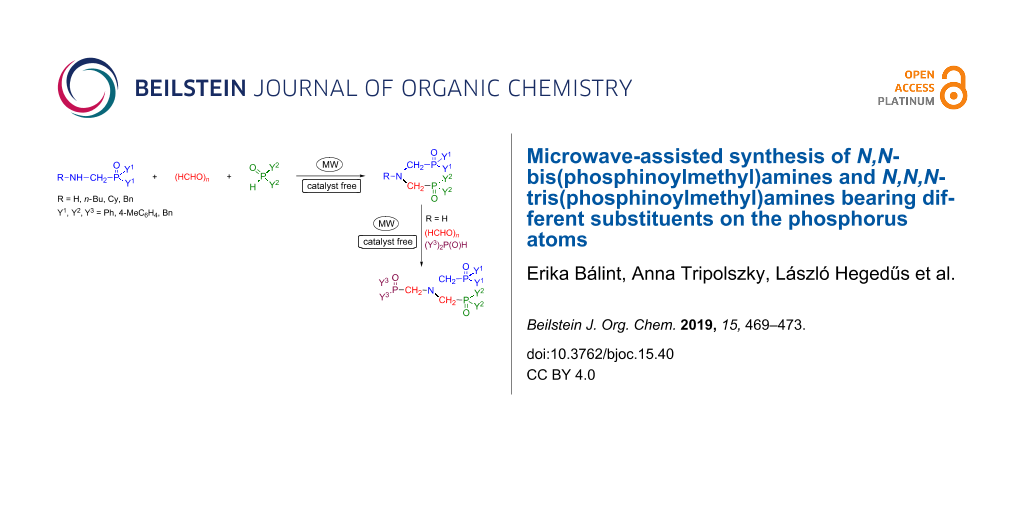
Abstract
A family of N,N-bis(phosphinoylmethyl)amines bearing different substituents on the phosphorus atoms was synthesized by the microwave-assisted and catalyst-free Kabachnik–Fields reaction of (aminomethyl)phosphine oxides with paraformaldehyde and diphenylphosphine oxide. The three-component condensation of N,N-bis(phosphinoylmethyl)amine, paraformaldehyde and a secondary phosphine oxide affording N,N,N-tris(phosphinoylmethyl)amine derivatives was also elaborated. This method is a novel approach for the synthesis of the target products.
Graphical Abstract
Introduction
α-Aminophosphine oxides are of considerable importance as potential precursors of α-aminophosphine ligands [1]. α-Aminophosphines play an important role in the synthesis of P(III)-transition metal complexes [2], which are often applied catalysts in homogeneous catalytic reactions [2-4]. In addition, a few Pt, Ru and Au complexes incorporating phosphine ligands show significant anticancer activity [5,6].
One of the most common synthetic routes to α-aminophosphine oxides is the Kabachnik–Fields (phospha-Mannich) reaction, where an amine, an oxo compound (aldehyde or ketone) and a secondary phosphine oxide react in a condensation reaction [1]. However, only a few papers deal with the synthesis of α-aminophosphine oxides. (Phenylaminomethyl)dibenzylphosphine oxide was prepared by the three-component reaction of aniline, paraformaldehyde and dibenzylphosphine oxide [7], as well as by the reaction of (hydroxymethyl)dibenzylphosphine oxide and aniline [8]. The condensation of butylamine, paraformaldehyde and di(p-tolyl)phosphine oxide to afford (butylaminomethyl)di(p-tolyl)phosphine oxide was also described [9]. A microwave (MW)-assisted, catalyst-free method was elaborated by us for the synthesis of several (aminomethyl)phosphine oxides [10,11].
As regards α-aminophosphine oxides with different P-substituents, only two different types were reported. Olszewski and co-workers synthesized chiral thiazole-substituted aminophosphine oxides 2 through the Pudovik reaction of alkylphenylphosphine oxides and the corresponding aldimine derivatives of thiazole 1 (Scheme 1) [12].
Scheme 1: Synthesis of chiral thiazole-substituted aminophosphine oxides.
Scheme 1: Synthesis of chiral thiazole-substituted aminophosphine oxides.
Cherkasov and his group applied the Kabachnik–Fields reaction to synthesize a P-chiral aminophosphine oxide with a 2-pyridyl substituent 3 (Scheme 2) [13].
Scheme 2: Synthesis of a P-chiral aminophosphine oxide containing a 2-pyridyl moiety.
Scheme 2: Synthesis of a P-chiral aminophosphine oxide containing a 2-pyridyl moiety.
Bis(aminophosphine oxide) derivatives were also prepared by the double Kabachnik–Fields reaction using primary amines [11,14,15], amino acids [16,17] or aminoethanol [14] as the amine component.
To the best of our knowledge, only one example can be found for a bis(α-aminophosphine oxide) containing different P-functions that was prepared by the condensation of (octylaminomethyl)dihexylphosphine oxide, paraformaldehyde and di(p-tolyl)phosphine oxide in the presence of p-toluenesulfonic acid in boiling acetonitrile (Scheme 3) [12].
Scheme 3: Condensation of (octylaminomethyl)dihexylphosphine oxide with paraformaldehyde and di(p-tolyl)phosphine oxide.
Scheme 3: Condensation of (octylaminomethyl)dihexylphosphine oxide with paraformaldehyde and di(p-tolyl)phosp...
Furthermore, tris(α-aminophosphine oxide) derivatives have not been described in the literature up to now. In this paper, we report the efficient, catalyst-free and MW-assisted synthesis of N,N-bis(phosphinoylmethyl)amine and N,N,N-tris(phosphinoylmethyl)amine derivatives bearing different substituents on the phosphorus atoms.
Results and Discussion
Synthesis of N,N-bis(phosphinoylmethyl)alkylamines containing different substituents on the phosphorus atoms
First, the (aminomethyl)phosphine oxide starting materials 5–7 were synthesized following our previous protocol [11]. Thus, the MW-assisted Kabachnik–Fields reaction of primary amines (butyl-, cyclohexyl- or benzylamine), paraformaldehyde and di(p-tolyl)- or dibenzylphosphine oxide was carried out in acetonitrile at 100 °C for 1 h affording the products with excellent yields (Scheme 4).
Scheme 4: Synthesis of (aminomethyl)phosphine oxides 5–7.
Scheme 4: Synthesis of (aminomethyl)phosphine oxides 5–7.
Then, (aminomethyl)diphenylphosphine oxide (9) was prepared through debenzylation of (benzylaminomethyl)diphenylphosphine oxide (8, Scheme 5). The reduction was carried out in the presence of a 10% palladium on carbon catalyst (Selcat Q), in methanol, at 75 °C for 3 h, and the (aminomethyl)diphenylphosphine oxide (9) was obtained in a yield of 47% after column chromatography.
Scheme 5: Synthesis of (aminomethyl)diphenylphosphine oxide (9).
Scheme 5: Synthesis of (aminomethyl)diphenylphosphine oxide (9).
In the next step, (aminomethyl)phosphine oxides 5–7 were converted to bis(phosphinoylmethyl)amine derivatives bearing different substituents at the phosphorous atoms (Y2P=O) by reacting them with one equivalent of paraformaldehyde and diphenylphosphine oxide under MW conditions (Scheme 6). The three-component condensations were performed in the absence of any catalyst in acetonitrile as the solvent to overcome the heterogeneity of the reaction mixture. After an irradiation of 1 h at 100 °C, the mixed N,N-bis(phosphinoylmethyl)amines 10a,b, 11a,b and 12a,b were obtained in yields of 92–97% and their structures were confirmed by 31P, 13C and 1H NMR, as well as HRMS measurements. Due to the two differently substituted phosphorous nuclei in the molecules, two signals were observed in the 31P NMR spectra.
Scheme 6: Synthesis of N,N-bis(phosphinoylmethyl)amines 10a,b, 11a,b and 12a,b bearing different substituents at the phosphorus atoms (Y2P=O).
Scheme 6: Synthesis of N,N-bis(phosphinoylmethyl)amines 10a,b, 11a,b and 12a,b bearing different substituents...
The valuable intermediate 9 was then utilized in the synthesis of N,N-bis(phosphinoylmethyl)amines 13a–c (Scheme 7). The condensation of (aminomethyl)diphenylphosphine oxide (9), paraformaldehyde and various secondary phosphine oxides, such as diphenyl, di(p-tolyl) or dibenzylphosphine oxide, at 100 °C for 40 min led to the corresponding N,N-bis(phosphinoylmethyl)amines containing identical (13a) or different substituents on the phosphorus atoms (13b and 13c) in excellent yields (95–97%).
Scheme 7: Synthesis of N,N-bis(phosphinoylmethyl)amines 13a–c.
Scheme 7: Synthesis of N,N-bis(phosphinoylmethyl)amines 13a–c.
Synthesis of N,N,N-tris(phosphinoylmethyl)amines
Finally, N,N-bis(phosphinoylmethyl)amines 13a and 13b were reacted further with paraformaldehyde and a secondary phosphine oxide (diphenyl-, di(p-tolyl)- or dibenzylphosphine oxide) to afford the N,N,N-tris(phosphinoylmethyl)amine derivatives bearing identical (14) and different Y2P=O groups (15–17) (Scheme 8). The condensations were performed as mentioned above. The introduction of a third phosphinoylmethyl moiety into the bis-derivatives containing an NH unit (13a and 13b) required a longer reaction time (2 h) at 100 °C. In these cases, the conversion was 70–95%, and the corresponding N,N,N-tris(phosphinoylmethyl)amine derivatives 14–17 were isolated in yields of 27–77%. However, applying a higher temperature and/or longer reaction time, lead to decomposition.
Scheme 8: Synthesis of N,N,N-tris(phosphinoylmethyl)amines 14–17.
Scheme 8: Synthesis of N,N,N-tris(phosphinoylmethyl)amines 14–17.
Conclusion
In summary, we have developed an efficient, catalyst-free and MW-assisted method for the synthesis of N,N-bis(phosphinoylmethyl)amines and N,N,N-tris(phosphinoylmethyl)amines bearing different substituents on the phosphorus atoms by the Kabachnik–Fields reaction. This method is a novel approach for the synthesis of the target products. In all, thirteen new derivatives were isolated in high yields and fully characterized.
Supporting Information
| Supporting Information File 1: Experimental procedures, characterization data, details of the NMR structural determination of all products and copies of 31P, 1H, and 13C NMR spectra for all compounds synthesized. | ||
| Format: PDF | Size: 2.0 MB | Download |
Acknowledgements
The above project was supported by the Hungarian Research Development and Innovation Fund (FK123961 and K119202), and in part (E. B.) by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences (BO/00278/17/7) and by the ÚNKP-18-4-BME-131 New National Excellence Program of the Ministry of Human Capacities. The authors thank for Zoltán Márta to the HRMS measurements.
References
-
Goud, E. V.; Sivaramakrishna, A.; Vijayakrishna, K. Top. Curr. Chem. 2017, 375, 10. doi:10.1007/s41061-016-0090-7
Return to citation in text: [1] [2] -
Bálint, E.; Tajti, Á.; Tripolszky, A.; Keglevich, G. Dalton Trans. 2018, 47, 4755–4778. doi:10.1039/c8dt00178b
Return to citation in text: [1] [2] -
Tsuji, J. Transition Metal Reagents and Catalysts; Wiley: Chichester, 2002. doi:10.1002/0470854766
Return to citation in text: [1] -
Beller, M.; Bolm, C. Transition Metals for Organic Synthesis: Building Blocks and Fine Chemicals; Wiley: Weinheim, 2004. doi:10.1002/9783527619405
Return to citation in text: [1] -
Berners-Price, S. J.; Sadler, P. J. Phosphines and metal phosphine complexes: Relationship of chemistry to anticancer and other biological activity. Bioinorganic Chemistry. Structure and Bonding; Springer: Berlin, Heidelberg, Germany, 1988; Vol. 70, pp 27–102. doi:10.1007/3-540-50130-4_2
Return to citation in text: [1] -
Nazarov, A. A.; Dyson, P. J. Metal Phosphorus Complexes as Antitumor Agents. In Phosphorus Compounds; Luca, G.; Peruzzini, M., Eds.; Springer: Dordrecht, Netherlands, 2011; Vol. 37, pp 445–461. doi:10.1007/978-90-481-3817-3_13
Return to citation in text: [1] -
Petrov, K. A.; Chauzov, V. A.; Erokhina, T. S.; Chernobrovkina, L. P. Zh. Obshch. Khim. 1976, 46, 493.
Return to citation in text: [1] -
Petrov, K. A.; Chauzov, V. A.; Erokhina, T. S. Khim. Elementoorg. Soedin. 1976, 200.
Return to citation in text: [1] -
Garifzyanov, A. R.; Vasil'ev, R. I.; Cherkasov, R. A. Russ. J. Gen. Chem. 2005, 75, 217–224. doi:10.1007/s11176-005-0201-6
Return to citation in text: [1] -
Keglevich, G.; Szekrényi, A. Lett. Org. Chem. 2008, 5, 616–622. doi:10.2174/157017808786857598
Return to citation in text: [1] -
Bálint, E.; Tripolszky, A.; Jablonkai, E.; Karaghiosoff, K.; Czugler, M.; Mucsi, Z.; Kollár, L.; Pongrácz, P.; Keglevich, G. J. Organomet. Chem. 2016, 801, 111–121. doi:10.1016/j.jorganchem.2015.10.029
Return to citation in text: [1] [2] [3] -
Olszewski, T. K.; Boduszek, B.; Sobek, S.; Kozłowski, H. Tetrahedron 2006, 62, 2183–2189. doi:10.1016/j.tet.2005.12.016
Return to citation in text: [1] [2] -
Cherkasov, R. A.; Garifzyanov, A. R.; Koshkin, S. A. Russ. J. Gen. Chem. 2011, 81, 773–774. doi:10.1134/s107036321104027x
Return to citation in text: [1] -
Cherkasov, R. A.; Garifzyanov, A. R.; Talan, A. S.; Davletshin, R. R.; Kurnosova, N. V. Russ. J. Gen. Chem. 2009, 79, 1835–1849. doi:10.1134/s1070363209090114
Return to citation in text: [1] [2] -
Bálint, E.; Fazekas, E.; Pongrácz, P.; Kollár, L.; Drahos, L.; Holczbauer, T.; Czugler, M.; Keglevich, G. J. Organomet. Chem. 2012, 717, 75–82. doi:10.1016/j.jorganchem.2012.07.031
Return to citation in text: [1] -
Bálint, E.; Fazekas, E.; Kóti, J.; Keglevich, G. Heteroat. Chem. 2015, 26, 106–115. doi:10.1002/hc.21221
Return to citation in text: [1] -
Zefirov, N. S.; Matveeva, E. D. ARKIVOC 2008, No. i, 1–17. doi:10.3998/ark.5550190.0009.101
Return to citation in text: [1]
| 1. | Goud, E. V.; Sivaramakrishna, A.; Vijayakrishna, K. Top. Curr. Chem. 2017, 375, 10. doi:10.1007/s41061-016-0090-7 |
| 1. | Goud, E. V.; Sivaramakrishna, A.; Vijayakrishna, K. Top. Curr. Chem. 2017, 375, 10. doi:10.1007/s41061-016-0090-7 |
| 12. | Olszewski, T. K.; Boduszek, B.; Sobek, S.; Kozłowski, H. Tetrahedron 2006, 62, 2183–2189. doi:10.1016/j.tet.2005.12.016 |
| 5. | Berners-Price, S. J.; Sadler, P. J. Phosphines and metal phosphine complexes: Relationship of chemistry to anticancer and other biological activity. Bioinorganic Chemistry. Structure and Bonding; Springer: Berlin, Heidelberg, Germany, 1988; Vol. 70, pp 27–102. doi:10.1007/3-540-50130-4_2 |
| 6. | Nazarov, A. A.; Dyson, P. J. Metal Phosphorus Complexes as Antitumor Agents. In Phosphorus Compounds; Luca, G.; Peruzzini, M., Eds.; Springer: Dordrecht, Netherlands, 2011; Vol. 37, pp 445–461. doi:10.1007/978-90-481-3817-3_13 |
| 11. | Bálint, E.; Tripolszky, A.; Jablonkai, E.; Karaghiosoff, K.; Czugler, M.; Mucsi, Z.; Kollár, L.; Pongrácz, P.; Keglevich, G. J. Organomet. Chem. 2016, 801, 111–121. doi:10.1016/j.jorganchem.2015.10.029 |
| 2. | Bálint, E.; Tajti, Á.; Tripolszky, A.; Keglevich, G. Dalton Trans. 2018, 47, 4755–4778. doi:10.1039/c8dt00178b |
| 3. | Tsuji, J. Transition Metal Reagents and Catalysts; Wiley: Chichester, 2002. doi:10.1002/0470854766 |
| 4. | Beller, M.; Bolm, C. Transition Metals for Organic Synthesis: Building Blocks and Fine Chemicals; Wiley: Weinheim, 2004. doi:10.1002/9783527619405 |
| 16. | Bálint, E.; Fazekas, E.; Kóti, J.; Keglevich, G. Heteroat. Chem. 2015, 26, 106–115. doi:10.1002/hc.21221 |
| 17. | Zefirov, N. S.; Matveeva, E. D. ARKIVOC 2008, No. i, 1–17. doi:10.3998/ark.5550190.0009.101 |
| 2. | Bálint, E.; Tajti, Á.; Tripolszky, A.; Keglevich, G. Dalton Trans. 2018, 47, 4755–4778. doi:10.1039/c8dt00178b |
| 14. | Cherkasov, R. A.; Garifzyanov, A. R.; Talan, A. S.; Davletshin, R. R.; Kurnosova, N. V. Russ. J. Gen. Chem. 2009, 79, 1835–1849. doi:10.1134/s1070363209090114 |
| 10. | Keglevich, G.; Szekrényi, A. Lett. Org. Chem. 2008, 5, 616–622. doi:10.2174/157017808786857598 |
| 11. | Bálint, E.; Tripolszky, A.; Jablonkai, E.; Karaghiosoff, K.; Czugler, M.; Mucsi, Z.; Kollár, L.; Pongrácz, P.; Keglevich, G. J. Organomet. Chem. 2016, 801, 111–121. doi:10.1016/j.jorganchem.2015.10.029 |
| 13. | Cherkasov, R. A.; Garifzyanov, A. R.; Koshkin, S. A. Russ. J. Gen. Chem. 2011, 81, 773–774. doi:10.1134/s107036321104027x |
| 9. | Garifzyanov, A. R.; Vasil'ev, R. I.; Cherkasov, R. A. Russ. J. Gen. Chem. 2005, 75, 217–224. doi:10.1007/s11176-005-0201-6 |
| 11. | Bálint, E.; Tripolszky, A.; Jablonkai, E.; Karaghiosoff, K.; Czugler, M.; Mucsi, Z.; Kollár, L.; Pongrácz, P.; Keglevich, G. J. Organomet. Chem. 2016, 801, 111–121. doi:10.1016/j.jorganchem.2015.10.029 |
| 14. | Cherkasov, R. A.; Garifzyanov, A. R.; Talan, A. S.; Davletshin, R. R.; Kurnosova, N. V. Russ. J. Gen. Chem. 2009, 79, 1835–1849. doi:10.1134/s1070363209090114 |
| 15. | Bálint, E.; Fazekas, E.; Pongrácz, P.; Kollár, L.; Drahos, L.; Holczbauer, T.; Czugler, M.; Keglevich, G. J. Organomet. Chem. 2012, 717, 75–82. doi:10.1016/j.jorganchem.2012.07.031 |
| 8. | Petrov, K. A.; Chauzov, V. A.; Erokhina, T. S. Khim. Elementoorg. Soedin. 1976, 200. |
| 7. | Petrov, K. A.; Chauzov, V. A.; Erokhina, T. S.; Chernobrovkina, L. P. Zh. Obshch. Khim. 1976, 46, 493. |
| 12. | Olszewski, T. K.; Boduszek, B.; Sobek, S.; Kozłowski, H. Tetrahedron 2006, 62, 2183–2189. doi:10.1016/j.tet.2005.12.016 |
© 2019 Bálint et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the authors and source are credited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)