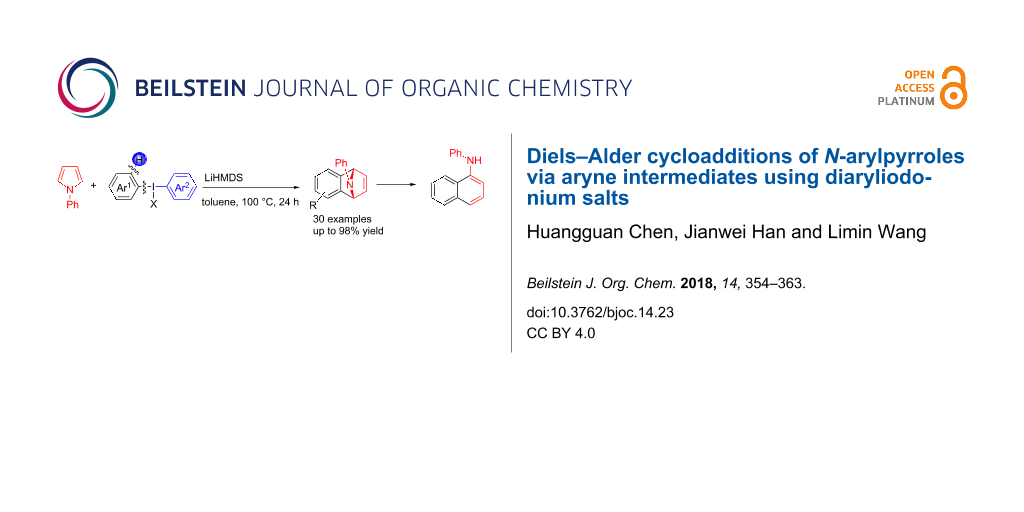
Abstract
With a strategy of the formation of benzynes by using diaryliodonium salts, a cycloaddition reaction of N-arylpyrroles with benzynes was reported. A wide range of bridge-ring amines with various substituents have been synthesized in moderate to excellent yields (35–96%). Furthermore, with a catalytic amount of TsOH·H2O, these amines can be converted into the corresponding N-phenylamine derivatives easily, which are potentially useful in photosensitive dyes.
Graphical Abstract
Introduction
Pyrrole is a very useful heterocyclic substrate to produce structural attributes of valuable chemicals, functional materials and pharmaceuticals [1-5]. Recently, arylation of pyrrole derivatives with diaryliodonium salts for pyrrole–aryl coupling products is generating tremendous academic interest in organic synthesis. In 2012, the Zhang and Yu group reported that sodium hydroxide promoted direct arylation of unprotected pyrroles with diaryliodonium salts at the temperature of 80 °C, the coupling products were obtained in moderate to good yields (Scheme 1a) [6]. Later in 2013, Xue and Xiao et al. developed a method of photoredox catalysis in the presence of [Ru(bpy)3]2+ with visible light for the coupling reaction of arenes with unprotected or N-substituted pyrroles, pyrrole substrates were well tolerated with N-methyl and N-phenyl groups (Scheme 1a) [7]. Ackermann et al. employed a 2,5-dimethylpyrrole derivative as substrate to deliver double arylated products at 3,4-positions of the pyrrole ring (Scheme 1b) [8]. Recently, the research group of Kita documented an oxidative biaryl coupling for pyrroles using a hypervalent iodine reagent and a stabilizer for pyrrolyliodonium intermediates (Scheme 1c) [9]. The reactions readily provided a variety of desired coupling products in good yields. In general, the mechanism of these arylations was postulated by generating aryl radicals with diaryiodonium salts in the literature.
Scheme 1: Arylations of pyrrole derivatives with diaryliodonium salts.
Scheme 1: Arylations of pyrrole derivatives with diaryliodonium salts.
In 1995, Kitamura prepared phenyl[o-(trimethylsilyl)phenyl]iodonium triflate which could be used as an efficient benzyne precursor in trapping furans [10]. Surprisingly, the research groups of Stuart [11,12] and Wang [13] independently discovered in 2016 that simple diaryliodonium salts can generate benzynes under severe basic conditions, the resulted benzynes were allowed to undergo cycloaddition reaction with furan or N-arylation of secondary amides and amines. Due to the easy accessibility of the diaryliodonium salts, this kind of benzyne precursor is attracting extensive attention [14-16]. Also as a five-membered heterocyclic ring, the cycloaddition reaction of N-substituted pyrroles is much less than that of furan [17-21]. As a matter of fact, the Diels–Alder adduct formation of pyrroles with benzyne has been postulated in 1965 as transient products under thermal conditions to afford arylamines [22]. Inspired by the pioneering work of Stuart and Wang [12,13], herein we reported the usage of diaryliodonium salts as aryne precursor for Diels–Alder cycloadditions of N-arylpyrroles (Scheme 1d).
Results and Discussion
We initially started the cycloaddition reaction of 1-phenylpyrrole (1a) using phenyl(mesityl)iodonium tosylate (2a) as benzyne precursor. To our delight, with LiHMDS as the base in toluene, the Diels–Alder adduct 3aa was obtained in 23% yield at room temperature (Table 1, entry 1). However, when the reaction temperature was increased to 100 oC or the solvent was changed to THF, we found a slight decrease in the yield of 3aa (Table 1, entries 2 and 3). Interestingly, the reaction stoichiometry of 1a and 2a had a significant influence on the yield of 3aa, which was similar to Stuart’s work [11,12] (Table 1, entry 4–8). Further examinations of bases did not lead to better results (Table 1, entries 9–14). We then chose LiHMDS as the optimal base for the reaction. The reaction yield could be improved to 85% when an excess amount of LiHMDS was used (Table 1, entries 15–17). However, a screening of reaction temperature, solvent, and reaction time did not improve the yield of 3aa (Table 1, entries 18–21).
Table 1: Optimization of reaction conditions.a
|
|
||||
| entry | 1a/2a (equiv) | base (equiv) | solvent | 3aa (%)b |
| 1 | 1:1.2 | LiHMDS (1.2) | toluene | 23 |
| 2c | 1:1.2 | LiHMDS (1.2) | toluene | 22 |
| 3 | 1:1.2 | LiHMDS (1.2) | THF | 20 |
| 4 | 1:3 | LiHMDS (3) | toluene | 40 |
| 5 | 3:1 | LiHMDS (1) | toluene | 59 |
| 6 | 4:1 | LiHMDS (1) | toluene | 73 |
| 7 | 5:1 | LiHMDS (1) | toluene | 80 |
| 8 | 6:1 | LiHMDS (1) | toluene | 74 |
| 9 | 5:1 | KHMDS (1.5) | toluene | 68 |
| 10 | 5:1 | KOt-Bu (1) | toluene | 60 |
| 11 | 5:1 | NaNH2 (1) | toluene | 39 |
| 12 | 5:1 | KOt-Bu (2) | toluene | 65 |
| 13 | 5:1 | NaOMe (2) | toluene | 40 |
| 14 | 5:1 | NaH (2) | toluene | n. r. |
| 15 | 5:1 | LiHMDS (1.2) | toluene | 79 |
| 16 | 5:1 | LiHMDS (1.5) | toluene | 85 |
| 17 | 5:1 | LiHMDS (2) | toluene | 74 |
| 18d | 5:1 | LiHMDS (1.5) | toluene | 70 |
| 19 | 5:1 | LiHMDS (1.5) | THF | 73 |
| 20 | 5:1 | LiHMDS (1.5) | MeCN | n. r. |
| 21e | 5:1 | LiHMDS (1.5) | toluene | 73 |
aReaction conditions: 1a or 2a (0.5 mmol, 1 equiv), base (0.5–0.75 mmol, 1–1.5 equiv), solvent (5 mL), 0 °C to rt, 9 h. bIsolated yield. cThe reaction temperature was 100 °C. dThe reaction temperature was 80 °C. eThe reaction was quenched after 13 hours. n. r. = no reaction.
With the optimal reaction conditions in hand, various aryl(mesityl)iodonium salts 2 were examined. As shown in Table 2, an extensive range of substituted aryl(mesityl)iodonium salts, bearing a wide variety of substituent groups, could react with 1a to afford the corresponding cycloaddition adducts 3. It was observed that the reaction gave the desired products 3ab and 3ac in moderate yields of 63% and 57% when iodonium salts 2 have electron-donating groups in the para-position of the aryl moiety, such as methyl groups and tert-butyl groups (Table 2, entries 2 and 3). For those bearing electron-withdrawing groups, such as halogens (F, Cl, Br), cyano, nitro, trifluoromethyl, and trifluoromethoxy groups, the corresponding products 3ad–3aj were obtained in good to excellent yields of 67–96% (Table 2, entries 4–10). It was found that the reactions underwent smoothly to give the products 3ak, 3al in good yields of 89% and 82%, respectively, when there was a phenyl group on the para- or ortho-positions of the aryl moiety for diaryliodonium salts (Table 2, entries 11 and 12). Analogous to previous work [11,12], when 2m and 2n were employed, the cycloaddition regioselectively afforded 3am and 3an in good yields of 80% and 71%, respectively (Table 2, entries 13 and 14). Of note, substituents at the ortho-position on the aryl moiety with 2, regardless of their electronic properties, had a negative effect on the reactivity (Table 2, entries 15–19).
Table 2: Scope of diaryliodonium salts 2.a
|
|
|||
| entry | aryl(mesityl)iodonium salts | product | yield (%)b |
| 1 |
2a |
3aa |
85 |
| 2 |
2b |
3ab |
63 |
| 3 |
2c |
3ac |
57 |
| 4 |
2d |
3ad |
77 |
| 5 |
2e |
3ae |
87 |
| 6 |
2f |
3af |
96 |
| 7 |
2g |
3ag |
71 |
| 8 |
2h |
3ah |
67 |
| 9 |
2i |
3ai |
77 |
| 10 |
2j |
3aj |
88 |
| 11 |
2k |
3ak |
89 |
| 12 |
2l |
3al |
82 |
| 13 |
2m |
3am |
80 |
| 14 |
2n |
3an |
71 |
| 15 |
2o |
3ao |
78 |
| 16 |
2p |
3ap |
60 |
| 17 |
2q |
3aq |
48 |
| 18 |
2r |
3ar |
62 |
| 19 |
2s |
3as |
58 |
aReaction conditions: 1a (2.5 mmol, 5 equiv), 2 (0.5 mmol), LiHMDS (1 M in toluene, 0.75 mL, 1.5 equiv), toluene (5 mL), 0 °C to rt, 9 h. bIsolated yield. Mes = 2,4,6-trimethylphenyl, OTs = 4-toluenesulfonate, OTf = trifluoromethansulfonate.
To further probe the scope of this reaction, a wide range of 1-arylpyrroles 1 was employed in the reaction under the standard conditions. Generally, the conditions proved to be efficient for this Diels–Alder cycloaddition. As shown in Table 3, the electronic properties of aryl substituents had a little influence on the reaction outcome. For example, 1-phenylpyrrole with electron-donating groups (Me, t-Bu, OMe) gave 3ba–3da in good yields of 62–83% (Table 3, entries 1–3). Meanwhile, 1-phenylpyrrole with electron-withdrawing groups (F, Cl, Br, CF3, OCF3, CN) also gave the corresponding products 3ea–3ja in good to excellent yields of 71–93% (Table 3, entry 4–9). However, when R was biphenyl, the desired product 3ka was only obtained in moderate yield of 35%, probably due to the poor solubility of the starting materials (Table 3, entry 10). In contrast, when N-substituents (R) were Ts, Boc, Bn or methyl, no desired product was detected by thin layer chromatography (TLC) experiments (Table 3, entries 11–13). Interestingly, the method of Lautens works with an N-Boc pyrrole [21].
Table 3: Scope of N-substituted pyrroles 1.a
|
|
|||
| entry | R | product | yield (%)b |
|---|---|---|---|
| 1 |
1b |
3ba |
77 |
| 2 |
1c |
3ca |
83 |
| 3 |
1d |
3da |
62 |
| 4 |
1e |
3ea |
82 |
| 5 |
1f |
3fa |
81 |
| 6 |
1g |
3ga |
71 |
| 7 |
1h |
3ha |
90 |
| 8 |
1i |
3ia |
83 |
| 9 |
1j |
3ja |
93 |
| 10 |
1k |
3ka |
35 |
| 11 |
1l |
3la |
0 |
| 12 |
1m |
3ma |
0 |
| 13 |
1n |
3na |
0 |
aReaction conditions: 1 (2.5 mmol, 5 equiv), 2a (0.5 mmol), LiHMDS (1 M in toluene, 0.75 mL, 1.5 equiv), toluene (5 mL), 0 °C to rt, 9 h. bIsolated yield. Mes = 2,4,6-trimethylphenyl. OTs = 4-toluenesulfonate.
To demonstrate the practical utility of this methodology, treatment of 3aa with 20 mol % TsOH·H2O in DCE at 80 °C resulted in N-phenylnaphthalen-1-ylamine (4) in 93% yield [23], which was widely used in dye-sensitized solar cells [24], hole transport materials [25,26] and organic light-emitting diodes (OLEDs, Scheme 2a) [27]. In another similar reaction with 3am, a novel N-phenylamine derivative 5 was synthesized in 75% yield (Scheme 2b), whose structure was determined by 2D-NMR analyses (see Supporting Information File 1). Furthermore, as a unique electron donor, the novel compound 5 may have potential applications in photosensitive dyes and OLEDs [28,29]. Interestingly, the bridged-ring compound 6 could be easily obtained in 63% yield with palladium on carbon catalyst under hydrogen atmosphere at room temperature (Scheme 3).
Scheme 2: Formation of N-phenylamine derivatives 4 and 5 via ring opening reactions.
Scheme 2: Formation of N-phenylamine derivatives 4 and 5 via ring opening reactions.
Scheme 3: Preparation of product 6 by hydrogenation.
Scheme 3: Preparation of product 6 by hydrogenation.
Conclusion
In summary, we have demonstrated a Diels–Alder cycloaddition of N-arylpyrroles by using diaryliodonium salts under mild conditions. The synthetic method was extended to a wide range of substrates. As such, various bridged-ring amines were prepared in moderate to excellent yields of 35–96%. Additionally, the resulting products could be easily converted to N-phenylamine derivatives and hydrogenated products in good yields. Further investigations on the application of this transformation are underway in our laboratory.
Supporting Information
| Supporting Information File 1: Experimental procedures and characterization data of all products, copies of 1H, 13C, 19F NMR and HRMS spectra of all compounds. | ||
| Format: PDF | Size: 4.4 MB | Download |
Acknowledgements
The work was supported by the National Nature Science Foundation of China (NSFC 21472213, 21272069), National Key Program (2016YFA0200302, Study on application and preparation of aroma nanocomposites), the Fundamental Research Funds for the Central Universities and Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences.
References
-
Baltazzi, E.; Krimen, L. I. Chem. Rev. 1963, 63, 511–556. doi:10.1021/cr60225a004
Return to citation in text: [1] -
Gholap, S. S. Eur. J. Med. Chem. 2016, 110, 13–31. doi:10.1016/j.ejmech.2015.12.017
Return to citation in text: [1] -
Gryko, D. T.; Vakuliuk, O.; Gryko, D.; Koszarna, B. J. Org. Chem. 2009, 74, 9517–9520. doi:10.1021/jo902124c
Return to citation in text: [1] -
Antilla, J. C.; Baskin, J. M.; Barder, T. E.; Buchwald, S. L. J. Org. Chem. 2004, 69, 5578–5587. doi:10.1021/jo049658b
Return to citation in text: [1] -
Honraedt, A.; Raux, M.-A.; Grognec, E. L.; Jacquemin, D.; Felpin, F.-X. Chem. Commun. 2014, 50, 5236–5238. doi:10.1039/C3CC45240A
Return to citation in text: [1] -
Wen, J.; Zhang, R.-Y.; Chen, S.-Y.; Zhang, J.; Yu, X.-Q. J. Org. Chem. 2012, 77, 766–771. doi:10.1021/jo202150t
Return to citation in text: [1] -
Liu, Y.-X.; Xue, D.; Wang, J.-D.; Zhao, C.-J.; Zou, Q.-Z.; Wang, C.; Xiao, J. Synlett 2013, 24, 507–513. doi:10.1055/s-0032-1318155
Return to citation in text: [1] -
Ackermann, L.; Dell’Acqua, M.; Fenner, S.; Vicente, R.; Sandmann, R. Org. Lett. 2011, 13, 2358–2360. doi:10.1021/ol200601e
Return to citation in text: [1] -
Morimoto, K.; Ohnishi, Y.; Koseki, D.; Nakamura, A.; Dohi, T.; Kita, Y. Org. Biomol. Chem. 2016, 14, 8947–8951. doi:10.1039/C6OB01764A
Return to citation in text: [1] -
Kitamura, T.; Yamane, M. J. Chem. Soc., Chem. Commun. 1995, 983–984. doi:10.1039/c39950000983
Return to citation in text: [1] -
Sundalam, S. K.; Nilova, A.; Seidl, T. L.; Stuart, D. R. Angew. Chem., Int. Ed. 2016, 55, 8431–8434. doi:10.1002/anie.201603222
Return to citation in text: [1] [2] [3] -
Stuart, D. R. Synlett 2017, 28, 275–279. doi:10.1055/s-0036-1588683
Return to citation in text: [1] [2] [3] [4] -
Wang, M.; Huang, Z. Org. Biomol. Chem. 2016, 14, 10185–10188. doi:10.1039/C6OB01649A
Return to citation in text: [1] [2] -
Stridfeldt, E.; Lindstedt, E.; Reitti, M.; Blid, J.; Norrby, P.-O.; Olofsson, B. Chem. – Eur. J. 2017, 23, 13249–13258. doi:10.1002/chem.201703057
Return to citation in text: [1] -
Yoshimura, A.; Fuchs, J. M.; Middleton, K. R.; Maskaev, A. V.; Rohde, G. T.; Saito, A.; Postnikov, P. S.; Yusubov, M. S.; Nemykin, V. N.; Zhdankin, V. V. Chem. – Eur. J. 2017, 23, 16738–16742. doi:10.1002/chem.201704393
Return to citation in text: [1] -
Robidas, R.; Guérin, V.; Provençal, L.; Echeverria, M.; Legault, C. Y. Org. Lett. 2017, 19, 6420–6423. doi:10.1021/acs.orglett.7b03307
Return to citation in text: [1] -
Caster, K. C.; Keck, C. G.; Walls, R. D. J. Org. Chem. 2001, 66, 2932–2936. doi:10.1021/jo001277k
Return to citation in text: [1] -
Davies, J. W.; Durrant, M. L.; Walker, M. P.; Belkacemi, D.; Malpass, J. R. Tetrahedron 1992, 48, 861–884. doi:10.1016/S0040-4020(01)88190-4
Return to citation in text: [1] -
Sumida, Y.; Kato, T.; Hosoya, T. Org. Lett. 2013, 15, 2806–2809. doi:10.1021/ol401140d
Return to citation in text: [1] -
Chen, J.; Baire, B.; Hoye, T. R. Heterocycles 2014, 88, 1191–1200. doi:10.3987/COM-13-S(S)83
Return to citation in text: [1] -
McManus, H. A.; Fleming, M. J.; Lautens, M. Angew. Chem., Int. Ed. 2007, 46, 433–436. doi:10.1002/anie.200603945
Return to citation in text: [1] [2] -
Wolthuis, E.; Jagt, D. V.; Mels, S.; De Boer, A. J. Org. Chem. 1965, 30, 190–193. doi:10.1021/jo01012a044
Return to citation in text: [1] -
Lin, C.; Zhen, L.; Cheng, Y.; Du, H.-J.; Zhao, H.; Wen, X.; Kong, L.-Y.; Xu, Q.-L.; Sun, H. Org. Lett. 2015, 17, 2684–2687. doi:10.1021/acs.orglett.5b01078
Return to citation in text: [1] -
Li, P.; Wang, Z.; Song, C.; Zhang, H. J. Mater. Chem. C 2017, 5, 11454–11465. doi:10.1039/C7TC03112B
Return to citation in text: [1] -
Shaikh, A. M.; Sharma, B. K.; Chacko, S.; Kamble, R. M. RSC Adv. 2016, 6, 94218–94227. doi:10.1039/C6RA20964E
Return to citation in text: [1] -
Piechowska, J.; Gryko, D. T. J. Org. Chem. 2011, 76, 10220–10228. doi:10.1021/jo202072d
Return to citation in text: [1] -
Chen, H.; Gao, C.-H.; Jiang, Z.-Q.; Zhang, L.; Cui, L.-S.; Ji, S.-J.; Liao, L.-S. Dyes Pigm. 2014, 107, 15–20. doi:10.1016/j.dyepig.2014.03.006
Return to citation in text: [1] -
Moorthy, J. N.; Venkatakrishnan, P.; Huang, D.-F.; Chow, T. J. Chem. Commun. 2008, 2146–2148. doi:10.1039/b718250c
Return to citation in text: [1] -
Hofmann, S.; Hummert, M.; Scholz, R.; Luschtinetz, R.; Murawski, C.; Will, P.-A.; Hintschich, S. I.; Alex, J.; Jankus, V.; Monkman, A. P.; Lüssem, B.; Leo, K.; Gather, M. C. Chem. Mater. 2014, 26, 2414–2426. doi:10.1021/cm500602y
Return to citation in text: [1]
| 27. | Chen, H.; Gao, C.-H.; Jiang, Z.-Q.; Zhang, L.; Cui, L.-S.; Ji, S.-J.; Liao, L.-S. Dyes Pigm. 2014, 107, 15–20. doi:10.1016/j.dyepig.2014.03.006 |
| 24. | Li, P.; Wang, Z.; Song, C.; Zhang, H. J. Mater. Chem. C 2017, 5, 11454–11465. doi:10.1039/C7TC03112B |
| 25. | Shaikh, A. M.; Sharma, B. K.; Chacko, S.; Kamble, R. M. RSC Adv. 2016, 6, 94218–94227. doi:10.1039/C6RA20964E |
| 26. | Piechowska, J.; Gryko, D. T. J. Org. Chem. 2011, 76, 10220–10228. doi:10.1021/jo202072d |
| 1. | Baltazzi, E.; Krimen, L. I. Chem. Rev. 1963, 63, 511–556. doi:10.1021/cr60225a004 |
| 2. | Gholap, S. S. Eur. J. Med. Chem. 2016, 110, 13–31. doi:10.1016/j.ejmech.2015.12.017 |
| 3. | Gryko, D. T.; Vakuliuk, O.; Gryko, D.; Koszarna, B. J. Org. Chem. 2009, 74, 9517–9520. doi:10.1021/jo902124c |
| 4. | Antilla, J. C.; Baskin, J. M.; Barder, T. E.; Buchwald, S. L. J. Org. Chem. 2004, 69, 5578–5587. doi:10.1021/jo049658b |
| 5. | Honraedt, A.; Raux, M.-A.; Grognec, E. L.; Jacquemin, D.; Felpin, F.-X. Chem. Commun. 2014, 50, 5236–5238. doi:10.1039/C3CC45240A |
| 9. | Morimoto, K.; Ohnishi, Y.; Koseki, D.; Nakamura, A.; Dohi, T.; Kita, Y. Org. Biomol. Chem. 2016, 14, 8947–8951. doi:10.1039/C6OB01764A |
| 21. | McManus, H. A.; Fleming, M. J.; Lautens, M. Angew. Chem., Int. Ed. 2007, 46, 433–436. doi:10.1002/anie.200603945 |
| 8. | Ackermann, L.; Dell’Acqua, M.; Fenner, S.; Vicente, R.; Sandmann, R. Org. Lett. 2011, 13, 2358–2360. doi:10.1021/ol200601e |
| 23. | Lin, C.; Zhen, L.; Cheng, Y.; Du, H.-J.; Zhao, H.; Wen, X.; Kong, L.-Y.; Xu, Q.-L.; Sun, H. Org. Lett. 2015, 17, 2684–2687. doi:10.1021/acs.orglett.5b01078 |
| 7. | Liu, Y.-X.; Xue, D.; Wang, J.-D.; Zhao, C.-J.; Zou, Q.-Z.; Wang, C.; Xiao, J. Synlett 2013, 24, 507–513. doi:10.1055/s-0032-1318155 |
| 11. | Sundalam, S. K.; Nilova, A.; Seidl, T. L.; Stuart, D. R. Angew. Chem., Int. Ed. 2016, 55, 8431–8434. doi:10.1002/anie.201603222 |
| 12. | Stuart, D. R. Synlett 2017, 28, 275–279. doi:10.1055/s-0036-1588683 |
| 6. | Wen, J.; Zhang, R.-Y.; Chen, S.-Y.; Zhang, J.; Yu, X.-Q. J. Org. Chem. 2012, 77, 766–771. doi:10.1021/jo202150t |
| 11. | Sundalam, S. K.; Nilova, A.; Seidl, T. L.; Stuart, D. R. Angew. Chem., Int. Ed. 2016, 55, 8431–8434. doi:10.1002/anie.201603222 |
| 12. | Stuart, D. R. Synlett 2017, 28, 275–279. doi:10.1055/s-0036-1588683 |
| 14. | Stridfeldt, E.; Lindstedt, E.; Reitti, M.; Blid, J.; Norrby, P.-O.; Olofsson, B. Chem. – Eur. J. 2017, 23, 13249–13258. doi:10.1002/chem.201703057 |
| 15. | Yoshimura, A.; Fuchs, J. M.; Middleton, K. R.; Maskaev, A. V.; Rohde, G. T.; Saito, A.; Postnikov, P. S.; Yusubov, M. S.; Nemykin, V. N.; Zhdankin, V. V. Chem. – Eur. J. 2017, 23, 16738–16742. doi:10.1002/chem.201704393 |
| 16. | Robidas, R.; Guérin, V.; Provençal, L.; Echeverria, M.; Legault, C. Y. Org. Lett. 2017, 19, 6420–6423. doi:10.1021/acs.orglett.7b03307 |
| 22. | Wolthuis, E.; Jagt, D. V.; Mels, S.; De Boer, A. J. Org. Chem. 1965, 30, 190–193. doi:10.1021/jo01012a044 |
| 13. | Wang, M.; Huang, Z. Org. Biomol. Chem. 2016, 14, 10185–10188. doi:10.1039/C6OB01649A |
| 12. | Stuart, D. R. Synlett 2017, 28, 275–279. doi:10.1055/s-0036-1588683 |
| 13. | Wang, M.; Huang, Z. Org. Biomol. Chem. 2016, 14, 10185–10188. doi:10.1039/C6OB01649A |
| 11. | Sundalam, S. K.; Nilova, A.; Seidl, T. L.; Stuart, D. R. Angew. Chem., Int. Ed. 2016, 55, 8431–8434. doi:10.1002/anie.201603222 |
| 12. | Stuart, D. R. Synlett 2017, 28, 275–279. doi:10.1055/s-0036-1588683 |
| 28. | Moorthy, J. N.; Venkatakrishnan, P.; Huang, D.-F.; Chow, T. J. Chem. Commun. 2008, 2146–2148. doi:10.1039/b718250c |
| 29. | Hofmann, S.; Hummert, M.; Scholz, R.; Luschtinetz, R.; Murawski, C.; Will, P.-A.; Hintschich, S. I.; Alex, J.; Jankus, V.; Monkman, A. P.; Lüssem, B.; Leo, K.; Gather, M. C. Chem. Mater. 2014, 26, 2414–2426. doi:10.1021/cm500602y |
| 10. | Kitamura, T.; Yamane, M. J. Chem. Soc., Chem. Commun. 1995, 983–984. doi:10.1039/c39950000983 |
| 17. | Caster, K. C.; Keck, C. G.; Walls, R. D. J. Org. Chem. 2001, 66, 2932–2936. doi:10.1021/jo001277k |
| 18. | Davies, J. W.; Durrant, M. L.; Walker, M. P.; Belkacemi, D.; Malpass, J. R. Tetrahedron 1992, 48, 861–884. doi:10.1016/S0040-4020(01)88190-4 |
| 19. | Sumida, Y.; Kato, T.; Hosoya, T. Org. Lett. 2013, 15, 2806–2809. doi:10.1021/ol401140d |
| 20. | Chen, J.; Baire, B.; Hoye, T. R. Heterocycles 2014, 88, 1191–1200. doi:10.3987/COM-13-S(S)83 |
| 21. | McManus, H. A.; Fleming, M. J.; Lautens, M. Angew. Chem., Int. Ed. 2007, 46, 433–436. doi:10.1002/anie.200603945 |
© 2018 Chen et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)