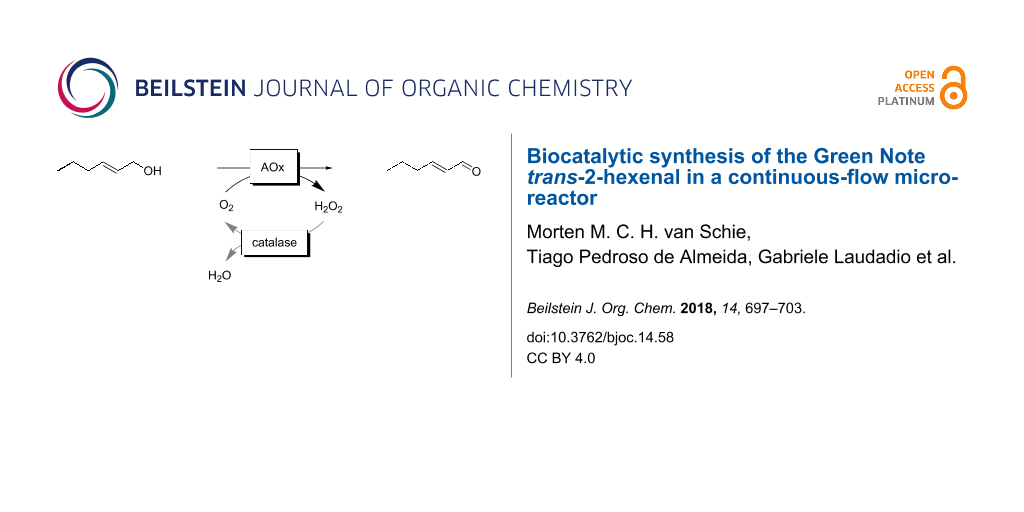
Abstract
The biocatalytic preparation of trans-hex-2-enal from trans-hex-2-enol using a novel aryl alcohol oxidase from Pleurotus eryngii (PeAAOx) is reported. As O2-dependent enzyme PeAAOx-dependent reactions are generally plagued by the poor solubility of O2 in aqueous media and mass transfer limitations resulting in poor reaction rates. These limitations were efficiently overcome by conducting the reaction in a flow-reactor setup reaching unpreceded catalytic activities for the enzyme in terms of turnover frequency (up to 38 s−1) and turnover numbers (more than 300000) pointing towards preparative usefulness of the proposed reaction scheme.
Graphical Abstract
Introduction
trans-2-Hexenal is well-known as a major component of the Green Notes of fruits and vegetables such as apples, strawberries, cherries and more. It is widely used in the flavour and fragrance industry as fresh flavour ingredient in foods and beverages.
One attractive access to trans-2-hex-2-enal is the oxidation of the corresponding allylic alcohol to the aldehyde. Though at first sight an oxidation of primary alcohols to the corresponding aldehydes does not appear to be a major challenge, the methods of the state-of-the-art are mostly plagued by undesired side reactions [1]. Also some of the stoichiometric oxidants used are questionable from an environmental and/or toxicological point of view and therefore are not compatible with consumer products such as Green Notes. Therefore, we turned our attention to biocatalytic oxidation methods. For clean conversion of primary alcohols to aldehydes principally two biocatalytic approaches are available (Scheme 1) [2-5]. Alcohol dehydrogenases catalyse the reversible oxidation of alcohols in a Meerwein–Ponndorf–Verley-type of reaction (Scheme 1A). The poor thermodynamic driving force of this reaction, however, necessitates significant molar surpluses of the stoichiometric oxidant (such as acetone). This not only negatively influences the environmental impact of the reaction [6] but also complicates downstream processing. Furthermore, the nicotinamide cofactor (even if used in catalytic amounts only) causes additional costs.
Scheme 1: Enzymatic reaction schemes for the selective oxidation of trans-hex-2-enol. A: Alcohol dehydrogenase (ADH)-catalysed oxidation producing stoichiometric amounts of NAD(P)H, which needs to be recycled in situ; the overall reaction is reversible requiring surpluses of the cosubstrate (e.g., acetone) to shift the overall equilibrium to the side of trans-hex-2-enal. B: Envisioned aerobic oxidation using alcohol oxidases (AOx). H2O2 is formed as byproduct and dismutated by catalase into H2O and O2.
Scheme 1: Enzymatic reaction schemes for the selective oxidation of trans-hex-2-enol. A: Alcohol dehydrogenas...
Therefore, we concentrated on alcohol oxidase-catalysed reaction schemes (Scheme 1B. Oxidases utilise O2 as terminal electron acceptor for the oxidation reaction yielding H2O2 as sole byproduct. The latter can be disproportionated easily by using catalase (Scheme 1B). Furthermore, O2 reduction adds sufficient thermodynamic driving force to the reaction to make it essentially irreversible.
The benefits of using O2, however, also come with the disadvantage of its very poor solubility in aqueous media (ca. 0.25 mM at room temperature). Hence, in the course of an oxidation reaction dissolved O2 is consumed rapidly and diffusion of O2 into the reaction medium can easily become overall rate-limiting. The O2 diffusion rate into the reaction medium directly correlates with the interfacial area between aqueous medium and the gas phase. Large interfacial surface areas can be achieved via heterogeneous intake, by bubbling, stirring, etc. Soluble enzymes, however, are often rather unstable under these conditions, possibly owing to the mechanical stress leading to irreversible inactivation of the biocatalyst [7,8]. Methods of bubble-free aeration have been described in the literature to alleviate the inactivation issue described above [9-12].
The continuous-flow microreactor technology has emerged as a safe and scalable way to approach oxidation reactions [13,14]. Due to its small dimensions, hazardous reactions can be easily controlled, owing to the large surface-to-volume ratio which can minimise hot-spot formation and allows for control over mixing and heating phenomena [15,16]. Furthermore, a well-defined gas–liquid regime can be easily maintained [17,18]. High mass-transfer coefficients are generally the consequence of small vortices induced by the segmented flow regime. This flow pattern guarantees an enhanced contact between the two phases and provides a uniform gas concentration in the liquid segment.
Therefore, it is not very astonishing that also the biocatalysis community is showing interest in flow chemistry. Several biocatalytic processes have been reported in flow reactors [19], mostly advocating easier process intensification in combination with enzyme immobilization [20-23]. Also the higher oxygen-transfer rates in flow reactions compared to batch reactions have been emphasised by several groups. Here, reactor designs ranging from simple flow reactors, tube-in-tube reactors [24], agitated tube reactors [25,26] and continuous agitated cell reactors [27] have been reported.
Encouraged by these contributions, we asked ourselves whether a slug-flow approach may combine mechanically less demanding conditions with high O2-transfer rates thereby enabling efficient and robust oxidase-catalysed oxidation reactions.
Results and Discussion
Selection and characterisation of the biocatalyst
As biocatalyst for this study we focussed on the recombinant aryl alcohol oxidase from Pleurotus eryngii (PeAAOx) [28-31]. Especially the availability as recombinant enzyme (enabling future at-scale production and protein engineering) and its promising activity on allylic alcohols make PeAAOx a promising starting point. Commercially available alcohol oxidases from Pichia pastoris and Candida boidinii showed no significant activity for the substrate under the same conditions. As trans-2-hex-enol had not been reported as substrate for PeAAOx we evaluated its catalytic properties, particularly the substrate concentration-dependency of the enzymatic oxidation. Initial rate measurements (performed in 1 mL cuvettes) revealed a Michaelis–Menten dependency of the enzyme activity (Figure 1). Apparent KM and kcat values of approximately 1 mM and 22 s−1 were estimated, respectively. These values are in the same order of magnitude as those for benzyl alcohol substrates reported previously [29]. The slightly decreasing enzyme activity at elevated substrate concentrations may be an indication for a slight substrate inhibition. Performing these initial rate measurements in the presence of varying product concentrations showed a pronounced product inhibition (Supporting Information File 1, Figure S2, vide infra).
Figure 1: Michaelis–Menten kinetics of the PeAAOx-catalysed oxidation of trans-hex-2-enol. Conditions: 50 mM KPi buffer (pH 7, 30 °C), [trans-hex-2-enol]0 = 3 mM, [PeAAOx] = 0.044 µM, [horseradish peroxidase] = 500 U mL−1, [ABTS] = 2 mM.
Figure 1: Michaelis–Menten kinetics of the PeAAOx-catalysed oxidation of trans-hex-2-enol. Conditions: 50 mM ...
Continuous-flow reactor enzymatic oxidation
Next, we performed the PeAAOx-catalysed oxidation of trans-hex-2-enol in a slug-flow reactor setup (Supporting Information File 1, Figure S1 and Figures S9–S11). In a first set of experiments we systematically varied the residence time of the reaction mixture in the flow reactor (and thereby the reaction time, Figure 2).
Figure 2: The influence of the residence time on the conversion of trans-hex-2-enol (red squares) to trans-2-hexenal (black diamonds) in a flow reactor. Conditions: 3 mL flow reactor, 50 mM KPi buffer (pH 7, 30 °C), [trans-hex-2-enol]0 = 10 mM, [PeAAOx] = 0.25 µM, [catalase] = 600 U mL−1.
Figure 2: The influence of the residence time on the conversion of trans-hex-2-enol (red squares) to trans-2-...
A full conversion of the starting material into the desired trans-hex-2-enal was observed at residence (reaction) times of approximately 40 min corresponding to a turnover number (TN) for the biocatalysts of 32400 and an average turnover frequency (TF) of 13.5 s−1. Even more interestingly, at higher flow rates apparent TF of up to 38 s−1 (RT = 5 min) were observed. This value exceeds the previously determined kcat(PeAAOx) (Figure 1) significantly. We attribute this observation to an increased oxygen-transfer rate at high flow rates. In the case of the 5 minutes residence time this corresponds to an O2-transfer rate of roughly 0.25 mM min−1. Similarly high values could be obtained previously only under mechanically demanding reaction conditions or using surfactant-stabilised emulsions [7]. Varying the ratio of gas to liquid had no significant effect on the overall rate of the reaction (Table 1).
Table 1: Effect of variation of the gas-to-liquid ratio on the rate of the PeAAOx-catalysed aerobic oxidation of trans-hex-2-enol.
|
ratio
(liquid:gas) |
liquid flow
[mL min−1] |
gas flow
[mL min−1] |
residence time [min] |
[product]
[mM] |
|---|---|---|---|---|
| 1:1 | 0.20 | 0.20 | 15 | 5.48 (± 0.01) |
| 1:3 | 0.10 | 0.30 | 15 | 5.18 (± 0.32) |
| 1:5 | 0.067 | 0.333 | 16 | 4.99 (± 0.49) |
Conditions: 3 mL flow reactor, 50 mM KPi buffer (pH 7, 30 °C), [trans-2-hexen-1-ol]0 = 10 mM, [PeAAOx] = 0.25 µM, [catalase] = 600 U mL−1.
Within the experimental error, the conversion in all experiments was identical indicating that even at a comparably low volumetric ratio of 1:1 the O2 availability was already sufficient not to be overall rate-limiting.
It is worth mentioning here that under batch reaction conditions, similar progression curves were only attainable under mechanically very demanding conditions (i.e., very vigorous stirring and bubbling of O2 directly into the reaction mixture, Supporting Information File 1, Figure S3). These conditions also caused a significant evaporation of the substrate at higher substrate concentration (Supporting Information File 1, Figure S4), which was much less the case in the flow-reaction setup.
From an economical point-of-view the catalyst performance in terms of turnover number (TN) is of utmost importance as it directly correlates with the cost-contribution of the catalyst to the production costs [32-34]. Therefore we evaluated the TN attainable for PeAAOx in the flow setup (Figure 3). For this lower PeAAOx concentrations as well as significantly increased residence times were applied. The increased residence times were achieved by decreasing the flow rates and using a longer flow reactor (6 mL volume instead of 3 mL).
Figure 3: Increasing the PeAAOx turnover numbers (TN) by increasing the residence time. Conditions: 6 mL flow reactor, 50 mM KPi buffer (pH 7, 30 °C), [trans-hex-2-enol]0 = 40 mM, [PeAAOx] = 0.02 µM, [catalase] = 600 U mL−1. The TN value was calculated based on the GC yield of every run. The TN was obtained by dividing the product concentration (as determined chromatographically) by the biocatalyst concentration.
Figure 3: Increasing the PeAAOx turnover numbers (TN) by increasing the residence time. Conditions: 6 mL flow...
Pleasingly, already in these first experiments a TN for the enzyme of more than 300000 was observed at long residence times. This also underlines the robustness of the enzyme under the flow conditions. Compared to Figure 2 somewhat lower TFs for PeAAOx were observed here, which again can be attributed to a lower O2-transfer rate at lower flow rates. The quasi-linear relationship shown in Figure 3 also suggests that even higher TN may be attainable – however at the expense of longer reaction times. Therefore, further investigations will focus on identifying conditions satisfying the demand for high TNs and short reaction times. Encouraged by these results, we also tried a semi-preparative scale reaction using 5 g L−1 (50 mM) substrate loading in a total of 50 mL with 0.75 μM PeAAOx. As a result, 90% conversion was achieved after 18 h of total reaction time (roughly 80 minutes of residence time in the 6 mL reactor). The product was purified chromatographically resulting in 200 mg of pure trans-hex-2-enal (as determined by NMR) in 81% isolated yield thereby demonstrating the preparative potential of the proposed reaction setup.
Conclusion
Alcohol oxidase-catalysed oxidation of alcohols to aldehydes bears a significant potential for preparative biocatalysis. The reaction is independent from expensive and instable nicotinamide cofactors (and the corresponding cosubstrates/coproducts as well as possible regeneration enzymes) and produces only water as byproduct. These advantages, however, are counteracted by the generally low reaction rates caused by the poor O2 availability. Flow chemistry is a promising technique to provide the aqueous reaction mixture with O2 needed for the oxidation. It enables high O2 transfer rates while avoiding enzyme robustness issues frequently observed with ‘traditional’ aeration methods.
Future developments in our laboratories will concentrate on the characterisation, extension and preparative demonstration of this powerful combination of oxidase catalysis and flow chemistry.
Experimental
General
Turnover numbers (TN) and turnover frequencies (TF) reported in this manuscript were calculated based on Equation 1 and Equation 2.
Production of PeAAOx
E. coli cultivation
For the production, activation and purification of PeAAOx, a slightly modified literature protocol was used [28]. Pre-cultures of LB media containing 100 μg mL−1 of ampicillin were inoculated with E. coli W3110 containing pFLAG1-AAO and incubated overnight at 37 °C and 180 rpm. Overexpression was carried out in 5 L flasks with 1 L of TB medium supplemented with 100 μg mL−1 of ampicillin. The medium was inoculated with the pre-culture to an OD of 0.05 and grown at 37 °C and 180 rpm. At an OD600 of 0.8, 1 mM isopropyl β-D-thiogalactopyranoside (IPTG) was added and the cultures were incubated for additional 4 h at 37 °C and 180 rpm. The bacterial pellets, obtained after harvesting the cells, were re-suspended in a total volume of 40 mL 50 mM Tris/HCl buffer, pH 8.0, containing 10 mM EDTA and 5 mM dithiothreitol (DTT).
Refolding
The re-suspended cells were disrupted by incubation with 2 mg mL−1 lysozyme for 1 h at 4 °C. Afterwards, 0.1 mg mL−1 DNase, 1 mM MgCl2 and 0.1 mM PMSF were added followed by sonication. The insoluble fraction was collected by centrifugation (30 min at 15,000 rpm and 4 °C), re-suspended and washed three times with 20 mL 20 mM Tris/HCl buffer, pH 8.0, containing 10 mM EDTA and 5 mM DTT using a potter homogenizing device. The pellets obtained after centrifugation (15 min at 15,000 rpm and 4 °C) were solubilized in a total volume of 30 mL 20 mM Tris/HCl buffer, pH 8.0, containing 2 mM EDTA, 50 mM DTT and 8 M urea. After incubation on ice for 30 min, the solution was cleared by centrifugation (15 min at 15,000 rpm and 4 °C). The obtained supernatant was used as stock solution for the in vitro refolding.
The PeAAOx was solubilized using 150 µg mL−1 protein in 20 mM Tris/HCl buffer, pH 9.0, containing 2.5 mM GSSG, 1 mM DTT, 0.02 mM FAD, 34% glycerol and 0.6 M urea at 4 °C for 80 h. After the incubation for PeAAOx activation/refolding, the refolding mixture was concentrated to 100 mL and the buffer exchanged against 10 mM sodium phosphate buffer, pH 5.5 by diafiltration (DV 20) and subsequently concentrated using an Amicon Ultra 15 mL centrifugal filter (MWCO 10 kDa). After centrifugation (overnight at 15,000 rpm and 4 °C), the soluble fraction was further purified using anion-exchange chromatography.
Purification
The concentrated PeAAOx solution was purified using a 58 mL Q Sepharose column (GE Healthcare). PeAAOx was eluted with a linear NaCl gradient (0–0.6 M over 6 CV) using 10 mM sodium phosphate buffer, pH 5.5. Fractions containing PeAAOx were pooled, concentrated and desalted using HiTrap desalting columns (GE Healthcare) and 10 mM sodium phosphate buffer, pH 5.5. The PeAAOx concentration was calculated based on the absorbance using the molar extinction coefficient of ε463 11,050 M−1 cm−1.
Activity assay
The activity of PeAAOx was determined by UV–vis spectroscopy, using an Agilent Cary 60 UV–vis spectrophotometer, following the oxidation of ABTS (ε405 = 36,800 M−1 cm−1) by horseradish peroxidase (POD) at the expense of hydrogen peroxide. In general, 0.044 µM PeAAOx was used to convert 3 mM of trans-2-hex-2-enol. The hydrogen peroxide formed in this reaction was subsequently used to convert 2 mM of ABTS to ABTS·+ by an excess of POD (500 U mL−1). The reactions were performed at 30 °C in oxygen-saturated 50 mM KPi buffer at pH 7.0.
Flow reactor experiments
PFA microreactor coils (750 μm ID) with a volume of 3 and 6 mL were constructed. The reaction mixture was introduced via a syringe pump (Fusion 200, Chemyx), while the pure oxygen flow was controlled by a mass flow controller (EL-FLOW, Bronkhorst), resulting in a segmented flow (Supporting Information File 1, Figure S8). Residence times were taken as the time between the solution entering and exiting the coil and were varied by altering the flow, keeping the ratio of oxygen to liquid at three to one. Samples were collected on ice and as soon as enough volume was collected, extracted with ethyl acetate and analysed by GC (vide infra).
GC analysis
The collected reaction mixtures were extracted into an equal volume of ethyl acetate, dried with magnesium sulphate and analysed on a CP-wax 52 CB GC column (50 m × 0.53 m × 2 µm) (GC method: 60 °C for 3 min; 30 °C/min to 105 °C; 105 °C for 7 min; 30 °C/min to 250 °C; 250 °C for 1 minute). Dodecane (5 mM) was added as standard.
Work-up semi-preparative scale
The reaction mixture was directly collected in deuterated chloroform at the end of the flow reactor followed by recording the NMR spectrum in order to evaluate the conversion (see Supporting Information File 1). The organic mixture was diluted and introduced into a separation funnel and washed with brine. The aqueous phase was backwashed once with DCM. The collected organic phase was dried over MgSO4, filtered and concentrated under reduced pressure. Purification of the isolated mixture was performed by flash chromatography on silica (pure DCM). The final product was obtained as colourless oil (200 mg).
(E)-Hex-2-enal
TLC (DCM) Rf 0.9; 1H NMR (399 MHz, CDCl3) δ 9.44 (d, J = 7.7 Hz, 1H), 6.78 (dt, J = 15.6, 6.8 Hz, 1H), 6.05 (ddq, J = 15.5, 7.8, 1.3 Hz, 1H), 2.33–2.18 (m, 2H), 1.48 (h, J = 7.4 Hz, 2H), 0.90 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 194.3, 158.9, 133.3, 34.8, 21.3, 13.8.
Supporting Information
| Supporting Information File 1: General information and supporting figures. | ||
| Format: PDF | Size: 984.9 KB | Download |
References
-
Alshammari, H.; Miedziak, P. J.; Morgan, D. J.; Knight, D. W.; Hutchings, G. J. Green Chem. 2013, 15, 1244–1254. doi:10.1039/C3GC36828A
Return to citation in text: [1] -
Turner, N. J. Chem. Rev. 2011, 111, 4073–4087. doi:10.1021/cr200111v
Return to citation in text: [1] -
Hollmann, F.; Arends, I. W. C. E.; Buehler, K.; Schallmey, A.; Bühler, B. Green Chem. 2011, 13, 226–265. doi:10.1039/c0gc00595a
Return to citation in text: [1] -
Kroutil, W.; Mang, H.; Edegger, K.; Faber, K. Curr. Opin. Chem. Biol. 2004, 8, 120–126. doi:10.1016/j.cbpa.2004.02.005
Return to citation in text: [1] -
Pickl, M.; Fuchs, M.; Glueck, S. M.; Faber, K. Appl. Microbiol. Biotechnol. 2015, 99, 6617–6642. doi:10.1007/s00253-015-6699-6
Return to citation in text: [1] -
Ni, Y.; Holtmann, D.; Hollmann, F. ChemCatChem 2014, 6, 930–943. doi:10.1002/cctc.201300976
Return to citation in text: [1] -
Churakova, E.; Arends, I. W. C. E.; Hollmann, F. ChemCatChem 2013, 5, 565–568. doi:10.1002/cctc.201200490
Return to citation in text: [1] [2] -
Bommarius, A. S.; Karau, A. Biotechnol. Prog. 2005, 21, 1663–1672. doi:10.1021/bp050249q
Return to citation in text: [1] -
Van Hecke, W.; Ludwig, R.; Dewulf, J.; Auly, M.; Messiaen, T.; Haltrich, D.; Van Langenhove, H. Biotechnol. Bioeng. 2009, 102, 122–131. doi:10.1002/bit.22042
Return to citation in text: [1] -
Van Hecke, W.; Bhagwat, A.; Ludwig, R.; Dewulf, J.; Haltrich, D.; Van Langenhove, H. Biotechnol. Bioeng. 2009, 102, 1475–1482. doi:10.1002/bit.22165
Return to citation in text: [1] -
Illner, S.; Hofmann, C.; Löb, P.; Kragl, U. ChemCatChem 2014, 6, 1748–1754. doi:10.1002/cctc.201400028
Return to citation in text: [1] -
Kaufhold, D.; Kopf, F.; Wolff, C.; Beutel, S.; Hilterhaus, L.; Hoffmann, M.; Scheper, T.; Schlüter, M.; Liese, A. J. Membr. Sci. 2012, 423–424, 342–347. doi:10.1016/j.memsci.2012.08.035
Return to citation in text: [1] -
Gemoets, H. P. L.; Su, Y.; Shang, M.; Hessel, V.; Luque, R.; Noël, T. Chem. Soc. Rev. 2016, 45, 83–117. doi:10.1039/C5CS00447K
Return to citation in text: [1] -
Plutschack, M. B.; Pieber, B.; Gilmore, K.; Seeberger, P. H. Chem. Rev. 2017, 117, 11796–11893. doi:10.1021/acs.chemrev.7b00183
Return to citation in text: [1] -
Kockmann, N.; Thenée, P.; Fleischer-Trebes, C.; Laudadio, G.; Noël, T. React. Chem. Eng. 2017, 2, 258–280. doi:10.1039/C7RE00021A
Return to citation in text: [1] -
Gutmann, B.; Cantillo, V.; Kappe, C. O. Angew. Chem., Int. Ed. 2015, 54, 6688–6728. doi:10.1002/anie.201409318
Return to citation in text: [1] -
Noël, T.; Su, Y.; Hessel, V. Beyond Organometallic Flow Chemistry: The Principles Behind the Use of Continuous-Flow Reactors for Synthesis. Organometallic Flow Chemistry; Topics in Organometallic Chemistry, Vol. 57; Springer, 2016; pp 1–41. doi:10.1007/3418_2015_152
Return to citation in text: [1] -
Mallia, C. J.; Baxendale, I. R. Org. Process Res. Dev. 2016, 20, 327–360. doi:10.1021/acs.oprd.5b00222
Return to citation in text: [1] -
Tamborini, L.; Fernandes, P.; Paradisi, F.; Molinari, F. Trends Biotechnol. 2018, 36, 73–88. doi:10.1016/j.tibtech.2017.09.005
Return to citation in text: [1] -
Peris, E.; Okafor, O.; Kulcinskaja, E.; Goodridge, R.; Luis, S. V.; Garcia-Verdugo, E.; O'Reilly, E.; Sans, V. Green Chem. 2017, 19, 5345–5349. doi:10.1039/C7GC02421E
Return to citation in text: [1] -
Bolivar, J. M.; Wiesbauer, J.; Nidetzky, B. Trends Biotechnol. 2011, 29, 333–342. doi:10.1016/j.tibtech.2011.03.005
Return to citation in text: [1] -
Zor, C.; Reeve, H. A.; Quinson, J.; Thompson, L. A.; Lonsdale, T. H.; Dillon, F.; Grobert, N.; Vincent, K. A. Chem. Commun. 2017, 53, 9839–9841. doi:10.1039/C7CC04465H
Return to citation in text: [1] -
Tang, X.; Allemann, R. K.; Wirth, T. Eur. J. Org. Chem. 2017, 414–418. doi:10.1002/ejoc.201601388
Return to citation in text: [1] -
Tomaszewski, B.; Lloyd, R. C.; Warr, A. J.; Buehler, K.; Schmid, A. ChemCatChem 2014, 6, 2567–2576. doi:10.1002/cctc.201402354
Return to citation in text: [1] -
Jones, E.; McClean, K.; Housden, S.; Gasparini, G.; Archer, I. Chem. Eng. Res. Des. 2012, 90, 726–731. doi:10.1016/j.cherd.2012.01.018
Return to citation in text: [1] -
Gasparini, G.; Archer, I.; Jones, E.; Ashe, R. Org. Process Res. Dev. 2012, 16, 1013–1016. doi:10.1021/op2003612
Return to citation in text: [1] -
Toftgaard Pedersen, A.; de Carvalho, T. M.; Sutherland, E.; Rehn, G.; Ashe, R.; Woodley, J. M. Biotechnol. Bioeng. 2017, 114, 1222–1230. doi:10.1002/bit.26267
Return to citation in text: [1] -
Ruiz-Dueñas, F. J.; Ferreira, P.; Martinez, M. J.; Martinez, A. T. Protein Expression Purif. 2006, 45, 191–199. doi:10.1016/j.pep.2005.06.003
Return to citation in text: [1] [2] -
Ferreira, P.; Medina, M.; Guillén, F.; Martínez, M. J.; Van Berkel, W. J. H.; Martínez, A. T. Biochem. J. 2005, 389, 731–738. doi:10.1042/bj20041903
Return to citation in text: [1] [2] -
Guillén, F.; Martinez, A. T.; Martinez, M. J. Eur. J. Biochem. 1992, 209, 603–611. doi:10.1111/j.1432-1033.1992.tb17326.x
Return to citation in text: [1] -
Guillén, F.; Martinez, A. T.; Martínez, M. J. Appl. Microbiol. Biotechnol. 1990, 32, 465–469. doi:10.1007/bf00903784
Return to citation in text: [1] -
Bormann, S.; Gomez Baraibar, A.; Ni, Y.; Holtmann, D.; Hollmann, F. Catal. Sci. Technol. 2015, 5, 2038–2052. doi:10.1039/C4CY01477D
Return to citation in text: [1] -
Toftgaard Pedersen, A.; Birmingham, W. R.; Rehn, G.; Charnock, S. J.; Turner, N. J.; Woodley, J. M. Org. Process Res. Dev. 2015, 19, 1580–1589. doi:10.1021/acs.oprd.5b00278
Return to citation in text: [1] -
Tufvesson, P.; Lima-Ramos, J.; Nordblad, M.; Woodley, J. M. Org. Process Res. Dev. 2011, 15, 266–274. doi:10.1021/op1002165
Return to citation in text: [1]
| 32. | Bormann, S.; Gomez Baraibar, A.; Ni, Y.; Holtmann, D.; Hollmann, F. Catal. Sci. Technol. 2015, 5, 2038–2052. doi:10.1039/C4CY01477D |
| 33. | Toftgaard Pedersen, A.; Birmingham, W. R.; Rehn, G.; Charnock, S. J.; Turner, N. J.; Woodley, J. M. Org. Process Res. Dev. 2015, 19, 1580–1589. doi:10.1021/acs.oprd.5b00278 |
| 34. | Tufvesson, P.; Lima-Ramos, J.; Nordblad, M.; Woodley, J. M. Org. Process Res. Dev. 2011, 15, 266–274. doi:10.1021/op1002165 |
| 28. | Ruiz-Dueñas, F. J.; Ferreira, P.; Martinez, M. J.; Martinez, A. T. Protein Expression Purif. 2006, 45, 191–199. doi:10.1016/j.pep.2005.06.003 |
| 1. | Alshammari, H.; Miedziak, P. J.; Morgan, D. J.; Knight, D. W.; Hutchings, G. J. Green Chem. 2013, 15, 1244–1254. doi:10.1039/C3GC36828A |
| 9. | Van Hecke, W.; Ludwig, R.; Dewulf, J.; Auly, M.; Messiaen, T.; Haltrich, D.; Van Langenhove, H. Biotechnol. Bioeng. 2009, 102, 122–131. doi:10.1002/bit.22042 |
| 10. | Van Hecke, W.; Bhagwat, A.; Ludwig, R.; Dewulf, J.; Haltrich, D.; Van Langenhove, H. Biotechnol. Bioeng. 2009, 102, 1475–1482. doi:10.1002/bit.22165 |
| 11. | Illner, S.; Hofmann, C.; Löb, P.; Kragl, U. ChemCatChem 2014, 6, 1748–1754. doi:10.1002/cctc.201400028 |
| 12. | Kaufhold, D.; Kopf, F.; Wolff, C.; Beutel, S.; Hilterhaus, L.; Hoffmann, M.; Scheper, T.; Schlüter, M.; Liese, A. J. Membr. Sci. 2012, 423–424, 342–347. doi:10.1016/j.memsci.2012.08.035 |
| 29. | Ferreira, P.; Medina, M.; Guillén, F.; Martínez, M. J.; Van Berkel, W. J. H.; Martínez, A. T. Biochem. J. 2005, 389, 731–738. doi:10.1042/bj20041903 |
| 7. | Churakova, E.; Arends, I. W. C. E.; Hollmann, F. ChemCatChem 2013, 5, 565–568. doi:10.1002/cctc.201200490 |
| 8. | Bommarius, A. S.; Karau, A. Biotechnol. Prog. 2005, 21, 1663–1672. doi:10.1021/bp050249q |
| 7. | Churakova, E.; Arends, I. W. C. E.; Hollmann, F. ChemCatChem 2013, 5, 565–568. doi:10.1002/cctc.201200490 |
| 6. | Ni, Y.; Holtmann, D.; Hollmann, F. ChemCatChem 2014, 6, 930–943. doi:10.1002/cctc.201300976 |
| 27. | Toftgaard Pedersen, A.; de Carvalho, T. M.; Sutherland, E.; Rehn, G.; Ashe, R.; Woodley, J. M. Biotechnol. Bioeng. 2017, 114, 1222–1230. doi:10.1002/bit.26267 |
| 2. | Turner, N. J. Chem. Rev. 2011, 111, 4073–4087. doi:10.1021/cr200111v |
| 3. | Hollmann, F.; Arends, I. W. C. E.; Buehler, K.; Schallmey, A.; Bühler, B. Green Chem. 2011, 13, 226–265. doi:10.1039/c0gc00595a |
| 4. | Kroutil, W.; Mang, H.; Edegger, K.; Faber, K. Curr. Opin. Chem. Biol. 2004, 8, 120–126. doi:10.1016/j.cbpa.2004.02.005 |
| 5. | Pickl, M.; Fuchs, M.; Glueck, S. M.; Faber, K. Appl. Microbiol. Biotechnol. 2015, 99, 6617–6642. doi:10.1007/s00253-015-6699-6 |
| 28. | Ruiz-Dueñas, F. J.; Ferreira, P.; Martinez, M. J.; Martinez, A. T. Protein Expression Purif. 2006, 45, 191–199. doi:10.1016/j.pep.2005.06.003 |
| 29. | Ferreira, P.; Medina, M.; Guillén, F.; Martínez, M. J.; Van Berkel, W. J. H.; Martínez, A. T. Biochem. J. 2005, 389, 731–738. doi:10.1042/bj20041903 |
| 30. | Guillén, F.; Martinez, A. T.; Martinez, M. J. Eur. J. Biochem. 1992, 209, 603–611. doi:10.1111/j.1432-1033.1992.tb17326.x |
| 31. | Guillén, F.; Martinez, A. T.; Martínez, M. J. Appl. Microbiol. Biotechnol. 1990, 32, 465–469. doi:10.1007/bf00903784 |
| 19. | Tamborini, L.; Fernandes, P.; Paradisi, F.; Molinari, F. Trends Biotechnol. 2018, 36, 73–88. doi:10.1016/j.tibtech.2017.09.005 |
| 24. | Tomaszewski, B.; Lloyd, R. C.; Warr, A. J.; Buehler, K.; Schmid, A. ChemCatChem 2014, 6, 2567–2576. doi:10.1002/cctc.201402354 |
| 17. | Noël, T.; Su, Y.; Hessel, V. Beyond Organometallic Flow Chemistry: The Principles Behind the Use of Continuous-Flow Reactors for Synthesis. Organometallic Flow Chemistry; Topics in Organometallic Chemistry, Vol. 57; Springer, 2016; pp 1–41. doi:10.1007/3418_2015_152 |
| 18. | Mallia, C. J.; Baxendale, I. R. Org. Process Res. Dev. 2016, 20, 327–360. doi:10.1021/acs.oprd.5b00222 |
| 25. | Jones, E.; McClean, K.; Housden, S.; Gasparini, G.; Archer, I. Chem. Eng. Res. Des. 2012, 90, 726–731. doi:10.1016/j.cherd.2012.01.018 |
| 26. | Gasparini, G.; Archer, I.; Jones, E.; Ashe, R. Org. Process Res. Dev. 2012, 16, 1013–1016. doi:10.1021/op2003612 |
| 15. | Kockmann, N.; Thenée, P.; Fleischer-Trebes, C.; Laudadio, G.; Noël, T. React. Chem. Eng. 2017, 2, 258–280. doi:10.1039/C7RE00021A |
| 16. | Gutmann, B.; Cantillo, V.; Kappe, C. O. Angew. Chem., Int. Ed. 2015, 54, 6688–6728. doi:10.1002/anie.201409318 |
| 13. | Gemoets, H. P. L.; Su, Y.; Shang, M.; Hessel, V.; Luque, R.; Noël, T. Chem. Soc. Rev. 2016, 45, 83–117. doi:10.1039/C5CS00447K |
| 14. | Plutschack, M. B.; Pieber, B.; Gilmore, K.; Seeberger, P. H. Chem. Rev. 2017, 117, 11796–11893. doi:10.1021/acs.chemrev.7b00183 |
| 20. | Peris, E.; Okafor, O.; Kulcinskaja, E.; Goodridge, R.; Luis, S. V.; Garcia-Verdugo, E.; O'Reilly, E.; Sans, V. Green Chem. 2017, 19, 5345–5349. doi:10.1039/C7GC02421E |
| 21. | Bolivar, J. M.; Wiesbauer, J.; Nidetzky, B. Trends Biotechnol. 2011, 29, 333–342. doi:10.1016/j.tibtech.2011.03.005 |
| 22. | Zor, C.; Reeve, H. A.; Quinson, J.; Thompson, L. A.; Lonsdale, T. H.; Dillon, F.; Grobert, N.; Vincent, K. A. Chem. Commun. 2017, 53, 9839–9841. doi:10.1039/C7CC04465H |
| 23. | Tang, X.; Allemann, R. K.; Wirth, T. Eur. J. Org. Chem. 2017, 414–418. doi:10.1002/ejoc.201601388 |
© 2018 van Schie et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)