Abstract
A synthesis efficiency algorithm, which must be based on concrete and reliable criteria, is essential for the evaluation and control of complex chemical synthesis, notably multicomponent reactions (MCRs). An algorithm has been developed to precisely evaluate even highly complex syntheses with regards to their synthesis efficiency Effsyn as a tool for strict compliance with green chemistry requirements, and for economic progress. The mathematical operations are highly suitable for electronic data processing (EDP). This algorithm is also suitable as a basis for fair cost assessment of complex chemical syntheses.
Graphical Abstract
Introduction
The ongoing upheavals in the sectors of information technology, energy and electromobility, which are in some cases extremely competitive, mean that ecological and economical aspects of chemistry, as applicable to humans, are increasingly being focused on during this socio-economic transformation. Comprehensive efforts are being undertaken in this field, including in large workshops, e.g., [1].
The efficiency of synthesis forms the core for the evaluation of innovations within synthesis chemistry [2-6] and is the indispensable requirement for a radical simplification of chemical synthesis [3]. Concrete and reliable criteria must be available for this purpose, criteria that can be easily determined and measured, and which can also form the basis for an algorithm. The standard evaluation of a chemical synthesis is traditionally based on the overall yield yoa. This is the product of all sequential synthesis steps yn (Equation 1).
An extreme example for the impact of the overall yield is the tropinone synthesis by Willstätter (yoa = 0.75%) [7,8] compared to the Robinson–Schöpf synthesis (yoa = 90%) [9,10]) using a double Mannich reaction, a multicomponent reaction (MCR) [11-13]. The Mannich-3CR is therefore 120 times better than the Willstätter synthesis.
Criterion overall yield yoa
This yoa directly influences the variable costs for the starting and other materials in each synthesis, but not most other (fixed) costs.
Criterion synthesis step number n
Such costs are significant and manifold, deriving from direct costs such as fixed employee and laboratory costs, laboratory rental and maintenance costs, operating costs, i.e., power, water, (gas), inert gas and disposal costs. Standard laboratory activities that are repetitive, such as reactor configuration, filling, reaction monitoring, draining, work-up, preparation of reaction mixture and product isolation, product purification (distillation, recrystallisation, chromatography) and product analysis apply to all synthesis steps. All these costs are similar for each synthesis step n and can be said to be constant in the first approximation in cumulo. This provides a second concrete criterion, the synthesis step n, which also encompasses and quantifies two factors – “waste prevention” and “energy efficiency” – as requirements for “green chemistry”.
The efficiency of a synthesis, Effsyn will be defined in Equation 2. The synthesis step n in the context of this paper is a practical unit of reactions with supplements that all are run in one pot in one working process without intermediate isolation and purification of the reaction participants. The synthesis step therefore differs somewhat from the normal definition of a reaction step.
Time influences reactions via their kinetics and is therefore not a primary factor but a soft criterion. This can usually be greatly minimised during cost generation through clever time management of the synthesis planning and can essentially be treated here as a fixed cost.
Table 1 indicates the major impact the synthesis steps n have on the efficiency Effsyn of the synthesis. The range of profitable to useful syntheses decreases drastically with increasing synthesis steps n. The detrimental impact of a greater number of steps n is shown in the above-mentioned tropinone synthesis by Willstätter (yoa = 0.75%, N = 20, Effsyn = 0.038% [7,8]), compared with the Robinson–Schöpf synthesis (yoa = 90%, N = 1, Effsyn = 90% [9,10]). The latter MCR is therefore 2368 times (!) more efficient than the original Willstätter synthesis. Further examples, including the comprehensive synthesis of complex natural substances, can be found in [2,3].
Table 1: Overall yields yoa and synthesis efficiency Effsyn.
|
Overall yields yoa [%]
geometric average yields yav |
Number
of steps n |
Synthesis efficiency Effsyn [%]
geometric average yields yav |
||||||||
| 95 | 90 | 80 | 70 | 60 | 95 | 90 | 80 | 70 | 60 | |
| 95 | 90 | 80 | 70 | 60 | 1 | 95 | 90 | 80 | 70 | 60 |
| 90 | 81 | 64 | 49 | 36 | 2 | 45 | 40 | 32 | 25 | 18 |
| 86 | 73 | 51 | 34 | 22 | 3 | 29 | 24 | 17 | 11 | 7.3 |
| 81 | 66 | 41 | 24 | 13 | 4 | 20 | 17 | 10 | 6.0 | 3.0 |
| 77 | 59 | 33 | 17 | 7.8 | 5 | 15 | 12 | 6.6 | 3.4 | 1.6 |
| 74 | 53 | 26 | 12 | 4.7 | 6 | 12 | 8.8 | 4.3 | 2.0 | 0.78 |
| 70 | 48 | 21 | 8.2 | 2.8 | 7 | 10 | 6.9 | 3.0 | 1.2 | 0.40 |
| 66 | 43 | 17 | 5.8 | 1.7 | 8 | 8.3 | 5.4 | 2.1 | 0.73 | 0.21 |
| 63 | 39 | 13 | 4.0 | 1.0 | 9 | 7.0 | 4.3 | 1.8 | 4.4 | 0.11 |
| 60 | 35 | 11 | 2.8 | 0.6 | 10 | 6.0 | 3.5 | 1.1 | 0.28 | 0.06 |
These figures may astound some people, but they are the clear results of an impartial analysis. Limitations need to be determined in order to delineate the scope of a meaningful application area.
Ignoring or omitting the number of steps as an essential criterion is a serious issue, for example, if one simply assumes that 4 reactions with 97% yield each are better than a 4CR with 90% yield. The fact that the outlay (fixed costs) during MCR drop by a massive 75% – compared to the 4 separate reactions – is often ignored. And those 4 separate reactions actually have an overall yield of 88.5%. This behaviour is unfortunately very common, culpably inefficient! The overall yield alone does not encompass such facts and any mathematical treatment needs to bring together all primary criteria.
MCRs have a high material and energy efficiency, and their atom balance is quite outstanding. Product purification is usually simple. All this reduces waste to a minimum. Due to the very weak negative reaction enthalpies, MCRs are also usually safe processes. The shortening of the synthesis through drastic reduction of the number of steps n leads to a strong waste prevention, which can be quantitatively measured through the synthesis efficiency in Equation 2 (Table 1).
Results and Discussion
In practice, there are problems with complete calculations of overall yields for complex syntheses, particularly when several precursors (2 or more) need to be included in the calculation, which is almost always the case with MCRs. All such reactions are parallel reactions and do not have any sequential character with respect to each other, instead they are cumulative, whereby the parallel reaction groups have different numbers of individual reactions n. The yields yn then need to be weighted with these m values and the arithmetic mean yam calculated as shown in Equation 3.
Algorithm
If you insert Equation 1 in Equation 3, and then insert this in Equation 2, you will obtain the efficiency algorithm Equation 4. This has already been described in a general manner in [2,3]. The weighting of the parallel reactions results in the precise value yoa.
The algorithm is broad in scope and can be used in many ways as required through the introduction of constants in the variables c1, c2 and invariable C. This permits the inclusion of soft criteria, such as suitable resources, time (see discussion above) and process control, in the cost analysis of complex syntheses (Equation 5).
An App based on this algorithm can offer an effective way to obtain a rapid overview of the total or partial synthesis. It can be used to evaluate and even control the synthesis from various aspects, including how it is affected by soft criteria.
Case study
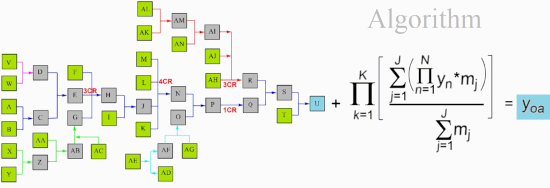
All listed and possible constellations of reactions and reaction groups in a complex synthesis are shown in a flow diagram (Scheme 1) and are presented in a detailed case study; the data were inserted into the general efficiency algorithm [2,3]. Although publications usually only show the synthesis path with the most spectacular molecules, such as the target molecule (TM), the total synthesis with all reactions is essential for production. The quantity of potential start molecule (STM) sets also rises strongly in complex syntheses. The example shows 5 STM sets (consisting of 10 STMs) with which the synthesis can be started, as well as 12 other STMs.
![[1860-5397-15-142-i1]](/bjoc/content/inline/1860-5397-15-142-i1.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 1: Case study of a complex synthesis, parallel reactions. 10 possible synthesis STMs in green, other 12 STMs in light green, TM U in blue.
Scheme 1: Case study of a complex synthesis, parallel reactions. 10 possible synthesis STMs in green, other 1...
To have a better overview in this study (Scheme 1), reactions are ordered to a main reaction set including two MCRs (reactants A-T, TM U), connected with 4 parallel reaction sets including a 3CR (Scheme 2).
![[1860-5397-15-142-i2]](/bjoc/content/inline/1860-5397-15-142-i2.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 2: Ordered reactions of the above complex synthesis to a main reaction set and connected parallel reaction sets.
Scheme 2: Ordered reactions of the above complex synthesis to a main reaction set and connected parallel reac...
Overall yield yoa and efficiency Effsyn calculation of J in case study
As a practical exercise, random numbers were added to the part A–J with both parallel reactions V–D and X–G in Scheme 3, and the overall yield yoa was then calculated incrementally with the algorithm in Equation 4. Expediently, a main reaction (set-1) to which the parallel reactions are linked (set-2, set-3) is set up. The weighted arithmetical mean of the yields for each reaction set is formed at the connection forms. The overall yield yoa of the total synthesis is determined through the sequential operation of the main reaction sets. The latter can be determined using the following calculation method.
![[1860-5397-15-142-i3]](/bjoc/content/inline/1860-5397-15-142-i3.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 3: Section A–J case study of Scheme 2 with operations of yoa calculation.
Scheme 3: Section A–J case study of Scheme 2 with operations of yoa calculation.
Calculation method
Split the main reaction set-1 at the connection points with the parallel reactions into set-1, set-2, set-3, then use Σ(Πyn)j to calculate the weighted mean yield values for the two branches C, D and E, G. The latter values, together with the values from Π[Σ(Πyn)j]k, deliver the overall yield yoa of the total synthesis according to Equations 6–8.
Parallel reactions set-2 to main reaction position (C), operation Σ(Πyn)j
Parallel reactions set-3 to main reaction position (E), operation Σ(Πyn)j
Sequential main reactions set-1, operation Π[Σ(Πyn)j]k
This synthesis consists of n = 8 synthesis steps, so the synthesis efficiency is
Modification of the calculation execution
Most chemists look exclusively at the interesting target molecule (TM) of a synthesis and only follow that path from STM to TM, while blanking out everything else. The second method for calculating yoa in complex syntheses is probably easier in this case. Here, the yield y(A–J) of the sequential main reaction set-1 is calculated (Equation 9) and added to the branches with the parallel reaction modification factors mf. This is equivalent to the quotients from the dividends C, D (from Equation 6) or E, G (from Equation 7) and the divisors A–C or E–G (Equations 10 and 11). The result from Equation 9 is multiplied with both modification factors mf to obtain the overall yield yoa (Equation 12).
Some useful strategic and practical applications demonstrate the enormous influence of branching on the overall yield yoa and synthesis efficiency Effsyn.
Fragment strategy: fragment linking in peptides synthesis
In synthetic peptide chemistry, amino acids are sequentially built up to form long oligo/polypeptides. For reasons of transparency, we have assumed the same yield of 80% in each step during the synthesis of a decapeptide in order to clearly indicate the effect of the branching (Scheme 4).
![[1860-5397-15-142-i4]](/bjoc/content/inline/1860-5397-15-142-i4.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 4: Sequential synthesis and fragment linking of a decapeptide with comparison of results in Equations 13–15.
Scheme 4: Sequential synthesis and fragment linking of a decapeptide with comparison of results in Equations ...
Three cases are discussed here:
- sequential linking of the 10 amino acids (Scheme 4a);
- sequential synthesis of two pentapeptides and the subsequent linking to form a linear decapeptide (Scheme 4b);
- sequential synthesis of two non-identical peptides (1 heptapeptide and 1 tripeptide) and their linking to form a linear decapeptide (Scheme 4c);
Calculation by means of the algorithm
The results are impressive. A up to 2.5-fold yield can be achieved depending on the configuration of the fragment linking, and the algorithm delivers rapid results. The number of steps is, however, only conditionally reduced (with identical steps) and this must be taken into consideration when calculating the synthesis efficiency Effsyn. The fragment strategy can of course be applied without limitations to other syntheses of this type.
MCR Strategy: Ugi-4CR in ecteinascidin-743 total synthesis
As described above, yields can be significantly increased by using the fragment strategy. However, the fragment strategy is limited to 2 components, while an MCR provides multiple components for linking, and also generates its own structure which is capable of extensions like the domino reaction types [5].
An Ugi reaction is used in the total synthesis of the extremely potent antitumor agent ecteinascidin-743 (Et-743, 1) by Fukuyama to form a large part of the skeleton 2 (Scheme 5). All data for the U-4CR are available in the literature [14]. The precursors of the Ugi reaction consist of 3 reaction chains of 6, 6 and 2 links, respectively.
Scheme 5: U-4CR with 17 precursors in the total synthesis of Et-743 (1) [14].
Scheme 5: U-4CR with 17 precursors in the total synthesis of Et-743 (1) [14].
Calculation by means of the algorithm
The calculation of the overall yield yoa of 2 is based on the data basis for the total synthesis of ecteinascidin-743 found in the literature [14], and the data for 4-methoxyphenylisocyanide is provided from the author, referred to Equations 16–20.
An outstanding result is shown for U-4CR, including the N = 14 (from real 17; three are double steps) precursors forming 2 with 50% overall yield. Synthesis efficiency is Effsyn = 2.9%, due to the high step number of 17 (real number of precursors). The same synthesis in linear architecture does not exist. A fictive comparison with the same dataset in a completely sequential reaction sequence results in a fictive yield of 2 yoa(fictive) = 0.192 (19%). The significant difference is due to the MCR itself and primarily the linked parallel reactions of the 3 precursors, as can be clearly seen in Scheme 5. These results favour the use of the MCR strategy with the Ugi reaction and provide an increase in yield by 2.6 times that of a linear solution.
MCR strategy: novel MCR as key step in total synthesis of (+)-20S-camptothecin
Another typical example for the simplification of a complex chemical synthesis [2,3] is the total synthesis of the extremely potent antitumor agent (+)-20S-camptothecin (3), which has been a highly effective agent for decades now. This total synthesis by Tietze uses a 4CR, specifically generated for this reaction from an aldehyde, meldrum’s acid, enol ether and methanol, as a key step in the synthesis to 4 (Scheme 6) [15]. This step-saving strategy for generating novel MCRs is a fast track towards the ubiquitous use of MCRs in complex syntheses. This algorithm (Equation 4) is an essential tool for the rational evaluation and synthesis control of MCRs.
Scheme 6: Synthesis of 4 as key step of (+)-20S-camptothecin (3) total synthesis.
Scheme 6: Synthesis of 4 as key step of (+)-20S-camptothecin (3) total synthesis.
Conclusion
The general efficiency algorithm [2,3], and the calculation methods (Equations 3–20) developed from that algorithm, can be used to precisely evaluate even highly complex syntheses and quantitatively compare them with alternative syntheses with regards to their synthesis efficiency Effsyn. The mathematical operations are highly suitable for electronic data processing (EDP), as is the algorithm itself. Due to the concrete criteria, this algorithm is also suitable as a basis for fair cost assessment of complex chemical syntheses. Fragment linking reactions, and an ecteinascidin-743 total syntheses including an Ugi reaction, as well as a 20S-camptothecin total synthesis based on a 4CR specifically generated for this purpose, were discussed. This demonstrates the high efficiency of the MCR application and its intrinsic suitability for green chemistry.
References
-
http://low-carbon-urban-development-germany-china.org/wp-content/uploads/2016/04/Weber-Sites-Consulting-Energy-Efficient-Technologies-in-Chemical-Industry-DE.pdf
Return to citation in text: [1] -
Eckert, H. Molecules 2017, 22, 349. doi:10.3390/molecules22030349
Return to citation in text: [1] [2] [3] [4] [5] [6] -
Eckert, H., Ed. Radical and Concerted Simplification of Chemical Synthesis; Wolff-Verlag: Berlin, Germany, 2017.
Return to citation in text: [1] [2] [3] [4] [5] [6] [7] -
Eckert, H. Radical simplification of chemical synthesis by MCRs Strategies. In Book of abstract, MCR-2018, Aug 26–31, 2018; 7th international conference on multicomponent reactions and related chemistry; p 71.
Return to citation in text: [1] -
Tietze, L. F., Ed. Domino Reactions: Concepts for Efficient Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2014. doi:10.1002/9783527671304
Return to citation in text: [1] [2] -
Bienaymé, H.; Hulme, C.; Oddon, G.; Schmitt, P. Chem. – Eur. J. 2000, 6, 3321–3329. doi:10.1002/1521-3765(20000915)6:18<3321::aid-chem3321>3.0.co;2-a
Return to citation in text: [1] -
Willstätter, R. Justus Liebigs Ann. Chem. 1901, 317, 204–265. doi:10.1002/jlac.19013170206
Return to citation in text: [1] [2] -
Smit, W. A.; Bochkov, A. F.; Caple, R. Organic Synthesis: The Science behind the Art; Royal Society of Chemistry: Cambridge, United Kingdom, 1998.
Return to citation in text: [1] [2] -
Robinson, R. J. Chem. Soc., Trans. 1917, 111, 762–768. doi:10.1039/ct9171100762
Return to citation in text: [1] [2] -
Schöpf, C. Angew. Chem. 1937, 50, 779–787. doi:10.1002/ange.19370504002
Return to citation in text: [1] [2] -
Zhu, J.; Bienaymé, H., Eds. Multicomponent Reactions; Wiley-VCH: Weinheim, Germany, 2005. doi:10.1002/3527605118
Return to citation in text: [1] -
Mueller, T. J. J., Ed. Multicomponent Reactions; Science of Synthesis, Vol. I + II; Thieme: Stuttgart, Germany, 2014.
Return to citation in text: [1] -
Huang, Y.; Yazbak, A.; Dömling, A. Multicomponent reactions. In Green Techniques for Organic Synthesis and Medicinal Chemistry; Zhang, W.; Cue, B. C., Jr., Eds.; John Wiley & Sons Ltd.: New York, U.S.A.; pp 497–522. doi:10.1002/9780470711828.ch18
Return to citation in text: [1] -
Endo, A.; Yanagisawa, A.; Abe, M.; Tohma, S.; Kan, T.; Fukuyama, T. J. Am. Chem. Soc. 2002, 124, 6552–6554. doi:10.1021/ja026216d
Return to citation in text: [1] [2] [3] -
Tietze, L. F.; Bischoff, M.; Khan, T. A.; Liu, D. Chem. Heterocycl. Compd. 2017, 53, 434–445. doi:10.1007/s10593-017-2070-4
Return to citation in text: [1]
| 15. | Tietze, L. F.; Bischoff, M.; Khan, T. A.; Liu, D. Chem. Heterocycl. Compd. 2017, 53, 434–445. doi:10.1007/s10593-017-2070-4 |
| 2. | Eckert, H. Molecules 2017, 22, 349. doi:10.3390/molecules22030349 |
| 3. | Eckert, H., Ed. Radical and Concerted Simplification of Chemical Synthesis; Wolff-Verlag: Berlin, Germany, 2017. |
| 9. | Robinson, R. J. Chem. Soc., Trans. 1917, 111, 762–768. doi:10.1039/ct9171100762 |
| 10. | Schöpf, C. Angew. Chem. 1937, 50, 779–787. doi:10.1002/ange.19370504002 |
| 14. | Endo, A.; Yanagisawa, A.; Abe, M.; Tohma, S.; Kan, T.; Fukuyama, T. J. Am. Chem. Soc. 2002, 124, 6552–6554. doi:10.1021/ja026216d |
| 7. | Willstätter, R. Justus Liebigs Ann. Chem. 1901, 317, 204–265. doi:10.1002/jlac.19013170206 |
| 8. | Smit, W. A.; Bochkov, A. F.; Caple, R. Organic Synthesis: The Science behind the Art; Royal Society of Chemistry: Cambridge, United Kingdom, 1998. |
| 2. | Eckert, H. Molecules 2017, 22, 349. doi:10.3390/molecules22030349 |
| 3. | Eckert, H., Ed. Radical and Concerted Simplification of Chemical Synthesis; Wolff-Verlag: Berlin, Germany, 2017. |
| 3. | Eckert, H., Ed. Radical and Concerted Simplification of Chemical Synthesis; Wolff-Verlag: Berlin, Germany, 2017. |
| 14. | Endo, A.; Yanagisawa, A.; Abe, M.; Tohma, S.; Kan, T.; Fukuyama, T. J. Am. Chem. Soc. 2002, 124, 6552–6554. doi:10.1021/ja026216d |
| 2. | Eckert, H. Molecules 2017, 22, 349. doi:10.3390/molecules22030349 |
| 3. | Eckert, H., Ed. Radical and Concerted Simplification of Chemical Synthesis; Wolff-Verlag: Berlin, Germany, 2017. |
| 4. | Eckert, H. Radical simplification of chemical synthesis by MCRs Strategies. In Book of abstract, MCR-2018, Aug 26–31, 2018; 7th international conference on multicomponent reactions and related chemistry; p 71. |
| 5. | Tietze, L. F., Ed. Domino Reactions: Concepts for Efficient Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2014. doi:10.1002/9783527671304 |
| 6. | Bienaymé, H.; Hulme, C.; Oddon, G.; Schmitt, P. Chem. – Eur. J. 2000, 6, 3321–3329. doi:10.1002/1521-3765(20000915)6:18<3321::aid-chem3321>3.0.co;2-a |
| 14. | Endo, A.; Yanagisawa, A.; Abe, M.; Tohma, S.; Kan, T.; Fukuyama, T. J. Am. Chem. Soc. 2002, 124, 6552–6554. doi:10.1021/ja026216d |
| 2. | Eckert, H. Molecules 2017, 22, 349. doi:10.3390/molecules22030349 |
| 3. | Eckert, H., Ed. Radical and Concerted Simplification of Chemical Synthesis; Wolff-Verlag: Berlin, Germany, 2017. |
| 2. | Eckert, H. Molecules 2017, 22, 349. doi:10.3390/molecules22030349 |
| 3. | Eckert, H., Ed. Radical and Concerted Simplification of Chemical Synthesis; Wolff-Verlag: Berlin, Germany, 2017. |
| 9. | Robinson, R. J. Chem. Soc., Trans. 1917, 111, 762–768. doi:10.1039/ct9171100762 |
| 10. | Schöpf, C. Angew. Chem. 1937, 50, 779–787. doi:10.1002/ange.19370504002 |
| 5. | Tietze, L. F., Ed. Domino Reactions: Concepts for Efficient Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2014. doi:10.1002/9783527671304 |
| 7. | Willstätter, R. Justus Liebigs Ann. Chem. 1901, 317, 204–265. doi:10.1002/jlac.19013170206 |
| 8. | Smit, W. A.; Bochkov, A. F.; Caple, R. Organic Synthesis: The Science behind the Art; Royal Society of Chemistry: Cambridge, United Kingdom, 1998. |
| 11. | Zhu, J.; Bienaymé, H., Eds. Multicomponent Reactions; Wiley-VCH: Weinheim, Germany, 2005. doi:10.1002/3527605118 |
| 12. | Mueller, T. J. J., Ed. Multicomponent Reactions; Science of Synthesis, Vol. I + II; Thieme: Stuttgart, Germany, 2014. |
| 13. | Huang, Y.; Yazbak, A.; Dömling, A. Multicomponent reactions. In Green Techniques for Organic Synthesis and Medicinal Chemistry; Zhang, W.; Cue, B. C., Jr., Eds.; John Wiley & Sons Ltd.: New York, U.S.A.; pp 497–522. doi:10.1002/9780470711828.ch18 |
| 2. | Eckert, H. Molecules 2017, 22, 349. doi:10.3390/molecules22030349 |
| 3. | Eckert, H., Ed. Radical and Concerted Simplification of Chemical Synthesis; Wolff-Verlag: Berlin, Germany, 2017. |
© 2019 Eckert; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the authors and source are credited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)