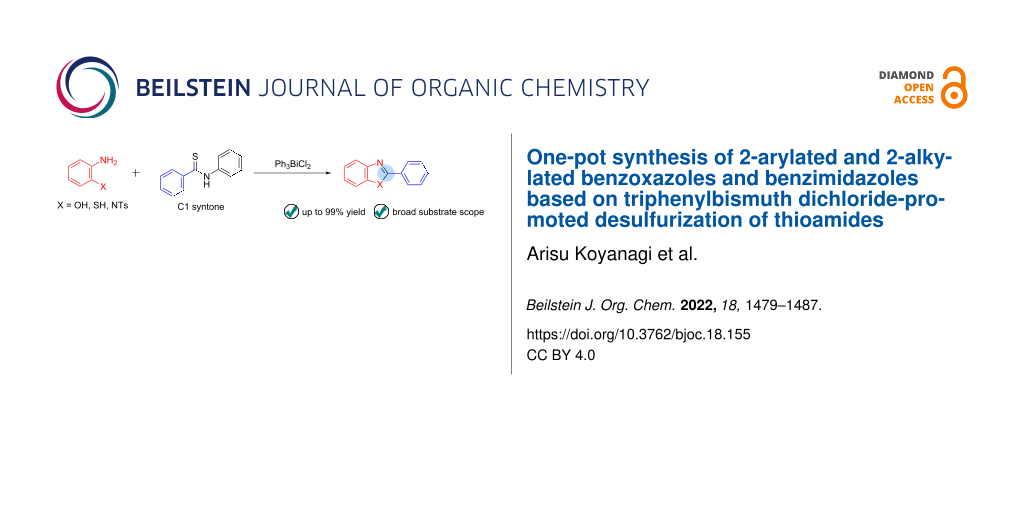
Abstract
The development of novel and efficient synthesis methods for 2-substituted benzazole derivatives is of interest as they are biologically active substances. Herein, a simple method for the synthesis of 2-aryl- and 2-alkyl-substituted benzazoles is described. The reaction of 2-aminophenols with thioamides at 60 °C in the presence of triphenylbismuth dichloride in 1,2-dichloroethane as a promoter afforded various 2-aryl- and 2-alkylbenzoxazoles in moderate to excellent yields under mild reaction conditions. This method could also be applied to the synthesis of benzimidazoles and benzothiazoles. This study presents the first use of triphenylbismuth dichloride to produce benzimidoyl chloride from thioamides by desulfurization and chlorination, as well as its application to the synthesis of 2-substituted benzazoles.
Graphical Abstract
Introduction
In the production of pharmacologically active compounds, 2-substituted benzazoles containing oxazole and imidazole skeletons are the most commonly used scaffolds [1-5]. Therefore, it is crucial for the drug industry to develop methods for their syntheses [1,2,4,6]. For example, conventional benzoxazole synthesis methods involve the condensation of 2-aminophenol with various carbonyl compounds, such as carboxylic acid derivatives or aldehydes [6-9]. These classic reactions require harsh conditions, such as using strong acids or high temperatures. The syntheses of azoles using one-pot reactions are attracting increasing attention as alternatives to conventional methods; a notable alternative is the cyclodesulfurization, where intramolecular cyclization is combined with desulfurization from thioamide analogs such as thioureas, thiosemicarbazides, and dithiocarbamate salts under mild reaction conditions [10]. These synthesis methods are effective for constructing azoles with an amine functional group at the 2-position, such as benzoxazole or benzoimidazole, but are unsuitable for the syntheses of 2-arylated or 2-alkylated benzoxazoles and benzimidazoles. In fact, only two reports cover the subject. Jackson et al. carried out the intramolecular ring-closure reaction of o-alkoxythiobenzamides with iodine in the presence of sodium hydride as the base [11]. Sugita et al. reported an unstable iodoalkyne, pentafluoro(iodoethynyl)benzene, which catalyzed the cyclization of thioamides with 2-aminophenol [12]. These approaches have some drawbacks, such as low yields, the need for bases, and limited substrate scope.
With the development of organobismuth chemistry, these compounds have been applied in various areas of science including biology and organic synthesis because they are normally non-toxic and exhibit unique biological activities [13-21]. Among them, triarylbismuth dichlorides (Ar3BiCl2) have been widely used as aryl group donors for the C- and O-arylation of phenol derivatives [22-24], N-arylation of pyridin-2-ones [25,26], α-arylation of α,β-unsaturated carbonyls [27,28], and tandem 1,3-bisarylation of cyclopropanes with arenes [29]. They have also been used in Pd-catalyzed cross-coupling reactions to react with hypervalent iodonium salts, organostananes, and vinyl epoxides [30-32]. Moreover, there are reports of them serving as oxidizing agents for alcohols [33]. Two papers have recently reported triphenylbismuth dichloride (Ph3BiCl2) to act efficiently in desulfurization reactions (Scheme 1-I and II). In 2018, we reported the preparation of 2-aminobenzoxazoles by the Ph3BiCl2-mediated cyclodesulfurization of thioureas, which were obtained from 2-aminophenols and isothiocyanates [34]. In 2019, Doris et al. performed the dehydrosulfurization of thioamides and thioureas that provided nitriles and cyanamides [35]. Using bismuth compounds as desulfurization reagents for synthesizing heteroazoles, this paper presents the syntheses of 2-aryl- and 2-alkylbenzoxazoles through the ring-closure reaction of 2-aminophenol with benzimidoyl chloride, which is produced by the desulfurization and chlorination of thioamides promoted by Ph3BiCl2 without a base. The developed protocol is also applied to prepare 2-substituted benzimidazoles using N-tosyl-1,2-phenylenediamines as substrates.
Scheme 1: Utilization of Ph3BiCl2 for organic reactions involving desulfurization.
Scheme 1: Utilization of Ph3BiCl2 for organic reactions involving desulfurization.
Results and Discussion
We initially focused on identifying the optimal experimental conditions for the synthesis of 2-phenylbenzoxazole (8a) using 2-aminophenol (1a) with benzothioamides 2–5 in the presence of organobismuth or organoantimony compounds 6 or 7. The results, including those for the screening of suitable ring-closure reagents, solvents, thioamides, and reagent ratios, are summarized in Table 1. We first performed the reaction of 1a (0.5 mmol) with N-phenylbenzothioamide (2a, 1.0 mmol) using organobismuth or organoantimony reagents 6 or 7 (1.0 mmol) in 1,2-dichloroethane (DCE) under aerobic conditions at 60 °C for 18 h (Table 1, entries 1–8). The use of Ph3BiCl2 6a resulted in the best yield (99%) of the expected product 8a. Screening of the solvents showed that the reaction proceeded effectively in 1,2-DCE, chloroform, and EtOH, among which 1,2-DCE afforded the highest yield of 8a (Table 1, entries 1, 9, and 10). In contrast, THF, toluene, DMF, and DMSO were inefficient reaction solvents (entries 11–14). Thus, 1,2-DCE was the best solvent for the reaction in terms of the product yield of 8a (99%), while chloroform posed a concern of acid contamination. Examination of the optimum amount of reagents 2a and 6a toward 1a proved that the reaction of 1a with 2a and Ph3BiCl2 6a in the ratio of 1:2:2 provided the best results, affording product 8a in the highest yield (99%) (Table 1, entries 1, 15, and 16). Moreover, in the presence of 30 mol % of 6a and a 1a:2a ratio of 1:2, the reaction was suppressed and the bismuth reagent did not catalyze it (entry 17). The addition of triethylamine as the base afforded 8a in a low yield (32%) (entry 18). At room temperature, the reaction hardly proceeded (entry 19). Screening various thioamides (2a–5) showed different behavior in the reaction with 1a and 6a; 2a afforded the desired product 8a in an excellent yield, whereas the N,N-disubstituted thioamide 5 did not react (Table 1, entries 1, 20–22). These results show that N-phenylbenzothioamide (2a) is superior as a C1 unit donor at the 2-position of benzoxazole. Consequently, the best result was obtained when 1a and 2a were reacted in the presence of 6a in 1,2-DCE at 60 °C under aerobic conditions. The optimal reagent, Ph3BiCl2 (6a), can be easily and inexpensively synthesized on a 10 g scale by scaling up the reported procedure (see Supporting Information File 1) [36,37].
Table 1: Screening of reaction conditionsa.
|
|
||||||
| Entry | Compound | R1 | R2 | Reagent | Solvent | Yield [%]b |
|---|---|---|---|---|---|---|
| 1 | 2a | Ph | H | Ph3BiCl2 6a | 1,2-DCE | 99 (94)c |
| 2 | 2a | Ph | H | Ph3Bi(OAc)2 6b | 1,2-DCE | 12 |
| 3 | 2a | Ph | H | Ph3Bi 6c | 1,2-DCE | 23 |
| 4 | 2a | Ph | H | BiCl3 6d | 1,2-DCE | 38 |
| 5 | 2a | Ph | H | Ph3SbCl2 7a | 1,2-DCE | 24 |
| 6 | 2a | Ph | H | Ph3Sb(OAc)2 7b | 1,2-DCE | 60 |
| 7 | 2a | Ph | H | Ph3Sb 7c | 1,2-DCE | – |
| 8 | 2a | Ph | H | SbCl3 7d | 1,2-DCE | 28 |
| 9 | 2a | Ph | H | Ph3BiCl2 6a | CHCl3 | 98 |
| 10 | 2a | Ph | H | Ph3BiCl2 6a | EtOH | 82 |
| 11 | 2a | Ph | H | Ph3BiCl2 6a | THF | 54 |
| 12 | 2a | Ph | H | Ph3BiCl2 6a | toluene | 49 |
| 13 | 2a | Ph | H | Ph3BiCl2 6a | DMF | 22 |
| 14 | 2a | Ph | H | Ph3BiCl2 6a | DMSO | 22 |
| 15d | 2a | Ph | H | Ph3BiCl2 6a | 1,2-DCE | 55 |
| 16e | 2a | Ph | H | Ph3BiCl2 6a | 1,2-DCE | 50 |
| 17f | 2a | Ph | H | Ph3BiCl2 6a | 1,2-DCE | 27 |
| 18g | 2a | Ph | H | Ph3BiCl2 6a | 1,2-DCE | 32 |
| 19h | 2a | Ph | H | Ph3BiCl2 6a | 1,2-DCE | 32 |
| 20 | 3 | H | H | Ph3BiCl2 6a | 1,2-DCE | 22 |
| 21 | 4 | Me | H | Ph3BiCl2 6a | 1,2-DCE | 62 |
| 22 | 5 | Ph | Me | Ph3BiCl2 6a | 1,2-DCE | – |
aReaction conditions: 1a (0.5 mmol), 2a–5 (1.0 mmol), pnictogen reagent (6 or 7: 1.0 mmol); bGC yield using dibenzyl as internal standard; cIsolated yield; d1a (0.5 mmol), 2a (0.75 mmol), 6a (0.75 mmol); e1a (0.5 mmol), 2a (0.5 mmol), 6a (0.5 mmol); f6a (30 mol %); gAddition of Et3N (1.0 mmol); hAt room temperature.
To investigate the efficiency and generality of the above-described cyclization, the reaction of various aminophenols 1 (0.5 mmol) and thioamides 2 (1.0 mmol) was investigated in the presence of Ph3BiCl2 6a (1.0 mmol) under the optimized conditions. The results are summarized in Table 2. The reaction of aminophenol (1a) with thioamides 2b–g bearing electron-donating or electron-withdrawing groups on the phenyl rings (R2) afforded the corresponding 2-arylbenzoxazoles 8b–g in good to excellent yields (79–99%). The nature of substituents on the benzene rings of the thioamides did not significantly affect the reaction outcome. Sterically hindered thioamides bearing ortho-substituted aryl groups readily reacted to furnish the corresponding benzoxazoles 8h–j; further, the reaction with a thioamide bearing a thiophene ring gave the expected product 8k in excellent yield (96%). Moreover, thioamides bearing alkyl groups (R2 = cyclohexyl, methyl) reacted with aminophenol 1a to afford the 2-alkylbenzoxazoles 8l and 8m. Various aminophenols bearing different electron-donating and electron-withdrawing groups at the 4-position of the benzene ring were treated with 2a and 6a to afford the corresponding products 8n–r in good to excellent yields. Subsequently, 2-aminophenols with methyl groups at the 3-, 4-, and 5-positions provided satisfactory yields of the products 8o, 8s, and 8t, respectively. On the other hand, an aminophenol with a methyl group at the 6-position provided the product 8u in a low yield. The reaction of 3-amino-2-naphthol with 2a furnished the tricyclic compound 8v in 84% yield. In contrast, the reaction with 3-amino-2-anthracenol resulted in a low yield of the tetracyclic compound 8w due to the low solubility of aminoanthracenol. The reaction of 2a with 2-aminothiophenol instead of 2-aminophenol proceeded smoothly, and the corresponding 2-phenylbenzothiazole (9) was isolated in 93% yield.
Table 2: Synthesis of 2-substituted benzazolesa.
|
|
|||
| Product | Yield (%)b | Product | Yield (%)b |
|---|---|---|---|
|
8b |
95 |
8c |
97 |
|
8d |
99 |
8e |
92 |
|
8f |
79 |
8g |
99 |
|
8h |
89 (5 h) |
8i |
78 (24 h) |
|
8j |
91 |
8k |
96 |
|
8l |
97 |
8m |
80 |
|
8n |
99 |
8o |
84 |
|
8p |
94 |
8q |
99 |
|
8r |
99 |
8s |
95 |
|
8t |
94 |
8u |
55 |
|
8v |
84 |
8w |
48 |
|
9 |
93 | ||
aReagents and conditions: 1 (0.5 mmol), 2 (1.0 mmol), and 6a (1.0 mmol) in 1,2-DCE at 60 °C; bIsolated yield.
Tafamidis (13), a compound with a 2-arylbenzoxazole skeleton is a clinically used drug for transthyretin amyloid inhibition [38,39], and was first synthesized by Kelly et al. [40]. We synthesized compound 13 by the developed cyclodesulfurization method (Scheme 2). The reaction of methyl 4-amino-3-hydroxybenzoate (10) with 3,5-dichloro-N-phenylbenzothioamide (11) afforded the benzoxazole 12 in 91% yield. The subsequent hydrolysis of compound 12 then afforded the desired product 13 in 92% yield (84% overall). On the other hand, an attempt at the direct synthesis of compound 13 from 4-amino-3-hydroxybenzoic acid, unfortunately, yielded a complex mixture and product 13 was not obtained.
In the next stage, the synthesis of 2-substituted benzimidazoles was examined using the same protocol (Table 3). However, the reaction of o-phenylenediamine (14) with 2a in the presence of 6a did not yield the desired 2-phenylbenzimidazole 16a and resulted in a complex mixture. Since the generation of acids such as hydrochloric acid was expected in the reaction system, the reaction was examined using N-tosyl-o-phenylenediamine (15) protected with tosyl group instead of the diamine 14, and the corresponding product 16c was obtained. The reaction of 15 with various thioamides 2 bearing electron-donating or electron-withdrawing groups on the phenyl rings, sterically hindered aryl groups, heteroaryl groups, and alkyl groups also proceeded efficiently, and the corresponding products 16b–i were obtained in 70–87% yields, similarly to the syntheses of benzoxazoles.
Table 3: Synthesis of 2-substituted N-tosylbenzimidazolesa.
|
|
|||
| Product | Yield (%)b | Product | Yield (%)b |
|---|---|---|---|
|
16a |
0 |
16b |
76 |
|
16c |
85 |
16d |
79 |
|
16e |
87 |
16f |
75 |
|
16g |
70 |
16h |
72 |
|
16i |
73 | ||
aReagents and conditions: 1 (0.5 mmol), 2 (1.0 mmol), and 6a (1.0 mmol) in 1,2-DCE at 60 °C; bIsolated yield.
A control experiment was carried out to investigate the reaction pathway and mechanism. When the reaction of benzothioamide (2a) with Ph3BiCl2 6a in chloroform-d at 60 °C was monitored by 1H NMR spectroscopy, phenylbenzimidoyl chloride (17) was observed to be generated (Scheme 3a) (see Supporting Information File 1 for details). When o-aminophenol (1a) was reacted with 17 [41] in a 1:2 ratio, product 8a and diphenylbenzamidine (18) were obtained in 81% and 86% yields, respectively, after purification by acid–base workup (Scheme 3b). A similar workup was performed for the reaction of 1a and 2a in the presence of 6a under standard conditions, and compounds 8a and 18 were isolated, respectively, in high yields (Scheme 3c). The reaction of 17 with aniline 19 afforded product 18 in 91% yield (Scheme 3d). These results suggest that the generation of benzamidine 18 by-produces aniline (19). On the other hand, aniline generation was not confirmed in the reaction between 1a and 2a without an acid–base workup (Table 1, entry 1). Based on the above control experiments and the reaction under study (which required no base; Table 1, entry 18), a possible mechanism for this cyclodesulfurization approach is shown in Scheme 4. The formation of intermediate A based on S···Bi [42,43] and Cl···H inter-coordination is anticipated from the reaction of thioamide 2 and Ph3BiCl2 6a as an initial step. With the elimination of hydrochloric acid, intermediate A is converted to intermediate B. When a base such as Et3N was added, hydrochloric acid was trapped and lowered the reaction yield (Table 1, entry 18). The nucleophilic attack of chloride ions on intermediate B produces D via C, which entails isomerization with the elimination of the sulfur-and-bismuth moiety. Aminophenol then reacts with D to generate intermediate F via E, which is converted to the benzoxazole 8, accompanied by the elimination of 19 by aromatization. The generation of hydrochloric acid was important in this reaction, and the addition of Et3N resulted in lower yield because the hydrochloric acid was trapped by the base (Table 1, entry 18). This reaction required two equivalents of thioamide 2 and Ph3BiCl2 6a for aminophenol (Table 1, entries 1, 15–17). The produced aniline 19 reacts with an excessive amount of D to form benzamidine hydrochloride G. The reaction of D with amines may require an excessive amount of D due to competition between aminophenol and the byproduct aniline. A similar mechanism is considered for the construction of benzimidazole and thiazole rings. On the other hand, the released bismuth moiety, Ph3Bi=S or (Ph3BiS)n, that was expected to be produced in this process, was not confirmed or isolated at this point.
Conclusion
We developed a novel method for the Ph3BiCl2-promoted cyclodesulfurization reaction between thioamides and 2-aminophenols, N-tosyl-1,2-phenylenediamines, and 2-aminobenzenethiol, and achieved the syntheses of 2-aryl- and 2-alkylbenzazoles, such as oxazoles, imidazoles, and thiazole, in satisfactory yields. The protocol was successfully applied to the synthesis of tafamidis, a clinically used drug for transthyretin amyloid inhibition. The reaction is simple, can be performed in aerobic conditions, and requires no bases. In this system, Ph3BiCl2 acts as a superior reagent for the desulfurization and chlorination of thioamides into benzimidoyl chloride as a reaction intermediate. On the other hand, the reaction still has the disadvantage of requiring excess amounts of Ph3BiCl2 and thioamide. Further investigations to expand the cyclodesulfurization reaction to other substrates and the development of a catalytic reaction using bismuth reagent are in progress, and the results will be reported in due course.
Experimental
General procedure for the synthesis of 2-arylazoles
A mixture of aminophenol derivatives (1, 10 or 15; 0.5 mmol), N-phenylthiobenzamide (2 or 11; 0.5 mmol), and Ph3BiCl2 (6a; 1.0 mmol) were well stirred at 60 °C in 1,2-DCE (3.0 mL) for 18 h. After completion of the reaction, the reaction mixture was diluted with H2O (20 mL) and CH2Cl2 (20 mL), and the aqueous phase was extracted with CH2Cl2 (3 × 30 mL). The combined organic phase was washed with brine (20 mL) and dried over MgSO4. Evaporation of the solvent furnished the crude product which was then purified by column chromatography on silica gel.
Supporting Information
| Supporting Information File 1: Experimental procedures, characterization data and copies of spectra. | ||
| Format: PDF | Size: 2.6 MB | Download |
References
-
Noël, S.; Cadet, S.; Gras, E.; Hureau, C. Chem. Soc. Rev. 2013, 42, 7747–7762. doi:10.1039/c3cs60086f
Return to citation in text: [1] [2] -
Singh, S.; Veeraswamy, G.; Bhattarai, D.; Goo, J.-I.; Lee, K.; Choi, Y. Asian J. Org. Chem. 2015, 4, 1338–1361. doi:10.1002/ajoc.201500235
Return to citation in text: [1] [2] -
Šlachtová, V.; Brulíková, L. ChemistrySelect 2018, 3, 4653–4662. doi:10.1002/slct.201800631
Return to citation in text: [1] -
Sattar, R.; Mukhtar, R.; Atif, M.; Hasnain, M.; Irfan, A. J. Heterocycl. Chem. 2020, 57, 2079–2107. doi:10.1002/jhet.3944
Return to citation in text: [1] [2] -
Bansal, Y.; Minhas, R.; Singhal, A.; Arora, R. K.; Bansal, G. Curr. Org. Chem. 2021, 25, 669–694. doi:10.2174/1385272825666210208141107
Return to citation in text: [1] -
Chanda, K.; Rajasekhar, S.; Maiti, B. Synlett 2017, 28, 521–541. doi:10.1055/s-0036-1588671
Return to citation in text: [1] [2] -
Hein, D. W.; Alheim, R. J.; Leavitt, J. J. J. Am. Chem. Soc. 1957, 79, 427–429. doi:10.1021/ja01559a053
Return to citation in text: [1] -
Terashima, M.; Ishii, M.; Kanaoka, Y. Synthesis 1982, 484–485. doi:10.1055/s-1982-29847
Return to citation in text: [1] -
Seijas, J. A.; Vázquez-Tato, M. P.; Carballido-Reboredo, M. R.; Crecente-Campo, J.; Romar-López, L. Synlett 2007, 313–317. doi:10.1055/s-2007-967994
Return to citation in text: [1] -
Kadagathur, M.; Shaikh, A. S.; Jadhav, G. S.; Sigalapalli, D. K.; Shankaraiah, N.; Tangellamudi, N. D. ChemistrySelect 2021, 6, 2621–2640. doi:10.1002/slct.202100201
Return to citation in text: [1] -
Downer-Riley, N. K.; Jackson, Y. A. Tetrahedron 2007, 63, 10276–10281. doi:10.1016/j.tet.2007.07.076
Return to citation in text: [1] -
Matsuzawa, A.; Takeuchi, S.; Sugita, K. Chem. – Asian J. 2016, 11, 2863–2866. doi:10.1002/asia.201601130
Return to citation in text: [1] -
Matano, Y. Antimony and Bismuth in Organic Synthesis. In Main Group Metals in Organic Synthesis; Yamamoto, H.; Oshima, K., Eds.; Wiley-VCH: Weinheim, 2004; Vol. 2, pp 753–811. doi:10.1002/3527602607.ch14
Return to citation in text: [1] -
Gagnon, A.; Benoit,, E.; Le Roch, A. Bismuth Compounds. In Sci. Synth., Knowl. Updates; Banert, K.; Clarke, P. A.; Drabowicz, J.; Oestreich, M., Eds.; Georg Thieme: Stuttgart, 2018; Vol. 4, pp 1–112.
Return to citation in text: [1] -
Condon, S.; Pichon, C.; Davi, M. Org. Prep. Proced. Int. 2014, 46, 89–131. doi:10.1080/00304948.2014.884369
Return to citation in text: [1] -
Gagnon, A.; Dansereau, J.; Le Roch, A. Synthesis 2017, 49, 1707–1745. doi:10.1055/s-0036-1589482
Return to citation in text: [1] -
Sun, H. Biological Chemistry of Arsenic, Antimony and Bismuth; John Wiley & Sons: West Sussex, 2011. doi:10.1002/9780470975503
Return to citation in text: [1] -
Andleeb, S.; Imtiaz‐ud‐Din; Rauf, M. K.; Azam, S. S.; Haq, I.-u.; Tahir, M. N.; Ahmad, S. Appl. Organomet. Chem. 2019, 10.1002/aoc.5061. doi:10.1002/aoc.5061
Return to citation in text: [1] -
Iuchi, K.; Shirai, S.; Tasaki, Y.; Hisatomi, H. Anti-Cancer Drugs 2020, 31, 55–59. doi:10.1097/cad.0000000000000841
Return to citation in text: [1] -
Voss, S.; Rademann, J.; Nitsche, C. Angew. Chem., Int. Ed. 2022, 61, 10.1002/anie.202113857. doi:10.1002/anie.202113857
Return to citation in text: [1] -
Takasawa, R.; Jona, A.; Inoue, M.; Azuma, M.; Akahane, H.; Nakagawa, Y.; Mano, Y.; Murata, Y.; Yasuike, S.; Kaji, T. J. Toxicol. Sci. 2022.
Submitted.
Return to citation in text: [1] -
Barton, D. H. R.; Bhatnagar, N. Y.; Blazejewski, J.-C.; Charpiot, B.; Finet, J.-P.; Lester, D. J.; Motherwell, W. B.; Papoula, M. T. B.; Stanforth, S. P. J. Chem. Soc., Perkin Trans. 1 1985, 2657–2665. doi:10.1039/p19850002657
Return to citation in text: [1] -
Barton, D. H. R.; Yadav-Bhatnagar, N.; Finet, J.-P.; Khamsi, J.; Motherwell, W. B.; Stanforth, S. P. Tetrahedron 1987, 43, 323–332. doi:10.1016/s0040-4020(01)89960-9
Return to citation in text: [1] -
Fedorov, A.; Combes, S.; Finet, J.-P. Tetrahedron 1999, 55, 1341–1352. doi:10.1016/s0040-4020(98)01185-5
Return to citation in text: [1] -
Ikegai, K.; Mukaiyama, T. Chem. Lett. 2005, 34, 1496–1497. doi:10.1246/cl.2005.1496
Return to citation in text: [1] -
Ikegai, K.; Nagata, Y.; Mukaiyama, T. Bull. Chem. Soc. Jpn. 2006, 79, 761–767. doi:10.1246/bcsj.79.761
Return to citation in text: [1] -
Arnauld, T.; Barton, D. H. R.; Normant, J.-F.; Doris, E. J. Org. Chem. 1999, 64, 6915–6917. doi:10.1021/jo9905928
Return to citation in text: [1] -
Koech, P. K.; Krische, M. J. J. Am. Chem. Soc. 2004, 126, 5350–5351. doi:10.1021/ja048987i
Return to citation in text: [1] -
Mondal, B.; Das, D.; Saha, J. Org. Lett. 2020, 22, 5115–5120. doi:10.1021/acs.orglett.0c01702
Return to citation in text: [1] -
Kang, S.-K.; Ryu, H.-C.; Kim, J.-W. Synth. Commun. 2001, 31, 1021–1026. doi:10.1081/scc-100103531
Return to citation in text: [1] -
Kang, S.-K.; Ryu, H.-C.; Lee, S.-W. Synth. Commun. 2001, 31, 1027–1034. doi:10.1081/scc-100103532
Return to citation in text: [1] -
Kang, S.-K.; Ryu, H.-C.; Hong, Y.-T.; Kim, M.-S.; Lee, S.-W.; Jung, J.-H. Synth. Commun. 2001, 31, 2365–2371. doi:10.1081/scc-100104838
Return to citation in text: [1] -
Matano, Y.; Hisanaga, T.; Yamada, H.; Kusakabe, S.; Nomura, H.; Imahori, H. J. Org. Chem. 2004, 69, 8676–8680. doi:10.1021/jo0485740
Return to citation in text: [1] -
Murata, Y.; Matsumoto, N.; Miyata, M.; Kitamura, Y.; Kakusawa, N.; Matsumura, M.; Yasuike, S. J. Organomet. Chem. 2018, 859, 18–23. doi:10.1016/j.jorganchem.2018.01.041
Return to citation in text: [1] -
Gopi, E.; Gravel, E.; Doris, E. Eur. J. Org. Chem. 2019, 4043–4045. doi:10.1002/ejoc.201900563
Return to citation in text: [1] -
Bresien, J.; Hering-Junghans, C.; Schulz, A.; Thomas, M.; Villinger, A. Organometallics 2018, 37, 2571–2580. doi:10.1021/acs.organomet.8b00318
Return to citation in text: [1] -
Moiseev, D. V.; Malysheva, Y. B.; Shavyrin, A. S.; Kurskii, Y. A.; Gushchin, A. V. J. Organomet. Chem. 2005, 690, 3652–3663. doi:10.1016/j.jorganchem.2005.04.051
Return to citation in text: [1] -
Lamb, Y. N.; Deeks, E. D. Drugs 2019, 79, 863–874. doi:10.1007/s40265-019-01129-6
Return to citation in text: [1] -
Park, J.; Egolum, U.; Parker, S.; Andrews, E.; Ombengi, D.; Ling, H. Ann. Pharmacother. 2020, 54, 470–477. doi:10.1177/1060028019888489
Return to citation in text: [1] -
Razavi, H.; Palaninathan, S. K.; Powers, E. T.; Wiseman, R. L.; Purkey, H. E.; Mohamedmohaideen, N. N.; Deechongkit, S.; Chiang, K. P.; Dendle, M. T. A.; Sacchettini, J. C.; Kelly, J. W. Angew. Chem., Int. Ed. 2003, 42, 2758–2761. doi:10.1002/anie.200351179
Return to citation in text: [1] -
Kawahara, K. P.; Matsuoka, W.; Ito, H.; Itami, K. Angew. Chem., Int. Ed. 2020, 59, 6383–6388. doi:10.1002/anie.201913394
Return to citation in text: [1] -
Briand, G. G.; Burford, N.; Eelman, M. D.; Aumeerally, N.; Chen, L.; Cameron, T. S.; Robertson, K. N. Inorg. Chem. 2004, 43, 6495–6500. doi:10.1021/ic049594n
Return to citation in text: [1] -
Andrews, P. C.; Ferrero, R. L.; Forsyth, C. M.; Junk, P. C.; Maclellan, J. G.; Peiris, R. M. Organometallics 2011, 30, 6283–6291. doi:10.1021/om2008869
Return to citation in text: [1]
| 41. | Kawahara, K. P.; Matsuoka, W.; Ito, H.; Itami, K. Angew. Chem., Int. Ed. 2020, 59, 6383–6388. doi:10.1002/anie.201913394 |
| 38. | Lamb, Y. N.; Deeks, E. D. Drugs 2019, 79, 863–874. doi:10.1007/s40265-019-01129-6 |
| 39. | Park, J.; Egolum, U.; Parker, S.; Andrews, E.; Ombengi, D.; Ling, H. Ann. Pharmacother. 2020, 54, 470–477. doi:10.1177/1060028019888489 |
| 40. | Razavi, H.; Palaninathan, S. K.; Powers, E. T.; Wiseman, R. L.; Purkey, H. E.; Mohamedmohaideen, N. N.; Deechongkit, S.; Chiang, K. P.; Dendle, M. T. A.; Sacchettini, J. C.; Kelly, J. W. Angew. Chem., Int. Ed. 2003, 42, 2758–2761. doi:10.1002/anie.200351179 |
| 1. | Noël, S.; Cadet, S.; Gras, E.; Hureau, C. Chem. Soc. Rev. 2013, 42, 7747–7762. doi:10.1039/c3cs60086f |
| 2. | Singh, S.; Veeraswamy, G.; Bhattarai, D.; Goo, J.-I.; Lee, K.; Choi, Y. Asian J. Org. Chem. 2015, 4, 1338–1361. doi:10.1002/ajoc.201500235 |
| 3. | Šlachtová, V.; Brulíková, L. ChemistrySelect 2018, 3, 4653–4662. doi:10.1002/slct.201800631 |
| 4. | Sattar, R.; Mukhtar, R.; Atif, M.; Hasnain, M.; Irfan, A. J. Heterocycl. Chem. 2020, 57, 2079–2107. doi:10.1002/jhet.3944 |
| 5. | Bansal, Y.; Minhas, R.; Singhal, A.; Arora, R. K.; Bansal, G. Curr. Org. Chem. 2021, 25, 669–694. doi:10.2174/1385272825666210208141107 |
| 11. | Downer-Riley, N. K.; Jackson, Y. A. Tetrahedron 2007, 63, 10276–10281. doi:10.1016/j.tet.2007.07.076 |
| 35. | Gopi, E.; Gravel, E.; Doris, E. Eur. J. Org. Chem. 2019, 4043–4045. doi:10.1002/ejoc.201900563 |
| 10. | Kadagathur, M.; Shaikh, A. S.; Jadhav, G. S.; Sigalapalli, D. K.; Shankaraiah, N.; Tangellamudi, N. D. ChemistrySelect 2021, 6, 2621–2640. doi:10.1002/slct.202100201 |
| 36. | Bresien, J.; Hering-Junghans, C.; Schulz, A.; Thomas, M.; Villinger, A. Organometallics 2018, 37, 2571–2580. doi:10.1021/acs.organomet.8b00318 |
| 37. | Moiseev, D. V.; Malysheva, Y. B.; Shavyrin, A. S.; Kurskii, Y. A.; Gushchin, A. V. J. Organomet. Chem. 2005, 690, 3652–3663. doi:10.1016/j.jorganchem.2005.04.051 |
| 6. | Chanda, K.; Rajasekhar, S.; Maiti, B. Synlett 2017, 28, 521–541. doi:10.1055/s-0036-1588671 |
| 7. | Hein, D. W.; Alheim, R. J.; Leavitt, J. J. J. Am. Chem. Soc. 1957, 79, 427–429. doi:10.1021/ja01559a053 |
| 8. | Terashima, M.; Ishii, M.; Kanaoka, Y. Synthesis 1982, 484–485. doi:10.1055/s-1982-29847 |
| 9. | Seijas, J. A.; Vázquez-Tato, M. P.; Carballido-Reboredo, M. R.; Crecente-Campo, J.; Romar-López, L. Synlett 2007, 313–317. doi:10.1055/s-2007-967994 |
| 33. | Matano, Y.; Hisanaga, T.; Yamada, H.; Kusakabe, S.; Nomura, H.; Imahori, H. J. Org. Chem. 2004, 69, 8676–8680. doi:10.1021/jo0485740 |
| 1. | Noël, S.; Cadet, S.; Gras, E.; Hureau, C. Chem. Soc. Rev. 2013, 42, 7747–7762. doi:10.1039/c3cs60086f |
| 2. | Singh, S.; Veeraswamy, G.; Bhattarai, D.; Goo, J.-I.; Lee, K.; Choi, Y. Asian J. Org. Chem. 2015, 4, 1338–1361. doi:10.1002/ajoc.201500235 |
| 4. | Sattar, R.; Mukhtar, R.; Atif, M.; Hasnain, M.; Irfan, A. J. Heterocycl. Chem. 2020, 57, 2079–2107. doi:10.1002/jhet.3944 |
| 6. | Chanda, K.; Rajasekhar, S.; Maiti, B. Synlett 2017, 28, 521–541. doi:10.1055/s-0036-1588671 |
| 34. | Murata, Y.; Matsumoto, N.; Miyata, M.; Kitamura, Y.; Kakusawa, N.; Matsumura, M.; Yasuike, S. J. Organomet. Chem. 2018, 859, 18–23. doi:10.1016/j.jorganchem.2018.01.041 |
| 25. | Ikegai, K.; Mukaiyama, T. Chem. Lett. 2005, 34, 1496–1497. doi:10.1246/cl.2005.1496 |
| 26. | Ikegai, K.; Nagata, Y.; Mukaiyama, T. Bull. Chem. Soc. Jpn. 2006, 79, 761–767. doi:10.1246/bcsj.79.761 |
| 29. | Mondal, B.; Das, D.; Saha, J. Org. Lett. 2020, 22, 5115–5120. doi:10.1021/acs.orglett.0c01702 |
| 22. | Barton, D. H. R.; Bhatnagar, N. Y.; Blazejewski, J.-C.; Charpiot, B.; Finet, J.-P.; Lester, D. J.; Motherwell, W. B.; Papoula, M. T. B.; Stanforth, S. P. J. Chem. Soc., Perkin Trans. 1 1985, 2657–2665. doi:10.1039/p19850002657 |
| 23. | Barton, D. H. R.; Yadav-Bhatnagar, N.; Finet, J.-P.; Khamsi, J.; Motherwell, W. B.; Stanforth, S. P. Tetrahedron 1987, 43, 323–332. doi:10.1016/s0040-4020(01)89960-9 |
| 24. | Fedorov, A.; Combes, S.; Finet, J.-P. Tetrahedron 1999, 55, 1341–1352. doi:10.1016/s0040-4020(98)01185-5 |
| 30. | Kang, S.-K.; Ryu, H.-C.; Kim, J.-W. Synth. Commun. 2001, 31, 1021–1026. doi:10.1081/scc-100103531 |
| 31. | Kang, S.-K.; Ryu, H.-C.; Lee, S.-W. Synth. Commun. 2001, 31, 1027–1034. doi:10.1081/scc-100103532 |
| 32. | Kang, S.-K.; Ryu, H.-C.; Hong, Y.-T.; Kim, M.-S.; Lee, S.-W.; Jung, J.-H. Synth. Commun. 2001, 31, 2365–2371. doi:10.1081/scc-100104838 |
| 13. | Matano, Y. Antimony and Bismuth in Organic Synthesis. In Main Group Metals in Organic Synthesis; Yamamoto, H.; Oshima, K., Eds.; Wiley-VCH: Weinheim, 2004; Vol. 2, pp 753–811. doi:10.1002/3527602607.ch14 |
| 14. | Gagnon, A.; Benoit,, E.; Le Roch, A. Bismuth Compounds. In Sci. Synth., Knowl. Updates; Banert, K.; Clarke, P. A.; Drabowicz, J.; Oestreich, M., Eds.; Georg Thieme: Stuttgart, 2018; Vol. 4, pp 1–112. |
| 15. | Condon, S.; Pichon, C.; Davi, M. Org. Prep. Proced. Int. 2014, 46, 89–131. doi:10.1080/00304948.2014.884369 |
| 16. | Gagnon, A.; Dansereau, J.; Le Roch, A. Synthesis 2017, 49, 1707–1745. doi:10.1055/s-0036-1589482 |
| 17. | Sun, H. Biological Chemistry of Arsenic, Antimony and Bismuth; John Wiley & Sons: West Sussex, 2011. doi:10.1002/9780470975503 |
| 18. | Andleeb, S.; Imtiaz‐ud‐Din; Rauf, M. K.; Azam, S. S.; Haq, I.-u.; Tahir, M. N.; Ahmad, S. Appl. Organomet. Chem. 2019, 10.1002/aoc.5061. doi:10.1002/aoc.5061 |
| 19. | Iuchi, K.; Shirai, S.; Tasaki, Y.; Hisatomi, H. Anti-Cancer Drugs 2020, 31, 55–59. doi:10.1097/cad.0000000000000841 |
| 20. | Voss, S.; Rademann, J.; Nitsche, C. Angew. Chem., Int. Ed. 2022, 61, 10.1002/anie.202113857. doi:10.1002/anie.202113857 |
| 21. |
Takasawa, R.; Jona, A.; Inoue, M.; Azuma, M.; Akahane, H.; Nakagawa, Y.; Mano, Y.; Murata, Y.; Yasuike, S.; Kaji, T. J. Toxicol. Sci. 2022.
Submitted. |
| 42. | Briand, G. G.; Burford, N.; Eelman, M. D.; Aumeerally, N.; Chen, L.; Cameron, T. S.; Robertson, K. N. Inorg. Chem. 2004, 43, 6495–6500. doi:10.1021/ic049594n |
| 43. | Andrews, P. C.; Ferrero, R. L.; Forsyth, C. M.; Junk, P. C.; Maclellan, J. G.; Peiris, R. M. Organometallics 2011, 30, 6283–6291. doi:10.1021/om2008869 |
| 12. | Matsuzawa, A.; Takeuchi, S.; Sugita, K. Chem. – Asian J. 2016, 11, 2863–2866. doi:10.1002/asia.201601130 |
| 27. | Arnauld, T.; Barton, D. H. R.; Normant, J.-F.; Doris, E. J. Org. Chem. 1999, 64, 6915–6917. doi:10.1021/jo9905928 |
| 28. | Koech, P. K.; Krische, M. J. J. Am. Chem. Soc. 2004, 126, 5350–5351. doi:10.1021/ja048987i |
© 2022 Koyanagi et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.