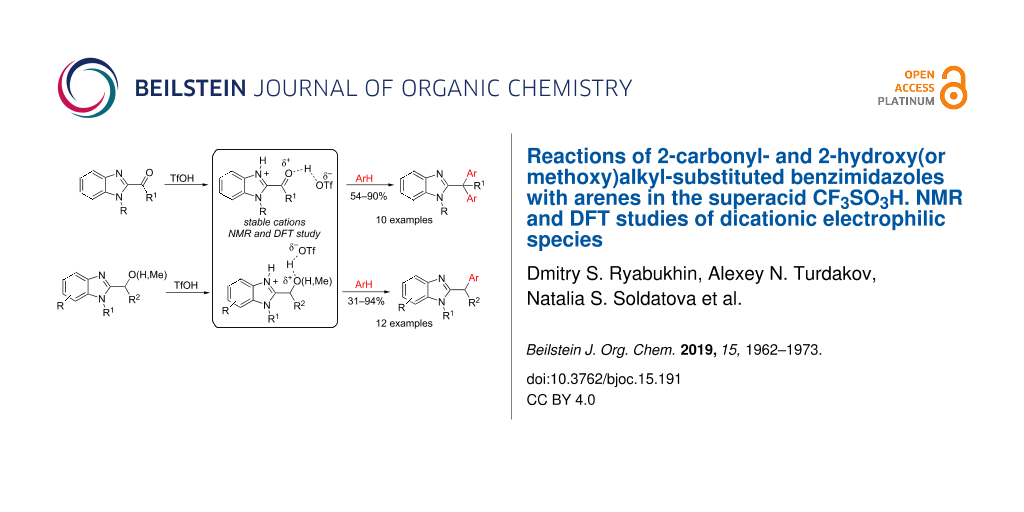
Abstract
Reactions of 2-carbonyl- and 2-hydroxy(or methoxy)alkylbenzimidazoles with arenes in the Brønsted superacid TfOH resulted in the formation of the corresponding Friedel–Crafts reaction products, 2-diarylmethyl and 2-arylmethyl-substituted benzimidazoles, in yields up to 90%. The reaction intermediates, protonated species derived from starting benzimidazoles in TfOH, were thoroughly studied by means of NMR and DFT calculations and plausible reaction mechanisms are discussed.
Graphical Abstract
Introduction
Imidazoles and benzimidazoles are important heterocyclic scaffolds in pharmaceuticals and agrochemicals [1-10]. They also have applications in the fields of dyes, chemo-sensing, and fluorescent materials [3]. (Benz)imidazoles are a common motif present in some components of human organisms, histidine, vitamin B12, purines, histamine, biotin, and in natural compounds such as lepidiline A and B [6].
Over the years of active research, benzimidazole derivatives have been involved in medicinal chemistry covering a wide range of biological activities including antiparasitic (albendazole, mebendazole), antiulcer (omeprazole), antihypertensive (candesartan, telmisartan, azilsartan, medoxomil, mebefradil), anticancer (bendamustine), antiemetic/antipsychotic (droperidole), antihistaminic (astemizole, emedastine) and many others (Figure 1) [1-10]. Benzimidazole fungicides (carbendazim, benomyl, thiabendazole and fuberidazole) have been widely used to fight against destructive plant pathogens (Figure 1) [7]. Interestingly, most of the above listed drugs are 2- or 1,2-disubstituted benzimidazole derivatives [2].
Figure 1: Examples of some commercially available pharmaceuticals and agrochemicals containing the benzimidazole scaffold.
Figure 1: Examples of some commercially available pharmaceuticals and agrochemicals containing the benzimidaz...
Thus, the development of further syntheses of benzimidazole derivatives and the study of their properties are important goals for chemistry, medicine and materials science.
Since George Olah proposed the concept of generating superelectrophilic intermediates through the protonation by Brønsted superacids or coordination by a Lewis superacid to produce a di- (tri- or higher) cationic species, the study of superelectrophilic activation became a very active area of research [11-13]. The acid-catalyzed condensation of ketones and aldehydes with aromatic compounds is known as the hydroxyalkylation reaction [14]. Recently, several hydroxyalkylation reactions followed by alkylation of arenes have been reported involving heterocycle-based superelectrophiles: pyridines, thiazoles, quinolines, isoquinolines, pyrazines, pyrazoles, imidazole and furans, bearing a formyl (carbonyl) group [15-23]. These carbonyl-substituted heteroarenes possess basic sites (nitrogen or oxygen atoms of the heterocyclic system), which are fully protonated in acid, so that upon subsequent protonation of the carbonyl oxygen, more reactive dicationic electrophiles can be generated.
Previously, superelectrophilic activation of the carbonyl group was achieved for 5-formyl and 5-acetylimidazoles in triflic acid CF3SO3H (TfOH) by Klumpp [19]. It was proposed that the triflic acid initially protonated the imidazole ring and an equilibrium was established with the O-protosolvated form or the dicationic superelectrophile. These two kinds of species may react with benzene leading to products of the transformation of the carbonyl group into a diphenylmethyl one (Figure 2) [19].
Figure 2: Formation of cationic species by protonation of 5-formyl-4-methylimidazole in TfOH and their reaction with benzene (data from ref. [19]).
Figure 2: Formation of cationic species by protonation of 5-formyl-4-methylimidazole in TfOH and their reacti...
To the best of our knowledge, no data on the generation of superelectrophilic species from carbonyl-substituted benzimidazoles and their reactions exist. Based on our previous studies on the chemistry of heterocycles under superelectrophilic activation [22-24], we undertook this research on reactions of benzimidazole derivatives in (super)acids.
The main goal of this work was the study of reactions of substituted benzimidazoles 1–8 (Figure 3) with arenes under the action of Brønsted (super)acids CF3SO3H (TfOH, triflic acid), H2SO4 and strong Lewis acids AlX3 (X = Cl, Br). One would expect the electrophilic activation of carbonyl or 2-hydroxyalkyl groups of these benzimidazoles in hydroxyalkylation and alkylation of arenes.
Figure 3: Benzimidazoles 1–8 used in this study.
Figure 3: Benzimidazoles 1–8 used in this study.
Results and Discussion
The protonation of formyl and acetylbenzimadazoles 1 and 2 gave N,O-diprotonated species I and II, respectively (see Table 1). Protonation of the hydroxy group of benzimidazoles 3–8 in strong acids gave dicationic species III, V, VII, and VIII, the dehydration of the latter resulted in the formation of heteroaromatic benzyl-type dications IV, VI, and IX, respectively. Electronic characteristics, energies of HOMO/LUMO, electrophilicity indices ω [25,26], charge distribution, and contribution of atomic orbital into the LUMO of species I–IX were calculated by DFT method to estimate their electrophilicity and electronic properties (Table 1). Apart from that, ΔG298 of the formations of cations I–IX from parent benzimidazoles 1–8 were calculated to estimate a thermodynamic possibility of the species formation (Table 1). The calculations were carried out for the solution phase in water.
Table 1: Selected calculated [DFT, 6-311+G(2d,2p) basis set] electronic characteristics of the protonated forms of benzimidazoles.
|
|
||||||||||
| Species |
EHOMO,
eV |
ELUMO,
eV |
ωa
eV |
q(Cα)b
e |
q(C2)b
e |
q(N1)b
e |
q(N3)b
e |
k(Cα)LUMOс
% |
k(C2)LUMOс
% |
∆Gd
kcal/mol |
|
I |
−9.53 | −6.39 | 4.9 | 0.47 | 0.33 | −0.29 | −0.44 | 22.1 | 2.3 | −18.6 |
|
II |
−9.38 | −4.16 | 4.4 | 0.65 | 0.35 | −0.30 | −0.44 | 35.4 | 4.7 | −21 |
|
III |
−9.11 | −2.02 | 2.2 | −0.06 | 0.46 | −0.47 | −0.48 | 17.3 | 26.3 | −21.5 |
|
IV |
−10.07 | −5.23 | 6.1 | −0.06 | 0.31 | −0.42 | −0.42 | 28.2 | 0.6 | 15.8 |
|
V |
−9.03 | −1.85 | 2.1 | 0.11 | 0.48 | −0.34 | −0.49 | 13.9 | 20.4 | −25.6 |
|
VI |
−9.77 | −4.92 | 5.6 | 0.14 | 0.33 | −0.28 | −0.43 | 30.5 | 1.4 | 7.6 |
|
VII |
−9.09 | −1.90 | 2.1 | −0.06 | 0.47 | −0.48 | −0.47 | 16.3 | 22.6 | −25.8 |
|
VIII |
−9.04 | −2.01 | 2.2 | −0.06 | 0.47 | −0.34 | −0.48 | 22.1 | 17.3 | −23.7 |
|
IX |
−9.96 | −5.14 | 5.9 | −0.08 | 0.33 | −0.29 | −0.43 | 26.5 | 0.6 | 14.4 |
aGlobal electrophilicity index ω = (EHOMO + ELUMO)2/8(ELUMO − EHOMO). bNatural charges. cContribution of atomic orbital into the molecular orbital. dGibbs energy of the species formation reaction.
According to DFT calculations, protonation of the benzimidazole nitrogen N3 and the oxygen of the carbonyl or hydroxymethyl group in 1–8 leading to dicationic species I–III, V, VII, and VIII is thermodynamically favorable (−18.6 to −25.8 kcal/mol, Table 1). On the other hand, the dehydration of species III, V, VII, VIII is rather unfavorable, since ΔG298 values for the formation of dications IV, VI, and IX are 7.2–15.8 kcal/mol (Table 1). These data reveal that, in the case of carbonyl-substituted benzimidazoles 1 and 2, the formation of dications I and II is very likely, and these species should be reactive electrophiles. While, in the case of hydroxyalkylbenzimidazoles 3–8, N,O-diprotonated species III, V, VII, VIII, the most probably, may be reactive intermediates.
The calculation of electrophilic properties of these cations show that species I and II have higher values of electrophilicity indices ω of 4.4 and 4.9 eV, respectively, compared to cations III, V, VII, and VIII with ω values of 2.1–2.2 eV. Among all studied species, the heteroaromatic benzyl dication IV, VI, IX have the highest electrophilicity indices ω = 5.6–6.1 eV (Table 1).
In the dications I and II, the carbon Cα bears a large positive charge (0.47 and 0.65 e) and gives a big contribution into the LUMO (22.1 and 35.4%, Table 1). It points out that this particular carbon should be an electrophilic reactive center by both charge and orbital factors. For comparison, the carbon C2 in I and II has a less positive charge (0.33 and 0.35 e) and a much less contribution in the LUMO (2.3 and 4.7%).
In hydroxonium type species III, V, VII, and VIII, the charge on the carbon Cα is from −0.06 to 0.11 e (Table 1). However, this carbon provides a big contribution into the LUMO, 13.9–22%. This reveals that electrophilic properties of this carbon are mainly ruled by orbital factors. Despite the carbon C2 in these species has a large positive charge (0.46–0.48 e) and a great orbital coefficient (17.3–26.3%), reactions of nucleophiles with this atom are less probable, since it leads to a loss of aromaticity of the benzimidazole system. The same applies to the reactivity of the carbon C2 in dications I and II.
Then we studied the protonation of benzimidazoles in the superacid TfOH by means of NMR. Selected 1H, 13C and 15N NMR data for starting neutral benzimidazoles 1, 3a, and 7 (in CDCl3, (CD3)2CO, CD3OD) and their corresponding protonated forms I', III', and VIII', respectively, in TfOH are presented in Table 2.
Table 2: 1H, 13C and 15N NMR data of benzimidazoles 1, 3a, 7 in the corresponding deuterated solvent and species I', III', VIII' in TfOH at room temperature.
| Compound or cation | Solvent | NMR, δ, ppm | |||||
| 1H | 13C | 15N | |||||
| Hα | N3H | C2 | Cα | N1 | N3 | ||
|
1 |
CDCl3 | 10.09 | – | 146.1 | 185.0 | 268.4 | 142.2 |
|
I‘ |
TfOH | 10.28 | 12.12 | 137.4 | 176.8 | 158.9 | 151.6 |
| ∆δa | 0.19 | −8.7 | −8.2 | 109.5 | 9.4 | ||
|
3a |
(CD3)2CO | 4.86 | – | 155.8 | 59.2 | –b | –b |
|
III‘ |
TfOH | 5.64 | 11.53 | 149.5 | 57.5 | 148.7 | 148.7 |
| ∆δa | 0.78 | −6.3 | −1.7 | – | – | ||
|
7 |
CD3OD | 4.85 | – | 142.5 | 57.7 | no data | no data |
|
VIII‘ |
TfOH | 5.57 | 11.37 | 148.4 | 56.9 | 152.5 | 149.1 |
| ∆δa | 0.72 | 5.9 | −0.8 | –b | –b | ||
a∆δ = δcation − δinitial. bNo correlation was observed in HSQC and HMBC N–H spectra.
The signals of the proton attached to the nitrogen N3 in 1H NMR spectra of all species I', III', and VIII' in TfOH appear in the range of 11.37–12.12 ppm (Table 2, see also the spectra given in Supporting Information File 1). However, the signals of the proton bound to the oxygen of the formyl or the hydroxy groups is not observed due to the fast proton exchange for these groups in TfOH at room temperature.
The obtained NMR data demonstrate the formation of N-protonated-O-protosolvated species I', III', and VIII' from benzimidazoles 1, 3a, and 7, respectively, in the superacid TfOH. However, it could not be excluded that the N,O-diprotonated species was formed in low concentration as a result of complete protonation of oxygen. These dicationic species may participate in further reactions with arenes.
Signals of Hα protons for species I', III', and VIII' in TfOH are downfield shifted relatively to the same signals in their neutral precursors 1, 3a, and 7 (in CDCl3, (CD3)2CO, CD3OD), respectively (see ∆δ values in Table 2). This reveals substantial solvatation of the formyl or the hydroxy oxygen of benzimidazoles 1, 3a, 7 in TfOH.
In the 13C NMR spectra, the signal of carbon Cα in species I', III', and VIII' in TfOH is slightly upfield shifted due to positive charge delocalization into the benzimidazole ring. Apart from that, the chemical shift of Cα in hydroxonium-type species III' and VIII' at 57.5 and 56.9 ppm, which are close to the shifts in starting neutral precursors 3a and 7, proves unambiguously that no dehydration of these species leading to heteroaromatic benzyl-type cations take place.
Based on HSQC and HMBC N–H correlations, we were able to measure 15N chemical shifts of nitrogen atoms for cations I', III', and VIII' in TfOH (Table 2). The peaks of the nitrogen atoms N1 and N3 appear in a rather narrow range of 148.7–158.9 ppm due to charge delocalization between these two nitrogen atoms for the corresponding resonance forms.
Then, we carried out Friedel–Crafts reactions of benzimidazoles 1–8 with arenes under the action of Brønsted acids (H2SO4, TfOH) or Lewis acids (AlX3, X = Cl, Br). Reactions of 2-formyl-1-methylbenzimidazole (1) with various arenes (benzene, and its methyl, methoxy or chloro-substituted derivatives) are given in Table 3. These reactions proceed on the formyl group of 1 and give rise to the corresponding 2-diarylmethylbenzimidazoles 9a–l. The best results were obtained in neat TfOH, which gave high yields of reaction products (54–86%) at room temperature after 2–3 h. Other acids were not so efficient. Thus, reactions under the action of sulfuric acid H2SO4 or AlCl3 and AlBr3 took longer time (24–27 h, Table 3, entries 1, 2, and 4). In some cases, the use of H2SO4 resulted in the formation of oligomeric reaction products in the reaction of 1 with electron donating p-xylene (Table 3, entry 10). Apart from that, the acidity of H2SO4 was not high enough to activate 1 in the reaction with poor π-nucleophilic 1,2-dichlorobenzene (Table 3, entry 13).
Table 3: Reactions of 2-formyl-1-methylbenzimidazole (1) with arenes under the action of various acids at room temperature.
|
|
||||
| Entry | Arene (equiv) | Reaction conditionsa | Reaction products 9, yield (%) | |
| Acid (equiv) | Time, h | |||
| 1 |
benzene
(18) |
H2SO4
(120) |
24 |
9a (69%) |
| 2 |
benzene
(18) |
TfOH
(35) |
2 | 9a (61%) |
| 3 |
benzene
(100) |
AlCl3
(5) |
25 | 9a (54%)b |
| 4 |
benzene
(100) |
AlBr3
(5) |
27 | 9a (80%) |
| 5 |
toluene
(2.2) |
TfOH
(35) |
2 |
ratio 9b/9c 6:1 |
| 6 |
anisole
(2.2) |
TfOH
(35) |
2 |
ratio 9d/9e 5:1 |
| 7 |
chlorobenzene
(2.2) |
TfOH
(35) |
2 |
ratio 9f/9g 3:1 |
| 8 |
o-xylene
(2.2) |
TfOH
(35) |
3 |
9h (75%) |
| 9 |
m-xylene
(2.2) |
TfOH
(35) |
3 |
9i (63%) |
| 10 |
p-xylene
(2.2) |
H2SO4
(120) |
24 | oligomeric materials |
| 11 |
p-xylene
(2.2) |
TfOH
(35) |
3 |
9j (86%) |
| 12 |
1,2-dichlorobenzene
(2.2) |
TfOH
(35) |
3 |
9k (61%) |
| 13 |
1,2-dichlorobenzene
(2.2) |
H2SO4
(120) |
24 | –c |
| 14 |
1,2,4-tremethylbenzene
(2.2) |
TfOH
(35) |
3 |
9l (78%) |
aAll reactions were carried out at room temperature. b26% of the starting benzimidazole 1 was recovered. c72% of the starting benzimidazole 1 was recovered.
2-Acetylbenzimidazole (2) reacted with benzene in TfOH in the same way, which gave two reaction products 10 and 11 (Scheme 1). Compound 10 was obtained as a result of the addition of two benzene molecules to the carbonyl group of 2. Alkenyl-substituted benzimidazole 11 was formed in an alternative way of transformation of intermediate cations (see reaction mechanism below in Scheme 3). It should be noted, that compound 11 in the reaction with benzene in TfOH at room temperature for 24 h afforded benzimidazole 10.
Scheme 1: Reaction of 2-acetylbenzimidazole (2) with TfOH and benzene.
Scheme 1: Reaction of 2-acetylbenzimidazole (2) with TfOH and benzene.
Then, we studied reactions of 2-hydroxyalkylbenzimidazoles 3a–c, 4, 7, and 8. It was found that these reactions needed extremely harsh reaction conditions, heating in neat TfOH at 140 °C in glass high pressure tubes (Table 4, Scheme 2). Only at this high temperature the formation of Friedel–Crafts reaction products 12a–h, 13, and 14 was achieved. No reactions took place at lower temperature or under the action of Lewis acids AlX3 (X = Cl, Br). For these 2-hydroxyalkylbenzimidazoles 3a–c, 4, 7, and 8, we were able to receive compounds 12–14 only in reactions with benzene, 1,2-dichlorobenzene and 1,3-dibromobenzene (Table 4). Transformations with methyl-substituted benzenes (xylenes) at these harsh conditions gave complex mixtures of oligomeric materials. The same interactions of 2-methoxyalkylbenzimidazoles 5a–c, and 6 with benzene in TfOH at 140 °C resulted in the formation of compounds 12a,f–h (Table 5).
Scheme 2: Reactions of hydroxymethyl-substituted benzimidazole 7 and 8 with TfOH and benzene.
Scheme 2: Reactions of hydroxymethyl-substituted benzimidazole 7 and 8 with TfOH and benzene.
Table 4: Reactions of 2-hydroxyalkylbenzimidazoles 3a–c and 4 with arenes in TfOH at 140 °C.
|
|
|||
| Entry | Starting benzimidazole | Starting arene (equiv) | Reaction products 12, yield (%) |
| 1 |
3a |
benzene
(18) |
12a (87%) |
| 2 | 3a |
1,2-dichlorobenzene
(4) |
ratio 12b/12c 1:0.5 |
| 3 | 3a |
1,3-dibromobenzene
(4) |
ratio 12d/12e 1:0.25 |
| 4 |
3b |
benzene
(18) |
12f (82%) |
| 5 |
3c |
benzene
(18) |
12g (88%) |
| 6 |
4 |
benzene
(18) |
12h (90%) |
Summarizing all data obtained by DFT calculations (Table 1) and NMR studies (Table 2) of intermediate cations generated from benzimidazoles 1–8 in TfOH, and their reactions with arenes (Tables 3–5, Scheme 1, and Scheme 2), the following reaction mechanisms are proposed (Scheme 3 and Scheme 4).
Scheme 3: Reaction mechanism of the formation of compounds 9–11.
Scheme 3: Reaction mechanism of the formation of compounds 9–11.
2-Carbonyl-substituted benzimidazoles 1 and 2 give rise to N-protonated-O-protosolvated forms I' and II'. These species in reactions with arenes furnish hydroxyalkylation products X, which are further transformed into cations XI. These species consequently react with arenes forming the target compounds 9 and 10 after final hydrolysis of superacidic reaction mixtures. There is another possible reaction pathway for 2-acetylbenzimidazole (2) on the stage of the formation of cation XI. The latter may undergo deprotonation at the methyl group, which results in the formation of alkenyl benzimidazole 11. Compound 11 may be also transformed into compound 10 by reaction with arenes in TfOH (see Scheme 2 and the corresponding discussion).
Hydroxy(or methoxy)alkyl-substituted benzimidazoles 3–8 form cations III', V', VII', and VIII', respectively. These species react with arenes, the most probably, in SN2 way to give benzimidazoles 12 (Scheme 4).
Scheme 4: Reaction mechanism of the formation of compounds 12.
Scheme 4: Reaction mechanism of the formation of compounds 12.
However, once again, in these reactions (Scheme 3 and Scheme 4) the formation of the corresponding N,O-diprotonated species I', II', III', V', VII', and VIII' in low concentration, which are not detected by NMR, could not be excluded. And these dications may react with arenes, as well.
It should be specially emphasized that 2-diarylmethyl and 2-arylmethyl-substituted benzimidazoles 9–12 are synthetically hardly available compounds [27,28]. For instance, they were investigated as AMP-activated protein kinase activators [27].
Conclusion
Reactions of 2-carbonyl and 2-hydroxy(or methoxy)alkyl-substituted benzimidazoles with arenes in the Brønsted superacid TfOH were studied for the first time. These reactions proceed at the carbon atom of the 2-carbonyl or 2-hydroxyalkyl group leading to the corresponding 2-diarylmethyl or 2-arylmethyl-substituted benzimidazoles. Based on this superelectrophilic activation approach, we have developed a useful synthetically procedure for the modification of the benzimidazole structure.
Supporting Information
| Supporting Information File 1: Experimental details, compound characterization data, copies of 1H and 13C NMR spectra, and details of DFT calculations. | ||
| Format: PDF | Size: 6.4 MB | Download |
References
-
Gaba, M.; Singh, S.; Mohan, C. Eur. J. Med. Chem. 2014, 76, 494–505. doi:10.1016/j.ejmech.2014.01.030
Return to citation in text: [1] [2] -
Largeron, M.; Nguyen, K. M. H. Synthesis 2018, 50, 241–253. doi:10.1055/s-0036-1590915
Return to citation in text: [1] [2] [3] -
Ke, F.; Zhang, P.; Xu, Y.; Lin, X.; Lin, J.; Lin, C.; Xu, J. Synlett 2018, 29, 2722–2726. doi:10.1055/s-0037-1610843
Return to citation in text: [1] [2] [3] -
Arulmurugan, S.; Kavitha, H. P.; Sathishkumar, S.; Arulmozhi, R. Mini-Rev. Org. Chem. 2015, 12, 178–195. doi:10.2174/1570193x1202150225153403
Return to citation in text: [1] [2] -
Rani, N.; Sharma, A.; Singh, R. Mini-Rev. Org. Chem. 2015, 12, 34–65. doi:10.2174/1570193x11666141028235010
Return to citation in text: [1] [2] -
Boulebd, H.; Zama, S.; Insaf, B.; Bouraiou, A.; Bouacida, S.; Merazig, H.; Romero, A.; Chioua, M.; Marco-Contelles, J.; Belfaitah, A. Monatsh. Chem. 2016, 147, 2209–2220. doi:10.1007/s00706-016-1733-7
Return to citation in text: [1] [2] [3] -
Wang, X.; Chen, Y.-F.; Yan, W.; Cao, L.-L.; Ye, Y.-H. Molecules 2016, 21, No. 1574. doi:10.3390/molecules21111574
Return to citation in text: [1] [2] [3] -
Orjales, A.; Alonso-Cires, L.; López-Tudanca, P.; Tapia, I.; Mosquera, R.; Labeaga, L. Eur. J. Med. Chem. 1999, 34, 415–422. doi:10.1016/s0223-5234(99)80091-9
Return to citation in text: [1] [2] -
Sharma, P.; Srinivasa Reddy, T.; Thummuri, D.; Senwar, K. R.; Praveen Kumar, N.; Naidu, V. G. M.; Bhargava, S. K.; Shankaraiah, N. Eur. J. Med. Chem. 2016, 124, 608–621. doi:10.1016/j.ejmech.2016.08.029
Return to citation in text: [1] [2] -
Woo, H. B.; Eom, Y. W.; Park, K.-S.; Ham, J.; Ahn, C. M.; Lee, S. Bioorg. Med. Chem. Lett. 2012, 22, 933–936. doi:10.1016/j.bmcl.2011.12.074
Return to citation in text: [1] [2] -
Olah, G. A. Angew. Chem., Int. Ed. Engl. 1993, 32, 767–788. doi:10.1002/anie.199307673
Return to citation in text: [1] -
Olah, G. A.; Prakash, G. K. S.; Molnár, A.; Sommer, J. Superacid Chemistry, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, U.S.A., 2009. doi:10.1002/9780470421604
Return to citation in text: [1] -
Olah, G. A.; Klumpp, D. A. Superelectrophiles and Their Chemistry; Wiley-Interscience: New Jersey, 2008. doi:10.1002/9780470185124
Return to citation in text: [1] -
Olah, G. A. Friedel-Crafts and related reactions; Wiley & Sons: New York, U.S.A., 1964; Vol. 2.
Return to citation in text: [1] -
Klumpp, D. A.; Lau, S. J. Org. Chem. 1999, 64, 7309–7311. doi:10.1021/jo9824908
Return to citation in text: [1] -
Klumpp, D. A.; Jones, A.; Lau, S.; de Leon, S.; Garza, M. Synthesis 2000, 1117–1120. doi:10.1055/s-2000-6322
Return to citation in text: [1] -
Klumpp, D. A.; Garza, M.; Sanchez, G. V.; Lau, S.; de Leon, S. J. Org. Chem. 2000, 65, 8997–9000. doi:10.1021/jo001035k
Return to citation in text: [1] -
Klumpp, D. A.; Kindelin, P. J.; Li, A. Tetrahedron Lett. 2005, 46, 2931–2935. doi:10.1016/j.tetlet.2005.02.152
Return to citation in text: [1] -
Sheets, M. R.; Li, A.; Bower, E. A.; Weigel, A. R.; Abbott, M. P.; Gallo, R. M.; Mitton, A. A.; Klumpp, D. A. J. Org. Chem. 2009, 74, 2502–2507. doi:10.1021/jo802798x
Return to citation in text: [1] [2] [3] [4] -
Prakash, G. K. S.; Paknia, F.; Chacko, S.; Mathew, T.; Olah, G. A. Heterocycles 2008, 76, 783–799. doi:10.3987/com-08-s(n)90
Return to citation in text: [1] -
O’Connor, M. J.; Boblak, K. N.; Topinka, M. J.; Kindelin, P. J.; Briski, J. M.; Zheng, C.; Klumpp, D. A. J. Am. Chem. Soc. 2010, 132, 3266–3267. doi:10.1021/ja1001482
Return to citation in text: [1] -
Gurskaya, L. Y.; Belyanskaya, D. S.; Ryabukhin, D. S.; Nilov, D. I.; Boyarskaya, I. A.; Vasilyev, A. V. Beilstein J. Org. Chem. 2016, 12, 950–956. doi:10.3762/bjoc.12.93
Return to citation in text: [1] [2] -
Ryabukhin, D. S.; Zakusilo, D. N.; Kompanets, M. O.; Tarakanov, A. A.; Boyarskaya, I. A.; Artamonova, T. O.; Khohodorkovskiy, M. A.; Opeida, I. O.; Vasilyev, A. V. Beilstein J. Org. Chem. 2016, 12, 2125–2135. doi:10.3762/bjoc.12.202
Return to citation in text: [1] [2] -
Ryabukhin, D. S.; Vasilyev, A. V. Russ. Chem. Rev. 2016, 85, 637–665. doi:10.1070/rcr4550
Return to citation in text: [1] -
Parr, R. G.; Szentpály, L. v.; Liu, S. J. Am. Chem. Soc. 1999, 121, 1922–1924. doi:10.1021/ja983494x
Return to citation in text: [1] -
Chattaraj, P. K.; Giri, S.; Duley, S. Chem. Rev. 2011, 111, PR43–PR75. doi:10.1021/cr100149p
Return to citation in text: [1] -
Charton, J.; Girault-Mizzi, S.; Debreu-Fontaine, M.-A.; Foufelle, F.; Hainault, I.; Bizot-Espiard, J.-G.; Caignard, D.-H.; Sergheraert, C. Bioorg. Med. Chem. 2006, 14, 4490–4518. doi:10.1016/j.bmc.2006.02.028
Return to citation in text: [1] [2] -
Charton, J.; Girault-Mizzi, S.; Sergheraert, C. Chem. Pharm. Bull. 2005, 53, 492–497. doi:10.1248/cpb.53.492
Return to citation in text: [1]
| 1. | Gaba, M.; Singh, S.; Mohan, C. Eur. J. Med. Chem. 2014, 76, 494–505. doi:10.1016/j.ejmech.2014.01.030 |
| 2. | Largeron, M.; Nguyen, K. M. H. Synthesis 2018, 50, 241–253. doi:10.1055/s-0036-1590915 |
| 3. | Ke, F.; Zhang, P.; Xu, Y.; Lin, X.; Lin, J.; Lin, C.; Xu, J. Synlett 2018, 29, 2722–2726. doi:10.1055/s-0037-1610843 |
| 4. | Arulmurugan, S.; Kavitha, H. P.; Sathishkumar, S.; Arulmozhi, R. Mini-Rev. Org. Chem. 2015, 12, 178–195. doi:10.2174/1570193x1202150225153403 |
| 5. | Rani, N.; Sharma, A.; Singh, R. Mini-Rev. Org. Chem. 2015, 12, 34–65. doi:10.2174/1570193x11666141028235010 |
| 6. | Boulebd, H.; Zama, S.; Insaf, B.; Bouraiou, A.; Bouacida, S.; Merazig, H.; Romero, A.; Chioua, M.; Marco-Contelles, J.; Belfaitah, A. Monatsh. Chem. 2016, 147, 2209–2220. doi:10.1007/s00706-016-1733-7 |
| 7. | Wang, X.; Chen, Y.-F.; Yan, W.; Cao, L.-L.; Ye, Y.-H. Molecules 2016, 21, No. 1574. doi:10.3390/molecules21111574 |
| 8. | Orjales, A.; Alonso-Cires, L.; López-Tudanca, P.; Tapia, I.; Mosquera, R.; Labeaga, L. Eur. J. Med. Chem. 1999, 34, 415–422. doi:10.1016/s0223-5234(99)80091-9 |
| 9. | Sharma, P.; Srinivasa Reddy, T.; Thummuri, D.; Senwar, K. R.; Praveen Kumar, N.; Naidu, V. G. M.; Bhargava, S. K.; Shankaraiah, N. Eur. J. Med. Chem. 2016, 124, 608–621. doi:10.1016/j.ejmech.2016.08.029 |
| 10. | Woo, H. B.; Eom, Y. W.; Park, K.-S.; Ham, J.; Ahn, C. M.; Lee, S. Bioorg. Med. Chem. Lett. 2012, 22, 933–936. doi:10.1016/j.bmcl.2011.12.074 |
| 7. | Wang, X.; Chen, Y.-F.; Yan, W.; Cao, L.-L.; Ye, Y.-H. Molecules 2016, 21, No. 1574. doi:10.3390/molecules21111574 |
| 27. | Charton, J.; Girault-Mizzi, S.; Debreu-Fontaine, M.-A.; Foufelle, F.; Hainault, I.; Bizot-Espiard, J.-G.; Caignard, D.-H.; Sergheraert, C. Bioorg. Med. Chem. 2006, 14, 4490–4518. doi:10.1016/j.bmc.2006.02.028 |
| 28. | Charton, J.; Girault-Mizzi, S.; Sergheraert, C. Chem. Pharm. Bull. 2005, 53, 492–497. doi:10.1248/cpb.53.492 |
| 1. | Gaba, M.; Singh, S.; Mohan, C. Eur. J. Med. Chem. 2014, 76, 494–505. doi:10.1016/j.ejmech.2014.01.030 |
| 2. | Largeron, M.; Nguyen, K. M. H. Synthesis 2018, 50, 241–253. doi:10.1055/s-0036-1590915 |
| 3. | Ke, F.; Zhang, P.; Xu, Y.; Lin, X.; Lin, J.; Lin, C.; Xu, J. Synlett 2018, 29, 2722–2726. doi:10.1055/s-0037-1610843 |
| 4. | Arulmurugan, S.; Kavitha, H. P.; Sathishkumar, S.; Arulmozhi, R. Mini-Rev. Org. Chem. 2015, 12, 178–195. doi:10.2174/1570193x1202150225153403 |
| 5. | Rani, N.; Sharma, A.; Singh, R. Mini-Rev. Org. Chem. 2015, 12, 34–65. doi:10.2174/1570193x11666141028235010 |
| 6. | Boulebd, H.; Zama, S.; Insaf, B.; Bouraiou, A.; Bouacida, S.; Merazig, H.; Romero, A.; Chioua, M.; Marco-Contelles, J.; Belfaitah, A. Monatsh. Chem. 2016, 147, 2209–2220. doi:10.1007/s00706-016-1733-7 |
| 7. | Wang, X.; Chen, Y.-F.; Yan, W.; Cao, L.-L.; Ye, Y.-H. Molecules 2016, 21, No. 1574. doi:10.3390/molecules21111574 |
| 8. | Orjales, A.; Alonso-Cires, L.; López-Tudanca, P.; Tapia, I.; Mosquera, R.; Labeaga, L. Eur. J. Med. Chem. 1999, 34, 415–422. doi:10.1016/s0223-5234(99)80091-9 |
| 9. | Sharma, P.; Srinivasa Reddy, T.; Thummuri, D.; Senwar, K. R.; Praveen Kumar, N.; Naidu, V. G. M.; Bhargava, S. K.; Shankaraiah, N. Eur. J. Med. Chem. 2016, 124, 608–621. doi:10.1016/j.ejmech.2016.08.029 |
| 10. | Woo, H. B.; Eom, Y. W.; Park, K.-S.; Ham, J.; Ahn, C. M.; Lee, S. Bioorg. Med. Chem. Lett. 2012, 22, 933–936. doi:10.1016/j.bmcl.2011.12.074 |
| 27. | Charton, J.; Girault-Mizzi, S.; Debreu-Fontaine, M.-A.; Foufelle, F.; Hainault, I.; Bizot-Espiard, J.-G.; Caignard, D.-H.; Sergheraert, C. Bioorg. Med. Chem. 2006, 14, 4490–4518. doi:10.1016/j.bmc.2006.02.028 |
| 6. | Boulebd, H.; Zama, S.; Insaf, B.; Bouraiou, A.; Bouacida, S.; Merazig, H.; Romero, A.; Chioua, M.; Marco-Contelles, J.; Belfaitah, A. Monatsh. Chem. 2016, 147, 2209–2220. doi:10.1007/s00706-016-1733-7 |
| 22. | Gurskaya, L. Y.; Belyanskaya, D. S.; Ryabukhin, D. S.; Nilov, D. I.; Boyarskaya, I. A.; Vasilyev, A. V. Beilstein J. Org. Chem. 2016, 12, 950–956. doi:10.3762/bjoc.12.93 |
| 23. | Ryabukhin, D. S.; Zakusilo, D. N.; Kompanets, M. O.; Tarakanov, A. A.; Boyarskaya, I. A.; Artamonova, T. O.; Khohodorkovskiy, M. A.; Opeida, I. O.; Vasilyev, A. V. Beilstein J. Org. Chem. 2016, 12, 2125–2135. doi:10.3762/bjoc.12.202 |
| 24. | Ryabukhin, D. S.; Vasilyev, A. V. Russ. Chem. Rev. 2016, 85, 637–665. doi:10.1070/rcr4550 |
| 3. | Ke, F.; Zhang, P.; Xu, Y.; Lin, X.; Lin, J.; Lin, C.; Xu, J. Synlett 2018, 29, 2722–2726. doi:10.1055/s-0037-1610843 |
| 25. | Parr, R. G.; Szentpály, L. v.; Liu, S. J. Am. Chem. Soc. 1999, 121, 1922–1924. doi:10.1021/ja983494x |
| 26. | Chattaraj, P. K.; Giri, S.; Duley, S. Chem. Rev. 2011, 111, PR43–PR75. doi:10.1021/cr100149p |
| 15. | Klumpp, D. A.; Lau, S. J. Org. Chem. 1999, 64, 7309–7311. doi:10.1021/jo9824908 |
| 16. | Klumpp, D. A.; Jones, A.; Lau, S.; de Leon, S.; Garza, M. Synthesis 2000, 1117–1120. doi:10.1055/s-2000-6322 |
| 17. | Klumpp, D. A.; Garza, M.; Sanchez, G. V.; Lau, S.; de Leon, S. J. Org. Chem. 2000, 65, 8997–9000. doi:10.1021/jo001035k |
| 18. | Klumpp, D. A.; Kindelin, P. J.; Li, A. Tetrahedron Lett. 2005, 46, 2931–2935. doi:10.1016/j.tetlet.2005.02.152 |
| 19. | Sheets, M. R.; Li, A.; Bower, E. A.; Weigel, A. R.; Abbott, M. P.; Gallo, R. M.; Mitton, A. A.; Klumpp, D. A. J. Org. Chem. 2009, 74, 2502–2507. doi:10.1021/jo802798x |
| 20. | Prakash, G. K. S.; Paknia, F.; Chacko, S.; Mathew, T.; Olah, G. A. Heterocycles 2008, 76, 783–799. doi:10.3987/com-08-s(n)90 |
| 21. | O’Connor, M. J.; Boblak, K. N.; Topinka, M. J.; Kindelin, P. J.; Briski, J. M.; Zheng, C.; Klumpp, D. A. J. Am. Chem. Soc. 2010, 132, 3266–3267. doi:10.1021/ja1001482 |
| 22. | Gurskaya, L. Y.; Belyanskaya, D. S.; Ryabukhin, D. S.; Nilov, D. I.; Boyarskaya, I. A.; Vasilyev, A. V. Beilstein J. Org. Chem. 2016, 12, 950–956. doi:10.3762/bjoc.12.93 |
| 23. | Ryabukhin, D. S.; Zakusilo, D. N.; Kompanets, M. O.; Tarakanov, A. A.; Boyarskaya, I. A.; Artamonova, T. O.; Khohodorkovskiy, M. A.; Opeida, I. O.; Vasilyev, A. V. Beilstein J. Org. Chem. 2016, 12, 2125–2135. doi:10.3762/bjoc.12.202 |
| 19. | Sheets, M. R.; Li, A.; Bower, E. A.; Weigel, A. R.; Abbott, M. P.; Gallo, R. M.; Mitton, A. A.; Klumpp, D. A. J. Org. Chem. 2009, 74, 2502–2507. doi:10.1021/jo802798x |
| 14. | Olah, G. A. Friedel-Crafts and related reactions; Wiley & Sons: New York, U.S.A., 1964; Vol. 2. |
| 19. | Sheets, M. R.; Li, A.; Bower, E. A.; Weigel, A. R.; Abbott, M. P.; Gallo, R. M.; Mitton, A. A.; Klumpp, D. A. J. Org. Chem. 2009, 74, 2502–2507. doi:10.1021/jo802798x |
| 11. | Olah, G. A. Angew. Chem., Int. Ed. Engl. 1993, 32, 767–788. doi:10.1002/anie.199307673 |
| 12. | Olah, G. A.; Prakash, G. K. S.; Molnár, A.; Sommer, J. Superacid Chemistry, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, U.S.A., 2009. doi:10.1002/9780470421604 |
| 13. | Olah, G. A.; Klumpp, D. A. Superelectrophiles and Their Chemistry; Wiley-Interscience: New Jersey, 2008. doi:10.1002/9780470185124 |
| 2. | Largeron, M.; Nguyen, K. M. H. Synthesis 2018, 50, 241–253. doi:10.1055/s-0036-1590915 |
| 19. | Sheets, M. R.; Li, A.; Bower, E. A.; Weigel, A. R.; Abbott, M. P.; Gallo, R. M.; Mitton, A. A.; Klumpp, D. A. J. Org. Chem. 2009, 74, 2502–2507. doi:10.1021/jo802798x |
© 2019 Ryabukhin et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the authors and source are credited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)