- and even the more congested ortho-position.
Scheme 1: Scope of substituted dioxazolones 2.
Scheme 1: Scope of substituted dioxazolones 2.
The versatile cobalt-catalyzed C–H amidation was not limited to mono-substituted ferrocenes 1 (Scheme 2). Indeed, the arylated ferrocenes 1b–d were identified as viable substrates likewise.
Scheme 2: C–H Amidation of arylated ferrocenes 1.
Scheme 2: C–H Amidation of arylated ferrocenes 1.
Moreover, differently substituted thiocarbonyls 1 were found to be amenable within the cobalt-catalyzed C–H amidation manifold by weak-coordination (Scheme 3).
Scheme 3: Thiocarbonyl-assisted C–H amidation.
Scheme 3: Thiocarbonyl-assisted C–H amidation.
Given the versatility of the cobalt-catalyzed C–H nitrogenation, we became intrigued to delineating its mode of action. To this end, C–H amidations in the presence of isotopically labelled co-solvents led to a significant H/D scrambling in proximity to the thiocarbonyl group. These findings are indicative of a reversible, thus facile organometallic C–H cobaltation regime (Scheme 4).
Next, intermolecular competition experiments revealed that electron-rich arylated thiocarbonylferrocene 1 reacted preferentially, which can be rationalized with a base-assisted internal electrophilic substitution (BIES) [24,105] C–H cobaltation mechanism. In addition, the electron-rich amidating reagent 2c was found to be inherently more reactive (Scheme 5).
Scheme 5: Intermolecular competition experiments.
Scheme 5: Intermolecular competition experiments.
As to further late-stage manipulation of the thus-obtained products, the amidated thiocarbonylferrocene 3aa could be easily transformed into the corresponding synthetically useful aminoketone 4aa (Scheme 6), illustrating the unique synthetic utility of our strategy.
Mechanochemical molecular synthesis has attracted recent renewed attention as an attractive alternative for facilitating sustainable organic syntheses [106]. Thus, we were delighted to observe that the mechanochemical C–H nitrogenations proved likewise viable by thiocarbonyl assistance in an effective manner (Scheme 7).
Scheme 7: Mechanochemical ferrocene C–H nitrogenation.
Scheme 7: Mechanochemical ferrocene C–H nitrogenation.
Conclusion
In conclusion, we have reported on the unprecedented cobalt-catalyzed C–H nitrogenation of ferrocenes by weakly-coordinating thiocarbonyls. The carboxylate-assisted cobalt catalysis was characterized by high functional group tolerance and ample substrate scope. Mechanistic studies provided evidence for a facile C–H activation. The C–H amidation was achieved in a thermal fashion as well as by means of mechanochemistry, providing access to synthetically meaningful aminoketones.
Supporting Information
| Supporting Information File 1: Experimental procedures, characterization data, and NMR spectra for new compounds. | ||
| Format: PDF | Size: 2.8 MB | Download |
References
-
Gandeepan, P.; Ackermann, L. Chem 2018, 4, 199–222. doi:10.1016/j.chempr.2017.11.002
Return to citation in text: [1] -
Wei, Y.; Hu, P.; Zhang, M.; Su, W. Chem. Rev. 2017, 117, 8864–8907. doi:10.1021/acs.chemrev.6b00516
Return to citation in text: [1] -
Ma, W.; Gandeepan, P.; Li, J.; Ackermann, L. Org. Chem. Front. 2017, 4, 1435–1467. doi:10.1039/C7QO00134G
Return to citation in text: [1] -
He, J.; Wasa, M.; Chan, K. S. L.; Shao, Q.; Yu, J.-Q. Chem. Rev. 2017, 117, 8754–8786. doi:10.1021/acs.chemrev.6b00622
Return to citation in text: [1] -
Zheng, Q.-Z.; Jiao, N. Chem. Soc. Rev. 2016, 45, 4590–4627. doi:10.1039/C6CS00107F
Return to citation in text: [1] -
Borie, C.; Ackermann, L.; Nechab, M. Chem. Soc. Rev. 2016, 45, 1368–1386. doi:10.1039/C5CS00622H
Return to citation in text: [1] -
Ye, B.; Cramer, N. Acc. Chem. Res. 2015, 48, 1308–1318. doi:10.1021/acs.accounts.5b00092
Return to citation in text: [1] -
Satoh, T.; Miura, M. Chem. – Eur. J. 2010, 16, 11212–11222. doi:10.1002/chem.201001363
Return to citation in text: [1] -
Ackermann, L.; Vicente, R.; Kapdi, A. R. Angew. Chem., Int. Ed. 2009, 121, 9976–10011. doi:10.1002/ange.200902996
Return to citation in text: [1] -
Hu, Y.; Zhou, B.; Wang, C. Acc. Chem. Res. 2018, 51, 816–827. doi:10.1021/acs.accounts.8b00028
Return to citation in text: [1] -
Liu, W.; Ackermann, L. ACS Catal. 2016, 6, 3743–3752. doi:10.1021/acscatal.6b00993
Return to citation in text: [1] -
Cera, G.; Ackermann, L. Top. Curr. Chem. 2016, 374, 191–224. doi:10.1007/s41061-016-0059-6
Return to citation in text: [1] -
Nakao, Y. Chem. Rec. 2011, 11, 242–251. doi:10.1002/tcr.201100023
Return to citation in text: [1] -
Castro, L. C. M.; Chatani, N. Chem. Lett. 2015, 44, 410–421. doi:10.1246/cl.150024
Return to citation in text: [1] -
Hirano, K.; Miura, M. Chem. Lett. 2015, 44, 868–873. doi:10.1246/cl.150354
Return to citation in text: [1] -
Yamaguchi, J.; Muto, K.; Itami, K. Eur. J. Org. Chem. 2013, 19–30. doi:10.1002/ejoc.201200914
Return to citation in text: [1] -
Kulkarni, A. A.; Daugulis, O. Synthesis 2009, 4087–4109. doi:10.1055/s-0029-1217131
Return to citation in text: [1] -
Yoshino, T.; Matsunaga, S. Adv. Synth. Catal. 2017, 359, 1245–1262. doi:10.1002/adsc.201700042
Return to citation in text: [1] -
Wei, D.; Zhu, X.; Niu, J.-L.; Song, M.-P. ChemCatChem 2016, 8, 1242–1263. doi:10.1002/cctc.201600040
Return to citation in text: [1] -
Moselage, M.; Li, J.; Ackermann, L. ACS Catal. 2016, 6, 498–525. doi:10.1021/acscatal.5b02344
Return to citation in text: [1] -
Gao, K.; Yoshikai, N. Acc. Chem. Res. 2014, 47, 1208–1219. doi:10.1021/ar400270x
Return to citation in text: [1] -
Ackermann, L. J. Org. Chem. 2014, 79, 8948–8954. doi:10.1021/jo501361k
Return to citation in text: [1] -
Zell, D.; Müller, V.; Dhawa, U.; Bursch, M.; Presa, R. R.; Grimme, S.; Ackermann, L. Chem. – Eur. J. 2017, 23, 12145–12148. doi:10.1002/chem.201702528
Return to citation in text: [1] -
Zell, D.; Bursch, M.; Müller, V.; Grimme, S.; Ackermann, L. Angew. Chem., Int. Ed. 2017, 56, 10378–10382. doi:10.1002/anie.201704196
Return to citation in text: [1] [2] -
Ikemoto, H.; Tanaka, R.; Sakata, K.; Kanai, M.; Yoshino, T.; Matsunaga, S. Angew. Chem., Int. Ed. 2017, 56, 7156–7160. doi:10.1002/anie.201703193
Return to citation in text: [1] -
Yan, Q.; Chen, Z.; Liu, Z.; Zhang, Y. Org. Chem. Front. 2016, 3, 678–682. doi:10.1039/C6QO00059B
Return to citation in text: [1] -
Lu, Q.; Vásquez-Céspedes, S.; Gensch, T.; Glorius, F. ACS Catal. 2016, 6, 2352–2356. doi:10.1021/acscatal.6b00367
Return to citation in text: [1] -
Kong, L.; Yu, S.; Zhou, X.; Li, X. Org. Lett. 2016, 18, 588–591. doi:10.1021/acs.orglett.5b03629
Return to citation in text: [1] -
Kalsi, D.; Laskar, R. A.; Barsu, N.; Premkumar, J. R.; Sundararaju, B. Org. Lett. 2016, 18, 4198–4201. doi:10.1021/acs.orglett.6b01845
Return to citation in text: [1] -
Bunno, Y.; Murakami, N.; Suzuki, Y.; Kanai, M.; Yoshino, T.; Matsunaga, S. Org. Lett. 2016, 18, 2216–2219. doi:10.1021/acs.orglett.6b00846
Return to citation in text: [1] -
Boerth, J. A.; Hummel, J. R.; Ellman, J. A. Angew. Chem., Int. Ed. 2016, 55, 12650–12654. doi:10.1002/anie.201603831
Return to citation in text: [1] -
Hummel, J. R.; Ellman, J. A. J. Am. Chem. Soc. 2015, 137, 490–498. doi:10.1021/ja5116452
Return to citation in text: [1] -
Wang, H.; Koeller, J.; Liu, W.; Ackermann, L. Chem. – Eur. J. 2015, 21, 15525–15528. doi:10.1002/chem.201503624
Return to citation in text: [1] -
Suzuki, Y.; Sun, B.; Sakata, K.; Yoshino, T.; Matsunaga, S.; Kanai, M. Angew. Chem., Int. Ed. 2015, 54, 9944–9947. doi:10.1002/anie.201503704
Return to citation in text: [1] -
Sen, M.; Kalsi, D.; Sundararaju, B. Chem. – Eur. J. 2015, 21, 15529–15533. doi:10.1002/chem.201503643
Return to citation in text: [1] -
Sauermann, N.; González, M. J.; Ackermann, L. Org. Lett. 2015, 17, 5316–5319. doi:10.1021/acs.orglett.5b02678
Return to citation in text: [1] -
Ma, W.; Ackermann, L. ACS Catal. 2015, 5, 2822–2825. doi:10.1021/acscatal.5b00322
Return to citation in text: [1] -
Li, J.; Ackermann, L. Angew. Chem., Int. Ed. 2015, 54, 3635–3638. doi:10.1002/anie.201409247
Return to citation in text: [1] -
Li, J.; Ackermann, L. Angew. Chem., Int. Ed. 2015, 54, 8551–8554. doi:10.1002/anie.201501926
Return to citation in text: [1] -
Ikemoto, H.; Yoshino, T.; Sakata, K.; Matsunaga, S.; Kanai, M. J. Am. Chem. Soc. 2014, 136, 5424–5431. doi:10.1021/ja5008432
Return to citation in text: [1] -
Yoshino, T.; Ikemoto, H.; Matsunaga, S.; Kanai, M. Angew. Chem., Int. Ed. 2013, 52, 2207–2211. doi:10.1002/anie.201209226
Return to citation in text: [1] -
Park, Y.; Kim, Y.; Chang, S. Chem. Rev. 2017, 117, 9247–9301. doi:10.1021/acs.chemrev.6b00644
Return to citation in text: [1] -
Jiao, J.; Murakami, K.; Itami, K. ACS Catal. 2016, 6, 610–633. doi:10.1021/acscatal.5b02417
Return to citation in text: [1] -
Borah, G.; Borah, P.; Patel, P. Org. Biomol. Chem. 2017, 15, 3854–3859. doi:10.1039/C7OB00540G
Return to citation in text: [1] -
Zhang, W.; Deng, H.; Li, H. Org. Chem. Front. 2017, 4, 2202–2206. doi:10.1039/C7QO00542C
Return to citation in text: [1] -
Xia, J.; Yang, X.; Li, Y.; Li, X. Org. Lett. 2017, 19, 3243–3246. doi:10.1021/acs.orglett.7b01356
Return to citation in text: [1] -
Huang, J.; Huang, Y.; Wang, T.; Huang, Q.; Wang, Z.; Chen, Z. Org. Lett. 2017, 19, 1128–1131. doi:10.1021/acs.orglett.7b00120
Return to citation in text: [1] -
Barsu, N.; Bolli, S. K.; Sundararaju, B. Chem. Sci. 2017, 8, 2431–2435. doi:10.1039/C6SC05026C
Return to citation in text: [1] -
Wang, X.; Lerchen, A.; Glorius, F. Org. Lett. 2016, 18, 2090–2093. doi:10.1021/acs.orglett.6b00716
Return to citation in text: [1] -
Wu, F.; Zhao, Y.; Chen, W. Tetrahedron 2016, 72, 8004–8008. doi:10.1016/j.tet.2016.10.032
Return to citation in text: [1] -
Wang, J.; Zha, S.; Chen, K.; Zhang, F.; Song, C.; Zhu, J. Org. Lett. 2016, 18, 2062–2065. doi:10.1021/acs.orglett.6b00691
Return to citation in text: [1] -
Wang, H.; Lorion, M. M.; Ackermann, L. Angew. Chem., Int. Ed. 2016, 55, 10386–10390. doi:10.1002/anie.201603260
Return to citation in text: [1] -
Park, Y.; Park, K. T.; Kim, J. G.; Chang, S. J. Am. Chem. Soc. 2015, 137, 4534–4542. doi:10.1021/jacs.5b01324
Return to citation in text: [1] -
Jeon, B.; Yeon, U.; Son, J.-Y.; Lee, P. H. Org. Lett. 2016, 18, 4610–4613. doi:10.1021/acs.orglett.6b02250
Return to citation in text: [1] -
Park, Y.; Jee, S.; Kim, J. G.; Chang, S. Org. Process Res. Dev. 2015, 19, 1024–1029. doi:10.1021/acs.oprd.5b00164
Return to citation in text: [1] -
Park, J.; Lee, J.; Chang, S. Angew. Chem., Int. Ed. 2017, 56, 4256–4260. doi:10.1002/anie.201701138
Return to citation in text: [1] -
Park, J.; Chang, S. Angew. Chem., Int. Ed. 2015, 54, 14103–14107. doi:10.1002/anie.201505820
Return to citation in text: [1] -
Mei, R.; Loup, J.; Ackermann, L. ACS Catal. 2016, 6, 793–797. doi:10.1021/acscatal.5b02661
Return to citation in text: [1] -
Liang, Y.; Liang, Y.-F.; Tang, C.; Yuan, Y.; Jiao, N. Chem. – Eur. J. 2015, 21, 16395–16399. doi:10.1002/chem.201503533
Return to citation in text: [1] -
Sauermann, N.; Mei, R.; Ackermann, L. Angew. Chem., Int. Ed. 2018, 57, 5090–5094. doi:10.1002/anie.201802206
Return to citation in text: [1] -
Tian, C.; Massignan, L.; Meyer, T. H.; Ackermann, L. Angew. Chem., Int. Ed. 2018, 57, 2383–2387. doi:10.1002/anie.201712647
Return to citation in text: [1] -
Sauermann, N.; Meyer, T. H.; Tian, C.; Ackermann, L. J. Am. Chem. Soc. 2017, 139, 18452–18455. doi:10.1021/jacs.7b11025
Return to citation in text: [1] -
Sauermann, N.; Loup, J.; Kootz, D.; Yatham, V. R.; Berkessel, A.; Ackermann, L. Synthesis 2017, 49, 3476–3484. doi:10.1055/s-0036-1590471
Return to citation in text: [1] -
Mei, R.; Ackermann, L. Adv. Synth. Catal. 2016, 358, 2443–2448. doi:10.1002/adsc.201600384
Return to citation in text: [1] -
Moselage, M.; Sauermann, N.; Richter, S. C.; Ackermann, L. Angew. Chem., Int. Ed. 2015, 54, 6352–6355. doi:10.1002/anie.201412319
Return to citation in text: [1] -
Li, J.; Ackermann, L. Chem. – Eur. J. 2015, 21, 5718–5722. doi:10.1002/chem.201500552
Return to citation in text: [1] -
Punji, B.; Song, W.; Shevchenko, G. A.; Ackermann, L. Chem. – Eur. J. 2013, 19, 10605–10610. doi:10.1002/chem.201301409
Return to citation in text: [1] -
Song, W.; Ackermann, L. Angew. Chem., Int. Ed. 2012, 51, 8251–8254. doi:10.1002/anie.201202466
Return to citation in text: [1] -
Sarkar, S. D.; Liu, W.; Kozhushkov, S. I.; Ackermann, L. Adv. Synth. Catal. 2014, 356, 1461–1479. doi:10.1002/adsc.201400110
Return to citation in text: [1] -
Cheng, H.; Hernández, J. G.; Bolm, C. Adv. Synth. Catal. 2018, 360, 1800–1804. doi:10.1002/adsc.201800161
Return to citation in text: [1] [2] -
Wang, S.-B.; Gu, Q.; You, S.-L. J. Catal. 2018, 361, 393–397. doi:10.1016/j.jcat.2018.03.007
Return to citation in text: [1] [2] -
Cai, Z.-J.; Liu, C.-X.; Gu, Q.; You, S.-L. Angew. Chem., Int. Ed. 2018, 57, 1296–1299. doi:10.1002/anie.201711451
Return to citation in text: [1] -
Xu, J.; Liu, Y.; Zhang, J.; Xu, X.; Jin, Z. Chem. Commun. 2018, 54, 689–692. doi:10.1039/C7CC09273C
Return to citation in text: [1] -
Gao, D.-W.; Gu, Q.; Zheng, C.; You, S.-L. Acc. Chem. Res. 2017, 50, 351–365. doi:10.1021/acs.accounts.6b00573
Return to citation in text: [1] -
Schmiel, D.; Butenschön, H. Eur. J. Org. Chem. 2017, 3041–3048. doi:10.1002/ejoc.201700358
Return to citation in text: [1] -
Schmiel, D.; Butenschön, H. Organometallics 2017, 36, 4979–4989. doi:10.1021/acs.organomet.7b00799
Return to citation in text: [1] -
Wang, S.-B.; Gu, Q.; You, S.-L. J. Org. Chem. 2017, 82, 11829–11835. doi:10.1021/acs.joc.7b00775
Return to citation in text: [1] -
Wang, S.-B.; Gu, Q.; You, S.-L. Organometallics 2017, 36, 4359–4362. doi:10.1021/acs.organomet.7b00691
Return to citation in text: [1] -
Cera, G.; Haven, T.; Ackermann, L. Angew. Chem., Int. Ed. 2016, 55, 1484–1488. doi:10.1002/anie.201509603
Return to citation in text: [1] -
Gao, D.-W.; Gu, Q.; You, S.-L. J. Am. Chem. Soc. 2016, 138, 2544–2547. doi:10.1021/jacs.6b00127
Return to citation in text: [1] -
Shibata, T.; Uno, N.; Sasaki, T.; Kanyiva, K. S. J. Org. Chem. 2016, 81, 6266–6272. doi:10.1021/acs.joc.6b00825
Return to citation in text: [1] -
Urbano, A.; Hernández-Torres, G.; del Hoyo, A. M.; Martínez-Carrión, A.; Carmen Carreño, M. Chem. Commun. 2016, 52, 6419–6422. doi:10.1039/C6CC02624A
Return to citation in text: [1] -
Wang, S.-B.; Zheng, J.; You, S.-L. Organometallics 2016, 35, 1420–1425. doi:10.1021/acs.organomet.6b00020
Return to citation in text: [1] -
Zhu, D.-Y.; Chen, P.; Xia, J.-B. ChemCatChem 2016, 8, 68–73. doi:10.1002/cctc.201500895
Return to citation in text: [1] -
Arae, S.; Ogasawara, M. Tetrahedron Lett. 2015, 56, 1751–1761. doi:10.1016/j.tetlet.2015.01.130
Return to citation in text: [1] -
López, L. A.; López, E. Dalton Trans. 2015, 44, 10128–10135. doi:10.1039/C5DT01373A
Return to citation in text: [1] -
Deng, R.; Huang, Y.; Ma, X.; Li, G.; Zhu, R.; Wang, B.; Kang, Y.-B.; Gu, Z. J. Am. Chem. Soc. 2014, 136, 4472–4475. doi:10.1021/ja500699x
Return to citation in text: [1] -
Gao, D.-W.; Yin, Q.; Gu, Q.; You, S.-L. J. Am. Chem. Soc. 2014, 136, 4841–4844. doi:10.1021/ja500444v
Return to citation in text: [1] -
Liu, L.; Zhang, A.-A.; Zhao, R.-J.; Li, F.; Meng, T.-J.; Ishida, N.; Murakami, M.; Zhao, W.-X. Org. Lett. 2014, 16, 5336–5338. doi:10.1021/ol502520b
Return to citation in text: [1] -
Pi, C.; Cui, X.; Liu, X.; Guo, M.; Zhang, H.; Wu, Y. Org. Lett. 2014, 16, 5164–5167. doi:10.1021/ol502509f
Return to citation in text: [1] -
Shibata, T.; Shizuno, T. Angew. Chem., Int. Ed. 2014, 53, 5410–5413. doi:10.1002/anie.201402518
Return to citation in text: [1] -
Xie, W.; Li, B.; Xu, S.; Song, H.; Wang, B. Organometallics 2014, 33, 2138–2141. doi:10.1021/om5002606
Return to citation in text: [1] -
Kornhaaß, C.; Kuper, C.; Ackermann, L. Adv. Synth. Catal. 2014, 356, 1619–1624. doi:10.1002/adsc.201301156
Return to citation in text: [1] -
Arrayás, R. G.; Adrio, J.; Carretero, J. C. Angew. Chem., Int. Ed. 2006, 45, 7674–7715. doi:10.1002/anie.200602482
Return to citation in text: [1] -
Fu, G. C. Acc. Chem. Res. 2004, 37, 542–547. doi:10.1021/ar030051b
Return to citation in text: [1] -
Dai, L.-X.; Tu, T.; You, S.-L.; Deng, W.-P.; Hou, X.-L. Acc. Chem. Res. 2003, 36, 659–667. doi:10.1021/ar020153m
Return to citation in text: [1] -
Fu, G. C. Acc. Chem. Res. 2000, 33, 412–420. doi:10.1021/ar990077w
Return to citation in text: [1] -
Temnikov, M. N.; Anisimov, A. A.; Zhemchugov, P. V.; Kholodkov, D. N.; Goloveshkin, A. S.; Naumkin, A. V.; Chistovalov, S. M.; Katsoulis, D.; Muzafarov, A. M. Green Chem. 2018, 20, 1962–1969. doi:10.1039/C7GC03862C
Return to citation in text: [1] -
Hermann, G. N.; Bolm, C. ACS Catal. 2017, 7, 4592–4596. doi:10.1021/acscatal.7b00582
Return to citation in text: [1] -
Cheng, H.; Hernández, J. G.; Bolm, C. Org. Lett. 2017, 19, 6284–6287. doi:10.1021/acs.orglett.7b02973
Return to citation in text: [1] -
Ackermann, L. Acc. Chem. Res. 2014, 47, 281–295. doi:10.1021/ar3002798
Return to citation in text: [1] -
Ackermann, L. Chem. Rev. 2011, 111, 1315–1345. doi:10.1021/cr100412j
Return to citation in text: [1] -
Lapointe, D.; Fagnou, K. Chem. Lett. 2010, 39, 1118–1126. doi:10.1246/cl.2010.1118
Return to citation in text: [1] -
Ackermann, L.; Novák, P.; Vicente, R.; Hofmann, N. Angew. Chem., Int. Ed. 2009, 48, 6045–6048. doi:10.1002/anie.200902458
Return to citation in text: [1] -
Ma, W.; Mei, R.; Tenti, G.; Ackermann, L. Chem. – Eur. J. 2014, 20, 15248–15251. doi:10.1002/chem.201404604
Return to citation in text: [1] -
Hernández, J. G. Chem. – Eur. J. 2017, 23, 17157–17165. doi:10.1002/chem.201703605
Return to citation in text: [1]
| 1. | Gandeepan, P.; Ackermann, L. Chem 2018, 4, 199–222. doi:10.1016/j.chempr.2017.11.002 |
| 2. | Wei, Y.; Hu, P.; Zhang, M.; Su, W. Chem. Rev. 2017, 117, 8864–8907. doi:10.1021/acs.chemrev.6b00516 |
| 3. | Ma, W.; Gandeepan, P.; Li, J.; Ackermann, L. Org. Chem. Front. 2017, 4, 1435–1467. doi:10.1039/C7QO00134G |
| 4. | He, J.; Wasa, M.; Chan, K. S. L.; Shao, Q.; Yu, J.-Q. Chem. Rev. 2017, 117, 8754–8786. doi:10.1021/acs.chemrev.6b00622 |
| 5. | Zheng, Q.-Z.; Jiao, N. Chem. Soc. Rev. 2016, 45, 4590–4627. doi:10.1039/C6CS00107F |
| 6. | Borie, C.; Ackermann, L.; Nechab, M. Chem. Soc. Rev. 2016, 45, 1368–1386. doi:10.1039/C5CS00622H |
| 7. | Ye, B.; Cramer, N. Acc. Chem. Res. 2015, 48, 1308–1318. doi:10.1021/acs.accounts.5b00092 |
| 8. | Satoh, T.; Miura, M. Chem. – Eur. J. 2010, 16, 11212–11222. doi:10.1002/chem.201001363 |
| 9. | Ackermann, L.; Vicente, R.; Kapdi, A. R. Angew. Chem., Int. Ed. 2009, 121, 9976–10011. doi:10.1002/ange.200902996 |
| 42. | Park, Y.; Kim, Y.; Chang, S. Chem. Rev. 2017, 117, 9247–9301. doi:10.1021/acs.chemrev.6b00644 |
| 43. | Jiao, J.; Murakami, K.; Itami, K. ACS Catal. 2016, 6, 610–633. doi:10.1021/acscatal.5b02417 |
| 24. | Zell, D.; Bursch, M.; Müller, V.; Grimme, S.; Ackermann, L. Angew. Chem., Int. Ed. 2017, 56, 10378–10382. doi:10.1002/anie.201704196 |
| 105. | Ma, W.; Mei, R.; Tenti, G.; Ackermann, L. Chem. – Eur. J. 2014, 20, 15248–15251. doi:10.1002/chem.201404604 |
| 23. | Zell, D.; Müller, V.; Dhawa, U.; Bursch, M.; Presa, R. R.; Grimme, S.; Ackermann, L. Chem. – Eur. J. 2017, 23, 12145–12148. doi:10.1002/chem.201702528 |
| 24. | Zell, D.; Bursch, M.; Müller, V.; Grimme, S.; Ackermann, L. Angew. Chem., Int. Ed. 2017, 56, 10378–10382. doi:10.1002/anie.201704196 |
| 25. | Ikemoto, H.; Tanaka, R.; Sakata, K.; Kanai, M.; Yoshino, T.; Matsunaga, S. Angew. Chem., Int. Ed. 2017, 56, 7156–7160. doi:10.1002/anie.201703193 |
| 26. | Yan, Q.; Chen, Z.; Liu, Z.; Zhang, Y. Org. Chem. Front. 2016, 3, 678–682. doi:10.1039/C6QO00059B |
| 27. | Lu, Q.; Vásquez-Céspedes, S.; Gensch, T.; Glorius, F. ACS Catal. 2016, 6, 2352–2356. doi:10.1021/acscatal.6b00367 |
| 28. | Kong, L.; Yu, S.; Zhou, X.; Li, X. Org. Lett. 2016, 18, 588–591. doi:10.1021/acs.orglett.5b03629 |
| 29. | Kalsi, D.; Laskar, R. A.; Barsu, N.; Premkumar, J. R.; Sundararaju, B. Org. Lett. 2016, 18, 4198–4201. doi:10.1021/acs.orglett.6b01845 |
| 30. | Bunno, Y.; Murakami, N.; Suzuki, Y.; Kanai, M.; Yoshino, T.; Matsunaga, S. Org. Lett. 2016, 18, 2216–2219. doi:10.1021/acs.orglett.6b00846 |
| 31. | Boerth, J. A.; Hummel, J. R.; Ellman, J. A. Angew. Chem., Int. Ed. 2016, 55, 12650–12654. doi:10.1002/anie.201603831 |
| 32. | Hummel, J. R.; Ellman, J. A. J. Am. Chem. Soc. 2015, 137, 490–498. doi:10.1021/ja5116452 |
| 33. | Wang, H.; Koeller, J.; Liu, W.; Ackermann, L. Chem. – Eur. J. 2015, 21, 15525–15528. doi:10.1002/chem.201503624 |
| 34. | Suzuki, Y.; Sun, B.; Sakata, K.; Yoshino, T.; Matsunaga, S.; Kanai, M. Angew. Chem., Int. Ed. 2015, 54, 9944–9947. doi:10.1002/anie.201503704 |
| 35. | Sen, M.; Kalsi, D.; Sundararaju, B. Chem. – Eur. J. 2015, 21, 15529–15533. doi:10.1002/chem.201503643 |
| 36. | Sauermann, N.; González, M. J.; Ackermann, L. Org. Lett. 2015, 17, 5316–5319. doi:10.1021/acs.orglett.5b02678 |
| 37. | Ma, W.; Ackermann, L. ACS Catal. 2015, 5, 2822–2825. doi:10.1021/acscatal.5b00322 |
| 38. | Li, J.; Ackermann, L. Angew. Chem., Int. Ed. 2015, 54, 3635–3638. doi:10.1002/anie.201409247 |
| 39. | Li, J.; Ackermann, L. Angew. Chem., Int. Ed. 2015, 54, 8551–8554. doi:10.1002/anie.201501926 |
| 40. | Ikemoto, H.; Yoshino, T.; Sakata, K.; Matsunaga, S.; Kanai, M. J. Am. Chem. Soc. 2014, 136, 5424–5431. doi:10.1021/ja5008432 |
| 41. | Yoshino, T.; Ikemoto, H.; Matsunaga, S.; Kanai, M. Angew. Chem., Int. Ed. 2013, 52, 2207–2211. doi:10.1002/anie.201209226 |
| 106. | Hernández, J. G. Chem. – Eur. J. 2017, 23, 17157–17165. doi:10.1002/chem.201703605 |
| 18. | Yoshino, T.; Matsunaga, S. Adv. Synth. Catal. 2017, 359, 1245–1262. doi:10.1002/adsc.201700042 |
| 19. | Wei, D.; Zhu, X.; Niu, J.-L.; Song, M.-P. ChemCatChem 2016, 8, 1242–1263. doi:10.1002/cctc.201600040 |
| 20. | Moselage, M.; Li, J.; Ackermann, L. ACS Catal. 2016, 6, 498–525. doi:10.1021/acscatal.5b02344 |
| 21. | Gao, K.; Yoshikai, N. Acc. Chem. Res. 2014, 47, 1208–1219. doi:10.1021/ar400270x |
| 22. | Ackermann, L. J. Org. Chem. 2014, 79, 8948–8954. doi:10.1021/jo501361k |
| 98. | Temnikov, M. N.; Anisimov, A. A.; Zhemchugov, P. V.; Kholodkov, D. N.; Goloveshkin, A. S.; Naumkin, A. V.; Chistovalov, S. M.; Katsoulis, D.; Muzafarov, A. M. Green Chem. 2018, 20, 1962–1969. doi:10.1039/C7GC03862C |
| 99. | Hermann, G. N.; Bolm, C. ACS Catal. 2017, 7, 4592–4596. doi:10.1021/acscatal.7b00582 |
| 100. | Cheng, H.; Hernández, J. G.; Bolm, C. Org. Lett. 2017, 19, 6284–6287. doi:10.1021/acs.orglett.7b02973 |
| 10. | Hu, Y.; Zhou, B.; Wang, C. Acc. Chem. Res. 2018, 51, 816–827. doi:10.1021/acs.accounts.8b00028 |
| 11. | Liu, W.; Ackermann, L. ACS Catal. 2016, 6, 3743–3752. doi:10.1021/acscatal.6b00993 |
| 12. | Cera, G.; Ackermann, L. Top. Curr. Chem. 2016, 374, 191–224. doi:10.1007/s41061-016-0059-6 |
| 13. | Nakao, Y. Chem. Rec. 2011, 11, 242–251. doi:10.1002/tcr.201100023 |
| 14. | Castro, L. C. M.; Chatani, N. Chem. Lett. 2015, 44, 410–421. doi:10.1246/cl.150024 |
| 15. | Hirano, K.; Miura, M. Chem. Lett. 2015, 44, 868–873. doi:10.1246/cl.150354 |
| 16. | Yamaguchi, J.; Muto, K.; Itami, K. Eur. J. Org. Chem. 2013, 19–30. doi:10.1002/ejoc.201200914 |
| 17. | Kulkarni, A. A.; Daugulis, O. Synthesis 2009, 4087–4109. doi:10.1055/s-0029-1217131 |
| 101. | Ackermann, L. Acc. Chem. Res. 2014, 47, 281–295. doi:10.1021/ar3002798 |
| 102. | Ackermann, L. Chem. Rev. 2011, 111, 1315–1345. doi:10.1021/cr100412j |
| 103. | Lapointe, D.; Fagnou, K. Chem. Lett. 2010, 39, 1118–1126. doi:10.1246/cl.2010.1118 |
| 104. | Ackermann, L.; Novák, P.; Vicente, R.; Hofmann, N. Angew. Chem., Int. Ed. 2009, 48, 6045–6048. doi:10.1002/anie.200902458 |
| 70. | Cheng, H.; Hernández, J. G.; Bolm, C. Adv. Synth. Catal. 2018, 360, 1800–1804. doi:10.1002/adsc.201800161 |
| 71. | Wang, S.-B.; Gu, Q.; You, S.-L. J. Catal. 2018, 361, 393–397. doi:10.1016/j.jcat.2018.03.007 |
| 94. | Arrayás, R. G.; Adrio, J.; Carretero, J. C. Angew. Chem., Int. Ed. 2006, 45, 7674–7715. doi:10.1002/anie.200602482 |
| 95. | Fu, G. C. Acc. Chem. Res. 2004, 37, 542–547. doi:10.1021/ar030051b |
| 96. | Dai, L.-X.; Tu, T.; You, S.-L.; Deng, W.-P.; Hou, X.-L. Acc. Chem. Res. 2003, 36, 659–667. doi:10.1021/ar020153m |
| 97. | Fu, G. C. Acc. Chem. Res. 2000, 33, 412–420. doi:10.1021/ar990077w |
| 69. | Sarkar, S. D.; Liu, W.; Kozhushkov, S. I.; Ackermann, L. Adv. Synth. Catal. 2014, 356, 1461–1479. doi:10.1002/adsc.201400110 |
| 70. | Cheng, H.; Hernández, J. G.; Bolm, C. Adv. Synth. Catal. 2018, 360, 1800–1804. doi:10.1002/adsc.201800161 |
| 71. | Wang, S.-B.; Gu, Q.; You, S.-L. J. Catal. 2018, 361, 393–397. doi:10.1016/j.jcat.2018.03.007 |
| 60. | Sauermann, N.; Mei, R.; Ackermann, L. Angew. Chem., Int. Ed. 2018, 57, 5090–5094. doi:10.1002/anie.201802206 |
| 61. | Tian, C.; Massignan, L.; Meyer, T. H.; Ackermann, L. Angew. Chem., Int. Ed. 2018, 57, 2383–2387. doi:10.1002/anie.201712647 |
| 62. | Sauermann, N.; Meyer, T. H.; Tian, C.; Ackermann, L. J. Am. Chem. Soc. 2017, 139, 18452–18455. doi:10.1021/jacs.7b11025 |
| 63. | Sauermann, N.; Loup, J.; Kootz, D.; Yatham, V. R.; Berkessel, A.; Ackermann, L. Synthesis 2017, 49, 3476–3484. doi:10.1055/s-0036-1590471 |
| 64. | Mei, R.; Ackermann, L. Adv. Synth. Catal. 2016, 358, 2443–2448. doi:10.1002/adsc.201600384 |
| 65. | Moselage, M.; Sauermann, N.; Richter, S. C.; Ackermann, L. Angew. Chem., Int. Ed. 2015, 54, 6352–6355. doi:10.1002/anie.201412319 |
| 66. | Li, J.; Ackermann, L. Chem. – Eur. J. 2015, 21, 5718–5722. doi:10.1002/chem.201500552 |
| 67. | Punji, B.; Song, W.; Shevchenko, G. A.; Ackermann, L. Chem. – Eur. J. 2013, 19, 10605–10610. doi:10.1002/chem.201301409 |
| 68. | Song, W.; Ackermann, L. Angew. Chem., Int. Ed. 2012, 51, 8251–8254. doi:10.1002/anie.201202466 |
| 44. | Borah, G.; Borah, P.; Patel, P. Org. Biomol. Chem. 2017, 15, 3854–3859. doi:10.1039/C7OB00540G |
| 45. | Zhang, W.; Deng, H.; Li, H. Org. Chem. Front. 2017, 4, 2202–2206. doi:10.1039/C7QO00542C |
| 46. | Xia, J.; Yang, X.; Li, Y.; Li, X. Org. Lett. 2017, 19, 3243–3246. doi:10.1021/acs.orglett.7b01356 |
| 47. | Huang, J.; Huang, Y.; Wang, T.; Huang, Q.; Wang, Z.; Chen, Z. Org. Lett. 2017, 19, 1128–1131. doi:10.1021/acs.orglett.7b00120 |
| 48. | Barsu, N.; Bolli, S. K.; Sundararaju, B. Chem. Sci. 2017, 8, 2431–2435. doi:10.1039/C6SC05026C |
| 49. | Wang, X.; Lerchen, A.; Glorius, F. Org. Lett. 2016, 18, 2090–2093. doi:10.1021/acs.orglett.6b00716 |
| 50. | Wu, F.; Zhao, Y.; Chen, W. Tetrahedron 2016, 72, 8004–8008. doi:10.1016/j.tet.2016.10.032 |
| 51. | Wang, J.; Zha, S.; Chen, K.; Zhang, F.; Song, C.; Zhu, J. Org. Lett. 2016, 18, 2062–2065. doi:10.1021/acs.orglett.6b00691 |
| 52. | Wang, H.; Lorion, M. M.; Ackermann, L. Angew. Chem., Int. Ed. 2016, 55, 10386–10390. doi:10.1002/anie.201603260 |
| 53. | Park, Y.; Park, K. T.; Kim, J. G.; Chang, S. J. Am. Chem. Soc. 2015, 137, 4534–4542. doi:10.1021/jacs.5b01324 |
| 54. | Jeon, B.; Yeon, U.; Son, J.-Y.; Lee, P. H. Org. Lett. 2016, 18, 4610–4613. doi:10.1021/acs.orglett.6b02250 |
| 55. | Park, Y.; Jee, S.; Kim, J. G.; Chang, S. Org. Process Res. Dev. 2015, 19, 1024–1029. doi:10.1021/acs.oprd.5b00164 |
| 56. | Park, J.; Lee, J.; Chang, S. Angew. Chem., Int. Ed. 2017, 56, 4256–4260. doi:10.1002/anie.201701138 |
| 57. | Park, J.; Chang, S. Angew. Chem., Int. Ed. 2015, 54, 14103–14107. doi:10.1002/anie.201505820 |
| 58. | Mei, R.; Loup, J.; Ackermann, L. ACS Catal. 2016, 6, 793–797. doi:10.1021/acscatal.5b02661 |
| 59. | Liang, Y.; Liang, Y.-F.; Tang, C.; Yuan, Y.; Jiao, N. Chem. – Eur. J. 2015, 21, 16395–16399. doi:10.1002/chem.201503533 |
| 72. | Cai, Z.-J.; Liu, C.-X.; Gu, Q.; You, S.-L. Angew. Chem., Int. Ed. 2018, 57, 1296–1299. doi:10.1002/anie.201711451 |
| 73. | Xu, J.; Liu, Y.; Zhang, J.; Xu, X.; Jin, Z. Chem. Commun. 2018, 54, 689–692. doi:10.1039/C7CC09273C |
| 74. | Gao, D.-W.; Gu, Q.; Zheng, C.; You, S.-L. Acc. Chem. Res. 2017, 50, 351–365. doi:10.1021/acs.accounts.6b00573 |
| 75. | Schmiel, D.; Butenschön, H. Eur. J. Org. Chem. 2017, 3041–3048. doi:10.1002/ejoc.201700358 |
| 76. | Schmiel, D.; Butenschön, H. Organometallics 2017, 36, 4979–4989. doi:10.1021/acs.organomet.7b00799 |
| 77. | Wang, S.-B.; Gu, Q.; You, S.-L. J. Org. Chem. 2017, 82, 11829–11835. doi:10.1021/acs.joc.7b00775 |
| 78. | Wang, S.-B.; Gu, Q.; You, S.-L. Organometallics 2017, 36, 4359–4362. doi:10.1021/acs.organomet.7b00691 |
| 79. | Cera, G.; Haven, T.; Ackermann, L. Angew. Chem., Int. Ed. 2016, 55, 1484–1488. doi:10.1002/anie.201509603 |
| 80. | Gao, D.-W.; Gu, Q.; You, S.-L. J. Am. Chem. Soc. 2016, 138, 2544–2547. doi:10.1021/jacs.6b00127 |
| 81. | Shibata, T.; Uno, N.; Sasaki, T.; Kanyiva, K. S. J. Org. Chem. 2016, 81, 6266–6272. doi:10.1021/acs.joc.6b00825 |
| 82. | Urbano, A.; Hernández-Torres, G.; del Hoyo, A. M.; Martínez-Carrión, A.; Carmen Carreño, M. Chem. Commun. 2016, 52, 6419–6422. doi:10.1039/C6CC02624A |
| 83. | Wang, S.-B.; Zheng, J.; You, S.-L. Organometallics 2016, 35, 1420–1425. doi:10.1021/acs.organomet.6b00020 |
| 84. | Zhu, D.-Y.; Chen, P.; Xia, J.-B. ChemCatChem 2016, 8, 68–73. doi:10.1002/cctc.201500895 |
| 85. | Arae, S.; Ogasawara, M. Tetrahedron Lett. 2015, 56, 1751–1761. doi:10.1016/j.tetlet.2015.01.130 |
| 86. | López, L. A.; López, E. Dalton Trans. 2015, 44, 10128–10135. doi:10.1039/C5DT01373A |
| 87. | Deng, R.; Huang, Y.; Ma, X.; Li, G.; Zhu, R.; Wang, B.; Kang, Y.-B.; Gu, Z. J. Am. Chem. Soc. 2014, 136, 4472–4475. doi:10.1021/ja500699x |
| 88. | Gao, D.-W.; Yin, Q.; Gu, Q.; You, S.-L. J. Am. Chem. Soc. 2014, 136, 4841–4844. doi:10.1021/ja500444v |
| 89. | Liu, L.; Zhang, A.-A.; Zhao, R.-J.; Li, F.; Meng, T.-J.; Ishida, N.; Murakami, M.; Zhao, W.-X. Org. Lett. 2014, 16, 5336–5338. doi:10.1021/ol502520b |
| 90. | Pi, C.; Cui, X.; Liu, X.; Guo, M.; Zhang, H.; Wu, Y. Org. Lett. 2014, 16, 5164–5167. doi:10.1021/ol502509f |
| 91. | Shibata, T.; Shizuno, T. Angew. Chem., Int. Ed. 2014, 53, 5410–5413. doi:10.1002/anie.201402518 |
| 92. | Xie, W.; Li, B.; Xu, S.; Song, H.; Wang, B. Organometallics 2014, 33, 2138–2141. doi:10.1021/om5002606 |
| 93. | Kornhaaß, C.; Kuper, C.; Ackermann, L. Adv. Synth. Catal. 2014, 356, 1619–1624. doi:10.1002/adsc.201301156 |
© 2018 Yetra et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)