Abstract
Macrocycles were designed to antagonize the protein–protein interaction p53-MDM2 based on the three-finger pharmacophore F19W23L25. The synthesis was accomplished by a rapid, one-pot synthesis of indole-based macrocycles based on Ugi macrocyclization. The reaction of 12 different α,ω-amino acids and different indole-3-carboxaldehyde derivatives afforded a unique library of macrocycles otherwise difficult to access. Screening of the library for p53-MDM2 inhibition by fluorescence polarization and 1H,15N HSQC NMR measurements confirm MDM2 binding.
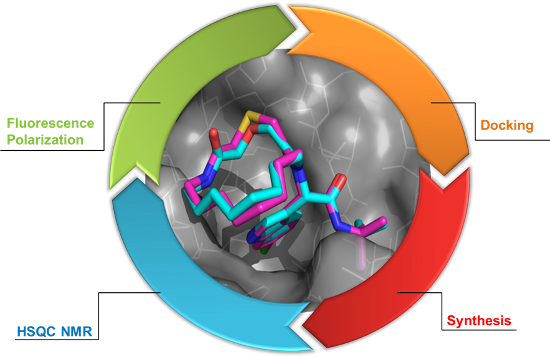
Graphical Abstract
Introduction
Macrocycles are the chemical entities that are consisting of a 12-membered or even bigger ring. It is estimated that 3% of the known natural products consists of a macrocyclic ring [1-5]. Compared to macrocycles in synthetic molecules, the aforementioned occurrence is still over proportional; for that reason, these compounds have delighted scientists worldwide due to their special physicochemical properties, their roles in biological systems and the associated synthetic challenges [6,7]. However, only few synthetic methods allow for the convergent and fast access to a large macrocyclic chemical space [8-10]; most of the times their synthesis is complex, multistep and sequential [11,12]. For this reason a great effort is ongoing to utilize multicomponent reactions for the synthesis of macrocycles [8,13-25].
The p53 protein is a well-studied protein which has a leading role in protecting our organism from cancer. It was found that most of the human cancers have either mutated the p53 itself or the p53 pathway is inhibited. The latter group of tumors retains the wild type p53 (wt-p53) but its pathway is inactivated by negative regulators, mainly the MDM2 and MDMX proteins. Thus, the design and synthesis of an inhibitor of the MDM2–p53 interaction could enable p53 and reverse tumor formation [26-28]. Based on our knowledge to antagonize the oncogenic protein–protein interaction p53–MDM2 [23,29-40] we designed macrocyclic inhibitors in continuation of our previous work [13,23]. Herein, an indole-based macrocycle synthesis is reported in a one-pot fashion based on Ugi macrocyclization with readily available α,ω-amino acids. Moreover, in continuation of our efforts in the design and synthesis of macrocycles targeting the p53–MDM2 interaction demonstrating the potential of these indole-based macrocycles, a subset of them was screened searching for MDM2 inhibitors. Compared to our previous indole-based macrocycles 1 following a different strategy (employing a classical Ugi-4C as the key reaction) [23], this one-pot Ugi macrocyclization leading to macrocycles 2 offers speed (one-pot procedure with one purification step), much better yields, no need of expensive catalysts as in ring-closing metathesis (RCM) reaction and higher complexity/diversity on the macrocyclic ring, e.g., insertion of heteroatoms that could improve the ADMET properties (Scheme 1) [4].
Scheme 1: MCR approach to indole-based macrocycles; a more effective strategy is proposed in this work, based on α,ω-amino acids and an Ugi macrocyclization.
Scheme 1: MCR approach to indole-based macrocycles; a more effective strategy is proposed in this work, based...
Results and Discussion
Synthesis
Based on our previous studies [13], unprotected diamines 3 were reacted in one-step with cyclic anhydrides 4 at rt affording the appropriate α,ω-amino acids 5 in excellent yields (see Supporting Information File 1). Elongated diamines (n = 2–4, 6, 8 and 10) and cyclic anhydrides that bear a heteroatom in the 4-position as oxygen or sulfur (Y = O, S, Scheme 2) were employed in order to enhance the diversity of our macrocycles [4]. Thus, in a parallel way, we readily synthesized 12 different amino acids which were subsequently subjected to the Ugi macrocyclization.
Scheme 2: Reaction of unprotected diamines 3 with cyclic anhydrides 4 at rt affording α,ω-amino acids 5 in quantitative yields.
Scheme 2: Reaction of unprotected diamines 3 with cyclic anhydrides 4 at rt affording α,ω-amino acids 5 in qu...
After quite some optimization, we improved the Ugi-macrocyclization procedure compared to our previous findings utilizing microwave irradiation (see Supporting Information File 1); Firstly, the corresponding amino acid was irradiated with indole-3-carboxaldehyde derivatives 6 using MeOH as solvent (5 mL) at 120 °C for 1 h. Then, tert-butyl isocyanide was added, diluted with more MeOH and irradiated again the reaction mixture at 120 °C for an additional 1 h in a final concentration of 0.1 M (Scheme 3). By this way, a rapid, one-pot access to macrocycles 2a–p was achieved otherwise very difficult to synthesize in relatively good yields (29–60%). 16 different indole-based macrocycles were synthesized with their size varying from 11–13, 15, 17 and 19 atoms (Scheme 3).
Scheme 3: Ugi macrocyclization in a one-pot fashion and synthesis of diverse indole-based macrocycles. The circle depicts the size number of the macrocycle.
Scheme 3: Ugi macrocyclization in a one-pot fashion and synthesis of diverse indole-based macrocycles. The ci...
Biological evaluation
Our previously introduced three-point pharmacophore model on mimicking the hot triad (Phe19, Trp23 and Leu26, F19W23L26) was the basis of the evaluation of the current derivatives as potent inhibitors [33]. The indole moiety could be used not only to constrain the two other substituents but also as an “anchor” mimicking the Trp23. The bulky tert-butyl group would mimic the Phe19 and the macrocyclic ring would fill the Leu26 sub-pocket as shown by our docking studies (Figure 1A,B, Figure S4 in Supporting Information File 1). Thus, extending our previous work [13], the Leu26 subpocket was probed by utilizing the different ring sizes and the different heteroatoms (oxygen or sulfur) of our macrocyclic library. In addition, the influence of the chlorine atom in the 6-position of the indole ring (Figure 1C) was examined. Macrocycles 2a–j consist of an oxygen linker whereas 2g–j bear also a chlorine atom in the 6-position in the indole ring. Macrocycles 2k–p incorporate both a sulfur linker and the chlorine on the indole ring (Scheme 3).
![[1860-5397-15-45-1]](/bjoc/content/figures/1860-5397-15-45-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: (A) Modeling of the macrocycle 2h (cyan sticks) and 2n (magenta sticks) into the MDM2 receptor (PDB ID: 1YCR); (B) 2D structure of 2h with the substituents targeting the subpockets of MDM2; (C) Analysis of the synthesized macrocycles probing the subpockets of MDM2 and expansion of the chemistry compared to previous studies [13].
Figure 1: (A) Modeling of the macrocycle 2h (cyan sticks) and 2n (magenta sticks) into the MDM2 receptor (PDB...
In order to exclude false positive hits, two biorthogonal assays were chosen; 1H,15N HSQC NMR and fluorescence polarization (FP, Table 1). FP assay was employed to determine the inhibitory affinities (Ki) of the derivatives against MDM2 as previously described [36]. Besides 2h (Ki = 2.3 μΜ, Kd = 12.1 μΜ), it was shown that 2i demonstrated a promising activity with a Ki of 5.5 μΜ. Furthermore, 1H,15N HSQC showed a Kd of 4.8 μΜ (Table 1, Figure 2). Moreover, macrocycles 2g and 2n demonstrated a Kd of 9 μΜ and 17 μΜ, respectively (Table 1). With this preliminary analysis, it was found that a ring size of 15–17 atoms and an oxygen as the heteroatom linker improves the binding affinity. All the active macrocycles have a 6-chloro-substituted indole core. It is well established that at the bottom of the Try23 pocket a hydrophobic small subpocket exists which is formed by Phe86, Ile103, Leu82 and Leu57. This pocket when filled with a smaller hydrophobic substituent such as -Cl boosts the inhibitor activity in accordance with literature [33].
Table 1: Measurement of Ki and Kd of the selected macrocycles based on FP and 1H,15N HSQC NMR assays, respectively.a
| Entry | Name | Structure | Ki MDM2 [µM] | Kd MDM2 [µM] |
| 1 | 2h |
|
2.3 | 12.1 ± 8.5 |
| 2 | 2i |
|
5.5 | 4.8 ± 1.5 |
| 3 | 2n |
|
316 | 17.2 ± 3.8 |
| 4 | 2g |
|
n.a. | 8.9 ± 1.2 |
an.a. no activity against MDM2 protein. Ki and Kd values were calculated based on fluorescence polarization binding and 1H,15N HSQC NMR assay, respectively.
![[1860-5397-15-45-2]](/bjoc/content/figures/1860-5397-15-45-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: (A) Overlay of 1 H,15N-HSQC spectra of the reference MDM2 (red) and the titration steps with the 2i inhibitor. MDM2/2i ratios 4:1 (orange), 4:2 (yellow), 4:3 (green), 1:1 (light blue), 1:2 (blue), 1:5 (purple). Examples of most perturbed residues are labeled on the spectrum. (B) Normalized chemical shift perturbations (δo) of MDM2 residue (calculated according to Stoll et al. [41]). Residue with δo equal 0 are either despairing from MDM2 spectrum upon titration or cannot be identified. (C) Chemical shift perturbations plotted onto the structure of MDM2 (wheat); orange (despairing – indicating stronger binding), light orange (>0.1 ppm), yellow (0.05–0.1 ppm). Residues which disappear upon titration experiment are labeled on the Mdm2 surface.
Figure 2: (A) Overlay of 1 H,15N-HSQC spectra of the reference MDM2 (red) and the titration steps with the 2i...
Conclusion
We effectively synthesized p53-MDM2 antagonists based on an artificial macrocyclic scaffold. 16 different derivatives were obtained and screened. The aforementioned artificial macrocycles combine the indole ring, a motif found in many bioactive molecules with the drug-like properties of a non-peptide macrocycle. We hypothesize that these chimeric derivatives of an indole and a macrocycle will offer new potential on specific PPIs and other postgenomic targets as it was demonstrated with the p53-MDM2 interaction.
Supporting Information
| Supporting Information File 1: Experimental procedures, analytical data, NMR spectra, fluorescence polarization binding assays, 1H,15N HSQC NMR spectra of 15N-labeled MDM2 and computational modeling studies. | ||
| Format: PDF | Size: 1.5 MB | Download |
Acknowledgements
This research has been supported to (AD) by the National Institute of Health (NIH) (2R01GM097082-05), the European Lead Factory (IMI) under grant agreement number 115489, the Qatar National Research Foundation (NPRP6-065-3-012) and to (TAH) by Grant UMO-2014/12/W/NZ1/00457 from the National Science Centre, Poland. Moreover funding was received through ITN “Accelerated Early stage drug dIScovery” (AEGIS, grant agreement No 675555) and COFUND ALERT (grant agreement No 665250), Hartstichting (ESCAPE-HF, 2018B012) and KWF Kankerbestrijding grant (grant agreement No 10504).
References
-
Giordanetto, F.; Kihlberg, J. J. Med. Chem. 2014, 57, 278–295. doi:10.1021/jm400887j
Return to citation in text: [1] -
Davis, A. M.; Plowright, A. T.; Valeur, E. Nat. Rev. Drug Discovery 2017, 16, 681–698. doi:10.1038/nrd.2017.146
Return to citation in text: [1] -
Scott, D. E.; Bayly, A. R.; Abell, C.; Skidmore, J. Nat. Rev. Drug Discovery 2016, 15, 533–550. doi:10.1038/nrd.2016.29
Return to citation in text: [1] -
Villar, E. A.; Beglov, D.; Chennamadhavuni, S.; Porco, J. A.; Kozakov, D.; Vajda, S.; Whitty, A. Nat. Chem. Biol. 2014, 10, 723–731. doi:10.1038/nchembio.1584
Return to citation in text: [1] [2] [3] -
Doak, B. C.; Zheng, J.; Dobritzsch, D.; Kihlberg, J. J. Med. Chem. 2016, 59, 2312–2327. doi:10.1021/acs.jmedchem.5b01286
Return to citation in text: [1] -
Yu, X.; Sun, D. Molecules 2013, 18, 6230–6268. doi:10.3390/molecules18066230
Return to citation in text: [1] -
White, C. J.; Yudin, A. K. Nat. Chem. 2011, 3, 509–524. doi:10.1038/nchem.1062
Return to citation in text: [1] -
Failli, A.; Immer, H.; Götz, M. Can. J. Chem. 1979, 57, 3257–3261. doi:10.1139/v79-533
Return to citation in text: [1] [2] -
Gartner, Z. J. Science 2004, 305, 1601–1605. doi:10.1126/science.1102629
Return to citation in text: [1] -
Jebrail, M. J.; Ng, A. H. C.; Rai, V.; Hili, R.; Yudin, A. K.; Wheeler, A. R. Angew. Chem., Int. Ed. 2010, 49, 8625–8629. doi:10.1002/anie.201001604
Return to citation in text: [1] -
Koopmanschap, G.; Ruijter, E.; Orru, R. V. A. Beilstein J. Org. Chem. 2014, 10, 544–598. doi:10.3762/bjoc.10.50
Return to citation in text: [1] -
Iyoda, M.; Yamakawa, J.; Rahman, M. J. Angew. Chem., Int. Ed. 2011, 50, 10522–10553. doi:10.1002/anie.201006198
Return to citation in text: [1] -
Madhavachary, R.; Abdelraheem, E. M. M.; Rossetti, A.; Twarda-Clapa, A.; Musielak, B.; Kurpiewska, K.; Kalinowska-Tłuścik, J.; Holak, T. A.; Dömling, A. Angew. Chem., Int. Ed. 2017, 56, 10725–10729. doi:10.1002/anie.201704426
Return to citation in text: [1] [2] [3] [4] [5] -
Abdelraheem, E.; Khaksar, S.; Dömling, A. Synthesis 2018, 50, 1027–1038. doi:10.1055/s-0036-1590946
Return to citation in text: [1] -
Janvier, P.; Bois-Choussy, M.; Bienaymé, H.; Zhu, J. Angew. Chem., Int. Ed. 2003, 42, 811–814. doi:10.1002/anie.200390216
Return to citation in text: [1] -
Wessjohann, L. A.; Voigt, B.; Rivera, D. G. Angew. Chem., Int. Ed. 2005, 44, 4785–4790. doi:10.1002/anie.200500019
Return to citation in text: [1] -
Wessjohann, L. A.; Rivera, D. G.; Vercillo, O. E. Chem. Rev. 2009, 109, 796–814. doi:10.1021/cr8003407
Return to citation in text: [1] -
Abdelraheem, E. M. M.; Kurpiewska, K.; Kalinowska-Tłuścik, J.; Dömling, A. J. Org. Chem. 2016, 81, 8789–8795. doi:10.1021/acs.joc.6b01430
Return to citation in text: [1] -
Beck, B.; Larbig, G.; Mejat, B.; Magnin-Lachaux, M.; Picard, A.; Herdtweck, E.; Dömling, A. Org. Lett. 2003, 5, 1047–1050. doi:10.1021/ol034077e
Return to citation in text: [1] -
Liao, G. P.; Abdelraheem, E. M. M.; Neochoritis, C. G.; Kurpiewska, K.; Kalinowska-Tłuścik, J.; McGowan, D. C.; Dömling, A. Org. Lett. 2015, 17, 4980–4983. doi:10.1021/acs.orglett.5b02419
Return to citation in text: [1] -
Abdelraheem, E. M. M.; Khaksar, S.; Kurpiewska, K.; Kalinowska-Tłuścik, J.; Shaabani, S.; Dömling, A. J. Org. Chem. 2018, 83, 1441–1447. doi:10.1021/acs.joc.7b02984
Return to citation in text: [1] -
Abdelraheem, E.; Shaabani, S.; Dömling, A. Synlett 2018, 29, 1136–1151. doi:10.1055/s-0036-1591975
Return to citation in text: [1] -
Estrada-Ortiz, N.; Neochoritis, C. G.; Twarda-Clapa, A.; Musielak, B.; Holak, T. A.; Dömling, A. ACS Med. Chem. Lett. 2017, 8, 1025–1030. doi:10.1021/acsmedchemlett.7b00219
Return to citation in text: [1] [2] [3] [4] -
Abdelraheem, E. M. M.; de Haan, M. P.; Patil, P.; Kurpiewska, K.; Kalinowska-Tłuścik, J.; Shaabani, S.; Dömling, A. Org. Lett. 2017, 19, 5078–5081. doi:10.1021/acs.orglett.7b02319
Return to citation in text: [1] -
Abdelraheem, E. M. M.; Madhavachary, R.; Rossetti, A.; Kurpiewska, K.; Kalinowska-Tłuścik, J.; Shaabani, S.; Dömling, A. Org. Lett. 2017, 19, 6176–6179. doi:10.1021/acs.orglett.7b03094
Return to citation in text: [1] -
Gu, J.; Wang, B.; Liu, Y.; Zhong, L.; Tang, Y.; Guo, H.; Jiang, T.; Wang, L.; Li, Y.; Cai, L. Eur. J. Cancer 2014, 50, 1184–1194. doi:10.1016/j.ejca.2013.12.027
Return to citation in text: [1] -
Brown, C. J.; Lain, S.; Verma, C. S.; Fersht, A. R.; Lane, D. P. Nat. Rev. Cancer 2009, 9, 862–873. doi:10.1038/nrc2763
Return to citation in text: [1] -
Cheok, C. F.; Verma, C. S.; Baselga, J.; Lane, D. P. Nat. Rev. Clin. Oncol. 2011, 8, 25–37. doi:10.1038/nrclinonc.2010.174
Return to citation in text: [1] -
Huang, Y.; Wolf, S.; Beck, B.; Köhler, L.-M.; Khoury, K.; Popowicz, G. M.; Goda, S. K.; Subklewe, M.; Twarda, A.; Holak, T. A.; Dömling, A. ACS Chem. Biol. 2014, 9, 802–811. doi:10.1021/cb400728e
Return to citation in text: [1] -
Bista, M.; Wolf, S.; Khoury, K.; Kowalska, K.; Huang, Y.; Wrona, E.; Arciniega, M.; Popowicz, G. M.; Holak, T. A.; Dömling, A. Structure 2013, 21, 2143–2151. doi:10.1016/j.str.2013.09.006
Return to citation in text: [1] -
Estrada-Ortiz, N.; Neochoritis, C. G.; Dömling, A. ChemMedChem 2016, 11, 757–772. doi:10.1002/cmdc.201500487
Return to citation in text: [1] -
Neochoritis, C. G.; Wang, K.; Estrada-Ortiz, N.; Herdtweck, E.; Kubica, K.; Twarda, A.; Zak, K. M.; Holak, T. A.; Dömling, A. Bioorg. Med. Chem. Lett. 2015, 25, 5661–5666. doi:10.1016/j.bmcl.2015.11.019
Return to citation in text: [1] -
Czarna, A.; Beck, B.; Srivastava, S.; Popowicz, G. M.; Wolf, S.; Huang, Y.; Bista, M.; Holak, T. A.; Dömling, A. Angew. Chem., Int. Ed. 2010, 49, 5352–5356. doi:10.1002/anie.201001343
Return to citation in text: [1] [2] [3] -
Neochoritis, C.; Estrada-Ortiz, N.; Khoury, K.; Dömling, A. Annu. Rep. Med. Chem. 2014, 49, 167–187. doi:10.1016/b978-0-12-800167-7.00012-2
Return to citation in text: [1] -
Popowicz, G. M.; Czarna, A.; Wolf, S.; Wang, K.; Wang, W.; Dömling, A.; Holak, T. A. Cell Cycle 2010, 9, 1104–1111. doi:10.4161/cc.9.6.10956
Return to citation in text: [1] -
Czarna, A.; Popowicz, G. M.; Pecak, A.; Wolf, S.; Dubin, G.; Holak, T. A. Cell Cycle 2009, 8, 1176–1184. doi:10.4161/cc.8.8.8185
Return to citation in text: [1] [2] -
Huang, Y.; Wolf, S.; Koes, D.; Popowicz, G. M.; Camacho, C. J.; Holak, T. A.; Dömling, A. ChemMedChem 2012, 7, 49–52. doi:10.1002/cmdc.201100428
Return to citation in text: [1] -
Surmiak, E.; Neochoritis, C. G.; Musielak, B.; Twarda-Clapa, A.; Kurpiewska, K.; Dubin, G.; Camacho, C.; Holak, T. A.; Dömling, A. Eur. J. Med. Chem. 2017, 126, 384–407. doi:10.1016/j.ejmech.2016.11.029
Return to citation in text: [1] -
Shaabani, S.; Neochoritis, C. G.; Twarda-Clapa, A.; Musielak, B.; Holak, T. A.; Dömling, A. Med. Chem. Commun. 2017, 8, 1046–1052. doi:10.1039/c7md00058h
Return to citation in text: [1] -
Koes, D. R.; Dömling, A.; Camacho, C. J. Protein Sci. 2018, 27, 229–232. doi:10.1002/pro.3303
Return to citation in text: [1] -
Stoll, R.; Renner, C.; Hansen, S.; Palme, S.; Klein, C.; Belling, A.; Zeslawski, W.; Kamionka, M.; Rehm, T.; Mühlhahn, P.; Schumacher, R.; Hesse, F.; Kaluza, B.; Voelter, W.; Engh, R. A.; Holak, T. A. Biochemistry 2001, 40, 336–344. doi:10.1021/bi000930v
Return to citation in text: [1]
| 33. | Czarna, A.; Beck, B.; Srivastava, S.; Popowicz, G. M.; Wolf, S.; Huang, Y.; Bista, M.; Holak, T. A.; Dömling, A. Angew. Chem., Int. Ed. 2010, 49, 5352–5356. doi:10.1002/anie.201001343 |
| 41. | Stoll, R.; Renner, C.; Hansen, S.; Palme, S.; Klein, C.; Belling, A.; Zeslawski, W.; Kamionka, M.; Rehm, T.; Mühlhahn, P.; Schumacher, R.; Hesse, F.; Kaluza, B.; Voelter, W.; Engh, R. A.; Holak, T. A. Biochemistry 2001, 40, 336–344. doi:10.1021/bi000930v |
| 1. | Giordanetto, F.; Kihlberg, J. J. Med. Chem. 2014, 57, 278–295. doi:10.1021/jm400887j |
| 2. | Davis, A. M.; Plowright, A. T.; Valeur, E. Nat. Rev. Drug Discovery 2017, 16, 681–698. doi:10.1038/nrd.2017.146 |
| 3. | Scott, D. E.; Bayly, A. R.; Abell, C.; Skidmore, J. Nat. Rev. Drug Discovery 2016, 15, 533–550. doi:10.1038/nrd.2016.29 |
| 4. | Villar, E. A.; Beglov, D.; Chennamadhavuni, S.; Porco, J. A.; Kozakov, D.; Vajda, S.; Whitty, A. Nat. Chem. Biol. 2014, 10, 723–731. doi:10.1038/nchembio.1584 |
| 5. | Doak, B. C.; Zheng, J.; Dobritzsch, D.; Kihlberg, J. J. Med. Chem. 2016, 59, 2312–2327. doi:10.1021/acs.jmedchem.5b01286 |
| 8. | Failli, A.; Immer, H.; Götz, M. Can. J. Chem. 1979, 57, 3257–3261. doi:10.1139/v79-533 |
| 13. | Madhavachary, R.; Abdelraheem, E. M. M.; Rossetti, A.; Twarda-Clapa, A.; Musielak, B.; Kurpiewska, K.; Kalinowska-Tłuścik, J.; Holak, T. A.; Dömling, A. Angew. Chem., Int. Ed. 2017, 56, 10725–10729. doi:10.1002/anie.201704426 |
| 14. | Abdelraheem, E.; Khaksar, S.; Dömling, A. Synthesis 2018, 50, 1027–1038. doi:10.1055/s-0036-1590946 |
| 15. | Janvier, P.; Bois-Choussy, M.; Bienaymé, H.; Zhu, J. Angew. Chem., Int. Ed. 2003, 42, 811–814. doi:10.1002/anie.200390216 |
| 16. | Wessjohann, L. A.; Voigt, B.; Rivera, D. G. Angew. Chem., Int. Ed. 2005, 44, 4785–4790. doi:10.1002/anie.200500019 |
| 17. | Wessjohann, L. A.; Rivera, D. G.; Vercillo, O. E. Chem. Rev. 2009, 109, 796–814. doi:10.1021/cr8003407 |
| 18. | Abdelraheem, E. M. M.; Kurpiewska, K.; Kalinowska-Tłuścik, J.; Dömling, A. J. Org. Chem. 2016, 81, 8789–8795. doi:10.1021/acs.joc.6b01430 |
| 19. | Beck, B.; Larbig, G.; Mejat, B.; Magnin-Lachaux, M.; Picard, A.; Herdtweck, E.; Dömling, A. Org. Lett. 2003, 5, 1047–1050. doi:10.1021/ol034077e |
| 20. | Liao, G. P.; Abdelraheem, E. M. M.; Neochoritis, C. G.; Kurpiewska, K.; Kalinowska-Tłuścik, J.; McGowan, D. C.; Dömling, A. Org. Lett. 2015, 17, 4980–4983. doi:10.1021/acs.orglett.5b02419 |
| 21. | Abdelraheem, E. M. M.; Khaksar, S.; Kurpiewska, K.; Kalinowska-Tłuścik, J.; Shaabani, S.; Dömling, A. J. Org. Chem. 2018, 83, 1441–1447. doi:10.1021/acs.joc.7b02984 |
| 22. | Abdelraheem, E.; Shaabani, S.; Dömling, A. Synlett 2018, 29, 1136–1151. doi:10.1055/s-0036-1591975 |
| 23. | Estrada-Ortiz, N.; Neochoritis, C. G.; Twarda-Clapa, A.; Musielak, B.; Holak, T. A.; Dömling, A. ACS Med. Chem. Lett. 2017, 8, 1025–1030. doi:10.1021/acsmedchemlett.7b00219 |
| 24. | Abdelraheem, E. M. M.; de Haan, M. P.; Patil, P.; Kurpiewska, K.; Kalinowska-Tłuścik, J.; Shaabani, S.; Dömling, A. Org. Lett. 2017, 19, 5078–5081. doi:10.1021/acs.orglett.7b02319 |
| 25. | Abdelraheem, E. M. M.; Madhavachary, R.; Rossetti, A.; Kurpiewska, K.; Kalinowska-Tłuścik, J.; Shaabani, S.; Dömling, A. Org. Lett. 2017, 19, 6176–6179. doi:10.1021/acs.orglett.7b03094 |
| 13. | Madhavachary, R.; Abdelraheem, E. M. M.; Rossetti, A.; Twarda-Clapa, A.; Musielak, B.; Kurpiewska, K.; Kalinowska-Tłuścik, J.; Holak, T. A.; Dömling, A. Angew. Chem., Int. Ed. 2017, 56, 10725–10729. doi:10.1002/anie.201704426 |
| 11. | Koopmanschap, G.; Ruijter, E.; Orru, R. V. A. Beilstein J. Org. Chem. 2014, 10, 544–598. doi:10.3762/bjoc.10.50 |
| 12. | Iyoda, M.; Yamakawa, J.; Rahman, M. J. Angew. Chem., Int. Ed. 2011, 50, 10522–10553. doi:10.1002/anie.201006198 |
| 36. | Czarna, A.; Popowicz, G. M.; Pecak, A.; Wolf, S.; Dubin, G.; Holak, T. A. Cell Cycle 2009, 8, 1176–1184. doi:10.4161/cc.8.8.8185 |
| 8. | Failli, A.; Immer, H.; Götz, M. Can. J. Chem. 1979, 57, 3257–3261. doi:10.1139/v79-533 |
| 9. | Gartner, Z. J. Science 2004, 305, 1601–1605. doi:10.1126/science.1102629 |
| 10. | Jebrail, M. J.; Ng, A. H. C.; Rai, V.; Hili, R.; Yudin, A. K.; Wheeler, A. R. Angew. Chem., Int. Ed. 2010, 49, 8625–8629. doi:10.1002/anie.201001604 |
| 33. | Czarna, A.; Beck, B.; Srivastava, S.; Popowicz, G. M.; Wolf, S.; Huang, Y.; Bista, M.; Holak, T. A.; Dömling, A. Angew. Chem., Int. Ed. 2010, 49, 5352–5356. doi:10.1002/anie.201001343 |
| 6. | Yu, X.; Sun, D. Molecules 2013, 18, 6230–6268. doi:10.3390/molecules18066230 |
| 7. | White, C. J.; Yudin, A. K. Nat. Chem. 2011, 3, 509–524. doi:10.1038/nchem.1062 |
| 13. | Madhavachary, R.; Abdelraheem, E. M. M.; Rossetti, A.; Twarda-Clapa, A.; Musielak, B.; Kurpiewska, K.; Kalinowska-Tłuścik, J.; Holak, T. A.; Dömling, A. Angew. Chem., Int. Ed. 2017, 56, 10725–10729. doi:10.1002/anie.201704426 |
| 23. | Estrada-Ortiz, N.; Neochoritis, C. G.; Twarda-Clapa, A.; Musielak, B.; Holak, T. A.; Dömling, A. ACS Med. Chem. Lett. 2017, 8, 1025–1030. doi:10.1021/acsmedchemlett.7b00219 |
| 13. | Madhavachary, R.; Abdelraheem, E. M. M.; Rossetti, A.; Twarda-Clapa, A.; Musielak, B.; Kurpiewska, K.; Kalinowska-Tłuścik, J.; Holak, T. A.; Dömling, A. Angew. Chem., Int. Ed. 2017, 56, 10725–10729. doi:10.1002/anie.201704426 |
| 13. | Madhavachary, R.; Abdelraheem, E. M. M.; Rossetti, A.; Twarda-Clapa, A.; Musielak, B.; Kurpiewska, K.; Kalinowska-Tłuścik, J.; Holak, T. A.; Dömling, A. Angew. Chem., Int. Ed. 2017, 56, 10725–10729. doi:10.1002/anie.201704426 |
| 23. | Estrada-Ortiz, N.; Neochoritis, C. G.; Twarda-Clapa, A.; Musielak, B.; Holak, T. A.; Dömling, A. ACS Med. Chem. Lett. 2017, 8, 1025–1030. doi:10.1021/acsmedchemlett.7b00219 |
| 4. | Villar, E. A.; Beglov, D.; Chennamadhavuni, S.; Porco, J. A.; Kozakov, D.; Vajda, S.; Whitty, A. Nat. Chem. Biol. 2014, 10, 723–731. doi:10.1038/nchembio.1584 |
| 23. | Estrada-Ortiz, N.; Neochoritis, C. G.; Twarda-Clapa, A.; Musielak, B.; Holak, T. A.; Dömling, A. ACS Med. Chem. Lett. 2017, 8, 1025–1030. doi:10.1021/acsmedchemlett.7b00219 |
| 29. | Huang, Y.; Wolf, S.; Beck, B.; Köhler, L.-M.; Khoury, K.; Popowicz, G. M.; Goda, S. K.; Subklewe, M.; Twarda, A.; Holak, T. A.; Dömling, A. ACS Chem. Biol. 2014, 9, 802–811. doi:10.1021/cb400728e |
| 30. | Bista, M.; Wolf, S.; Khoury, K.; Kowalska, K.; Huang, Y.; Wrona, E.; Arciniega, M.; Popowicz, G. M.; Holak, T. A.; Dömling, A. Structure 2013, 21, 2143–2151. doi:10.1016/j.str.2013.09.006 |
| 31. | Estrada-Ortiz, N.; Neochoritis, C. G.; Dömling, A. ChemMedChem 2016, 11, 757–772. doi:10.1002/cmdc.201500487 |
| 32. | Neochoritis, C. G.; Wang, K.; Estrada-Ortiz, N.; Herdtweck, E.; Kubica, K.; Twarda, A.; Zak, K. M.; Holak, T. A.; Dömling, A. Bioorg. Med. Chem. Lett. 2015, 25, 5661–5666. doi:10.1016/j.bmcl.2015.11.019 |
| 33. | Czarna, A.; Beck, B.; Srivastava, S.; Popowicz, G. M.; Wolf, S.; Huang, Y.; Bista, M.; Holak, T. A.; Dömling, A. Angew. Chem., Int. Ed. 2010, 49, 5352–5356. doi:10.1002/anie.201001343 |
| 34. | Neochoritis, C.; Estrada-Ortiz, N.; Khoury, K.; Dömling, A. Annu. Rep. Med. Chem. 2014, 49, 167–187. doi:10.1016/b978-0-12-800167-7.00012-2 |
| 35. | Popowicz, G. M.; Czarna, A.; Wolf, S.; Wang, K.; Wang, W.; Dömling, A.; Holak, T. A. Cell Cycle 2010, 9, 1104–1111. doi:10.4161/cc.9.6.10956 |
| 36. | Czarna, A.; Popowicz, G. M.; Pecak, A.; Wolf, S.; Dubin, G.; Holak, T. A. Cell Cycle 2009, 8, 1176–1184. doi:10.4161/cc.8.8.8185 |
| 37. | Huang, Y.; Wolf, S.; Koes, D.; Popowicz, G. M.; Camacho, C. J.; Holak, T. A.; Dömling, A. ChemMedChem 2012, 7, 49–52. doi:10.1002/cmdc.201100428 |
| 38. | Surmiak, E.; Neochoritis, C. G.; Musielak, B.; Twarda-Clapa, A.; Kurpiewska, K.; Dubin, G.; Camacho, C.; Holak, T. A.; Dömling, A. Eur. J. Med. Chem. 2017, 126, 384–407. doi:10.1016/j.ejmech.2016.11.029 |
| 39. | Shaabani, S.; Neochoritis, C. G.; Twarda-Clapa, A.; Musielak, B.; Holak, T. A.; Dömling, A. Med. Chem. Commun. 2017, 8, 1046–1052. doi:10.1039/c7md00058h |
| 40. | Koes, D. R.; Dömling, A.; Camacho, C. J. Protein Sci. 2018, 27, 229–232. doi:10.1002/pro.3303 |
| 26. | Gu, J.; Wang, B.; Liu, Y.; Zhong, L.; Tang, Y.; Guo, H.; Jiang, T.; Wang, L.; Li, Y.; Cai, L. Eur. J. Cancer 2014, 50, 1184–1194. doi:10.1016/j.ejca.2013.12.027 |
| 27. | Brown, C. J.; Lain, S.; Verma, C. S.; Fersht, A. R.; Lane, D. P. Nat. Rev. Cancer 2009, 9, 862–873. doi:10.1038/nrc2763 |
| 28. | Cheok, C. F.; Verma, C. S.; Baselga, J.; Lane, D. P. Nat. Rev. Clin. Oncol. 2011, 8, 25–37. doi:10.1038/nrclinonc.2010.174 |
| 4. | Villar, E. A.; Beglov, D.; Chennamadhavuni, S.; Porco, J. A.; Kozakov, D.; Vajda, S.; Whitty, A. Nat. Chem. Biol. 2014, 10, 723–731. doi:10.1038/nchembio.1584 |
© 2019 Neochoritis et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the authors and source are credited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)