Abstract
Capsular polysaccharides of pathogenic bacteria have been reported to be effective vaccines against diseases caused by them. Providencia stuartii is a class of enterobacteria of the family Providencia that is responsible for several antibiotic resistant infections, particularly urinary tract infections of patients with prolonged catheterization in hospital settings. Towards the goal of development of vaccine candidates against this pathogen, we herein report the total synthesis of a trisaccharide repeating unit of the O-antigen polysaccharide of the P. stuartii O49 serotype containing the →6)-β-ᴅ-Galp-(1→3)-β-ᴅ-GalpNAc(1→4)-α-ᴅ-Galp(1→ linkage. The synthesis of the trisaccharide repeating unit was carried out first by a linear strategy involving the [1 + (1 + 1 = 2)] assembly, followed by a one-pot synthesis involving [1 + 1 + 1] strategy from the corresponding monosaccharides. The one-pot method provided a higher yield of the protected trisaccharide intermediate (73%) compared to the two step synthesis (66%). The protected trisaccharide was then deprotected and N-acetylated to finally afford the desired trisaccharide repeating unit as its α-p-methoxyphenyl glycoside.
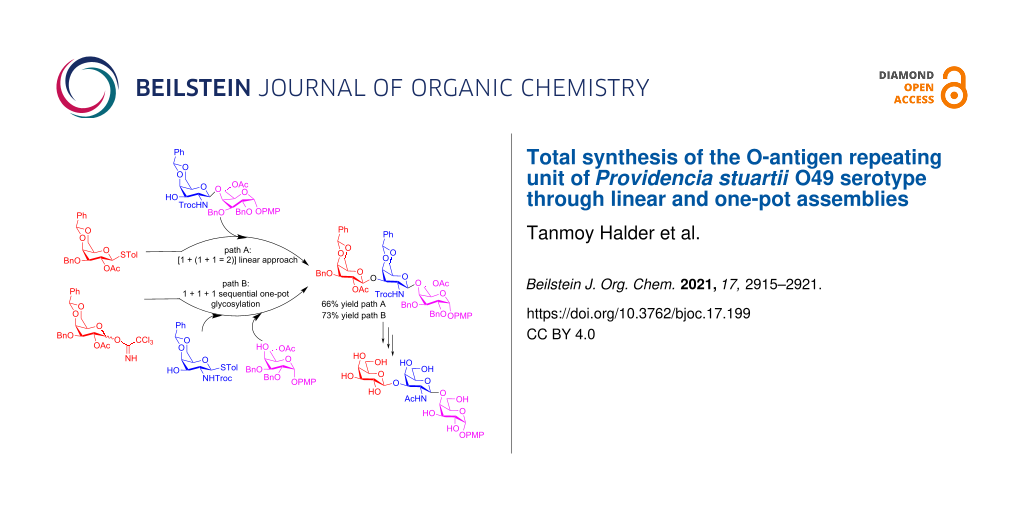
Graphical Abstract
Introduction
O-antigens or O-specific polysaccharides are one of the important constituents of the surface lipopolysaccharides (LPS) of the cell wall of Gram-negative bacteria. These antigens are responsible for several functions that include adhesion to the host cells and are also found to contribute to the evasion of the host immune responses. Structurally, the O-antigens consist of polysaccharide repeating units bearing several different monosaccharides. Due to their importance in regulating the host’s immune system, the bacterial cell surface LPS in general and the O-antigens in particular have been proposed and reported as candidates for vaccine development [1-8]. This objective has been proposed to be achieved by the synthesis of chemically homogeneous glycoconjugates bearing the O-antigen oligosaccharide conjugated to peptides for eliciting the desired immune response through vaccines [9-21]. For the above purposes, large scale access to pure, defined, and homogeneous samples of the desired LPS oligosaccharides are essential for realization of the goal towards vaccine development against these pathogens [1-21].
Providencia is a genus of Gram-negative bacteria that belongs to the enterobacteria family and is responsible for causing several enteric infections including urinary tract infections. Providencia includes mainly five virulent species – P. alcalifaciens, P. rettgeri, P. rustigianii, P. stuartii, and P. heimbachae [22]. The Providencia species has been isolated from urine, stool, blood, throat, axilla, and perineum of infected patients as also from polluted soil and wastewater [22-24]. Of the five species, the clinically important ones are Providencia rettgeri and P. stuartii which are found to be particularly responsible for antibiotic resistant infections in hospitalized patients with long term urinary catheters, particularly immuno-compromised patients [25]. A total 61% of urinary region specimens in the infected populace consist of either Providencia stuartii or Proteus mirabilis, and the infections may even lead to fatal bacteremia [22,25].
The structure of repeating oligosaccharide units of O-polysaccharides of several Providencia O-serogroups as well as P. stuartii O4 [26], O18 [27], O20 [28] O33 [29], O43 [30], O44 [31], O47 [32], and O57 [33] have been reported. With respect to the synthesis of the O-antigens, that of Providencia rustigianii O34 was reported by Mukhopadhyay et al. in 2013 [34]. Chheda and co-worker, in 2015, reported the total synthesis of the pentasaccharide repeating unit of the O-specific polysaccharide of Providencia alcalifaciens O28 [35]. In 2017, Kulkarni and co-worker accomplished the total synthesis of a O-polysaccharide of Providencia alcalifaciens O22 via a one pot assembly of the oligosaccharide unit [36]. Recently in 2020 Werz and co-workers completed the total synthesis of a tri-, hexa-, and heptasaccharide of the O-polysaccharide of Providencia rustigianii O34 [37]. In the context of the O-antigen repeating units of various P. stuartii serotypes, in 2004 Bushmarinov et al. [38] reported the O-antigen of the O49 serotype as consisting of the →6)-β-ᴅ-Galp-(1→3)-β-ᴅ-GalpNAc(1→4)-α-ᴅ-Galp(1→ linkage (Figure 1). Herein, we report the total synthesis of the hitherto not synthesized above trisaccharide repeating unit containing two ᴅ-galactose and one N-acetyl-ᴅ-galactosamine moieties. The linear synthesis of the target oligosaccharide was first carried out via a linear [1 + (1 + 1 = 2)] assembly of a galactopyranose donor with a GalpNTroc–Galp acceptor. We also demonstrated a follow-up one-pot synthesis involving a [1 + 1 + 1] strategy using the corresponding appropriately protected monosaccharides, providing the opportunity for rapid access to the desired target molecule.
Figure 1: Structure of the repeating unit of the lipopolysaccharide of Providencia stuartii O49 serotype.
Figure 1: Structure of the repeating unit of the lipopolysaccharide of Providencia stuartii O49 serotype.
Results and Discussion
The retrosynthesis of the target trisaccharide 1, led to the monosaccharides 3, 6, and 7 (Scheme 1). The choice of the p-methoxyphenyl (PMP) group at the reducing end was based on the fact that it could be easily synthesized stereoselectively to mimic the α-glycosidic linkage in the native oligosaccharide. Further, the trisaccharide could be adapted for further conjugation to other moieties towards the synthesis of vaccine conjugates by removal of the anomeric PMP group. This has been previously demonstrated by removal of the anomeric PMP group, conversion to a glycosidic donor, and further conjugation to either linkers or amino acids by several authors [39-42]. The starting materials 3, 6, and 7 were also amenable to a [1 + 1 + 1] one-pot synthetic strategy by adopting minor synthetic modifications.
Scheme 1: Retrosynthetic analysis for the synthesis of the target trisaccharide 1.
Scheme 1: Retrosynthetic analysis for the synthesis of the target trisaccharide 1.
The monosaccharide building blocks 3, 6, and 7 [43] were synthesized from previously reported compounds 8 [36], 9 [44,45], and 10 [46] as described in Scheme 2.
Scheme 2: Synthesis of the monosaccharide building blocks 3, 6, and 7.
Scheme 2: Synthesis of the monosaccharide building blocks 3, 6, and 7.
With the monosaccharide building blocks in hand, the galactosamine donor 6 was coupled with galactose acceptor 7 by activation of the thioglycoside using N-iodosuccinimide (NIS) in the presence of TMSOTf to afford the desired disaccharide β-ᴅ-GalpNHTroc-(1→4)-α-ᴅ-Galp (4) in 85% yield, as a single isomer (Scheme 3). Then, the chloroacetyl group was selectively removed using thiourea and 2,4,6-collidine [47] to afford the 3’-OH acceptor 5 in 87% yield. Finally, NIS/TMSOTf-promoted coupling of donor 3 with disaccharide acceptor 5 provided the desired β-linked trisaccharide 2 in 89% yield (Scheme 3).
Scheme 3: Linear synthesis of trisaccharide derivative 2.
Scheme 3: Linear synthesis of trisaccharide derivative 2.
The linear synthesis of oligosaccharides is associated with several disadvantages such as multiple steps involving multiple work-up procedures, purifications requiring long time, manpower and the resulting high cost of synthesis and production. Consequently one-pot strategies have recently attracted a lot of attention and several methodologies have been developed as a potential solution that can reduce cost via bringing down solvent and time consumption [48-50]. In view of this, we next attempted the one-pot synthesis of the trisaccharide derivative 2 via a [1 + 1 + 1] approach. Initial studies using the thioglycoside donor 3 were not very fruitful, affording a complex mixture of products. Therefore, we modified our strategy to include its trichloroacetimidate derivative as the donor. The thioglycoside 3 was converted to the anomeric hydroxide using trichloroisocyanuric acid (TCCA) in aqueous acetone [51] resulting in compound 11. Treatment of compound 11 with trichloroacetonitrile and DBU in dry DCM resulted in the formation of the desired trichloroacetimidate donor 12 in 93% yield (Scheme 4).
Scheme 4: Synthesis of ᴅ-galactose donor 12.
Scheme 4: Synthesis of ᴅ-galactose donor 12.
The first stage of the one-pot synthesis was carried out using donor 12 and acceptor 9 in the presence of TMSOTf as promoter (Scheme 5). After 1 h of reaction, TLC monitoring indicated the full consumption of the starting materials. Analysis of a small aliquot of the reaction mixture by HRMS confirmed the formation of the desired disaccharide. Then, to the same pot, the second monosaccharide acceptor 7 was added, followed by the addition of NIS and TMSOTf. After 15 min of reaction, TLC monitoring showed complete consumption of the donor. Work-up of the reaction mixture followed by chromatographic purification afforded the pure trisaccharide 2 as a single isomer in an overall yield of 73%. The structure of the trisaccharide was confirmed by comparison of its NMR and HRMS spectral data with that of the previously synthesized product by the linear strategy.
Scheme 5: One-pot synthesis of trisaccharide derivative 2.
Scheme 5: One-pot synthesis of trisaccharide derivative 2.
With the protected trisaccharide 2 in hand, it remained to carry out the N-acetylation and the removal of the protecting groups on the hydroxy groups. First, the concomitant removal of the Troc group and the N-acetylation was achieved using Zn/AcOH/Ac2O 3:2:1 as reagent in one pot (Scheme 6) [52]. Then, O-deacetylation was accomplished by using a catalytic amount of NaOMe in MeOH at room temperature. Finally, the benzylidene and the benzyl groups were removed by hydrogenolysis using 10% Pd(OH)2/C in methanol at ambient temperature with a H2 balloon, which afforded the target trisaccharide 1 in 68% yield over three steps (Scheme 6). The structure of 1 was confirmed by several NMR spectroscopic techniques such as 1H NMR, DEPT-135, 13C NMR, COSY, and HSQC as well as mass spectrometry using HRMS. The NMR data were found to correlate well with the data reported for the natural polysaccharide [38].
Scheme 6: Synthesis of trisaccharide derivative 1.
Scheme 6: Synthesis of trisaccharide derivative 1.
Conclusion
In conclusion, the trisaccharide repeating unit of the O-polysaccharide of Providencia stuartii O49 in its p-methoxyphenyl glycoside form 1 was synthesized through a linear [1 + (1 + 1 = 2)] strategy. The target trisaccharide was synthesized as its p-methoxyphenyl glycoside that offered the unaltered stereochemistry of the sugar at the reducing end to mimic the glycosidic linkage of the natural polysaccharide. The target protected trisaccharide was also synthesized through a [1 + 1 + 1] one-pot strategy involving sequential glycosylations from the reducing end to the non-reducing end. The one-pot synthesis provided the final trisaccharide in an overall yield of 73% compared to the overall yield of 66% from the two step synthesis, though the former involved two extra steps for the synthesis of the first glycosidic donor and one chromatographic separation. The synthesis of the desired product was achieved through manipulations of the appropriate protecting group on the monosaccharides and subsequent realization of stereoselective glycosylations. The work provides an access to the trisaccharide repeating unit of the O-polysaccharide of Providencia stuartii O49 with the stereospecific α-p-methoxyphenyl glycoside.
References
-
Wertz, D. B.; Vidal, S. Modern Synthetic Methods in Carbohydrate Chemistry; Wiley-VCH: Weinheim, Germany, 2014. doi:10.1002/9783527658947
Return to citation in text: [1] [2] -
Oberli, M. A.; Tamborrini, M.; Tsai, Y.-H.; Werz, D. B.; Horlacher, T.; Adibekian, A.; Gauss, D.; Möller, H. M.; Pluschke, G.; Seeberger, P. H. J. Am. Chem. Soc. 2010, 132, 10239–10241. doi:10.1021/ja104027w
Return to citation in text: [1] [2] -
Micoli, F.; Del Bino, L.; Alfini, R.; Carboni, F.; Romano, M. R.; Adamo, R. Expert Rev. Vaccines 2019, 18, 881–895. doi:10.1080/14760584.2019.1657012
Return to citation in text: [1] [2] -
Mettu, R.; Chen, C.-Y.; Wu, C.-Y. J. Biomed. Sci. (London, U. K.) 2020, 27, No. 9. doi:10.1186/s12929-019-0591-0
Return to citation in text: [1] [2] -
Costantino, P.; Rappuoli, R.; Berti, F. Expert Opin. Drug Discovery 2011, 6, 1045–1066. doi:10.1517/17460441.2011.609554
Return to citation in text: [1] [2] -
Vella, M.; Pace, D. Expert Opin. Biol. Ther. 2015, 15, 529–546. doi:10.1517/14712598.2015.993375
Return to citation in text: [1] [2] -
Méndez, Y.; Chang, J.; Humpierre, A. R.; Zanuy, A.; Garrido, R.; Vasco, A. V.; Pedroso, J.; Santana, D.; Rodríguez, L. M.; García-Rivera, D.; Valdés, Y.; Vérez-Bencomo, V.; Rivera, D. G. Chem. Sci. 2018, 9, 2581–2588. doi:10.1039/c7sc05467j
Return to citation in text: [1] [2] -
Adamo, R. Acc. Chem. Res. 2017, 50, 1270–1279. doi:10.1021/acs.accounts.7b00106
Return to citation in text: [1] [2] -
Hu, Z.; Benkoulouche, M.; Barel, L.-A.; Le Heiget, G.; Ben Imeddourene, A.; Le Guen, Y.; Monties, N.; Guerreiro, C.; Remaud-Siméon, M.; Moulis, C.; André, I.; Mulard, L. A. J. Org. Chem. 2021, 86, 2058–2075. doi:10.1021/acs.joc.0c00777
Return to citation in text: [1] [2] -
Nishi, N.; Seki, K.; Takahashi, D.; Toshima, K. Angew. Chem., Int. Ed. 2021, 60, 1789–1796. doi:10.1002/anie.202013729
Return to citation in text: [1] [2] -
Domínguez-Medina, C. C.; Pérez-Toledo, M.; Schager, A. E.; Marshall, J. L.; Cook, C. N.; Bobat, S.; Hwang, H.; Chun, B. J.; Logan, E.; Bryant, J. A.; Channell, W. M.; Morris, F. C.; Jossi, S. E.; Alshayea, A.; Rossiter, A. E.; Barrow, P. A.; Horsnell, W. G.; MacLennan, C. A.; Henderson, I. R.; Lakey, J. H.; Gumbart, J. C.; López-Macías, C.; Bavro, V. N.; Cunningham, A. F. Nat. Commun. 2020, 11, 851. doi:10.1038/s41467-020-14655-9
Return to citation in text: [1] [2] -
Tian, G.; Hu, J.; Qin, C.; Li, L.; Zou, X.; Cai, J.; Seeberger, P. H.; Yin, J. Angew. Chem., Int. Ed. 2020, 59, 13362–13370. doi:10.1002/anie.202004267
Return to citation in text: [1] [2] -
Tian, G.; Qin, C.; Liu, Z.; Shen, D.; Zou, X.; Fu, J.; Hu, J.; Seeberger, P. H.; Yin, J. Chem. Commun. 2020, 56, 344–347. doi:10.1039/c9cc07915g
Return to citation in text: [1] [2] -
Behera, A.; Rai, D.; Kulkarni, S. S. J. Am. Chem. Soc. 2020, 142, 456–467. doi:10.1021/jacs.9b11309
Return to citation in text: [1] [2] -
Huo, C.-X.; Dhara, D.; Baliban, S. M.; Tahmasebi Nick, S.; Tan, Z.; Simon, R.; Misra, A. K.; Huang, X. Chem. Commun. 2019, 55, 4519–4522. doi:10.1039/c8cc08622b
Return to citation in text: [1] [2] -
Cloutier, M.; Delar, E.; Muru, K.; Ndong, S.; Hoyeck, R. R.; Kaewarpai, T.; Chantratita, N.; Burtnick, M. N.; Brett, P. J.; Gauthier, C. Org. Biomol. Chem. 2019, 17, 8878–8901. doi:10.1039/c9ob01711a
Return to citation in text: [1] [2] -
Pfister, H. B.; Kelly, M.; Qadri, F.; Ryan, E. T.; Kováč, P. Org. Biomol. Chem. 2019, 17, 4049–4060. doi:10.1039/c9ob00368a
Return to citation in text: [1] [2] -
Qin, C.; Schumann, B.; Zou, X.; Pereira, C. L.; Tian, G.; Hu, J.; Seeberger, P. H.; Yin, J. J. Am. Chem. Soc. 2018, 140, 3120–3127. doi:10.1021/jacs.8b00148
Return to citation in text: [1] [2] -
Pennini, M. E.; De Marco, A.; Pelletier, M.; Bonnell, J.; Cvitkovic, R.; Beltramello, M.; Cameroni, E.; Bianchi, S.; Zatta, F.; Zhao, W.; Xiao, X.; Camara, M. M.; DiGiandomenico, A.; Semenova, E.; Lanzavecchia, A.; Warrener, P.; Suzich, J.; Wang, Q.; Corti, D.; Stover, C. K. Nat. Commun. 2017, 8, 1991. doi:10.1038/s41467-017-02223-7
Return to citation in text: [1] [2] -
Grayson, E. J.; Bernardes, G. J. L.; Chalker, J. M.; Boutureira, O.; Koeppe, J. R.; Davis, B. G. Angew. Chem., Int. Ed. 2011, 50, 4127–4132. doi:10.1002/anie.201006327
Return to citation in text: [1] [2] -
Sundgren, A.; Lahmann, M.; Oscarson, S. Beilstein J. Org. Chem. 2010, 6, 704–708. doi:10.3762/bjoc.6.80
Return to citation in text: [1] [2] -
O'Hara, C. M.; Brenner, F. W.; Miller, J. M. Clin. Microbiol. Rev. 2000, 13, 534–546. doi:10.1128/cmr.13.4.534
Return to citation in text: [1] [2] [3] -
Ovchinnikova, O. G.; Rozalski, A.; Liu, B.; Knirel, Y. A. Biochemistry 2013, 78, 798–817. doi:10.1134/s0006297913070110
Return to citation in text: [1] -
Penner, J. L.; Hinton, N. A.; Duncan, I. B.; Hennessy, J. N.; Whiteley, G. R. J. Clin. Microbiol. 1979, 9, 11–14. doi:10.1128/jcm.9.1.11-14.1979
Return to citation in text: [1] -
Warren, J. W. Rev. Infect. Dis. 1986, 8, 61–67. doi:10.1093/clinids/8.1.61
Return to citation in text: [1] [2] -
Kocharova, N. A.; Torzewska, A.; Zatonsky, G. V.; Błaszczyk, A.; Bystrova, O. V.; Shashkov, A. S.; Knirel, Y. A.; Rozalski, A. Carbohydr. Res. 2004, 339, 195–200. doi:10.1016/j.carres.2003.10.017
Return to citation in text: [1] -
Kocharova, N. A.; Błaszczyk, A.; Zatonsky, G. V.; Torzewska, A.; Bystrova, O. V.; Shashkov, A. S.; Knirel, Y. A.; Rozalski, A. Carbohydr. Res. 2004, 339, 409–413. doi:10.1016/j.carres.2003.10.022
Return to citation in text: [1] -
Shashkov, A. S.; Kocharova, N. A.; Zatonsky, G. V.; Błaszczyk, A.; Knirel, Y. A.; Rozalski, A. Carbohydr. Res. 2007, 342, 653–658. doi:10.1016/j.carres.2006.08.005
Return to citation in text: [1] -
Torzewska, A.; Kocharova, N. A.; Zatonsky, G. V.; Blaszczyk, A.; Bystrova, O. V.; Shashkov, A. S.; Knirel, Y. A.; Rozalski, A. FEMS Immunol. Med. Microbiol. 2004, 41, 133–139. doi:10.1016/j.femsim.2004.02.007
Return to citation in text: [1] -
Ovchinnikova, O. G.; Kocharova, N. A.; Torzewska, A.; Blaszczyk, A.; Shashkov, A. S.; Knirel, Y. A.; Rozalski, A. Carbohydr. Res. 2005, 340, 1407–1411. doi:10.1016/j.carres.2005.03.011
Return to citation in text: [1] -
Kocharova, N. A.; Ovchinnikova, O. G.; Toukach, F. V.; Torzewska, A.; Shashkov, A. S.; Knirel, Y. A.; Rozalski, A. Carbohydr. Res. 2005, 340, 1419–1423. doi:10.1016/j.carres.2005.02.020
Return to citation in text: [1] -
Ovchinnikova, O. G.; Kocharova, N. A.; Bakinovskiy, L. V.; Torzewska, A.; Shashkov, A. S.; Knirel, Y. A.; Rozalski, A. Carbohydr. Res. 2004, 339, 2621–2626. doi:10.1016/j.carres.2004.08.011
Return to citation in text: [1] -
Kocharova, N. A.; Ovchinnikova, O. G.; Bushmarinov, I. S.; Toukach, F. V.; Torzewska, A.; Shashkov, A. S.; Knirel, Y. A.; Rozalski, A. Carbohydr. Res. 2005, 340, 775–780. doi:10.1016/j.carres.2005.01.007
Return to citation in text: [1] -
Verma, P. R.; Mukhopadhyay, B. RSC Adv. 2013, 3, 201–207. doi:10.1039/c2ra22407k
Return to citation in text: [1] -
Mandal, P. K.; Chheda, P. R. Tetrahedron Lett. 2015, 56, 900–902. doi:10.1016/j.tetlet.2014.12.143
Return to citation in text: [1] -
Podilapu, A. R.; Kulkarni, S. S. Org. Lett. 2017, 19, 5466–5469. doi:10.1021/acs.orglett.7b02791
Return to citation in text: [1] [2] -
Ahadi, S.; Awan, S. I.; Werz, D. B. Chem. – Eur. J. 2020, 26, 6264–6270. doi:10.1002/chem.202000496
Return to citation in text: [1] -
Bushmarinov, I. S.; Ovchinnikova, O. G.; Kocharova, N. A.; Blaszczyk, A.; Toukach, F. V.; Torzewska, A.; Shashkov, A. S.; Knirel, Y. A.; Rozalski, A. Carbohydr. Res. 2004, 339, 1557–1560. doi:10.1016/j.carres.2004.03.021
Return to citation in text: [1] [2] -
Zhang, P.; Wang, K.; Zhang, J.; Li, C.; Guan, H. Eur. J. Org. Chem. 2015, 570–583. doi:10.1002/ejoc.201403296
Return to citation in text: [1] -
Solera, C.; Macchione, G.; Maza, S.; Kayser, M. M.; Corzana, F.; de Paz, J. L.; Nieto, P. M. Chem. – Eur. J. 2016, 22, 2356–2369. doi:10.1002/chem.201504440
Return to citation in text: [1] -
Macchione, G.; Maza, S.; Mar Kayser, M.; de Paz, J. L.; Nieto, P. M. Eur. J. Org. Chem. 2014, 3868–3884. doi:10.1002/ejoc.201402222
Return to citation in text: [1] -
Schwörer, R.; Zubkova, O. V.; Turnbull, J. E.; Tyler, P. C. Chem. – Eur. J. 2013, 19, 6817–6823. doi:10.1002/chem.201204519
Return to citation in text: [1] -
Mukherjee, M. M.; Basu, N.; Nandi, S.; Ghosh, R. Carbohydr. Res. 2019, 476, 36–43. doi:10.1016/j.carres.2019.03.002
Return to citation in text: [1] -
Sun, B.; Yang, B.; Huang, X. Sci. China: Chem. 2012, 55, 31–35. doi:10.1007/s11426-011-4449-x
Return to citation in text: [1] -
Wu, X.; McFall-Boegeman, H.; Rashidijahanabad, Z.; Liu, K.; Pett, C.; Yu, J.; Schorlemer, M.; Ramadan, S.; Behren, S.; Westerlind, U.; Huang, X. Org. Biomol. Chem. 2021, 19, 2448–2455. doi:10.1039/d1ob00007a
Return to citation in text: [1] -
Emmadi, M.; Kulkarni, S. S. Org. Biomol. Chem. 2013, 11, 3098–3102. doi:10.1039/c3ob40615f
Return to citation in text: [1] -
Pal, K. B.; Mukhopadhyay, B. ChemistrySelect 2017, 2, 7378–7381. doi:10.1002/slct.201701082
Return to citation in text: [1] -
Wang, C.-C.; Lee, J.-C.; Luo, S.-Y.; Kulkarni, S. S.; Huang, Y.-W.; Lee, C.-C.; Chang, K.-L.; Hung, S.-C. Nature 2007, 446, 896–899. doi:10.1038/nature05730
Return to citation in text: [1] -
Kulkarni, S. S.; Wang, C.-C.; Sabbavarapu, N. M.; Podilapu, A. R.; Liao, P.-H.; Hung, S.-C. Chem. Rev. 2018, 118, 8025–8104. doi:10.1021/acs.chemrev.8b00036
Return to citation in text: [1] -
Huang, T.-Y.; Zulueta, M. M. L.; Hung, S.-C. Org. Biomol. Chem. 2014, 12, 376–382. doi:10.1039/c3ob42097c
Return to citation in text: [1] -
Basu, N.; Maity, S. K.; Chaudhury, A.; Ghosh, R. Carbohydr. Res. 2013, 369, 10–13. doi:10.1016/j.carres.2013.01.001
Return to citation in text: [1] -
Ghosh, S.; Nishat, S.; Andreana, P. R. J. Org. Chem. 2016, 81, 4475–4484. doi:10.1021/acs.joc.6b00195
Return to citation in text: [1]
| 51. | Basu, N.; Maity, S. K.; Chaudhury, A.; Ghosh, R. Carbohydr. Res. 2013, 369, 10–13. doi:10.1016/j.carres.2013.01.001 |
| 52. | Ghosh, S.; Nishat, S.; Andreana, P. R. J. Org. Chem. 2016, 81, 4475–4484. doi:10.1021/acs.joc.6b00195 |
| 38. | Bushmarinov, I. S.; Ovchinnikova, O. G.; Kocharova, N. A.; Blaszczyk, A.; Toukach, F. V.; Torzewska, A.; Shashkov, A. S.; Knirel, Y. A.; Rozalski, A. Carbohydr. Res. 2004, 339, 1557–1560. doi:10.1016/j.carres.2004.03.021 |
| 1. | Wertz, D. B.; Vidal, S. Modern Synthetic Methods in Carbohydrate Chemistry; Wiley-VCH: Weinheim, Germany, 2014. doi:10.1002/9783527658947 |
| 2. | Oberli, M. A.; Tamborrini, M.; Tsai, Y.-H.; Werz, D. B.; Horlacher, T.; Adibekian, A.; Gauss, D.; Möller, H. M.; Pluschke, G.; Seeberger, P. H. J. Am. Chem. Soc. 2010, 132, 10239–10241. doi:10.1021/ja104027w |
| 3. | Micoli, F.; Del Bino, L.; Alfini, R.; Carboni, F.; Romano, M. R.; Adamo, R. Expert Rev. Vaccines 2019, 18, 881–895. doi:10.1080/14760584.2019.1657012 |
| 4. | Mettu, R.; Chen, C.-Y.; Wu, C.-Y. J. Biomed. Sci. (London, U. K.) 2020, 27, No. 9. doi:10.1186/s12929-019-0591-0 |
| 5. | Costantino, P.; Rappuoli, R.; Berti, F. Expert Opin. Drug Discovery 2011, 6, 1045–1066. doi:10.1517/17460441.2011.609554 |
| 6. | Vella, M.; Pace, D. Expert Opin. Biol. Ther. 2015, 15, 529–546. doi:10.1517/14712598.2015.993375 |
| 7. | Méndez, Y.; Chang, J.; Humpierre, A. R.; Zanuy, A.; Garrido, R.; Vasco, A. V.; Pedroso, J.; Santana, D.; Rodríguez, L. M.; García-Rivera, D.; Valdés, Y.; Vérez-Bencomo, V.; Rivera, D. G. Chem. Sci. 2018, 9, 2581–2588. doi:10.1039/c7sc05467j |
| 8. | Adamo, R. Acc. Chem. Res. 2017, 50, 1270–1279. doi:10.1021/acs.accounts.7b00106 |
| 22. | O'Hara, C. M.; Brenner, F. W.; Miller, J. M. Clin. Microbiol. Rev. 2000, 13, 534–546. doi:10.1128/cmr.13.4.534 |
| 23. | Ovchinnikova, O. G.; Rozalski, A.; Liu, B.; Knirel, Y. A. Biochemistry 2013, 78, 798–817. doi:10.1134/s0006297913070110 |
| 24. | Penner, J. L.; Hinton, N. A.; Duncan, I. B.; Hennessy, J. N.; Whiteley, G. R. J. Clin. Microbiol. 1979, 9, 11–14. doi:10.1128/jcm.9.1.11-14.1979 |
| 33. | Kocharova, N. A.; Ovchinnikova, O. G.; Bushmarinov, I. S.; Toukach, F. V.; Torzewska, A.; Shashkov, A. S.; Knirel, Y. A.; Rozalski, A. Carbohydr. Res. 2005, 340, 775–780. doi:10.1016/j.carres.2005.01.007 |
| 22. | O'Hara, C. M.; Brenner, F. W.; Miller, J. M. Clin. Microbiol. Rev. 2000, 13, 534–546. doi:10.1128/cmr.13.4.534 |
| 34. | Verma, P. R.; Mukhopadhyay, B. RSC Adv. 2013, 3, 201–207. doi:10.1039/c2ra22407k |
| 1. | Wertz, D. B.; Vidal, S. Modern Synthetic Methods in Carbohydrate Chemistry; Wiley-VCH: Weinheim, Germany, 2014. doi:10.1002/9783527658947 |
| 2. | Oberli, M. A.; Tamborrini, M.; Tsai, Y.-H.; Werz, D. B.; Horlacher, T.; Adibekian, A.; Gauss, D.; Möller, H. M.; Pluschke, G.; Seeberger, P. H. J. Am. Chem. Soc. 2010, 132, 10239–10241. doi:10.1021/ja104027w |
| 3. | Micoli, F.; Del Bino, L.; Alfini, R.; Carboni, F.; Romano, M. R.; Adamo, R. Expert Rev. Vaccines 2019, 18, 881–895. doi:10.1080/14760584.2019.1657012 |
| 4. | Mettu, R.; Chen, C.-Y.; Wu, C.-Y. J. Biomed. Sci. (London, U. K.) 2020, 27, No. 9. doi:10.1186/s12929-019-0591-0 |
| 5. | Costantino, P.; Rappuoli, R.; Berti, F. Expert Opin. Drug Discovery 2011, 6, 1045–1066. doi:10.1517/17460441.2011.609554 |
| 6. | Vella, M.; Pace, D. Expert Opin. Biol. Ther. 2015, 15, 529–546. doi:10.1517/14712598.2015.993375 |
| 7. | Méndez, Y.; Chang, J.; Humpierre, A. R.; Zanuy, A.; Garrido, R.; Vasco, A. V.; Pedroso, J.; Santana, D.; Rodríguez, L. M.; García-Rivera, D.; Valdés, Y.; Vérez-Bencomo, V.; Rivera, D. G. Chem. Sci. 2018, 9, 2581–2588. doi:10.1039/c7sc05467j |
| 8. | Adamo, R. Acc. Chem. Res. 2017, 50, 1270–1279. doi:10.1021/acs.accounts.7b00106 |
| 9. | Hu, Z.; Benkoulouche, M.; Barel, L.-A.; Le Heiget, G.; Ben Imeddourene, A.; Le Guen, Y.; Monties, N.; Guerreiro, C.; Remaud-Siméon, M.; Moulis, C.; André, I.; Mulard, L. A. J. Org. Chem. 2021, 86, 2058–2075. doi:10.1021/acs.joc.0c00777 |
| 10. | Nishi, N.; Seki, K.; Takahashi, D.; Toshima, K. Angew. Chem., Int. Ed. 2021, 60, 1789–1796. doi:10.1002/anie.202013729 |
| 11. | Domínguez-Medina, C. C.; Pérez-Toledo, M.; Schager, A. E.; Marshall, J. L.; Cook, C. N.; Bobat, S.; Hwang, H.; Chun, B. J.; Logan, E.; Bryant, J. A.; Channell, W. M.; Morris, F. C.; Jossi, S. E.; Alshayea, A.; Rossiter, A. E.; Barrow, P. A.; Horsnell, W. G.; MacLennan, C. A.; Henderson, I. R.; Lakey, J. H.; Gumbart, J. C.; López-Macías, C.; Bavro, V. N.; Cunningham, A. F. Nat. Commun. 2020, 11, 851. doi:10.1038/s41467-020-14655-9 |
| 12. | Tian, G.; Hu, J.; Qin, C.; Li, L.; Zou, X.; Cai, J.; Seeberger, P. H.; Yin, J. Angew. Chem., Int. Ed. 2020, 59, 13362–13370. doi:10.1002/anie.202004267 |
| 13. | Tian, G.; Qin, C.; Liu, Z.; Shen, D.; Zou, X.; Fu, J.; Hu, J.; Seeberger, P. H.; Yin, J. Chem. Commun. 2020, 56, 344–347. doi:10.1039/c9cc07915g |
| 14. | Behera, A.; Rai, D.; Kulkarni, S. S. J. Am. Chem. Soc. 2020, 142, 456–467. doi:10.1021/jacs.9b11309 |
| 15. | Huo, C.-X.; Dhara, D.; Baliban, S. M.; Tahmasebi Nick, S.; Tan, Z.; Simon, R.; Misra, A. K.; Huang, X. Chem. Commun. 2019, 55, 4519–4522. doi:10.1039/c8cc08622b |
| 16. | Cloutier, M.; Delar, E.; Muru, K.; Ndong, S.; Hoyeck, R. R.; Kaewarpai, T.; Chantratita, N.; Burtnick, M. N.; Brett, P. J.; Gauthier, C. Org. Biomol. Chem. 2019, 17, 8878–8901. doi:10.1039/c9ob01711a |
| 17. | Pfister, H. B.; Kelly, M.; Qadri, F.; Ryan, E. T.; Kováč, P. Org. Biomol. Chem. 2019, 17, 4049–4060. doi:10.1039/c9ob00368a |
| 18. | Qin, C.; Schumann, B.; Zou, X.; Pereira, C. L.; Tian, G.; Hu, J.; Seeberger, P. H.; Yin, J. J. Am. Chem. Soc. 2018, 140, 3120–3127. doi:10.1021/jacs.8b00148 |
| 19. | Pennini, M. E.; De Marco, A.; Pelletier, M.; Bonnell, J.; Cvitkovic, R.; Beltramello, M.; Cameroni, E.; Bianchi, S.; Zatta, F.; Zhao, W.; Xiao, X.; Camara, M. M.; DiGiandomenico, A.; Semenova, E.; Lanzavecchia, A.; Warrener, P.; Suzich, J.; Wang, Q.; Corti, D.; Stover, C. K. Nat. Commun. 2017, 8, 1991. doi:10.1038/s41467-017-02223-7 |
| 20. | Grayson, E. J.; Bernardes, G. J. L.; Chalker, J. M.; Boutureira, O.; Koeppe, J. R.; Davis, B. G. Angew. Chem., Int. Ed. 2011, 50, 4127–4132. doi:10.1002/anie.201006327 |
| 21. | Sundgren, A.; Lahmann, M.; Oscarson, S. Beilstein J. Org. Chem. 2010, 6, 704–708. doi:10.3762/bjoc.6.80 |
| 31. | Kocharova, N. A.; Ovchinnikova, O. G.; Toukach, F. V.; Torzewska, A.; Shashkov, A. S.; Knirel, Y. A.; Rozalski, A. Carbohydr. Res. 2005, 340, 1419–1423. doi:10.1016/j.carres.2005.02.020 |
| 9. | Hu, Z.; Benkoulouche, M.; Barel, L.-A.; Le Heiget, G.; Ben Imeddourene, A.; Le Guen, Y.; Monties, N.; Guerreiro, C.; Remaud-Siméon, M.; Moulis, C.; André, I.; Mulard, L. A. J. Org. Chem. 2021, 86, 2058–2075. doi:10.1021/acs.joc.0c00777 |
| 10. | Nishi, N.; Seki, K.; Takahashi, D.; Toshima, K. Angew. Chem., Int. Ed. 2021, 60, 1789–1796. doi:10.1002/anie.202013729 |
| 11. | Domínguez-Medina, C. C.; Pérez-Toledo, M.; Schager, A. E.; Marshall, J. L.; Cook, C. N.; Bobat, S.; Hwang, H.; Chun, B. J.; Logan, E.; Bryant, J. A.; Channell, W. M.; Morris, F. C.; Jossi, S. E.; Alshayea, A.; Rossiter, A. E.; Barrow, P. A.; Horsnell, W. G.; MacLennan, C. A.; Henderson, I. R.; Lakey, J. H.; Gumbart, J. C.; López-Macías, C.; Bavro, V. N.; Cunningham, A. F. Nat. Commun. 2020, 11, 851. doi:10.1038/s41467-020-14655-9 |
| 12. | Tian, G.; Hu, J.; Qin, C.; Li, L.; Zou, X.; Cai, J.; Seeberger, P. H.; Yin, J. Angew. Chem., Int. Ed. 2020, 59, 13362–13370. doi:10.1002/anie.202004267 |
| 13. | Tian, G.; Qin, C.; Liu, Z.; Shen, D.; Zou, X.; Fu, J.; Hu, J.; Seeberger, P. H.; Yin, J. Chem. Commun. 2020, 56, 344–347. doi:10.1039/c9cc07915g |
| 14. | Behera, A.; Rai, D.; Kulkarni, S. S. J. Am. Chem. Soc. 2020, 142, 456–467. doi:10.1021/jacs.9b11309 |
| 15. | Huo, C.-X.; Dhara, D.; Baliban, S. M.; Tahmasebi Nick, S.; Tan, Z.; Simon, R.; Misra, A. K.; Huang, X. Chem. Commun. 2019, 55, 4519–4522. doi:10.1039/c8cc08622b |
| 16. | Cloutier, M.; Delar, E.; Muru, K.; Ndong, S.; Hoyeck, R. R.; Kaewarpai, T.; Chantratita, N.; Burtnick, M. N.; Brett, P. J.; Gauthier, C. Org. Biomol. Chem. 2019, 17, 8878–8901. doi:10.1039/c9ob01711a |
| 17. | Pfister, H. B.; Kelly, M.; Qadri, F.; Ryan, E. T.; Kováč, P. Org. Biomol. Chem. 2019, 17, 4049–4060. doi:10.1039/c9ob00368a |
| 18. | Qin, C.; Schumann, B.; Zou, X.; Pereira, C. L.; Tian, G.; Hu, J.; Seeberger, P. H.; Yin, J. J. Am. Chem. Soc. 2018, 140, 3120–3127. doi:10.1021/jacs.8b00148 |
| 19. | Pennini, M. E.; De Marco, A.; Pelletier, M.; Bonnell, J.; Cvitkovic, R.; Beltramello, M.; Cameroni, E.; Bianchi, S.; Zatta, F.; Zhao, W.; Xiao, X.; Camara, M. M.; DiGiandomenico, A.; Semenova, E.; Lanzavecchia, A.; Warrener, P.; Suzich, J.; Wang, Q.; Corti, D.; Stover, C. K. Nat. Commun. 2017, 8, 1991. doi:10.1038/s41467-017-02223-7 |
| 20. | Grayson, E. J.; Bernardes, G. J. L.; Chalker, J. M.; Boutureira, O.; Koeppe, J. R.; Davis, B. G. Angew. Chem., Int. Ed. 2011, 50, 4127–4132. doi:10.1002/anie.201006327 |
| 21. | Sundgren, A.; Lahmann, M.; Oscarson, S. Beilstein J. Org. Chem. 2010, 6, 704–708. doi:10.3762/bjoc.6.80 |
| 32. | Ovchinnikova, O. G.; Kocharova, N. A.; Bakinovskiy, L. V.; Torzewska, A.; Shashkov, A. S.; Knirel, Y. A.; Rozalski, A. Carbohydr. Res. 2004, 339, 2621–2626. doi:10.1016/j.carres.2004.08.011 |
| 27. | Kocharova, N. A.; Błaszczyk, A.; Zatonsky, G. V.; Torzewska, A.; Bystrova, O. V.; Shashkov, A. S.; Knirel, Y. A.; Rozalski, A. Carbohydr. Res. 2004, 339, 409–413. doi:10.1016/j.carres.2003.10.022 |
| 29. | Torzewska, A.; Kocharova, N. A.; Zatonsky, G. V.; Blaszczyk, A.; Bystrova, O. V.; Shashkov, A. S.; Knirel, Y. A.; Rozalski, A. FEMS Immunol. Med. Microbiol. 2004, 41, 133–139. doi:10.1016/j.femsim.2004.02.007 |
| 26. | Kocharova, N. A.; Torzewska, A.; Zatonsky, G. V.; Błaszczyk, A.; Bystrova, O. V.; Shashkov, A. S.; Knirel, Y. A.; Rozalski, A. Carbohydr. Res. 2004, 339, 195–200. doi:10.1016/j.carres.2003.10.017 |
| 30. | Ovchinnikova, O. G.; Kocharova, N. A.; Torzewska, A.; Blaszczyk, A.; Shashkov, A. S.; Knirel, Y. A.; Rozalski, A. Carbohydr. Res. 2005, 340, 1407–1411. doi:10.1016/j.carres.2005.03.011 |
| 22. | O'Hara, C. M.; Brenner, F. W.; Miller, J. M. Clin. Microbiol. Rev. 2000, 13, 534–546. doi:10.1128/cmr.13.4.534 |
| 25. | Warren, J. W. Rev. Infect. Dis. 1986, 8, 61–67. doi:10.1093/clinids/8.1.61 |
| 28. | Shashkov, A. S.; Kocharova, N. A.; Zatonsky, G. V.; Błaszczyk, A.; Knirel, Y. A.; Rozalski, A. Carbohydr. Res. 2007, 342, 653–658. doi:10.1016/j.carres.2006.08.005 |
| 37. | Ahadi, S.; Awan, S. I.; Werz, D. B. Chem. – Eur. J. 2020, 26, 6264–6270. doi:10.1002/chem.202000496 |
| 35. | Mandal, P. K.; Chheda, P. R. Tetrahedron Lett. 2015, 56, 900–902. doi:10.1016/j.tetlet.2014.12.143 |
| 36. | Podilapu, A. R.; Kulkarni, S. S. Org. Lett. 2017, 19, 5466–5469. doi:10.1021/acs.orglett.7b02791 |
| 47. | Pal, K. B.; Mukhopadhyay, B. ChemistrySelect 2017, 2, 7378–7381. doi:10.1002/slct.201701082 |
| 48. | Wang, C.-C.; Lee, J.-C.; Luo, S.-Y.; Kulkarni, S. S.; Huang, Y.-W.; Lee, C.-C.; Chang, K.-L.; Hung, S.-C. Nature 2007, 446, 896–899. doi:10.1038/nature05730 |
| 49. | Kulkarni, S. S.; Wang, C.-C.; Sabbavarapu, N. M.; Podilapu, A. R.; Liao, P.-H.; Hung, S.-C. Chem. Rev. 2018, 118, 8025–8104. doi:10.1021/acs.chemrev.8b00036 |
| 50. | Huang, T.-Y.; Zulueta, M. M. L.; Hung, S.-C. Org. Biomol. Chem. 2014, 12, 376–382. doi:10.1039/c3ob42097c |
| 44. | Sun, B.; Yang, B.; Huang, X. Sci. China: Chem. 2012, 55, 31–35. doi:10.1007/s11426-011-4449-x |
| 45. | Wu, X.; McFall-Boegeman, H.; Rashidijahanabad, Z.; Liu, K.; Pett, C.; Yu, J.; Schorlemer, M.; Ramadan, S.; Behren, S.; Westerlind, U.; Huang, X. Org. Biomol. Chem. 2021, 19, 2448–2455. doi:10.1039/d1ob00007a |
| 46. | Emmadi, M.; Kulkarni, S. S. Org. Biomol. Chem. 2013, 11, 3098–3102. doi:10.1039/c3ob40615f |
| 43. | Mukherjee, M. M.; Basu, N.; Nandi, S.; Ghosh, R. Carbohydr. Res. 2019, 476, 36–43. doi:10.1016/j.carres.2019.03.002 |
| 36. | Podilapu, A. R.; Kulkarni, S. S. Org. Lett. 2017, 19, 5466–5469. doi:10.1021/acs.orglett.7b02791 |
| 38. | Bushmarinov, I. S.; Ovchinnikova, O. G.; Kocharova, N. A.; Blaszczyk, A.; Toukach, F. V.; Torzewska, A.; Shashkov, A. S.; Knirel, Y. A.; Rozalski, A. Carbohydr. Res. 2004, 339, 1557–1560. doi:10.1016/j.carres.2004.03.021 |
| 39. | Zhang, P.; Wang, K.; Zhang, J.; Li, C.; Guan, H. Eur. J. Org. Chem. 2015, 570–583. doi:10.1002/ejoc.201403296 |
| 40. | Solera, C.; Macchione, G.; Maza, S.; Kayser, M. M.; Corzana, F.; de Paz, J. L.; Nieto, P. M. Chem. – Eur. J. 2016, 22, 2356–2369. doi:10.1002/chem.201504440 |
| 41. | Macchione, G.; Maza, S.; Mar Kayser, M.; de Paz, J. L.; Nieto, P. M. Eur. J. Org. Chem. 2014, 3868–3884. doi:10.1002/ejoc.201402222 |
| 42. | Schwörer, R.; Zubkova, O. V.; Turnbull, J. E.; Tyler, P. C. Chem. – Eur. J. 2013, 19, 6817–6823. doi:10.1002/chem.201204519 |
© 2021 Halder and Yadav; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.