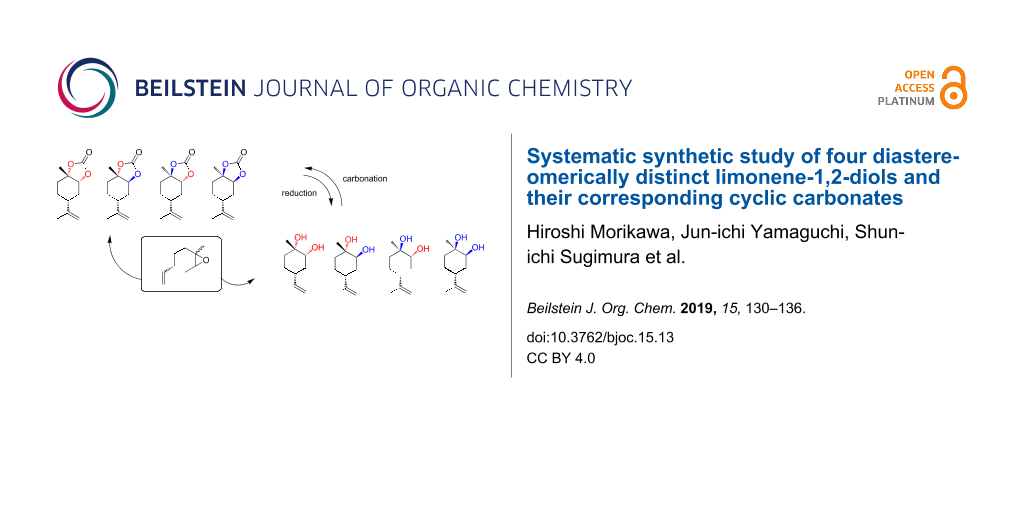
Abstract
In order to produce versatile and potentially functional terpene-based compounds, a (R)-limonene-derived diol and its corresponding five-membered cyclic carbonate were prepared. The diol (cyclic carbonate) comprises four diastereomers based on the stereochemical configuration of the diol (and cyclic carbonate) moiety. By choosing the appropriate starting compounds (trans- and cis-limonene oxide) and conditions, the desired diastereomers were synthesised in moderate to high yields with, in most cases, high stereoselectivity. Comparison of the NMR data of the obtained diols and carbonates revealed that the four different diastereomers of each compound could be distinguished by reference to their characteristic signals.
Graphical Abstract
Introduction
(R)-Limonene (LM) is a naturally occurring terpene, and therefore a very attractive and renewable resource [1]. Its derivatives have versatilely and widely been studied [1-4].
Otherwise, syntheses of five-membered cyclic carbonates (5CCs) have been intensively investigated [5-7] in terms of utilisation of CO2 and the further reactions to produce functional chemicals such as oxazolizin-2-ones [8] and polyurethanes [9]. For (R)-limonene-derived 5CC (LM5CC), four diastereomers are considered from the different stereochemical configurations at the 1- and 2-positions of LM (Figure 1). A few studies on 1a and 1d have been recently reported [10,11], whereas 1b and 1c have never been synthesised. Consequently, the systematic and stereoselective syntheses of these four LM5CCs are of deep significance, largely because LM derivatives with their versatile functionality can be effectively utilised in organic and polymer chemistry.
(4R)-Limonene-1,2-diols (LMdiols) are some of the most important LM derivatives because they act as precursors of bioactive molecules [12-14]. Furthermore, LMdiol is detected as metabolite in vivo in biochemistry [15], and is known to react in the atmosphere to afford the secondary organic aerosol as air pollutants [16]. Among the four diastereomers of LMdiols (Figure 2), 2b and 2c have already been reported [14,17-20]. Conversely, the distinct characterisations of 2a and 2d remain unreported.
Figure 2: Diastereomers of LMdiols, 2a–d.
Figure 2: Diastereomers of LMdiols, 2a–d.
Very recently, Wang et al. synthesised 2a and 2d using OsO4, and exclusively 2d using Sharpless AD-mix-β [21] as a catalyst (Scheme 1a) [16]. In contrast to that, Mori reported that (R)-dihydrolimonene, the structure of which is similar to LM, was oxidised using Sharpless AD-mix-β to afford a 2a-type main product (Scheme 1b) [22]. Hao et al. also reported the synthesis of 2a and 2d using OsO4 (Scheme 1a) [23]. However, the NMR data of 2a and 2d were not consistent with those [16] reported by Wang. Moreover, they referred to a published paper [24] for the assignment of 2d. However, the reaction mechanism in the referred paper indicates the formation of 2b. A rational explanation for the syntheses of 2a and 2d is not available at the present stage.
Scheme 1: Dihydroxylation of (a) (R)-limonene and (b) (R)-dihydrolimonene.
Scheme 1: Dihydroxylation of (a) (R)-limonene and (b) (R)-dihydrolimonene.
Since Royals et al. presented a pioneering study despite insufficient data for three diastereomers of LMdiol in 1966 [25], no further reports on the systematic synthesis and comparison of the four LMdiols have been published. The stereochemical structures of LMdiols are not depicted in several reports [26] presumably due to a lack of reliable characterisation data for the four LMdiols. Therefore, reliable characterisation data of LMdiols and LM5CCs are required, particularly if the confusion surrounding LMdiols should be resolved.
Herein, we focus on two compounds, LMdiol and LM5CC, as versatile and functional LM derivatives, and report the systematic synthesis of each of their four diastereomers and their characterisation using spectroscopic methods, mainly NMR. Comparing the data, unambiguous distinction of the four diastereomers is achieved in both cases.
Results and Discussion
Syntheses of LM5CC 1a and 1d and LMdiol 2a and 2d
Facile syntheses of two LM5CCs 1a and 1d and one LMdiol 2d have been preliminarily reported in our previous study [27]. Of these, 1a has already been synthesised by other groups [10,11] as well as our group. Particularly, Kleij et al. revealed the solid-state structure of 1a via X-ray analysis [11], but the isolation and characterisation of 1d has only been reported by our group.
Herein, we report the synthesis of 1a from the trans-isomer of (R)-limonene oxide (LO) and 1d from cis-LO using CO2 and tetrabutylammonium chloride (TBAC) as a catalyst. Both reactions proceeded without any side reactions and produced no unwanted isomers, such as 1c. Though the CO2 pressure employed in our previous study was 3 MPa [27], 5 MPa CO2 was applied in the present study to promote a higher consumption of LO. The result showed the isolated yields were improved to 84% yield for 1a and 30% yield for 1d.
In our previous report, 1d was reduced by lithium aluminium hydride (LAH) to obtain the corresponding LMdiol 2d [27]. Notably, the structure of 2d was confirmed by X-ray analysis. It has been reported that reduction of the 5CC moiety with LAH gives the corresponding diol with the same stereochemical configuration at the carbon atoms as of the original 5CC moiety [28,29]. Such a reduction with LAH was initially applied in this study to 1a (Scheme 2) as an analogy to the reaction of 1d. The reaction proceeded smoothly giving the corresponding LMdiol 2a in an excellent yield (Scheme 3).
Scheme 2: Reduction of LM5CC (1a) and carbonation of LMdiol (2a).
Scheme 2: Reduction of LM5CC (1a) and carbonation of LMdiol (2a).
Scheme 3: Overall synthetic routes to LM5CCs and LMdiols. The 93% value (standing for *) from 1d to 2d was cited from ref [27]. Reagents and conditions: (a) 5 MPa CO2, 10 mol % TBAC, 100 °C, 72 h; (b) LAH, rt, 2 h; (c) triphosgene, rt, 2 h; (d) H2O/dioxane (1:1, v/v), 120 °C, 72 h; (e) H2O, 90 °C, 12 h.
Scheme 3: Overall synthetic routes to LM5CCs and LMdiols. The 93% value (standing for *) from 1d to 2d was ci...
It is also possible to synthesise 5CCs from diols by reaction with triphosgene (Scheme 2). It is well known that this carbonation reaction maintains the stereochemical configuration of the original diol [30,31]. Accordingly, the reactions of 2a and 2d with triphosgene successfully afforded 1a and 1d, respectively, in this study (Scheme 3). The two carbonates obtained from the diols and triphosgene were spectroscopically identical to those synthesised from LO and CO2, as confirmed by 1H and 13C NMR analyses.
Syntheses of LM5CC 1b and 1c and LMdiol 2b and 2c
The reaction of trans-LO and water in dioxane afforded a mixture of two LMdiols 2b/2c. After purification, 2b and 2c were isolated in 58% and 19% yields, respectively (Scheme 3). Conversely, the reaction of cis-LO with water afforded only 2b, which was obtained in 70% yield after purification. These results are in good agreement with those of previous reports [18,20,32].
Similarly to the aforementioned carbonation of 2a (Scheme 2), 2b and 2c were also reacted with triphosgene. Neither of the desired products 1b and 1c have been synthesised until now. When 2c was used, the carbonation proceeded smoothly to afford the corresponding carbonate 1c exclusively. The isolated yield after purification was moderate (58%). Conversely, the reaction of 2b was accompanied by side reactions. Although the starting material 2b was completely consumed, two major products were detected by thin-layer chromatography. After purification, the desired 1b was obtained in a relatively low yield of 24%.
Two conformations (A and B in Scheme 4) are possible for the intermediate when 2b is reacted with triphosgene. The conformation A is expected to be more stable because the isopropenyl group remains in the equatorial position on the cyclohexane ring. In this conformation, the remaining hydroxy group is located in the axial position and away from the carbonyl group of the chloroformate moiety. This conformation makes the formation of the five-membered cyclic structure by ring-closing very difficult, leading to the low yield. However, although the yield of 1b was low, both 1b and 1c were successfully synthesised for the first time in the present study.
Scheme 4: Plausible conformations of the intermediate in the reaction of LMdiol (2b) with triphosgene.
Scheme 4: Plausible conformations of the intermediate in the reaction of LMdiol (2b) with triphosgene.
The carbonates 1b and 1c were also reacted with LAH in the same manner as 1a (Scheme 2). After reaction and purification, 2b and 2c were afforded in high yields (Scheme 3). The two resultant compounds were characterised by 1H and 13C NMR analyses, and the spectroscopic data were identical to those of 2b and 2c obtained from LO and water. Therefore, these results confirm the formation of the two carbonates 1b and 1c.
Overview of synthetic routes to the LM5CCs and LMdiols
The overall results for the syntheses of the four LM5CCs and the four LMdiols are shown in Scheme 3. All the reactions do not lead to the formation of other diastereomers as byproducts except for the reaction of trans-LO with water (Scheme 3, conditions (d)). The reduction under conditions (b) and carbonation under conditions (a) and (c) retain the diastereomeric conformations around the epoxy, 5CC and diol moieties.
In all the reactions, the yields for the reduction of LM5CC with LAH were high at 84–94%. Conversely, the yields of the carbonations to LM5CC differ depending on both the starting material and reaction conditions, i.e., (a) and (c) in Scheme 3. Thus, all the LM5CCs and LMdiols can be synthesised by choosing the appropriate starting LO and reaction conditions with high diastereomeric stereoselectivity and, in most cases, moderate to high yields.
To compare the four LMdiols and the four LM5CCs, a set of the characteristic analytical data such as melting points, optical rotation values and Kovats retention index values in gas chromatography was listed in Tables S1 and S2 in Supporting Information File 1.
Analysis and comparison of the four LM5CCs
In our previous study, the assignments of the NMR signals for 1a and 1d were not adequate [27]. Table 1 lists the 13C NMR chemical shift values for all LM5CCs, showing the assignments for each of the carbon atoms. The multiplicities were ascertained using DEPT135 analysis (Supporting Information File 1, pages S13, S18, S23, and S28).
Table 1: Chemical shift values (ppm) of four LM5CCs with the assignmentsa.
|
|
|||||||
| 1a | 1b | 1c | 1d | ||||
| 154.6 | (C11) | 155.1 | (C11) | 155.2 | (C11) | 154.4 | (C11) |
| 147.2 | (C7) | 145.7 | (C7) | 146.4 | (C7) | 147.3 | (C7) |
| 110.0 | (C8) | 111.9 | (C8) | 110.7 | (C8) | 109.8 | (C8) |
| 82.0 | (C1) | 86.2 | (C1) | 85.4 | (C1) | 82.6 | (C1) |
| 80.4 | (C2) | 81.6 | (C2) | 84.8 | (C2) | 81.7 | (C2) |
| 39.8 | (C4) | 38.5 | (C4) | 43.0 | (C4) | 37.3 | (C4) |
| 33.9 | (C3) | 31.7 | (C6) | 33.0 | (C6) | 34.1 | (C6) |
| 32.9 | (C6) | 25.8 | (C3) | 28.9 | (C3) | 30.5 | (C3) |
| 26.1 | (C10) | 25.1 | (C5) | 27.9 | (C5) | 26.2 | (C5) |
| 25.6 | (C5) | 22.5 | (C9) | 20.8 | (C9) | 22.2 | (C10) |
| 20.5 | (C9) | 16.8 | (C10) | 16.9 | (C10) | 20.8 | (C9) |
aIn CDCl3.
As characteristic signals, the C9 values are observed at higher magnetic fields than the C10 values for 1a and 1d. However, the relationship is reversed for 1b and 1c. In addition, the values for C10 in 1b and 1c are observed at the higher magnetic fields (16.8 and 16.9 ppm, respectively) compared with those for 1a and 1d. The order of C3, C6 and C5 signals for 1a is also different from that (C6, C3, C5) of the other three LM5CCs.
1H NMR spectra of the four LM5CCs are shown in Figure 3 (selected range) and Supporting Information File 1, page S32 (full range) with the assignments of signals a–h. Three characteristic signals f, a and c are clearly observed at 5.0–4.7, 4.4–4.2 and 2.4–1.9 ppm, respectively. Obviously, the respective diastereomers can be distinguished by comparison of the characteristic signals. The determination of all the signals in 1H and 13C NMR is also supported by 1H,1H COSY and HETCOR (Supporting Information File 1).
![[1860-5397-15-13-3]](/bjoc/content/figures/1860-5397-15-13-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: 1H NMR spectra of LM5CCs 1a–d in CDCl3.
Figure 3: 1H NMR spectra of LM5CCs 1a–d in CDCl3.
Analysis and comparison for the four LMdiols
Table 2 lists the chemical shifts in the 13C NMR spectra with the assignments for all LMdiols. The listed values for 2a and 2c are close, but distinguishable. Conversely, 2b and 2d present rather similar values for all the signals. In the 28–24 ppm region, two characteristic signals ascribed to C5 and C10, which are clearly distinguishable by DEPT 135, are observed. The two values are approximately 4 ppm apart for 2d, but less than 1 ppm apart for 2b. Furthermore, the order is reversed for the two samples; the signal of C10 appears at higher field than that for C5 in case of 2d. This demonstrates that 2b and 2d are similar but can be differentiated by their characteristic NMR signals.
Table 2: Chemical shift values (ppm) of four LMdiols with the assignmentsa.
|
|
|||||||
| 2a | 2b | 2c | 2d | ||||
| 149.0 | (C7) | 149.1 | (C7) | 148.4 | (C7) | 149.0 | (C7) |
| 108.8 | (C8) | 109.0 | (C8) | 109.1 | (C8) | 108.9 | (C8) |
| 75.1 | (C2) | 73.8 | (C2) | 77.2 | (C2) | 73.7 | (C2) |
| 70.8 | (C1) | 71.4 | (C1) | 74.0 | (C1) | 71.9 | (C1) |
| 43.7 | (C4) | 37.4 | (C4) | 43.6 | (C4) | 37.5 | (C4) |
| 37.3 | (C6) | 33.9 | (C3) | 38.5 | (C6) | 34.5 | (C3) |
| 35.5 | (C3) | 33.7 | (C6) | 36.1 | (C3) | 34.1 | (C6) |
| 27.1 | (C10) | 26.5 | (C10) | 28.7 | (C5) | 28.2 | (C5) |
| 26.1 | (C5) | 26.1 | (C5) | 20.9 | (C9) | 24.4 | (C10) |
| 20.8 | (C9) | 21.1 | (C9) | 18.9 | (C10) | 21.2 | (C9) |
aIn CDCl3.
In 1H NMR spectra of the four LMdiols (Figure 4 and Supporting Information File 1, page S61), characteristic signals a are observed at 3.41 for 2a and 3.58 ppm for 2c, demonstrating that they are different. However, signals a and c as well as others show similar values for 2b and 2d. The most significant difference is observed for the signal at 1.3 ppm of 2d (Supporting Information File 1, page S55), allowing the two samples to be distinguished.
![[1860-5397-15-13-4]](/bjoc/content/figures/1860-5397-15-13-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: 1H NMR spectra of LMdiols 2a–d in CDCl3.
Figure 4: 1H NMR spectra of LMdiols 2a–d in CDCl3.
The assignments in the 1H and 13C NMR signals were supported by DEPT135, 1H,1H COSY, HETCOR and HMBC (heteronuclear multiple bond correlation) analyses. Two samples of 2a and 2b were also subjected to 1,1-ADEQUATE (adequate double quantum transfer experiment) analysis.
Structures of four LMdiols and four LM5CCs
Plausible conformations for the LM5CCs and LMdiols in CDCl3 are proposed in Figure 5. In Figure 3, large coupling constants (J > ca. 10 Hz) for the signal a are observed for 1a–c. Conversely, the signal for 1d exhibits a small coupling constant (J < ca. 3 Hz), showing a largely singlet shape. For the former three LM5CCs, this means that the proton a locates at the axial position on the cyclohexane ring and interacts strongly with the neighbouring proton b. Conversely, for 1d, the proton locates at the equatorial position; thus, the interaction with proton b is weak. These interactions support the structures shown in Figure 5. For 1b, two oxygen atoms located at the equatorial positions form a 5CC cyclic structure; then, the isopropenyl group is located at the axial position. This speculation is also supported by the low 24% yield in the synthesis of 1b from 2b, as shown in Scheme 3 and Scheme 4.
Figure 5: Plausible conformations of LM5CCs and LMdiols in CDCl3.
Figure 5: Plausible conformations of LM5CCs and LMdiols in CDCl3.
In these conformations, proton c is expected to be located in the axial position for 1a, 1c and 1d, and in the equatorial position for 1b. In fact, the coupling constants match well the conformations in Figure 5 although the signal c for 1d unfortunately overlaps with other signals. The structure of 1a is in good agreement with the solid-state structure reported previously [11].
Conformations of the four LMdiols were also considered in the same manner as those of LM5CCs by reference to the a and c signals in Figure 4. The coupling constants for the signal a support the structures shown in Figure 5. Indeed, the equatorial position of the isopropenyl group for 2c and 2d in the solid state was confirmed by X-ray diffraction analysis, as reported previously [20,27]. The c protons are expected to stay in the axial positions for all the LMdiols. These considerations are consistent with the fact that the signals exhibit large coupling constants for 2a, 2c and 2d. For 2b in CDCl3, the coupling constants could not be estimated because of the multiplet shape; however, a large coupling constant was clearly observed in CD3OD and in benzene-d6 (Supporting Information File 1, page S42).
Conclusion
Four diastereomers of (R)-limonene-derived diols (LMdiols) and the corresponding five-membered cyclic carbonates (LM5CCs) were synthesised and characterised by NMR analysis. In particular, the NMR data for 2b and 2d exhibit rather similar values. The assignments of 1H and 13C NMR signals were found to provide a means of differentiating the respective diastereomers for the first time. These fundamental studies will contribute to the organic, bioorganic and environmental chemistry of (R)-limonene-based compounds as well as promoting their potential application.
Supporting Information
| Supporting Information File 1: Experimental, synthesis, and NMR and FTIR spectra of all the compounds. | ||
| Format: PDF | Size: 3.6 MB | Download |
References
-
Ciriminna, R.; Lomeli-Rodriguez, M.; Demma Carà, P.; Lopez-Sanchez, J. A.; Pagliaro, M. Chem. Commun. 2014, 50, 15288–15296. doi:10.1039/c4cc06147k
Return to citation in text: [1] [2] -
Gunam Resul, M. F. M.; López Fernández, A. M.; Rehman, A.; Harvey, A. P. React. Chem. Eng. 2018, 3, 747–756. doi:10.1039/c8re00094h
Return to citation in text: [1] -
Schimpf, V.; Ritter, B. S.; Weis, P.; Parison, K.; Mülhaupt, R. Macromolecules 2017, 50, 944–955. doi:10.1021/acs.macromol.6b02460
Return to citation in text: [1] -
Vandresen, F.; Falzirolli, H.; Almeida Batista, S. A.; da Silva-Giardini, A. P. B.; de Oliveira, D. N.; Catharino, R. R.; Ruiz, A. L. T. G.; de Carvalho, J. E.; Foglio, M. A.; da Silva, C. C. Eur. J. Med. Chem. 2014, 79, 110–116. doi:10.1016/j.ejmech.2014.03.086
Return to citation in text: [1] -
Maeda, C.; Sasaki, S.; Takaishi, K.; Ema, T. Catal. Sci. Technol. 2018, 8, 4193–4198. doi:10.1039/c8cy00941d
Return to citation in text: [1] -
Murayama, T.; Asano, M.; Ohmura, T.; Usuki, A.; Yasui, T.; Yamamoto, Y. Bull. Chem. Soc. Jpn. 2018, 91, 383–390. doi:10.1246/bcsj.20170371
Return to citation in text: [1] -
Fujihara, T.; Inokuchi, M.; Mizoe, T.; Nogi, K.; Terao, J.; Tsuji, Y. Chem. Lett. 2017, 46, 968–969. doi:10.1246/cl.170252
Return to citation in text: [1] -
Wang, B.; Yang, S.; Min, L.; Gu, Y.; Zhang, Y.; Wu, X.; Zhang, L.; Elageed, E. H. M.; Wu, S.; Gao, G. Adv. Synth. Catal. 2014, 356, 3125–3134. doi:10.1002/adsc.201400026
Return to citation in text: [1] -
Datta, J.; Włoch, M. Polym. Bull. 2016, 73, 1459–1496. doi:10.1007/s00289-015-1546-6
Return to citation in text: [1] -
Martínez, J.; Fernández-Baeza, J.; Sánchez-Barba, L. F.; Castro-Osma, J. A.; Lara-Sánchez, A.; Otero, A. ChemSusChem 2017, 10, 2886–2890. doi:10.1002/cssc.201700898
Return to citation in text: [1] [2] -
Fiorani, G.; Stuck, M.; Martín, C.; Belmonte, M. M.; Martin, E.; Escudero-Adán, E. C.; Kleij, A. W. ChemSusChem 2016, 9, 1304–1311. doi:10.1002/cssc.201600238
Return to citation in text: [1] [2] [3] [4] -
Leung, A. E.; Blair, M.; Forsyth, C. M.; Tuck, K. L. Org. Lett. 2013, 15, 2198–2201. doi:10.1021/ol400754e
Return to citation in text: [1] -
Blair, M.; Tuck, K. L. Tetrahedron: Asymmetry 2009, 20, 2149–2153. doi:10.1016/j.tetasy.2009.08.009
Return to citation in text: [1] -
Leung, A. E.; Rubbiani, R.; Gasser, G.; Tuck, K. L. Org. Biomol. Chem. 2014, 12, 8239–8246. doi:10.1039/c4ob01662a
Return to citation in text: [1] [2] -
Schmidt, L.; Göen, T. Arch. Toxicol. 2017, 91, 1175–1185. doi:10.1007/s00204-016-1751-6
Return to citation in text: [1] -
Wang, Y.; Ren, J.; Huang, X. H. H.; Tong, R.; Yu, J. Z. Environ. Sci. Technol. 2017, 51, 6791–6801. doi:10.1021/acs.est.7b01179
Return to citation in text: [1] [2] [3] -
Plummer, C. M.; Kraft, P.; Froese, J.; Hudlický, T.; Rook, T. J.; Jones, O. A. H.; Hügel, H. M. Asian J. Org. Chem. 2015, 4, 1075–1084. doi:10.1002/ajoc.201500233
Return to citation in text: [1] -
Águila, S.; Vazquez-Duhalt, R.; Tinoco, R.; Rivera, M.; Pecchi, G.; Alderete, J. B. Green Chem. 2008, 10, 647–653. doi:10.1039/b719992a
Return to citation in text: [1] [2] -
Wang, Z.; Cui, Y.-T.; Xu, Z.-B.; Qu, J. J. Org. Chem. 2008, 73, 2270–2274. doi:10.1021/jo702401t
Return to citation in text: [1] -
Blair, M.; Andrews, P. C.; Fraser, B. H.; Forsyth, C. M.; Junk, P. C.; Massi, M.; Tuck, K. L. Synthesis 2007, 1523–1527. doi:10.1055/s-2007-966033
Return to citation in text: [1] [2] [3] -
Kolb, H. C.; VanNieuwenhze, M. S.; Sharpless, K. B. Chem. Rev. 1994, 94, 2483–2547. doi:10.1021/cr00032a009
Return to citation in text: [1] -
Mori, K. Tetrahedron: Asymmetry 2006, 17, 2133–2142. doi:10.1016/j.tetasy.2006.07.030
Return to citation in text: [1] -
Hao, B.; Gunaratna, M. J.; Zhang, M.; Weerasekara, S.; Seiwald, S. N.; Nguyen, V. T.; Meier, A.; Hua, D. H. J. Am. Chem. Soc. 2016, 138, 16839–16848. doi:10.1021/jacs.6b12113
Return to citation in text: [1] -
Kumar, S. C. S.; Manjunatha, J. R.; Srinivas, P.; Bettadaiah, B. K. J. Chem. Sci. 2014, 126, 875–880. doi:10.1007/s12039-014-0633-9
Return to citation in text: [1] -
Royals, E. E.; Leffingwell, J. C. J. Org. Chem. 1966, 31, 1937–1944. doi:10.1021/jo01344a062
Return to citation in text: [1] -
Wróblewska, A. Molecules 2014, 19, 19907–19922. doi:10.3390/molecules191219907
Return to citation in text: [1] -
Morikawa, H.; Minamoto, M.; Gorou, Y.; Yamaguchi, J.-i.; Morinaga, H.; Motokucho, S. Bull. Chem. Soc. Jpn. 2018, 91, 92–94. doi:10.1246/bcsj.20170300
Return to citation in text: [1] [2] [3] [4] [5] [6] -
Wender, P. A.; Buschmann, N.; Cardin, N. B.; Jones, L. R.; Kan, C.; Kee, J.-M.; Kowalski, J. A.; Longcore, K. E. Nat. Chem. 2011, 3, 615–619. doi:10.1038/nchem.1074
Return to citation in text: [1] -
Marcos, I. S.; Castañeda, L.; Basabe, P.; Díez, D.; Urones, J. G. Tetrahedron 2008, 64, 10860–10866. doi:10.1016/j.tet.2008.09.007
Return to citation in text: [1] -
Paterson, I.; Anderson, E. A.; Dalby, S. M.; Lim, J. H.; Maltas, P. Org. Biomol. Chem. 2012, 10, 5873–5886. doi:10.1039/c2ob25101a
Return to citation in text: [1] -
Superchi, S.; Donnoli, M. I.; Proni, G.; Spada, G. P.; Rosini, C. J. Org. Chem. 1999, 64, 4762–4767. doi:10.1021/jo990038y
Return to citation in text: [1] -
Palumbo, C.; Ferrandi, E. E.; Marchesi, C.; Monti, D.; Riva, S.; Psaro, R.; Guidotti, M. ChemistrySelect 2016, 1, 1795–1798. doi:10.1002/slct.201600529
Return to citation in text: [1]
| 27. | Morikawa, H.; Minamoto, M.; Gorou, Y.; Yamaguchi, J.-i.; Morinaga, H.; Motokucho, S. Bull. Chem. Soc. Jpn. 2018, 91, 92–94. doi:10.1246/bcsj.20170300 |
| 11. | Fiorani, G.; Stuck, M.; Martín, C.; Belmonte, M. M.; Martin, E.; Escudero-Adán, E. C.; Kleij, A. W. ChemSusChem 2016, 9, 1304–1311. doi:10.1002/cssc.201600238 |
| 20. | Blair, M.; Andrews, P. C.; Fraser, B. H.; Forsyth, C. M.; Junk, P. C.; Massi, M.; Tuck, K. L. Synthesis 2007, 1523–1527. doi:10.1055/s-2007-966033 |
| 27. | Morikawa, H.; Minamoto, M.; Gorou, Y.; Yamaguchi, J.-i.; Morinaga, H.; Motokucho, S. Bull. Chem. Soc. Jpn. 2018, 91, 92–94. doi:10.1246/bcsj.20170300 |
| 1. | Ciriminna, R.; Lomeli-Rodriguez, M.; Demma Carà, P.; Lopez-Sanchez, J. A.; Pagliaro, M. Chem. Commun. 2014, 50, 15288–15296. doi:10.1039/c4cc06147k |
| 9. | Datta, J.; Włoch, M. Polym. Bull. 2016, 73, 1459–1496. doi:10.1007/s00289-015-1546-6 |
| 16. | Wang, Y.; Ren, J.; Huang, X. H. H.; Tong, R.; Yu, J. Z. Environ. Sci. Technol. 2017, 51, 6791–6801. doi:10.1021/acs.est.7b01179 |
| 8. | Wang, B.; Yang, S.; Min, L.; Gu, Y.; Zhang, Y.; Wu, X.; Zhang, L.; Elageed, E. H. M.; Wu, S.; Gao, G. Adv. Synth. Catal. 2014, 356, 3125–3134. doi:10.1002/adsc.201400026 |
| 24. | Kumar, S. C. S.; Manjunatha, J. R.; Srinivas, P.; Bettadaiah, B. K. J. Chem. Sci. 2014, 126, 875–880. doi:10.1007/s12039-014-0633-9 |
| 5. | Maeda, C.; Sasaki, S.; Takaishi, K.; Ema, T. Catal. Sci. Technol. 2018, 8, 4193–4198. doi:10.1039/c8cy00941d |
| 6. | Murayama, T.; Asano, M.; Ohmura, T.; Usuki, A.; Yasui, T.; Yamamoto, Y. Bull. Chem. Soc. Jpn. 2018, 91, 383–390. doi:10.1246/bcsj.20170371 |
| 7. | Fujihara, T.; Inokuchi, M.; Mizoe, T.; Nogi, K.; Terao, J.; Tsuji, Y. Chem. Lett. 2017, 46, 968–969. doi:10.1246/cl.170252 |
| 22. | Mori, K. Tetrahedron: Asymmetry 2006, 17, 2133–2142. doi:10.1016/j.tetasy.2006.07.030 |
| 1. | Ciriminna, R.; Lomeli-Rodriguez, M.; Demma Carà, P.; Lopez-Sanchez, J. A.; Pagliaro, M. Chem. Commun. 2014, 50, 15288–15296. doi:10.1039/c4cc06147k |
| 2. | Gunam Resul, M. F. M.; López Fernández, A. M.; Rehman, A.; Harvey, A. P. React. Chem. Eng. 2018, 3, 747–756. doi:10.1039/c8re00094h |
| 3. | Schimpf, V.; Ritter, B. S.; Weis, P.; Parison, K.; Mülhaupt, R. Macromolecules 2017, 50, 944–955. doi:10.1021/acs.macromol.6b02460 |
| 4. | Vandresen, F.; Falzirolli, H.; Almeida Batista, S. A.; da Silva-Giardini, A. P. B.; de Oliveira, D. N.; Catharino, R. R.; Ruiz, A. L. T. G.; de Carvalho, J. E.; Foglio, M. A.; da Silva, C. C. Eur. J. Med. Chem. 2014, 79, 110–116. doi:10.1016/j.ejmech.2014.03.086 |
| 23. | Hao, B.; Gunaratna, M. J.; Zhang, M.; Weerasekara, S.; Seiwald, S. N.; Nguyen, V. T.; Meier, A.; Hua, D. H. J. Am. Chem. Soc. 2016, 138, 16839–16848. doi:10.1021/jacs.6b12113 |
| 16. | Wang, Y.; Ren, J.; Huang, X. H. H.; Tong, R.; Yu, J. Z. Environ. Sci. Technol. 2017, 51, 6791–6801. doi:10.1021/acs.est.7b01179 |
| 21. | Kolb, H. C.; VanNieuwenhze, M. S.; Sharpless, K. B. Chem. Rev. 1994, 94, 2483–2547. doi:10.1021/cr00032a009 |
| 15. | Schmidt, L.; Göen, T. Arch. Toxicol. 2017, 91, 1175–1185. doi:10.1007/s00204-016-1751-6 |
| 16. | Wang, Y.; Ren, J.; Huang, X. H. H.; Tong, R.; Yu, J. Z. Environ. Sci. Technol. 2017, 51, 6791–6801. doi:10.1021/acs.est.7b01179 |
| 12. | Leung, A. E.; Blair, M.; Forsyth, C. M.; Tuck, K. L. Org. Lett. 2013, 15, 2198–2201. doi:10.1021/ol400754e |
| 13. | Blair, M.; Tuck, K. L. Tetrahedron: Asymmetry 2009, 20, 2149–2153. doi:10.1016/j.tetasy.2009.08.009 |
| 14. | Leung, A. E.; Rubbiani, R.; Gasser, G.; Tuck, K. L. Org. Biomol. Chem. 2014, 12, 8239–8246. doi:10.1039/c4ob01662a |
| 10. | Martínez, J.; Fernández-Baeza, J.; Sánchez-Barba, L. F.; Castro-Osma, J. A.; Lara-Sánchez, A.; Otero, A. ChemSusChem 2017, 10, 2886–2890. doi:10.1002/cssc.201700898 |
| 11. | Fiorani, G.; Stuck, M.; Martín, C.; Belmonte, M. M.; Martin, E.; Escudero-Adán, E. C.; Kleij, A. W. ChemSusChem 2016, 9, 1304–1311. doi:10.1002/cssc.201600238 |
| 14. | Leung, A. E.; Rubbiani, R.; Gasser, G.; Tuck, K. L. Org. Biomol. Chem. 2014, 12, 8239–8246. doi:10.1039/c4ob01662a |
| 17. | Plummer, C. M.; Kraft, P.; Froese, J.; Hudlický, T.; Rook, T. J.; Jones, O. A. H.; Hügel, H. M. Asian J. Org. Chem. 2015, 4, 1075–1084. doi:10.1002/ajoc.201500233 |
| 18. | Águila, S.; Vazquez-Duhalt, R.; Tinoco, R.; Rivera, M.; Pecchi, G.; Alderete, J. B. Green Chem. 2008, 10, 647–653. doi:10.1039/b719992a |
| 19. | Wang, Z.; Cui, Y.-T.; Xu, Z.-B.; Qu, J. J. Org. Chem. 2008, 73, 2270–2274. doi:10.1021/jo702401t |
| 20. | Blair, M.; Andrews, P. C.; Fraser, B. H.; Forsyth, C. M.; Junk, P. C.; Massi, M.; Tuck, K. L. Synthesis 2007, 1523–1527. doi:10.1055/s-2007-966033 |
| 27. | Morikawa, H.; Minamoto, M.; Gorou, Y.; Yamaguchi, J.-i.; Morinaga, H.; Motokucho, S. Bull. Chem. Soc. Jpn. 2018, 91, 92–94. doi:10.1246/bcsj.20170300 |
| 25. | Royals, E. E.; Leffingwell, J. C. J. Org. Chem. 1966, 31, 1937–1944. doi:10.1021/jo01344a062 |
| 26. | Wróblewska, A. Molecules 2014, 19, 19907–19922. doi:10.3390/molecules191219907 |
| 30. | Paterson, I.; Anderson, E. A.; Dalby, S. M.; Lim, J. H.; Maltas, P. Org. Biomol. Chem. 2012, 10, 5873–5886. doi:10.1039/c2ob25101a |
| 31. | Superchi, S.; Donnoli, M. I.; Proni, G.; Spada, G. P.; Rosini, C. J. Org. Chem. 1999, 64, 4762–4767. doi:10.1021/jo990038y |
| 18. | Águila, S.; Vazquez-Duhalt, R.; Tinoco, R.; Rivera, M.; Pecchi, G.; Alderete, J. B. Green Chem. 2008, 10, 647–653. doi:10.1039/b719992a |
| 20. | Blair, M.; Andrews, P. C.; Fraser, B. H.; Forsyth, C. M.; Junk, P. C.; Massi, M.; Tuck, K. L. Synthesis 2007, 1523–1527. doi:10.1055/s-2007-966033 |
| 32. | Palumbo, C.; Ferrandi, E. E.; Marchesi, C.; Monti, D.; Riva, S.; Psaro, R.; Guidotti, M. ChemistrySelect 2016, 1, 1795–1798. doi:10.1002/slct.201600529 |
| 28. | Wender, P. A.; Buschmann, N.; Cardin, N. B.; Jones, L. R.; Kan, C.; Kee, J.-M.; Kowalski, J. A.; Longcore, K. E. Nat. Chem. 2011, 3, 615–619. doi:10.1038/nchem.1074 |
| 29. | Marcos, I. S.; Castañeda, L.; Basabe, P.; Díez, D.; Urones, J. G. Tetrahedron 2008, 64, 10860–10866. doi:10.1016/j.tet.2008.09.007 |
| 27. | Morikawa, H.; Minamoto, M.; Gorou, Y.; Yamaguchi, J.-i.; Morinaga, H.; Motokucho, S. Bull. Chem. Soc. Jpn. 2018, 91, 92–94. doi:10.1246/bcsj.20170300 |
| 27. | Morikawa, H.; Minamoto, M.; Gorou, Y.; Yamaguchi, J.-i.; Morinaga, H.; Motokucho, S. Bull. Chem. Soc. Jpn. 2018, 91, 92–94. doi:10.1246/bcsj.20170300 |
| 27. | Morikawa, H.; Minamoto, M.; Gorou, Y.; Yamaguchi, J.-i.; Morinaga, H.; Motokucho, S. Bull. Chem. Soc. Jpn. 2018, 91, 92–94. doi:10.1246/bcsj.20170300 |
| 10. | Martínez, J.; Fernández-Baeza, J.; Sánchez-Barba, L. F.; Castro-Osma, J. A.; Lara-Sánchez, A.; Otero, A. ChemSusChem 2017, 10, 2886–2890. doi:10.1002/cssc.201700898 |
| 11. | Fiorani, G.; Stuck, M.; Martín, C.; Belmonte, M. M.; Martin, E.; Escudero-Adán, E. C.; Kleij, A. W. ChemSusChem 2016, 9, 1304–1311. doi:10.1002/cssc.201600238 |
| 11. | Fiorani, G.; Stuck, M.; Martín, C.; Belmonte, M. M.; Martin, E.; Escudero-Adán, E. C.; Kleij, A. W. ChemSusChem 2016, 9, 1304–1311. doi:10.1002/cssc.201600238 |
© 2019 Morikawa et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the authors and source are credited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)