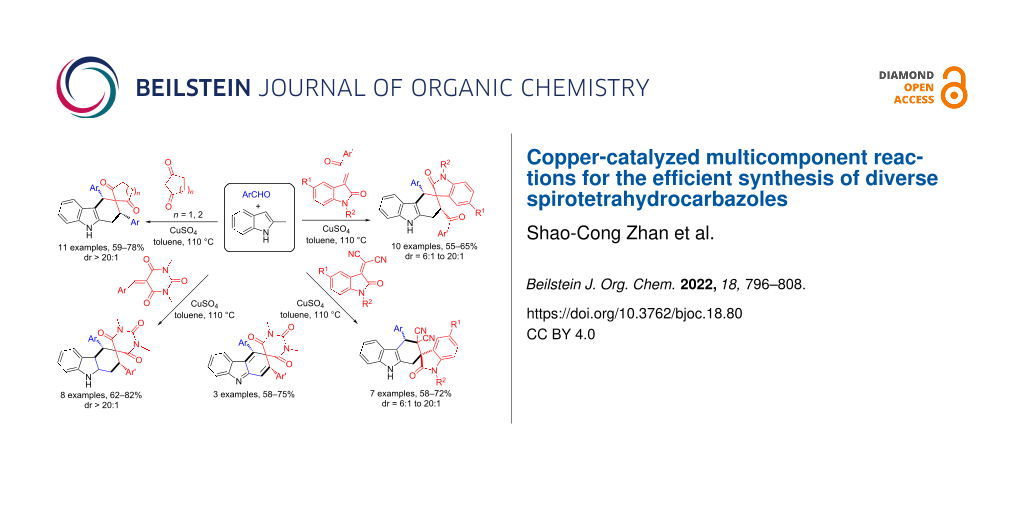
Abstract
In the presence of copper sulfate, three- or four-component reactions of 2-methylindole, aromatic aldehydes and various cyclic dienophiles in refluxing toluene afforded diverse spirotetrahydrocarbazoles. This reaction is an important development of the Levy reaction by using 2-methylindole to replace ethyl indole-2-acetate and successfully provides facile access to important polysubstituted spiro[carbazole-3,3'-indolines], spiro[carbazole-2,3'-indolines], spiro[carbazole-3,5'-pyrimidines] and spiro[carbazole-3,1'-cycloalkanes] in satisfactory yields and with high diastereoselectivity.
Graphical Abstract
Introduction
Tetrahydrocarbazole is one of the most privileged heterocyclic core structures. It widely exists in various naturally occurring alkaloids and pharmacologically active compounds exhibiting important bioactivities such as antitumor activity and antiprotein kinase C activity [1-3]. Additionally, the corresponding carbazole derivatives also show important applications in various functional materials [4-7]. Owing to their remarkable significance, developing convenient synthetic protocols for functionalized tetrahydrocarbazoles has attracted continual attention in synthetic and pharmaceutical chemistry [8-15]. Among many well-designed strategies for the synthesis of tetrahydrocarbazoles, the direct assembly of the tetrahydrocyclohexenyl ring with readily available functionalized indoles as precursors has proven to be one of the most efficient synthetic protocols [16-21]. Therefore, many [4 + 2] reactions of 3-vinylindolines or 2-vinylindolines with diverse dienophiles have been successfully developed for the synthesis of many tetrahydrocarbazole and carbazole derivatives [22-50]. On the other hand, the Diels–Alder reaction of the in situ generated indole-2,3-quinodimethanes with various dienophiles is also a powerful method for rapid construction of functionalized tetrahydrocarbazoles [51-68]. In this respect, Lévy reported a copper-catalyzed three-component reaction of aromatic aldehydes, ethyl indole-2-acetate and N-alkylmaleimides for the efficient construction of polycyclic tetrahydrocarbazoles, in which indolo-2,3-quinodimethane intermediate was initially generated and sequentially underwent a [4 + 2] cycloaddition reaction (reaction 1 in Scheme 1) [69-74]. This metal-catalyzed one-pot reaction not only combined the advantages of a traditional Diels–Alder reaction and the recently developed multicomponent reactions, but also meets the goal of green and sustainable chemistry. Recently, we have reported the efficient construction of a series of heterocyclic spiro compounds including tetrahydrospiro[carbazole-3,3’-indolines] by using the Levy three-component reaction of indole-2-acetate, aromatic aldehydes and various cyclic dienophiles such as 3-phenacylideneoxindoles (reaction 2 in Scheme 1) [75-77]. It was noticed that only ethyl indole-2-acetate was successfully employed as the precursor to generate the active indolo-2,3-quinodimethane intermediate in the Levy reaction, which greatly limited its practical synthetic value. We envisioned that other substituted indoles without electron-withdrawing activating groups could be employed in the Levy reaction, which will greatly develop the potential synthetic applications of the Levy-type reaction. Herein, we wish to report a Levy-type reaction by using readily available 2-methylindole to replace ethyl indole-2-acetate for the efficient synthesis of diverse spiro tetrahydrocarbazoles. In the presence of CuSO4, the multicomponent reactions of aromatic aldehydes, 2-methylindole and various cyclic dieneophiles such as 3-phenacylideneoxindoles, isatylidene malononitriles and the in situ generated 5-arylidene-1,3-dimethylbarbituric acids or 2-arylidene-1,3-cycloketones efficiently afforded diverse cyclic spirotetrahydrocarbazoles in good yields and with high diastereoselectivity (reaction 3 in Scheme 1).
Scheme 1: Construction of diverse tetrahydrocarbazoles via Levy-type reaction.
Scheme 1: Construction of diverse tetrahydrocarbazoles via Levy-type reaction.
Results and Discussion
Initially, (E)-1-benzyl-3-(2-oxo-2-phenylethylidene)indolin-2-one, benzaldehyde, and 2-methylindole served as model substrates for the optimization of reaction conditions. Inspired by our previous work [75-77], we first screened different catalysts (Table 1, entries 1−5) with toluene as the solvent and found that CuSO4 was the best choice, providing 1a in 58% isolated yield. Notably, the yield of the byproduct 1a’ was also obtained in less than 5% yield, which indicated the high diastereoselectivity of this reaction. Several other solvents such as EtOH and MeCN were explored, the product 1a was formed in lower yields (Table 1, entries 6 and 7). Importantly, the reaction also proceeded smoothly when the temperature was reduced to 90 °C and with better yield at 110 °C (Table 1, entries 8 and 9). Moreover, different reaction times and the catalyst loadings were also examined (Table 1, entries 10–12), but no better results were observed. Therefore, a mixture of 2-methylindole (0.5 mmol), benzaldehyde (0.6 equiv), and 3-methyleneoxindole (1.0 equiv) with CuSO4 (0.1 mmol) as the catalyst in toluene (10.0 mL) reacting at 110 °C for 3 h were selected as the optimal reaction conditions.
Table 1: Optimization of reaction conditionsa.
|
|
|||||
| Entry | Catalyst | Solvent | Time (h) | Temp. (°C) | Yield (%)b |
| 1 | FeCl3 | toluene | 3 | 130 | <10 |
| 2 | CuI | toluene | 3 | 130 | <10 |
| 3 | Cu(OTf)2 | toluene | 3 | 130 | 30 |
| 4 | Cu(OAc)2 | toluene | 3 | 130 | 25 |
| 5 | CuSO4 | toluene | 3 | 130 | 58 |
| 6 | CuSO4 | EtOH | 3 | 130 | 30 |
| 7 | CuSO4 | MeCN | 3 | 130 | 40 |
| 8 | CuSO4 | toluene | 3 | 110 | 61 |
| 9 | CuSO4 | toluene | 3 | 90 | 50 |
| 10 | CuSO4 | toluene | 6 | 110 | 61 |
| 11c | CuSO4 | toluene | 3 | 110 | 58 |
| 12d | CuSO4 | toluene | 3 | 110 | 61 |
aReaction conditions: 2-methylindole (0.5 mmol), aldehyde (0.6 mmol), 3-phenacylideneoxindole (0.5 mmol), catalyst (0.1 mmol), solvent (10.0 mL). bIsolated yields. cCuSO4 (0.05 mmol). dCuSO4 (0.2 mmol).
Having established the optimal reaction conditions, we first sought to determine the generality of the aromatic aldehydes. As illustrated in Scheme 2, the reaction usually resulted in a mixture of major isomers 1a–j and the minor isomers 1a’–j’ with the molecular ratios of 6:1 to 20:1. Aromatic aldehydes with both electron-donating and electron-withdrawing groups were compatible. This reaction tolerated many kinds of functional groups, including Me (1b), OMe (1c and 1j), Cl (1d, 1e and 1f), and NO2 (1g, 1h and 1i) groups. Besides different aromatic aldehydes, we further tested the generality of various 3-phenacylideneoxindoles. Unsurprisingly, a wide range of 3-methyleneoxindoles containing different substituents at various positions (1e–j) reacted smoothly to give the corresponding products with good to excellent diastereoselectivity. A protecting n-butyl group (1e) also could promote such a transformation with good yield and high diastereoselectivity. All major isomers 1a–j and some minor isomers 1a’, 1b’, 1f’, 1g’, 1j’ were successfully isolated and fully characterized. Notably, the relative configuration of major isomer 1f (CCDC 2109575) was determined by X-ray crystallographic analysis, in which the m-chlorophenyl, benzoyl and the phenyl group in oxindole are in cis-configuration. It has been known that the benzoyl group and the phenyl group in oxindole stand in cis-position in the starting 3-phenacylideneoxindoles [75]. Therefore, a concerted Diels–Alder reaction should be involved in this three-component reaction.
Scheme 2: Synthesis of spiro[carbazole-3,3'-inolines]. Reaction conditions: 2-methylindole (0.5 mmol), aromatic aldehydes (0.6 mmol), 3-phenacylideneoxindole (0.5 mmol), CuSO4 (0.10 mmol), toluene (6.0 mL), 110 °C, 3 h. Isolated yields are shown. The dr values were determined by 1H NMR.
Scheme 2: Synthesis of spiro[carbazole-3,3'-inolines]. Reaction conditions: 2-methylindole (0.5 mmol), aromat...
On the basis of this success, we further considered whether other dienophiles could be applied in such catalytic system. Under the same reaction conditions, the three-component reaction of 2-methylindole, benzaldehyde and 2-(1-benzyl-2-oxoindolin-3-ylidene)malononitrile reacted smoothly to give the expected spiro[carbazole-2,3'indolines] in 51% yield with a diastereometric ratio (dr) value of 7:1. As shown in Scheme 3, a wide range of aromatic aldehydes with different substituents on the aromatic ring reacted smoothly to give the desired products 2a–e in good yields and with good to excellent diastereoselectivity. On the other hand, we also tested various isatylidene malononitriles, substrates with a differently substituted indole ring or with different protecting groups on the indole N-1 position (2f, 2g). All substrates reacted smoothly to give the corresponding products in moderate to good yields and with high stereoselectivity. It should be pointed out that the minor products 2a’–e’ and 2g’ were also isolated and fully characterized.
Scheme 3: Synthesis of spiro[carbazole-2,3'-indolines]. Reaction conditions: 2-methylindole (0.5 mmol), aromatic aldehyde (0.6 mmol), isatylidene malononitrile (0.5 mmol), CuSO4 (0.10 mmol), toluene (6.0 mL), 110 °C, 3 h. Isolated yields are shown. The dr values were determined by 1H NMR.
Scheme 3: Synthesis of spiro[carbazole-2,3'-indolines]. Reaction conditions: 2-methylindole (0.5 mmol), aroma...
Moreover, the relative configuration of major isomer 2b (CCDC 2109576) and minor isomer 2g’ (CCDC 2109577) were determined by X-ray crystallographic analysis. In the major isomer 2b, the phenyl group at C4-position is in cis-position to the aryl group in the oxindole scaffold, while these two groups exist in trans-configuration in the minor isomer 2g’.
5-Arylidene-1,3-dimethylbarbituric acids, as good dienophiles, could also react smoothly in such a catalytic system (Scheme 4a). Firstly, the three-component reaction of 2-methylindole, 5-arylidene-1,3-dimethylbarbituric acid and 4-methylbenzaldehyde under the standard reaction conditions and further oxidation with DDQ generated the final aromatized product 3a in 75% yield. Under the same reaction conditions, similar aromatized products 3b and 3c were also produced in good yields with different kinds of aromatic aldehydes and 5-arylidene-1,3-dimethylbarbituric acids. 5-Arylidene-1,3-dimethylbarbituric acids could be easily generated through Knoevenagel condensation of aromatic aldehydes and 1,3-dimethylbarbituric acid under the catalysis of Lewis acid. We envisioned whether the desired dienophiles, 5-arylidene-1,3-dimethylbarbituric acids, could be in situ generated by aromatic aldehydes and 1,3-dimethylbarbituric acid under the standard conditions. Therefore, the four-component reaction was carried out by using two molecular aromatic aldehydes, 1,3- dimethylbarbituric acid and 2-methylindole utilizing CuSO4 as the catalyst in toluene at 110 °C. The desired product 4a was obtained in 82% with excellent diastereoselectivity (dr > 20:1). In this reaction, both dienes (o-QDMs) and dienophiles were in situ formed from the starting material. As shown in Scheme 4b, a wide range of aromatic aldehydes that bore different substituents at the ortho- (4c), meta- (4e, 4g) and para-position (4b, 4d, 4f and 4h) of the phenyl ring reacted smoothly to give the corresponding products 4a–h in moderate to good yields with excellent diastereoselectivity (dr > 20:1). The single crystal structures of the compound 3a (CCDC 2109578) and 4e (CCDC 2109579) were successfully determined by X-ray diffraction. It is pleased to find that the obtained spiro[carbazole-3,5'-pyrimidines] 4a–h have same cis-configuration to that of the above prepared spiro[carbazole-3,3'-indolines] 1a–j and spiro[carbazole-2,3'-indolines] 2a–h, in which the two phenyl groups exist in cis-position. This result clearly indicated that this reaction has a similar outcome of stereochemistry.
Scheme 4: Synthesis of tetrahydrospiro[carbazole-3,5'-pyrimidines]. Reaction conditions: 2-methylindole (0.5 mmol), aromatic aldehyde (0.6 mmol), 5-arylidene-1,3-dimethylbarbituric acid (0.5 mmol), CuSO4 (0.1 mmol), toluene (6.0 mL), 110 °C, 3 h, and then DDQ (1.0 mmol), CH3CN (10. 0 mL), rt, 2 h. Isolated yields are shown. The dr values were determined by 1H NMR. aReaction conditions: 2-methylindole (0.5 mmol), aromatic aldehyde (1.2 mmol), 1,3-dimethylbarbituric acid (0.5 mmol), CuSO4 (0.1 mmol), toluene (6.0 mL), 110 °C, 3 h.
Scheme 4: Synthesis of tetrahydrospiro[carbazole-3,5'-pyrimidines]. Reaction conditions: 2-methylindole (0.5 ...
Based on the success of the four-component reaction through in situ generation of dienes and dienophiles, we further considered whether more common cyclic 1,3-diones could be applied in this catalytic system. To our delight, when cyclopentane-1,3-dione was used to replace 1,3-dimethylbarbituiric acid under the same conditions, the desired product 5a was obtained in 74% yield with excellent diastereoselectivity (dr > 20:1). Furthermore, we examined the scope of aromatic aldehydes (Scheme 5), different substituents on the aromatic ring showed marginal effect on the yields. The spiro compounds 5a–g were successfully produced in moderate to excellent yields with excellent diastereoselectivity (dr > 20:1). Cyclohexane-1,3-dione also reacted smoothly to produce the corresponding products 5h–k in high yields with high diastereoselectivity. The single crystal structure of compound 5b (CCDC 2109580) clearly indicated that the two aryl groups are in cis-position.
Scheme 5: Synthesis of tetrahydrospiro[carbazole-3,1'-cycloalkane]-diones. Reaction conditions: 2-methylindole (0.5 mmol), aromatic aldehyde (1.0 mmol), cyclic 1,3-dione (0.5 mmol), CuSO4 (0.1 mmol), toluene (6.0 mL), 110 °C, 3 h. Isolated yields are shown. The dr values were determined by 1H NMR.
Scheme 5: Synthesis of tetrahydrospiro[carbazole-3,1'-cycloalkane]-diones. Reaction conditions: 2-methylindol...
When we did not add the dienophiles to this system, 4-methylbenzaldehyde could react with two molecules of 2-methylindole under the same reaction conditions to generate the well-known 3,3'-(p-tolylmethylene)bis(2-methylindole) (6a) in 75% yield. Subsequently, we sought to determine the generality of aromatic aldehydes. As illustrated in Scheme 6, a diversity of functional groups, which include Me (6a), OMe (6b, 6c), NMe2 (6d), NO2 (6e), and Cl (6f, 6g), could be well tolerated in this reaction. Importantly, the reaction is not sensitive to the electronic properties of the arenes, as the substrates bearing either electron-donating (6a–d), or electron-withdrawing (6e–g) groups on the aryl ring were all transformed smoothly into the corresponding desired products 6a–g with good to excellent yields. Though 3,3'-(p-tolylmethylene)bisindoles have been previously prepared by acid-catalyzed reaction of aromatic aldehydes and various indoles. Here we also provided an alternative synthetic protocol by Lewis acid CuSO4 catalyzed reaction.
Scheme 6: Synthesis of 3,3'-(arylmethylene)bis(2-methyl-1H-indole). Reaction conditions: 2-methylindole (1.0 mmol), aromatic aldehyde (0.5 mmol), CuSO4 (0.1 mmol), toluene (6.0 mL), 110 °C, 3 h. Isolated yields are shown.
Scheme 6: Synthesis of 3,3'-(arylmethylene)bis(2-methyl-1H-indole). Reaction conditions: 2-methylindole (1.0 ...
Based on the above experimental results and the previously works [75-77], a plausible reaction pathway is illustrated in Scheme 7. At first, 2-methylindole reacts with aromatic aldehydes in the presence of the catalyst CuSO4 to generate the intermediate 3-substituted indole, which undergoes dehydration to form the key intermediate indole-based ortho-quinodimethanes (o-QDMs, A). In the meantime, the cyclic 1,3-diones and aromatic aldehyde undergo Knoevenagel condensation to afford the different kinds of dienophiles. Subsequently, the Diels–Alder cycloaddition between the indole-based ortho-quinodimethanes (o-QDMs, A) and dienophiles affords the final spiro compounds 1, 2, 4 and 5 as major isomers through an endo-transition state. Due to the different polarity, 3-phenacylideneoxindole and isatylidene malononitrile resulted in regioisomeric spiro[carbazole-3,3'-indoline] 1 and spiro[carbazole-2,3'-indoline] 2 as the final products. It can be seen that the two aryl groups at 1,3-positions actually exist on the e-bonds in the newly formed cyclohexyl ring in the spiro compounds 4 and 5. This also means that the major isomers 4 and 5 are the thermodynamically stable isomers. In the major isomer 1, the aryl group and the benzoyl group at 1,3-position also stand on the e-bonds, which indicated that the major isomer 1 is also belonging to the thermodynamically stable isomer. This result showed that this reaction is a thermodynamically controlled reaction. On the other hand, the tetrahydrospiro[carbazole-3,5'-pyrimidine] 4 can be converted to aromatized spiro[carbazole-3,5'-pyrimidine] 3 through the oxidation of DDQ. In the absence of the effective dienophile, the normal Friedel–Crafts alkylation of 2-methylindole with aromatic aldehyde gives the well-known 3,3'-(arylmethylene)bis(2-methylindoles) 6.
Scheme 7: Proposed reaction mechanism for the multicomponent reaction.
Scheme 7: Proposed reaction mechanism for the multicomponent reaction.
Conclusion
In summary, we have developed a copper-catalyzed multicomponent Diels–Alder reaction of 2-methylindole, aromatic aldehydes and cyclic 1,3-diones through in situ generated dienes and dienophiles under the same conditions. These strategies are sustainable, general and practical, which providing facile access to important polysubstituted spiro[carbazole-3,3'-indolines], spiro[carbazole-2,3'-indolines], spiro[carbazole-3,5'-pyrimidines] and spiro[carbazole-3,1'-cycloalkanes] with good yields and high diastereoselectivity. This reaction actually developed the practical synthetic values of the well-known Levy reaction by using inactivated indole derivatives. The outcome of diastereoselectivity of the reaction was clearly elucidated by determination of the several single crystal structures and the analysis of the reaction mechanism. The experimental results indicate the in situ generation of both indole-based ortho-quinodimethanes (o-QDMs) as active dienes and cyclic dienophiles are the key intermediates in these reactions. Moreover, this protocol exhibited good functional group tolerance, broad substrate scope and facile scalability. It would provide great potential for applications in organic synthesis, pharmaceutical chemistry and materials science.
Experimental
1. General procedure for the preparation of the spiro[carbazole-3,3'-inolines] 1a–j and 1a’–j’: A mixture of 2-methyl-1H-indole (0.5 mmol, 1.0 equiv), aldehyde (0.6 mmol, 1.2 equiv), 3-methyleneoxindole (0.5 mmol, 1.0 equiv) and CuSO4 (0.1 mmol, 0.2 equiv) in dry toluene (6.0 mL) was stirred at 110 °C for about three hours. After removing the solvent by evaporating at reduced pressure, the residue was subjected to column chromatography with ethyl acetate and light petroleum (v/v = 1:5–1:8) as eluent to give pure 1a–j and 1a’–j’.
2-Benzoyl-1'-benzyl-4-phenyl-1,2,4,9-tetrahydrospiro[carbazole-3,3'-indolin]-2'-one (1a): Purple solid, 61%, mp 182–185 °C; 1H NMR (400 MHz, CDCl3) δ 8.23 (s, 1H, NH), 7.89 (d, J = 7.2 Hz, 1H, ArH), 7.54 (t, J = 7.2 Hz, 1H, ArH), 7.43–7.39 (m, 3H, ArH), 7.30 (d, J = 8.0 Hz, 1H, ArH), 7.23–7.15 (m, 2H, ArH), 7.14–7.08 (m, 5H, ArH), 6.99 (t, J = 7.6 Hz, 1H, ArH), 6.83–6.73 (m, 3H, ArH), 6.63–6.62 (m, 2H, ArH), 6.36 (d, J = 7.6 Hz, 1H, ArH), 6.28 (t, J = 7.2 Hz, 2H, ArH), 5.00 (s, 1H, CH), 4.83 (dd, J1 = 12.4 Hz, J2 = 5.2 Hz, 1H, CH), 4.60 (d, J = 16.0 Hz, 1H, CH), 4.46 (d, J = 16.0 Hz, 1H, CH), 3.49 (t, J = 12.4 Hz, 1H, CH), 3.26 (dd, J1 = 16.8 Hz, J2 = 5.2 Hz, 1H, CH); 13C NMR (100 MHz, CDCl3) δ 199.3, 178.0, 143.6, 136.6, 136.5, 136.2, 135.2, 133.3, 132.3, 130.9, 130.0, 128.7, 128.6, 128.4, 128.2, 127.9, 127.7, 127.0, 126.9, 126.7, 126.5, 125.6, 121.8, 121.5, 120.2, 119.1, 110.7, 110.7, 109.0, 56.1, 50.0, 48.8, 43.6, 25.4; IR(KBr) υ: 3367, 3210, 3155, 3017, 2980, 2831, 2864, 1877, 1623, 1611, 1507, 1456, 1355, 1241, 1178, 1143, 955, 931, 849, 789 cm−1; HRMS–ESI (m/z): [M + Na]+ calcd for C39H30N2O2, 581.2199; found, 581.2191.
2. General procedure for the preparation of the spiro[carbazole-2,3'-indolines] 2a–g and 2a’–g’: A mixture of 2-methyl-1H-indole (0.5 mmol, 1.0 equiv), aldehyde (0.6 mmol, 1.2 equiv), 2-(1-benzyl-2-oxoindolin-3-ylidene)malononitrile (0.5 mmol, 1.0 equiv) and CuSO4 (0.1 mmol, 0.2 equiv) in dry toluene (6.0 mL) was stirred at 110 °C for about three hours. After removing the solvent by evaporating at reduced pressure, the residue was subjected to column chromatography with ethyl acetate and light petroleum (v/v =1:5–1:8) as eluent to give pure 2a–g and 2a’–g’.
1'-Benzyl-2'-oxo-4-phenyl-4,9-dihydrospiro[carbazole-2,3'-indoline]-3,3(1H)-dicarbonitrile (2a): White solid, 51%, mp 201–204 °C; 1H NMR (400 MHz, CDCl3) δ 8.27 (s, 1H, NH), 7.52–7.46 (m, 3H, ArH), 7.43–7.38 (m, 4H, ArH), 7.35–7.28 (m, 5H, ArH), 7.20 (t, J = 7.6 Hz, 1H, CH), 6.95–6.90 (m, 4H, ArH), 6.54 (d, J = 8.0 Hz, 1H, CH), 5.14 (d, J = 15.6 Hz, 1H, CH), 4.96 (d, J = 15.6 Hz, 1H, CH), 4.89 (s, 1H, CH), 4.10 (dd, J1 = 16.4 Hz, J2 = 2.4 Hz, 1H, CH), 2.96 (d, J = 16.4 Hz, 1H, CH); 13C NMR (100 MHz, CDCl3) δ 173.2, 136.6, 134.7, 133.6, 130.9, 129.4, 128.8, 128.0, 127.6, 125.0, 123.6, 122.7, 120.2, 120.1, 112.7, 111.0, 110.4, 106.9, 51.8, 47.6, 46.2, 44.7, 29.1; IR(KBr) υ: 3355, 3207, 3117, 3048, 2963, 2831, 2167, 1871, 1641, 1633, 1554, 1431, 1370, 1240, 1131, 1100, 972, 961, 881, 764 cm−1; HRMS–ESI (m/z): [M + Na]+ calcd for C34H24N4O, 527.1842; found, 527.1849.
3. General procedure for the preparation of the tetrahydrospiro[carbazole-3,5'-pyrimidines] 3a–c: A mixture of 2-methyl-1H-indole (0.5 mmol, 1.0 equiv), aldehyde (0.6 mmol, 1.2 equiv), 5-arylidene-1,3-dimethylbaribituric acid (0.5 mmol, 1.0 equiv) and CuSO4 (0.1 mmol, 0.2 equiv) in dry toluene (6.0 mL) was stirred at 110 °C for about three hours. After removing the solvent by evaporating at reduced pressure, the mixture of the above obtained product and DDQ (1.0 mmol, 0.227 g, 2.0 equiv) in dry acetonitrile (10.0 mL) was stirred at room temperature for about four hours. After removing the solvent by evaporating at reduced pressure, the residue was subjected to column chromatography with ethyl acetate and light petroleum (v/v = 1:3–1:6) as eluent to give pure products 3a–c.
1',3'-Dimethyl-2,4-di-p-tolyl-2'H-spiro[carbazole-3,5'-pyrimidine]-2',4',6'(1'H,3'H)-trione (3a): Yellow solid, 75%, mp 190–192 °C; 1H NMR (400 MHz, CDCl3) δ 7.59 (d, J = 7.6 Hz, 1H, ArH), 7.35–7.30 (m, 4H, ArH), 7.25–7.17 (m, 2H, ArH), 7.04 (s, 1H, ArH), 6.96–6.91 (m, 4H, ArH), 6.35 (d, J = 7.6 Hz, 1H, CH), 3.10 (s, 3H, CH3), 3.07 (s, 3H, CH3), 2.37 (s, 3H, CH3), 2.34 (s, 3H, CH3); 13C NMR (100 MHz, CDCl3) δ 165.3, 162.7, 157.9, 149.7, 149.0, 147.3, 142.6, 139.1, 138.7, 137.1, 136.0, 133.7, 130.6, 130.3, 130.0, 129.1, 128.7, 128.3, 128.0, 126.3, 126.1, 125.5, 124.7, 124.2, 123.1, 120.8, 29.6, 28.8, 21.4, 21.3; IR (KBr) υ: 3219, 3158, 3043, 2966, 2900, 1843, 1755, 1648, 1617, 1537, 1466, 1358, 1318, 1266, 1150, 987, 899, 765 cm−1; HRMS–ESI (m/z): [M + Na]+ calcd for C31H25N3O3, 510.1788; found, 510.1788.
4. General procedure for the preparation of the spiro[carbazole-3,3'-inolines] 4a–h: A mixture of 2-methyl-1H-indole (0.5 mmol, 1.0 equiv), aldehyde (1.2 mmol, 1.2 equiv), 1,3-dimethylbaribituric acid (0.5 mmol, 1.0 equiv) and CuSO4 (0.1 mmol, 0.2 equiv) in dry toluene (6.0 mL) was stirred at 110 °C for about three hours. After removing the solvent by evaporating at reduced pressure, the residue was subjected to column chromatography with ethyl acetate and light petroleum (v/v = 1:5–1:8) as eluent to give pure 4a–h.
1',3'-Dimethyl-2,4-diphenyl-1,2,4,9-tetrahydro-2'H-spiro[carbazole-3,5'-pyrimidine]-2',4',6'(1'H,3'H)-trione (4a): Purple solid, 82%, mp 205–208 °C; 1H NMR (400 MHz, CDCl3) δ 8.02 (s, 1H, NH), 7.30–7.27 (m, 2H, ArH), 7.25–7.24 (m, 2H, ArH), 7.24–7.22 (m, 2H, ArH), 7.19–7.15 (m, 3H, ArH), 7.15–7.10 (m, 1H, ArH), 7.06 (t, J = 7.2 Hz, 1H, ArH), 6.94 (d, J = 7.2 Hz, 1H, ArH), 6.80 (t, J = 8.0 Hz, 1H, ArH), 6.41 (d, J = 8.0 Hz, 1H, ArH), 5.24 (s, 1H, CH), 4.13 (dd, J1 = 12.0 Hz, J2 = 5.6 Hz, 1H, CH), 3.90–3.82 (m, 1H, CH), 3.05 (dd, J1 = 16.4 Hz, J2 = 5.6 Hz, 1H, CH), 2.97 (s, 3H, CH3), 2.81 (s, 3H, CH3); 13C NMR (100 MHz, CDCl3) δ 171.7, 167.8, 150.0, 138.9, 137.2, 136.0, 134.6, 129.3, 128.9, 128.7, 128.2, 128.2, 128.1, 128.1, 128.1, 126.3, 121.0, 119.7, 118.9, 110.6, 107.5, 62.5, 51.1, 48.2, 28.3, 27.8, 27.7; IR (KBr) υ: 3407, 3078, 2981, 1873, 1744, 1658, 1667, 1582, 1466, 1356, 1321, 1221, 1180, 912, 833 cm−1; HRMS–ESI (m/z): [M + Na]+ calcd for C29H25N3O3, 486.1788; found, 486.1794.
5. General procedure for the preparation of the tetrahydrospiro[carbazole-3,1'-cycloalkane]-diones 5a–k: A mixture of 2-methyl-1H-indole (0.5 mmol, 1.0 equiv), aldehyde (1.2 mmol, 2.4 equiv), 1,3-diones (0.5 mmol, 1.0 equiv) and CuSO4 (0.1 mmol, 0.2 equiv) in dry toluene (6.0 mL) was stirred at 110 °C for about three hours. After removing the solvent by evaporating at reduced pressure, the residue was subjected to column chromatography with ethyl acetate and light petroleum (v/v = 1:5–1:8) as eluent to give pure 5a–k.
2,4-Diphenyl-1,2,4,9-tetrahydrospiro[carbazole-3,1'-cyclopentane]-2',5'-dione (5a): Purple solid, 74%, mp 178–180 °C; 1H NMR (400 MHz, CDCl3) δ 8.02 (s, 1H, NH), 7.30 (d, J = 8.4 Hz, 1H, ArH), 7.28–7.27 (m, 2H, ArH), 7.26–7.25 (m, 1H, ArH), 7.22 (d, J = 8.0 Hz, 1H, ArH), 7.19–7.17 (m, 2H, ArH), 7.14 (t, J = 7.6 Hz, 1H, ArH), 7.06 (t, J = 7.2 Hz, 1H, ArH), 6.92 (d, J = 8.0 Hz, 1H, ArH), 6.79 (t, J = 7.2 Hz, 1H, ArH), 6.39 (d, J = 8.0 Hz, 1H, ArH), 4.87 (s, 1H, CH), 3.97–3.90 (m, 1H, CH), 3.66 (dd, J1 = 12.4 Hz, J2 = 4.8 Hz, 1H, CH), 2.95 (dd, J1 = 16.0 Hz, J2 = 4.8 Hz, 1H, CH), 2.01–1.90 (m, 2H, CH2), 1.39–1.31 (m, 1H, CH), 1.26–1.22 (m, 1H, CH); 13C NMR (100 MHz, CDCl3) δ 218.2, 216.7, 139.1, 137.8, 136.1, 134.7, 129.9, 129.5, 128.8, 128.7, 128.5, 128.2, 127.8, 127.5, 126.3, 121.0, 119.7, 118.9, 110.7, 107.8, 65.9, 48.8, 47.4, 37.3, 36.5, 26.5; IR (KBr) υ: 3200, 3173, 3064, 2978, 1861, 1745, 1677, 1631, 1567, 1467, 1382, 1311, 1254, 1132, 960, 841, 781 cm−1; HRMS–ESI (m/z): [M + Na]+ calcd for C28H23NO2, 428.1621; found, 428.1626.
Supporting Information
The crystallographic data of the compounds 1f (CCDC 2109575), 2b (CCDC 2109576), 2g’ (CCDC 2109577), 3a (CCDC 2109578), 4e (CCDC 2109579) and 5b (CCDC 2109580) have been deposited at the Cambridge Crystallographic Database Center (http://www.ccdc.cam.ac.uk).
| Supporting Information File 1: Characterization data and 1H NMR, 13C NMR, HRMS spectra of the compounds. | ||
| Format: PDF | Size: 11.6 MB | Download |
References
-
Shaikh, M. S.; Karpoormath, R.; Thapliyal, N.; Rane, R. A.; Palkar, M. B.; Faya, A. M.; Patel, H. M.; Alwan, W. S.; Jain, K.; Hampannavar, G. A. Anti-Cancer Agents Med. Chem. 2015, 15, 1049–1065. doi:10.2174/1871520615666150113105405
Return to citation in text: [1] -
Dhara, K.; Mandal, T.; Das, J.; Dash, J. Angew. Chem., Int. Ed. 2015, 54, 15831–15835. doi:10.1002/anie.201508746
Return to citation in text: [1] -
Lin, S.; Liu, J.; Li, H.; Liu, Y.; Chen, Y.; Luo, J.; Liu, S. J. Med. Chem. 2020, 63, 9284–9299. doi:10.1021/acs.jmedchem.0c00433
Return to citation in text: [1] -
Jiang, H.; Sun, J.; Zhang, J. Curr. Org. Chem. 2012, 16, 2014–2025. doi:10.2174/138527212803251604
Return to citation in text: [1] -
Kamtekar, K. T.; Monkman, A. P.; Bryce, M. R. Adv. Mater. (Weinheim, Ger.) 2010, 22, 572–582. doi:10.1002/adma.200902148
Return to citation in text: [1] -
Yang, M.; Park, I. S.; Yasuda, T. J. Am. Chem. Soc. 2020, 142, 19468–19472. doi:10.1021/jacs.0c10081
Return to citation in text: [1] -
An, J.; Yang, X.; Cai, B.; Zhang, L.; Yang, K.; Yu, Z.; Wang, X.; Hagfeldt, A.; Sun, L. ACS Appl. Mater. Interfaces 2020, 12, 46397–46405. doi:10.1021/acsami.0c14952
Return to citation in text: [1] -
Milcendeau, P.; Sabat, N.; Ferry, A.; Guinchard, X. Org. Biomol. Chem. 2020, 18, 6006–6017. doi:10.1039/d0ob01245a
Return to citation in text: [1] -
Sheng, F.-T.; Wang, J.-Y.; Tan, W.; Zhang, Y.-C.; Shi, F. Org. Chem. Front. 2020, 7, 3967–3998. doi:10.1039/d0qo01124j
Return to citation in text: [1] -
Chaudhari, T. Y.; Tandon, V. Org. Biomol. Chem. 2021, 19, 1926–1939. doi:10.1039/d0ob02274h
Return to citation in text: [1] -
Banerjee, A.; Kundu, S.; Bhattacharyya, A.; Sahu, S.; Maji, M. S. Org. Chem. Front. 2021, 8, 2710–2771. doi:10.1039/d1qo00092f
Return to citation in text: [1] -
Kumar, V. P.; Gruner, K. K.; Kataeva, O.; Knölker, H.-J. Angew. Chem., Int. Ed. 2013, 52, 11073–11077. doi:10.1002/anie.201305993
Return to citation in text: [1] -
Cho, S. H.; Yoon, J.; Chang, S. J. Am. Chem. Soc. 2011, 133, 5996–6005. doi:10.1021/ja111652v
Return to citation in text: [1] -
Hernandez-Perez, A. C.; Collins, S. K. Angew. Chem., Int. Ed. 2013, 52, 12696–12700. doi:10.1002/anie.201306920
Return to citation in text: [1] -
Gao, H.; Xu, Q.-L.; Yousufuddin, M.; Ess, D. H.; Kürti, L. Angew. Chem., Int. Ed. 2014, 53, 2701–2705. doi:10.1002/anie.201309973
Return to citation in text: [1] -
Zhao, F.; Li, N.; Zhu, Y.-F.; Han, Z.-Y. Org. Lett. 2016, 18, 1506–1509. doi:10.1021/acs.orglett.6b00012
Return to citation in text: [1] -
Song, L.; Ni, D.; Jia, S.; Pi, R.; Dong, S.; Yang, F.; Tang, J.; Liu, S. Org. Lett. 2020, 22, 1846–1851. doi:10.1021/acs.orglett.0c00145
Return to citation in text: [1] -
Mou, C.; Zhou, L.; Song, R.; Chai, H.; Hao, L.; Chi, Y. R. Org. Lett. 2020, 22, 2542–2547. doi:10.1021/acs.orglett.0c00418
Return to citation in text: [1] -
Zahara, A. J.; Hinds, E. M.; Nguyen, A. L.; Wilkerson-Hill, S. M. Org. Lett. 2020, 22, 8065–8069. doi:10.1021/acs.orglett.0c03007
Return to citation in text: [1] -
Chen, C.; Jiao, H.; Chen, D.; Tang, T.; Xu, Z.-F.; Duan, S.; Li, C.-Y. Org. Biomol. Chem. 2022, 20, 2802–2807. doi:10.1039/d2ob00164k
Return to citation in text: [1] -
Mei, H.; Yu, Y.; Wang, C.; Liu, A.; Han, J. Org. Chem. Front. 2022, 9, 2516–2521. doi:10.1039/d2qo00247g
Return to citation in text: [1] -
Rossi, E.; Abbiati, G.; Pirovano, V. Eur. J. Org. Chem. 2017, 4512–4529. doi:10.1002/ejoc.201700120
Return to citation in text: [1] -
Ren, J.-W.; Zhou, Z.-F.; Xiao, J.-A.; Chen, X.-Q.; Yang, H. Eur. J. Org. Chem. 2016, 1264–1268. doi:10.1002/ejoc.201501619
Return to citation in text: [1] -
Deng, S.; Qu, C.; Jiao, Y.; Liu, W.; Shi, F. J. Org. Chem. 2020, 85, 11641–11653. doi:10.1021/acs.joc.0c01123
Return to citation in text: [1] -
Guo, T.; Han, L.; Wang, T.; Lei, L.; Zhang, J.; Xu, D. J. Org. Chem. 2020, 85, 9117–9128. doi:10.1021/acs.joc.0c01056
Return to citation in text: [1] -
Kuo, C.-W.; Konala, A.; Lin, L.; Chiang, T.-T.; Huang, C.-Y.; Yang, T.-H.; Kavala, V.; Yao, C.-F. Chem. Commun. 2016, 52, 7870–7873. doi:10.1039/c6cc03124b
Return to citation in text: [1] -
Rathore, K. S.; Lad, B. S.; Chennamsetti, H.; Katukojvala, S. Chem. Commun. 2016, 52, 5812–5815. doi:10.1039/c5cc10637k
Return to citation in text: [1] -
Sharma, P.; Kumar, N. P.; Krishna, N. H.; Prasanna, D.; Sridhar, B.; Shankaraiah, N. Org. Chem. Front. 2016, 3, 1503–1508. doi:10.1039/c6qo00430j
Return to citation in text: [1] -
Saha, S.; Maji, M. S. Org. Biomol. Chem. 2020, 18, 1765–1768. doi:10.1039/c9ob02751c
Return to citation in text: [1] -
Wang, T.; Hoye, T. R. J. Am. Chem. Soc. 2016, 138, 13870–13873. doi:10.1021/jacs.6b09628
Return to citation in text: [1] -
Chen, S.; Li, Y.; Ni, P.; Yang, B.; Huang, H.; Deng, G.-J. J. Org. Chem. 2017, 82, 2935–2942. doi:10.1021/acs.joc.6b02892
Return to citation in text: [1] -
Banerjee, A.; Sahu, S.; Maji, M. S. Adv. Synth. Catal. 2017, 359, 1860–1866. doi:10.1002/adsc.201700092
Return to citation in text: [1] -
Banerjee, A.; Maji, M. S. Chem. – Eur. J. 2019, 25, 11521–11527. doi:10.1002/chem.201902268
Return to citation in text: [1] -
Liu, Y.; Nappi, M.; Arceo, E.; Vera, S.; Melchiorre, P. J. Am. Chem. Soc. 2011, 133, 15212–15218. doi:10.1021/ja206517s
Return to citation in text: [1] -
Wang, Y.; Tu, M.-S.; Yin, L.; Sun, M.; Shi, F. J. Org. Chem. 2015, 80, 3223–3232. doi:10.1021/acs.joc.5b00198
Return to citation in text: [1] -
Ren, J.-W.; Wang, J.; Xiao, J.-A.; Li, J.; Xiang, H.-Y.; Chen, X.-Q.; Yang, H. J. Org. Chem. 2017, 82, 6441–6449. doi:10.1021/acs.joc.7b00733
Return to citation in text: [1] -
Huang, L.-J.; Weng, J.; Wang, S.; Lu, G. Adv. Synth. Catal. 2015, 357, 993–1003. doi:10.1002/adsc.201400780
Return to citation in text: [1] -
Sharma, P.; Kumar, N. P.; Krishna, N. H.; Prasanna, D.; Sridhar, B.; Shankaraiah, N. Org. Chem. Front. 2016, 3, 1503–1508. doi:10.1039/c6qo00430j
Return to citation in text: [1] -
You, Z.-H.; Chen, Y.-H.; Tang, Y.; Liu, Y.-K. Org. Lett. 2018, 20, 6682–6686. doi:10.1021/acs.orglett.8b02731
Return to citation in text: [1] -
Yang, R.-Y.; Sun, J.; Tao, Y.; Sun, Q.; Yan, C.-G. J. Org. Chem. 2017, 82, 13277–13287. doi:10.1021/acs.joc.7b02397
Return to citation in text: [1] -
Yang, R.-Y.; Sun, J.; Sun, Q.; Yan, C.-G. J. Org. Chem. 2018, 83, 5909–5919. doi:10.1021/acs.joc.8b00196
Return to citation in text: [1] -
Yang, R.-Y.; Sun, J.; Yan, C.-G. ACS Omega 2018, 3, 5406–5416. doi:10.1021/acsomega.8b00464
Return to citation in text: [1] -
Ye, R.; Yan, C.-G. Eur. J. Org. Chem. 2019, 5882–5886. doi:10.1002/ejoc.201900955
Return to citation in text: [1] -
Wang, D.-Q.; Sun, J.; Yan, C.-G. ChemistrySelect 2019, 4, 10550–10554. doi:10.1002/slct.201902407
Return to citation in text: [1] -
Wang, D.; Sun, J.; Liu, R.-Z.; Wang, Y.; Yan, C.-G. J. Org. Chem. 2021, 86, 5616–5629. doi:10.1021/acs.joc.1c00103
Return to citation in text: [1] -
Yan, C.; Sun, J.; Yan, C.-G. Chin. Chem. Lett. 2021, 32, 1253–1256. doi:10.1016/j.cclet.2020.08.052
Return to citation in text: [1] -
Ye, R.; Sun, J.; Han, Y.; Yan, C.-G. New J. Chem. 2021, 45, 5075–5080. doi:10.1039/d0nj06036d
Return to citation in text: [1] -
Zhan, S.-C.; Fang, R.-J.; Yang, R.-Y.; Zhao, R.-F.; Wang, Y.; Sun, J.; Yan, C.-G. New J. Chem. 2021, 45, 15423–15428. doi:10.1039/d1nj02836g
Return to citation in text: [1] -
Fang, R.-J.; Yan, C.; Sun, J.; Han, Y.; Yan, C.-G. Beilstein J. Org. Chem. 2021, 17, 2425–2432. doi:10.3762/bjoc.17.159
Return to citation in text: [1] -
Yan, C.; Sun, J.; Han, Y.; Yan, C.-G. J. Org. Chem. 2021, 86, 9263–9279. doi:10.1021/acs.joc.1c00044
Return to citation in text: [1] -
Pindur, U.; Erfanian-Abdoust, H. Chem. Rev. 1989, 89, 1681–1689. doi:10.1021/cr00098a002
Return to citation in text: [1] -
Magnus, P.; Gallagher, T.; Brown, P.; Pappalardo, P. Acc. Chem. Res. 1984, 17, 35–41. doi:10.1021/ar00097a006
Return to citation in text: [1] -
Laronze, M.; Sapi, J. Tetrahedron Lett. 2002, 43, 7925–7928. doi:10.1016/s0040-4039(02)01907-x
Return to citation in text: [1] -
Miambo, R. F.; Laronze-Cochard, M.; Lawson, A.-M.; Guillot, R.; Baldeyrou, B.; Lansiaux, A.; Supuran, C. T.; Sapi, J. Tetrahedron 2014, 70, 8286–8302. doi:10.1016/j.tet.2014.09.015
Return to citation in text: [1] -
Rinderspacher, A.; Gribble, G. W. Molecules 2020, 25, 261. doi:10.3390/molecules25020261
Return to citation in text: [1] -
Terzidis, M.; Tsoleridis, C. A.; Stephanidou-Stephanatou, J. Tetrahedron Lett. 2005, 46, 7239–7242. doi:10.1016/j.tetlet.2005.08.048
Return to citation in text: [1] -
Rassu, G.; Curti, C.; Zambrano, V.; Pinna, L.; Brindani, N.; Pelosi, G.; Zanardi, F. Chem. – Eur. J. 2016, 22, 12637–12640. doi:10.1002/chem.201602793
Return to citation in text: [1] -
Gu, B.-Q.; Zhang, H.; Su, R.-H.; Deng, W.-P. Tetrahedron 2016, 72, 6595–6602. doi:10.1016/j.tet.2016.08.077
Return to citation in text: [1] -
Liu, T.-X.; Ma, J.; Chao, D.; Zhang, P.; Ma, N.; Liu, Q.; Shi, L.; Zhang, Z.; Zhang, G. Org. Lett. 2016, 18, 4044–4047. doi:10.1021/acs.orglett.6b01902
Return to citation in text: [1] -
Banerjee, A.; Maji, M. S. Chem. – Eur. J. 2019, 25, 11521–11527. doi:10.1002/chem.201902268
Return to citation in text: [1] -
Kuroda, N.; Takahashi, Y.; Yoshinaga, K.; Mukai, C. Org. Lett. 2006, 8, 1843–1845. doi:10.1021/ol060418n
Return to citation in text: [1] -
Inagaki, F.; Mizutani, M.; Kuroda, N.; Mukai, C. J. Org. Chem. 2009, 74, 6402–6405. doi:10.1021/jo901325d
Return to citation in text: [1] -
Xiao, Y.-C.; Zhou, Q.-Q.; Dong, L.; Liu, T.-Y.; Chen, Y.-C. Org. Lett. 2012, 14, 5940–5943. doi:10.1021/ol302853m
Return to citation in text: [1] -
Liu, Y.; Nappi, M.; Escudero-Adán, E. C.; Melchiorre, P. Org. Lett. 2012, 14, 1310–1313. doi:10.1021/ol300192p
Return to citation in text: [1] -
Xu, J.; Yuan, S.; Miao, M. Org. Lett. 2016, 18, 3822–3825. doi:10.1021/acs.orglett.6b01831
Return to citation in text: [1] -
Chen, X.; Yang, S.; Song, B.-A.; Chi, Y. R. Angew. Chem., Int. Ed. 2013, 52, 11134–11137. doi:10.1002/anie.201305861
Return to citation in text: [1] -
Zhou, L.; Xu, B.; Zhang, J. Angew. Chem., Int. Ed. 2015, 54, 9092–9096. doi:10.1002/anie.201503549
Return to citation in text: [1] -
Wu, X.; Zhu, H.-J.; Zhao, S.-B.; Chen, S.-S.; Luo, Y.-F.; Li, Y.-G. Org. Lett. 2018, 20, 32–35. doi:10.1021/acs.orglett.7b03251
Return to citation in text: [1] -
Diker, K.; Döéde Maindreville, M.; Royer, D.; Le Provost, F.; Lévy, J. Tetrahedron Lett. 1999, 40, 7463–7467. doi:10.1016/s0040-4039(99)01349-0
Return to citation in text: [1] -
Royer, D.; Wong, Y.-S.; Plé, S.; Chiaroni, A.; Diker, K.; Lévy, J. Tetrahedron 2008, 64, 9607–9618. doi:10.1016/j.tet.2008.07.045
Return to citation in text: [1] -
Laudet, B.; Moucadel, V.; Prudent, R.; Filhol, O.; Wong, Y.-S.; Royer, D.; Cochet, C. Mol. Cell. Biochem. 2008, 316, 63–69. doi:10.1007/s11010-008-9821-6
Return to citation in text: [1] -
Kröger, L.; Wünsch, B. Eur. J. Org. Chem. 2018, 6297–6303. doi:10.1002/ejoc.201801162
Return to citation in text: [1] -
Cochard, F.; Laronze, M.; Sigaut, P.; Sapi, J.; Laronze, J.-Y. Tetrahedron Lett. 2004, 45, 1703–1707. doi:10.1016/j.tetlet.2003.12.099
Return to citation in text: [1] -
Dagar, A.; Biswas, S.; Mobin, S. M.; Samanta, S. ChemistrySelect 2016, 1, 6362–6367. doi:10.1002/slct.201601611
Return to citation in text: [1] -
Zhan, S.-C.; Sun, J.; Liu, R.-Z.; Yan, C.-G. Org. Biomol. Chem. 2020, 18, 163–168. doi:10.1039/c9ob02013f
Return to citation in text: [1] [2] [3] [4] -
Zhan, S.-C.; Fang, R.-J.; Sun, J.; Yan, C.-G. J. Org. Chem. 2021, 86, 8726–8741. doi:10.1021/acs.joc.1c00538
Return to citation in text: [1] [2] [3] -
Zhan, S.-C.; Fang, R.-J.; Sun, J.; Yan, C.-G. Org. Biomol. Chem. 2021, 19, 6322–6327. doi:10.1039/d1ob01113h
Return to citation in text: [1] [2] [3]
| 1. | Shaikh, M. S.; Karpoormath, R.; Thapliyal, N.; Rane, R. A.; Palkar, M. B.; Faya, A. M.; Patel, H. M.; Alwan, W. S.; Jain, K.; Hampannavar, G. A. Anti-Cancer Agents Med. Chem. 2015, 15, 1049–1065. doi:10.2174/1871520615666150113105405 |
| 2. | Dhara, K.; Mandal, T.; Das, J.; Dash, J. Angew. Chem., Int. Ed. 2015, 54, 15831–15835. doi:10.1002/anie.201508746 |
| 3. | Lin, S.; Liu, J.; Li, H.; Liu, Y.; Chen, Y.; Luo, J.; Liu, S. J. Med. Chem. 2020, 63, 9284–9299. doi:10.1021/acs.jmedchem.0c00433 |
| 22. | Rossi, E.; Abbiati, G.; Pirovano, V. Eur. J. Org. Chem. 2017, 4512–4529. doi:10.1002/ejoc.201700120 |
| 23. | Ren, J.-W.; Zhou, Z.-F.; Xiao, J.-A.; Chen, X.-Q.; Yang, H. Eur. J. Org. Chem. 2016, 1264–1268. doi:10.1002/ejoc.201501619 |
| 24. | Deng, S.; Qu, C.; Jiao, Y.; Liu, W.; Shi, F. J. Org. Chem. 2020, 85, 11641–11653. doi:10.1021/acs.joc.0c01123 |
| 25. | Guo, T.; Han, L.; Wang, T.; Lei, L.; Zhang, J.; Xu, D. J. Org. Chem. 2020, 85, 9117–9128. doi:10.1021/acs.joc.0c01056 |
| 26. | Kuo, C.-W.; Konala, A.; Lin, L.; Chiang, T.-T.; Huang, C.-Y.; Yang, T.-H.; Kavala, V.; Yao, C.-F. Chem. Commun. 2016, 52, 7870–7873. doi:10.1039/c6cc03124b |
| 27. | Rathore, K. S.; Lad, B. S.; Chennamsetti, H.; Katukojvala, S. Chem. Commun. 2016, 52, 5812–5815. doi:10.1039/c5cc10637k |
| 28. | Sharma, P.; Kumar, N. P.; Krishna, N. H.; Prasanna, D.; Sridhar, B.; Shankaraiah, N. Org. Chem. Front. 2016, 3, 1503–1508. doi:10.1039/c6qo00430j |
| 29. | Saha, S.; Maji, M. S. Org. Biomol. Chem. 2020, 18, 1765–1768. doi:10.1039/c9ob02751c |
| 30. | Wang, T.; Hoye, T. R. J. Am. Chem. Soc. 2016, 138, 13870–13873. doi:10.1021/jacs.6b09628 |
| 31. | Chen, S.; Li, Y.; Ni, P.; Yang, B.; Huang, H.; Deng, G.-J. J. Org. Chem. 2017, 82, 2935–2942. doi:10.1021/acs.joc.6b02892 |
| 32. | Banerjee, A.; Sahu, S.; Maji, M. S. Adv. Synth. Catal. 2017, 359, 1860–1866. doi:10.1002/adsc.201700092 |
| 33. | Banerjee, A.; Maji, M. S. Chem. – Eur. J. 2019, 25, 11521–11527. doi:10.1002/chem.201902268 |
| 34. | Liu, Y.; Nappi, M.; Arceo, E.; Vera, S.; Melchiorre, P. J. Am. Chem. Soc. 2011, 133, 15212–15218. doi:10.1021/ja206517s |
| 35. | Wang, Y.; Tu, M.-S.; Yin, L.; Sun, M.; Shi, F. J. Org. Chem. 2015, 80, 3223–3232. doi:10.1021/acs.joc.5b00198 |
| 36. | Ren, J.-W.; Wang, J.; Xiao, J.-A.; Li, J.; Xiang, H.-Y.; Chen, X.-Q.; Yang, H. J. Org. Chem. 2017, 82, 6441–6449. doi:10.1021/acs.joc.7b00733 |
| 37. | Huang, L.-J.; Weng, J.; Wang, S.; Lu, G. Adv. Synth. Catal. 2015, 357, 993–1003. doi:10.1002/adsc.201400780 |
| 38. | Sharma, P.; Kumar, N. P.; Krishna, N. H.; Prasanna, D.; Sridhar, B.; Shankaraiah, N. Org. Chem. Front. 2016, 3, 1503–1508. doi:10.1039/c6qo00430j |
| 39. | You, Z.-H.; Chen, Y.-H.; Tang, Y.; Liu, Y.-K. Org. Lett. 2018, 20, 6682–6686. doi:10.1021/acs.orglett.8b02731 |
| 40. | Yang, R.-Y.; Sun, J.; Tao, Y.; Sun, Q.; Yan, C.-G. J. Org. Chem. 2017, 82, 13277–13287. doi:10.1021/acs.joc.7b02397 |
| 41. | Yang, R.-Y.; Sun, J.; Sun, Q.; Yan, C.-G. J. Org. Chem. 2018, 83, 5909–5919. doi:10.1021/acs.joc.8b00196 |
| 42. | Yang, R.-Y.; Sun, J.; Yan, C.-G. ACS Omega 2018, 3, 5406–5416. doi:10.1021/acsomega.8b00464 |
| 43. | Ye, R.; Yan, C.-G. Eur. J. Org. Chem. 2019, 5882–5886. doi:10.1002/ejoc.201900955 |
| 44. | Wang, D.-Q.; Sun, J.; Yan, C.-G. ChemistrySelect 2019, 4, 10550–10554. doi:10.1002/slct.201902407 |
| 45. | Wang, D.; Sun, J.; Liu, R.-Z.; Wang, Y.; Yan, C.-G. J. Org. Chem. 2021, 86, 5616–5629. doi:10.1021/acs.joc.1c00103 |
| 46. | Yan, C.; Sun, J.; Yan, C.-G. Chin. Chem. Lett. 2021, 32, 1253–1256. doi:10.1016/j.cclet.2020.08.052 |
| 47. | Ye, R.; Sun, J.; Han, Y.; Yan, C.-G. New J. Chem. 2021, 45, 5075–5080. doi:10.1039/d0nj06036d |
| 48. | Zhan, S.-C.; Fang, R.-J.; Yang, R.-Y.; Zhao, R.-F.; Wang, Y.; Sun, J.; Yan, C.-G. New J. Chem. 2021, 45, 15423–15428. doi:10.1039/d1nj02836g |
| 49. | Fang, R.-J.; Yan, C.; Sun, J.; Han, Y.; Yan, C.-G. Beilstein J. Org. Chem. 2021, 17, 2425–2432. doi:10.3762/bjoc.17.159 |
| 50. | Yan, C.; Sun, J.; Han, Y.; Yan, C.-G. J. Org. Chem. 2021, 86, 9263–9279. doi:10.1021/acs.joc.1c00044 |
| 16. | Zhao, F.; Li, N.; Zhu, Y.-F.; Han, Z.-Y. Org. Lett. 2016, 18, 1506–1509. doi:10.1021/acs.orglett.6b00012 |
| 17. | Song, L.; Ni, D.; Jia, S.; Pi, R.; Dong, S.; Yang, F.; Tang, J.; Liu, S. Org. Lett. 2020, 22, 1846–1851. doi:10.1021/acs.orglett.0c00145 |
| 18. | Mou, C.; Zhou, L.; Song, R.; Chai, H.; Hao, L.; Chi, Y. R. Org. Lett. 2020, 22, 2542–2547. doi:10.1021/acs.orglett.0c00418 |
| 19. | Zahara, A. J.; Hinds, E. M.; Nguyen, A. L.; Wilkerson-Hill, S. M. Org. Lett. 2020, 22, 8065–8069. doi:10.1021/acs.orglett.0c03007 |
| 20. | Chen, C.; Jiao, H.; Chen, D.; Tang, T.; Xu, Z.-F.; Duan, S.; Li, C.-Y. Org. Biomol. Chem. 2022, 20, 2802–2807. doi:10.1039/d2ob00164k |
| 21. | Mei, H.; Yu, Y.; Wang, C.; Liu, A.; Han, J. Org. Chem. Front. 2022, 9, 2516–2521. doi:10.1039/d2qo00247g |
| 8. | Milcendeau, P.; Sabat, N.; Ferry, A.; Guinchard, X. Org. Biomol. Chem. 2020, 18, 6006–6017. doi:10.1039/d0ob01245a |
| 9. | Sheng, F.-T.; Wang, J.-Y.; Tan, W.; Zhang, Y.-C.; Shi, F. Org. Chem. Front. 2020, 7, 3967–3998. doi:10.1039/d0qo01124j |
| 10. | Chaudhari, T. Y.; Tandon, V. Org. Biomol. Chem. 2021, 19, 1926–1939. doi:10.1039/d0ob02274h |
| 11. | Banerjee, A.; Kundu, S.; Bhattacharyya, A.; Sahu, S.; Maji, M. S. Org. Chem. Front. 2021, 8, 2710–2771. doi:10.1039/d1qo00092f |
| 12. | Kumar, V. P.; Gruner, K. K.; Kataeva, O.; Knölker, H.-J. Angew. Chem., Int. Ed. 2013, 52, 11073–11077. doi:10.1002/anie.201305993 |
| 13. | Cho, S. H.; Yoon, J.; Chang, S. J. Am. Chem. Soc. 2011, 133, 5996–6005. doi:10.1021/ja111652v |
| 14. | Hernandez-Perez, A. C.; Collins, S. K. Angew. Chem., Int. Ed. 2013, 52, 12696–12700. doi:10.1002/anie.201306920 |
| 15. | Gao, H.; Xu, Q.-L.; Yousufuddin, M.; Ess, D. H.; Kürti, L. Angew. Chem., Int. Ed. 2014, 53, 2701–2705. doi:10.1002/anie.201309973 |
| 4. | Jiang, H.; Sun, J.; Zhang, J. Curr. Org. Chem. 2012, 16, 2014–2025. doi:10.2174/138527212803251604 |
| 5. | Kamtekar, K. T.; Monkman, A. P.; Bryce, M. R. Adv. Mater. (Weinheim, Ger.) 2010, 22, 572–582. doi:10.1002/adma.200902148 |
| 6. | Yang, M.; Park, I. S.; Yasuda, T. J. Am. Chem. Soc. 2020, 142, 19468–19472. doi:10.1021/jacs.0c10081 |
| 7. | An, J.; Yang, X.; Cai, B.; Zhang, L.; Yang, K.; Yu, Z.; Wang, X.; Hagfeldt, A.; Sun, L. ACS Appl. Mater. Interfaces 2020, 12, 46397–46405. doi:10.1021/acsami.0c14952 |
| 75. | Zhan, S.-C.; Sun, J.; Liu, R.-Z.; Yan, C.-G. Org. Biomol. Chem. 2020, 18, 163–168. doi:10.1039/c9ob02013f |
| 76. | Zhan, S.-C.; Fang, R.-J.; Sun, J.; Yan, C.-G. J. Org. Chem. 2021, 86, 8726–8741. doi:10.1021/acs.joc.1c00538 |
| 77. | Zhan, S.-C.; Fang, R.-J.; Sun, J.; Yan, C.-G. Org. Biomol. Chem. 2021, 19, 6322–6327. doi:10.1039/d1ob01113h |
| 75. | Zhan, S.-C.; Sun, J.; Liu, R.-Z.; Yan, C.-G. Org. Biomol. Chem. 2020, 18, 163–168. doi:10.1039/c9ob02013f |
| 76. | Zhan, S.-C.; Fang, R.-J.; Sun, J.; Yan, C.-G. J. Org. Chem. 2021, 86, 8726–8741. doi:10.1021/acs.joc.1c00538 |
| 77. | Zhan, S.-C.; Fang, R.-J.; Sun, J.; Yan, C.-G. Org. Biomol. Chem. 2021, 19, 6322–6327. doi:10.1039/d1ob01113h |
| 75. | Zhan, S.-C.; Sun, J.; Liu, R.-Z.; Yan, C.-G. Org. Biomol. Chem. 2020, 18, 163–168. doi:10.1039/c9ob02013f |
| 76. | Zhan, S.-C.; Fang, R.-J.; Sun, J.; Yan, C.-G. J. Org. Chem. 2021, 86, 8726–8741. doi:10.1021/acs.joc.1c00538 |
| 77. | Zhan, S.-C.; Fang, R.-J.; Sun, J.; Yan, C.-G. Org. Biomol. Chem. 2021, 19, 6322–6327. doi:10.1039/d1ob01113h |
| 69. | Diker, K.; Döéde Maindreville, M.; Royer, D.; Le Provost, F.; Lévy, J. Tetrahedron Lett. 1999, 40, 7463–7467. doi:10.1016/s0040-4039(99)01349-0 |
| 70. | Royer, D.; Wong, Y.-S.; Plé, S.; Chiaroni, A.; Diker, K.; Lévy, J. Tetrahedron 2008, 64, 9607–9618. doi:10.1016/j.tet.2008.07.045 |
| 71. | Laudet, B.; Moucadel, V.; Prudent, R.; Filhol, O.; Wong, Y.-S.; Royer, D.; Cochet, C. Mol. Cell. Biochem. 2008, 316, 63–69. doi:10.1007/s11010-008-9821-6 |
| 72. | Kröger, L.; Wünsch, B. Eur. J. Org. Chem. 2018, 6297–6303. doi:10.1002/ejoc.201801162 |
| 73. | Cochard, F.; Laronze, M.; Sigaut, P.; Sapi, J.; Laronze, J.-Y. Tetrahedron Lett. 2004, 45, 1703–1707. doi:10.1016/j.tetlet.2003.12.099 |
| 74. | Dagar, A.; Biswas, S.; Mobin, S. M.; Samanta, S. ChemistrySelect 2016, 1, 6362–6367. doi:10.1002/slct.201601611 |
| 51. | Pindur, U.; Erfanian-Abdoust, H. Chem. Rev. 1989, 89, 1681–1689. doi:10.1021/cr00098a002 |
| 52. | Magnus, P.; Gallagher, T.; Brown, P.; Pappalardo, P. Acc. Chem. Res. 1984, 17, 35–41. doi:10.1021/ar00097a006 |
| 53. | Laronze, M.; Sapi, J. Tetrahedron Lett. 2002, 43, 7925–7928. doi:10.1016/s0040-4039(02)01907-x |
| 54. | Miambo, R. F.; Laronze-Cochard, M.; Lawson, A.-M.; Guillot, R.; Baldeyrou, B.; Lansiaux, A.; Supuran, C. T.; Sapi, J. Tetrahedron 2014, 70, 8286–8302. doi:10.1016/j.tet.2014.09.015 |
| 55. | Rinderspacher, A.; Gribble, G. W. Molecules 2020, 25, 261. doi:10.3390/molecules25020261 |
| 56. | Terzidis, M.; Tsoleridis, C. A.; Stephanidou-Stephanatou, J. Tetrahedron Lett. 2005, 46, 7239–7242. doi:10.1016/j.tetlet.2005.08.048 |
| 57. | Rassu, G.; Curti, C.; Zambrano, V.; Pinna, L.; Brindani, N.; Pelosi, G.; Zanardi, F. Chem. – Eur. J. 2016, 22, 12637–12640. doi:10.1002/chem.201602793 |
| 58. | Gu, B.-Q.; Zhang, H.; Su, R.-H.; Deng, W.-P. Tetrahedron 2016, 72, 6595–6602. doi:10.1016/j.tet.2016.08.077 |
| 59. | Liu, T.-X.; Ma, J.; Chao, D.; Zhang, P.; Ma, N.; Liu, Q.; Shi, L.; Zhang, Z.; Zhang, G. Org. Lett. 2016, 18, 4044–4047. doi:10.1021/acs.orglett.6b01902 |
| 60. | Banerjee, A.; Maji, M. S. Chem. – Eur. J. 2019, 25, 11521–11527. doi:10.1002/chem.201902268 |
| 61. | Kuroda, N.; Takahashi, Y.; Yoshinaga, K.; Mukai, C. Org. Lett. 2006, 8, 1843–1845. doi:10.1021/ol060418n |
| 62. | Inagaki, F.; Mizutani, M.; Kuroda, N.; Mukai, C. J. Org. Chem. 2009, 74, 6402–6405. doi:10.1021/jo901325d |
| 63. | Xiao, Y.-C.; Zhou, Q.-Q.; Dong, L.; Liu, T.-Y.; Chen, Y.-C. Org. Lett. 2012, 14, 5940–5943. doi:10.1021/ol302853m |
| 64. | Liu, Y.; Nappi, M.; Escudero-Adán, E. C.; Melchiorre, P. Org. Lett. 2012, 14, 1310–1313. doi:10.1021/ol300192p |
| 65. | Xu, J.; Yuan, S.; Miao, M. Org. Lett. 2016, 18, 3822–3825. doi:10.1021/acs.orglett.6b01831 |
| 66. | Chen, X.; Yang, S.; Song, B.-A.; Chi, Y. R. Angew. Chem., Int. Ed. 2013, 52, 11134–11137. doi:10.1002/anie.201305861 |
| 67. | Zhou, L.; Xu, B.; Zhang, J. Angew. Chem., Int. Ed. 2015, 54, 9092–9096. doi:10.1002/anie.201503549 |
| 68. | Wu, X.; Zhu, H.-J.; Zhao, S.-B.; Chen, S.-S.; Luo, Y.-F.; Li, Y.-G. Org. Lett. 2018, 20, 32–35. doi:10.1021/acs.orglett.7b03251 |
| 75. | Zhan, S.-C.; Sun, J.; Liu, R.-Z.; Yan, C.-G. Org. Biomol. Chem. 2020, 18, 163–168. doi:10.1039/c9ob02013f |
© 2022 Zhan et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.