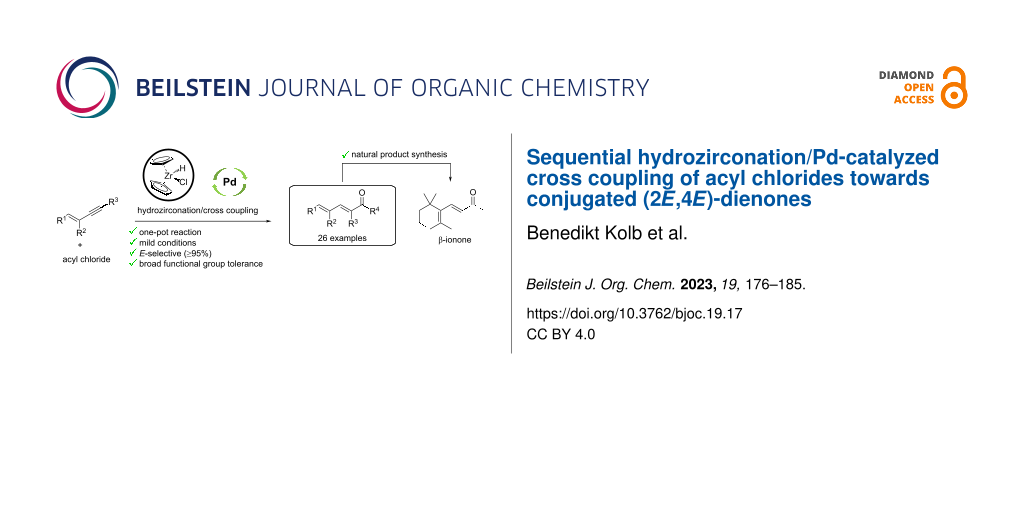
Abstract
Dienones are challenging building blocks in natural product synthesis due to their high reactivity and complex synthesis. Based on previous work and own initial results, a new stereospecific sequential hydrozirconation/Pd-catalyzed acylation of enynes with acyl chlorides towards conjugated (2E,4E)-dienones is reported. We investigated a number of substrates with different alkyl and aryl substituents in the one-pot reaction and showed that regardless of the substitution pattern, the reactions lead to the stereoselective formation (≥95% (2E,4E)) of the respective dienones under mild conditions. It was found that enynes with alkyl chains gave higher yields than the corresponding aryl-substituted analogues, whereas the variation of the acyl chlorides did not affect the reaction significantly. The synthetic application is demonstrated by formation of non-natural and natural dienone-containing terpenes such as β-ionone which was available in 4 steps and 6% overall yield.
Graphical Abstract
Introduction
Conjugated dienones are recurring structural motifs in natural products. Several biologically relevant compounds carry (2Ε,4E)-unsaturated ketones or the corresponding esters or amides. Selected examples are clifednamide H (1) which displays pronounced cytotoxicity against two human cancer cell lines [1], epicocconone (2), a fluorescent compound from the fungus Epicoccum nigrum [2], β-ionone (3) from many plant-derived sources [3], epoxysorbicillinol (4) from the saltwater culture of the fungus Trichoderma longibrachiatum separated from a haliclona marine sponge [4], and vertinolide (5) from Verticillium intertextum [5] (Scheme 1).
Scheme 1: Examples of biologically active compounds with (2Ε,4E)-unsaturated ketone units.
Scheme 1: Examples of biologically active compounds with (2Ε,4E)-unsaturated ketone units.
As outlined in Scheme 2, a variety of methods has been reported for the synthesis of conjugated dienones, mostly via addition/elimination reactions such as Knoevenagel condensation or Claisen–Schmidt condensation of enals 6 with aldehydes 7a or ketones 7b [6-11], isomerization of alkynones 8 [12-15], Horner–Wadsworth–Emmons reaction of unsaturated phosphonates 9 and aldehydes 10 [16,17], and dehydrogenation of enones 11 [18]. Further, Claisen rearrangement of vinyl propargylic ethers 12 [19] and metal-catalyzed cross coupling of alkenes 13 and enones 14 [20,21] have been reported.
Scheme 2: Selected examples for the synthesis of conjugated dienones from the literature [6-21].
Scheme 2: Selected examples for the synthesis of conjugated dienones from the literature [6-21].
However, these reactions face multiple disadvantages such as limited substrate scope, use of hazardous solvents and harsh reaction conditions such as high temperatures or acidic/basic conditions, which might be incompatible with existing functional groups and/or the stereochemical integrity [18,22]. Especially in natural product synthesis, dienone functional groups suffer from isomerization and polymerization [23]. Therefore, a late stage introduction of dienone units is advantageous [24].
Since the early work by Wailes, Schwartz and Buchwald on the Schwartz reagent Cp2Zr(H)Cl and its reactivity towards alkynes, alkenes, and C–X double bonds particularly hydrozirconation has gained much attention [25-30]. It has been successfully employed in methodology studies [31-40] as well as in several total syntheses of natural products [41-46]. Especially the combination of hydrozirconation and Pd or Ni-catalyzed cross coupling was elaborated by several groups (Scheme 3) [47-54].
Scheme 3: Previous work of hydrozirconations with Schwartz's reagent and our work [54,55,57,58,61,62].
Scheme 3: Previous work of hydrozirconations with Schwartz's reagent and our work [54,55,57,58,61,62].
Negishi extended Crombie’s work on dienamides and prepared dienoates 15 by hydrozirconation of terminal alkynes 16 followed by Pd-catalyzed cross coupling with enoates 17 [55,56]. A repetitive approach gave rise to oligoenoates [57]. Hydrozirconations were also combined with carbonylations to install carbonyl groups. For example, the sequential hydrozirconation/carbonylation of propargylic ethers 18 reported by Donato [58] yielded α,β-unsaturated lactones 19. Beside the hydrozirconation/acylation sequence of nitriles utilizing acid chlorides published by Majoral/Floreancig [59,60], Cox revealed that terminal alkynes 16 could be converted to enones 20 by hydrozirconation followed by Pd-catalyzed acylation with acyl chlorides 21 [61]. The substrate scope required aryl units at either alkyne or acid chloride unit. Recently, we could extend this method to alkyl-substituted alkynes 16 and acetyl chloride (22), providing enone building blocks 23 for the synthesis of clifednamides [54,62]. The addition of Schwartz's reagent proceeds as a syn-addition affording (E)-alkenylzirconocenes 24 from terminal alkynes [28]. Based on these precedents from the literature, we surmised that it might be possible to establish a related approach to convert (E)-enynes 25 via hydrozirconation to the corresponding (E)-alkenylzirconocenes 24 and subsequent Pd-catalyzed acylation to conjugated (2E,4E)-dienones 27.
Results and Discussion
The investigation of the hydrozirconation/acylation sequence required first the synthesis of (E)-enynes 25 via Corey–Fuchs reaction (Table 1) [63]. Starting from different substituted, (E)-configurated enals 28, the respective dibromo-olefines 29 were formed by reaction of 28 with 4 equiv of PPh3 and 2 equiv of CBr4 for 3 h. Treating 29 with 2.2 equiv of n-butyllithium for 1 h resulted in the desired enynes 25a–e in yields up to 77%. Unfortunately, in the case of nitro-substituted enyne 25d only 4% were isolated due to rapid decomposition and instability issues (Table 1, entry 4).
With enynes 25a–e in hand, the influence of solvent, reaction temperature, time, and Pd source on the hydrozirconation and subsequent coupling were examined by using phenylenyne 25a and benzoyl chloride (26a) as benchmark substrates. The results are summarized in Table 2. For example, treatment of phenylenyne 25a with 1.12 equiv of commercially available Schwartz reagent in THF at 50 °C for 1 h and subsequent treatment with benzoyl chloride (26a) in the presence of 5 mol % of Pd(PPh3)2Cl2 for 20 h at room temperature yielded 49% of the desired (2E,4E)-configurated dienone 27a (Table 2, entry 1). No other stereoisomer was detected in the crude product, suggesting an all E-configuration ≥95% (via 1H NMR). Accordingly, the internal double bond in 25a stayed unaffected whereas the triple bond, as expected, selectively formed the double bond with (E)-configuration. Note that a reaction temperature of 50 °C is required in the first step of the sequence to obtain rapid solubility of the Schwartz reagent in the solvent but is not necessarily required in the subsequent steps. When the reaction was carried out in benzene, CH2Cl2 or dioxane, much lower yields of 28%, 31%, and 8%, respectively, were obtained (Table 2, entries 2–4). Toluene gave the best yield with 55% (Table 2, entry 5). Therefore, further optimization steps were performed with toluene. By running the reaction at room temperature and decreased reaction times (3 h), the yield decreased to 28% (Table 2, entry 6). On increasing the reaction temperature to 50 °C instead, the product was isolated in 31% yield (Table 2, entry 7). Longer reaction times of 20 h at 50 °C led only to 8% yield (Table 2, entry 8).
Table 2: Hydrozirconation and Pd-catalyzed cross coupling of 25a and 26a with various solvents, reaction times, and temperatures.
|
|
||||
| entry | solvent | temp. [°C] | time [h] | yield 27a [%] |
| 1 | THF | rt | 20 | 49 |
| 2 | benzene | rt | 20 | 28 |
| 3 | CH2Cl2 | rt | 20 | 31 |
| 4 | dioxane | rt | 20 | 8 |
| 5 | toluene | rt | 20 | 55 |
| 6 | toluene | rt | 3 | 28 |
| 7 | toluene | 50 | 3 | 31 |
| 8 | toluene | 50 | 20 | 8 |
Trapping experiments of the in situ-formed (E)-alkenylzirconocene 24 revealed quantitative conversion of the starting material 25a and only traces of chain walking (less than 4%) (for details see Supporting Information File 1, chapter 2.1). To optimize the second step of the reaction sequence, several Pd complexes were tested in the reaction of 25a with 26a. However, the yields of the dienone 27a decreased considerably, when (Ph3P)2PdCl2 was replaced by other Pd complexes (Table 3, entries 2–5). In particular, (AntPhos)2Pd(dba) and (XPhos)2Pd(dba) were catalytically inactive (Table 3, entries 6 and 7). Furthermore, in situ-formed Schwartz reagent was found to be less effective as compared to the use of isolated Cp2Zr(H)Cl and thus further experiments in this direction were abandoned.
In order to explore the substrate scope of the sequential reaction, different enynes 25 and acid chlorides 26 were studied (Table 4). Fortunately, all desired products 27 were formed in ≥95% (2E,4E)-configuration (via 1H NMR). First, phenylenyne 25a (R1 = Ph) was chosen as the starting material and reacted with different acid chlorides 26a–s (Table 4). While 4-methyl-benzoic acid chloride (26b) behaved similarly to benzoyl chloride (26a) giving 27ab in a slightly higher yield of 57% (Table 4, entry 2), the yield of 27ac dropped to only 17% upon use of the corresponding 2-methylbenzoyl chloride (26c) (Table 4, entry 3). By attaching further electron-donating or electron-withdrawing groups at the benzoyl chloride 26 (Table 4, entries 4–8), the yields of the desired products decreased to 10–30% compared to 27ab. Benzoyl chlorides carrying multiple substituents, such as 2,4,6-trichlorobenzoyl chloride (26i), 3,5-dinitrobenzoyl chloride (26j), 3,4,5-trimethoxybenzoyl chloride (26k), and pentafluorobenzoyl chloride (26l) were also tested, but did not give any trace of the respective dienone 27 (Table 4, entries 9–12). Consequently, the hydrozirconation and Pd-catalyzed cross coupling is rather sensitive towards both electron-donating and electron-withdrawing substituents at the benzoyl moiety of 25. The analysis of crude 1H NMR spectra of 27 indicated decomposition and side product formation (for details see Supporting Information File 1, chapter 2.2). Unfortunately, due to the poor amount of side products, these compounds could not be isolated by chromatography and HPLC purification steps. However, GC–MS analysis of the crude product of one exemplary dienone 27ac (with only 17% yield) indicated only decomposition in the reaction sequence.
Table 4: Hydrozirconation and Pd-catalyzed cross coupling of enyne 25 and acyl-chlorides 26.
|
|
||||||
| entry | R1 | 25 | R2 | 26 | yield 27 [%] | 27 |
| 1 | Ph | a | Ph | a | 55 | aa |
| 2 | Ph | a | 4-Me-C6H4 | b | 57 | ab |
| 3 | Ph | a | 2-Me-C6H4 | c | 17 | ac |
| 4 | Ph | a | 4-F-C6H4 | d | 19 | ad |
| 5 | Ph | a | 4-Cl-C6H4 | e | 10 | ae |
| 6 | Ph | a | 4-Br-C6H4 | f | 20 | af |
| 7 | Ph | a | 4-NO2-C6H4 | g | 30 | ag |
| 8 | Ph | a | 4-MeO-C6H4 | h | 14 | ah |
| 9 | Ph | a | 2,4,6-Cl3-C6H2 | i | – | ai |
| 10 | Ph | a | 2,4-NO2-C6H3 | j | – | aj |
| 11 | Ph | a | 3,4,5-OMe-C6H2 | k | – | ak |
| 12 | Ph | a | C6F5 | l | – | al |
| 13 | Ph | a | C2H2-Ph | m | 12 | am |
| 14 | Ph | a | C8H17 | n | 43 | an |
| 15 | Ph | a | C2H2-Me | o | 40 | ao |
| 16 | Ph | a | t-Bu | p | 32 | ap |
| 17 | Ph | a | Me | 22 | 32 | aq |
| 18 | Ph | a | OEt | r | 22 | ar |
| 19 | Ph | a | CH2Cl | s | 23 | as |
| 20 | 4-Me-C6H4 | b | Ph | a | 9 | ba |
| 21 | 4-Cl-C6H4 | c | Ph | a | 17 | ca |
| 22 | 4-NO2-C6H4 | d | Ph | a | 10 | da |
| 23 | n-C7H15 | e | Ph | a | 34 | ea |
| 24 | n-C7H15 | e | Me | 22 | 65 | eq |
Further, phenylenyne 25a was treated with different conjugated and aliphatic acyl chlorides 26m–p (Table 4, entries 13–16). While the reaction with cinnamoyl chloride (26m) gave only 12% of desired dienone 27am (Table 4, entry 13), the yield increased up to 40% by using crotonoyl chloride 26o instead (Table 4, entry 15). In general, by using aliphatic acyl chlorides, the yields increased, e.g., nonanoyl chloride (26n) yielded 43% of the desired product 27an (Table 4, entry 14) and even the sterically hindered pivaloyl chloride (26p) gave 32% of dienone 27ap (Table 4, entry 16). Due to these promising results, we tested the combination of phenylenyne 25a with acetyl chloride (22), which however provided 32% of the corresponding dienone 27aq (Table 4, entry 17). The reaction with ethyl chloroformate (26r) and chloroacetyl chloride (26s) gave decreased yields with 22% and 23% (Table 4, entries 18 and 19).
Next, the variation of enynes 25 was investigated (Table 4, entries 20–24). The sequence again showed its sensitivity towards both electron-donating and electron-withdrawing substituents on the phenyl group of the enyne 25. The reaction of 4-methylphenylenyne 25b with benzoyl chloride (26a, Table 4, entry 20) led to the desired product 27ba in only 9% yield. With 4-chlorophenylenyne 25c as well as 4-nitrophenylenyne 25d, 17% and 10% yield of product were obtained, respectively (Table 4, entries 21 and 22). In contrast, aliphatic enynes showed promising results. Due to their high volatility, we limited the following experiments to enyne 25e with a long alkyl chain. Reaction of 25e with benzoyl chloride (26a) gave 34% of the dienone 27ea (Table 4, entry 23), whereas the reaction with acetyl chloride (22) gave 65% yield of the desired product 27eq (Table 4, entry 24).
In the following series of experiments, substituted enynes 25f–o were employed, which were synthesized beforehand via Corey–Fuchs reaction [63] starting from (E)-configurated enals 28a and 28f forming 29a and 29f in 95% and 86% yield, respectively (Scheme 4). The dibromo-olefines 29a and 29f were then each treated with 2.2 equiv of n-butyllithium for 1 h to form enynes 25a and 25f in 48% yield. Whereas treating 29a and 29f with 2.2 equiv of n-butyllithium and 5 equiv of alkyl iodide led to isolation of the alkyl-substituted compounds 25g–j with up to 54% yield. The reaction of 29a and 29f with TBAF·H2O gave bromoenynes 25m in 13% and 25n in 52% yield. Deprotonation of 25a and 25f with n-butyllithium and reaction with ethyl chloroformate yielded 25k and 25l in 55% and 82% yield, respectively.
Scheme 4: Synthesis of substituted enynes 25f–o via Corey–Fuchs reaction and Hunsdiecker reaction.
Scheme 4: Synthesis of substituted enynes 25f–o via Corey–Fuchs reaction and Hunsdiecker reaction.
Furthermore, methylated enyne 25o was obtained via Hunsdiecker reaction [64] with subsequent Pd-catalyzed Kumada coupling [65]. Therefore, 4-methylcinnamic acid (30) was treated with triethylamine and NBS first. After isolating the respective bromide 31 in 22% yield, it was subsequently coupled with ethynylmagnesium bromide to form enyne 25o in 41% yield.
With the substituted enynes 25f–o in hand, the hydrozirconation and cross coupling was investigated. Therefore, 4-phenyl-3-methylenyne 25f reacted with benzoyl chloride (26a) smoothly to the dienone 27fa in 55% yield (Table 5, entry 1). In agreement with the previous observations, methyl substituents at the aryl moiety and/or the alkyne terminus compromised the yield (Table 5, entries 3 and 10). Furthermore, dienones 27g,i–n with bromo-, ethyl-, and ethoxycarbonyl substituents were not accessible through this approach.
Table 5: Hydrozirconation and Pd-catalyzed cross coupling of substituted enynes 25f–o and acyl chloride 26a.
|
|
||||||
| entry | 25 | R1 | R2 | R3 | 27 | yield [%] |
| 1 | f | Ph | Me | H | fa | 55 |
| 2 | g | Ph | H | Me | ga | 0 |
| 3 | h | Ph | Me | Me | ha | 17 |
| 4 | i | Ph | H | Et | ia | 0 |
| 5 | j | Ph | Me | Et | ja | 0 |
| 6 | k | Ph | H | COOEt | ka | 0 |
| 7 | l | Ph | Me | COOEt | la | 0 |
| 8 | m | Ph | H | Br | ma | 0 |
| 9 | n | Ph | Me | Br | na | 0 |
| 10 | o | 4-Me-C6H4 | H | Me | oa | 10 |
As the sequential hydrozirconation/Pd-catalyzed acylation worked reasonably well for aliphatic substrates, we surmised that terpene-derived enynes might be suitable starting materials for natural product synthesis. For this purpose, two terpene enynes 25p and 25q were synthesized and investigated in the hydrozirconation/acylation sequence (Scheme 5).
Scheme 5: Synthesis of non-natural (a) and natural (b) dienone-containing terpenes: synthesis of β-ionone (3).
Scheme 5: Synthesis of non-natural (a) and natural (b) dienone-containing terpenes: synthesis of β-ionone (3)....
Following the Corey–Fuchs procedure as described above, enyne 25p was synthesized using (−)-myrtenal (28p) as starting material in 77% yield. The synthesis of enyne 25q started from 2,6-dimethylcyclohexanone (32), which was deprotonated with LDA at −78 °C in THF and subsequently methylated to give 34 in 99%, followed by treatment with alkynyl Grignard reagent to give the tertiary alcohol 35 in 71% yield. Final elimination with MsCl and NEt3 yielded the desired enyne 25q (49%). When terpene enynes 25p and 25q were submitted to the hydrozirconation/Pd-catalyzed acylation sequence with benzoyl chloride (26a), the corresponding dienones 27pa and 27qa could be isolated in 47% and 25% yield, respectively. The reaction of 25q with acetyl chloride (22) gave the fragrant β-ionone (3) in 18% yield.
Conclusion
Based on initial results by Cox on the sequential hydrozirconation/Pd-catalyzed acylation of alkynes with acyl chlorides, the scope was extended towards a novel stereospecific dienone synthesis starting from enynes 25 and acyl chlorides 26. Our results revealed that the reaction sequence formed selectively (2E,4E)-dienones 27 (≥95% (2E,4E)) under mild conditions, as the acetylene moiety in substrates 25 only reacted to the (E)-olefin while the internal double bond stayed unaffected. Compared to the reaction with benzoyl chloride (26a), which led to the desired dienone 27aa in 55% yield, aliphatic or conjugated acyl chlorides did not affect the reaction with phenylenyne 25a significantly. However, in case of substituted aromatic acyl chlorides, both electron-donating and electron-withdrawing substituents at the aryl unit, decreased the yield remarkably up to 10%. The same effect was observed in the reaction of substituted phenylenynes 25b–d with benzoyl chloride (26a). The best results were obtained in the reaction of aliphatic enyne 25e with benzoyl chloride (26a) and acetyl chloride (22) in yields of 34% and 65%, respectively. Methyl substitution on the alkene functionality of enyne 25 did not affect the yield, however, methyl substitution at the alkyne terminus significantly decreased the yield. Finally, non-natural and natural dienone-containing terpenes were synthesized such as β-ionone (3), which was available in 4 steps (6% overall yield). Thereby, the synthetic utility was demonstrated by a late-stage introduction of the dienone unit by a hydrozirconation/acylation sequence.
Supporting Information
| Supporting Information File 1: Experimental part. | ||
| Format: PDF | Size: 3.1 MB | Download |
Funding
Generous financial support by the Deutsche Forschungsgemeinschaft (shared instrumentation grant no INST 41/897-1 FUGG for 700 MHz), the Ministerium für Wissenschaft, Forschung und Kunst des Landes Baden-Württemberg, the Fonds der Chemischen Industrie, the Merck KGaA, Darmstadt, is gratefully acknowledged.
References
-
Jiao, Y.-J.; Liu, Y.; Wang, H.-X.; Zhu, D.-Y.; Shen, Y.-M.; Li, Y.-Y. J. Nat. Prod. 2020, 83, 2803–2808. doi:10.1021/acs.jnatprod.0c00900
Return to citation in text: [1] -
Bell, P. J. L.; Karuso, P. J. Am. Chem. Soc. 2003, 125, 9304–9305. doi:10.1021/ja035496+
Return to citation in text: [1] -
Sabetay, S. Compt. Rend. 1930, 189, 808–809.
Return to citation in text: [1] -
Sperry, S.; Samuels, G. J.; Crews, P. J. Org. Chem. 1998, 63, 10011–10014. doi:10.1021/jo9808122
Return to citation in text: [1] -
Trifonov, L. S.; Dreiding, A. S.; Hoesch, L.; Rast, D. M. Helv. Chim. Acta 1981, 64, 1843–1846. doi:10.1002/hlca.19810640616
Return to citation in text: [1] -
Crouch, I. T.; Dreier, T.; Frantz, D. E. Angew. Chem., Int. Ed. 2011, 50, 6128–6132. doi:10.1002/anie.201101820
Return to citation in text: [1] [2] -
Li, C.; Li, M.; Zhong, W.; Jin, Y.; Li, J.; Wu, W.; Jiang, H. Org. Lett. 2019, 21, 872–875. doi:10.1021/acs.orglett.8b03606
Return to citation in text: [1] [2] -
Yuan, F.-Q.; Han, F.-S. Org. Lett. 2012, 14, 1218–1221. doi:10.1021/ol203444g
Return to citation in text: [1] [2] -
He, Y.-H.; Hu, Y.; Guan, Z. Synth. Commun. 2011, 41, 1617–1628. doi:10.1080/00397911.2010.490626
Return to citation in text: [1] [2] -
Liu, C.; Zhou, H.; Sheng, X. B.; Liu, X. H.; Chen, F. H. Bioorg. Chem. 2020, 102, 104077. doi:10.1016/j.bioorg.2020.104077
Return to citation in text: [1] [2] -
Polaquini, C. R.; Torrezan, G. S.; Santos, V. R.; Nazaré, A. C.; Campos, D. L.; Almeida, L. A.; Silva, I. C.; Ferreira, H.; Pavan, F. R.; Duque, C.; Regasini, L. Molecules 2017, 22, 1685. doi:10.3390/molecules22101685
Return to citation in text: [1] [2] -
Trost, B. M.; Biannic, B. Org. Lett. 2015, 17, 1433–1436. doi:10.1021/acs.orglett.5b00279
Return to citation in text: [1] [2] -
Trost, B. M.; Kazmaier, U. J. Am. Chem. Soc. 1992, 114, 7933–7935. doi:10.1021/ja00046a062
Return to citation in text: [1] [2] -
Trost, B. M.; Li, C.-J. J. Am. Chem. Soc. 1994, 116, 10819–10820. doi:10.1021/ja00102a071
Return to citation in text: [1] [2] -
Kwong, C. K.-W.; Fu, M. Y.; Lam, C. S.-L.; Toy, P. H. Synthesis 2008, 2307–2317. doi:10.1055/s-2008-1067173
Return to citation in text: [1] [2] -
Poulsen, P. H.; Vergura, S.; Monleón, A.; Jørgensen, D. K. B.; Jørgensen, K. A. J. Am. Chem. Soc. 2016, 138, 6412–6415. doi:10.1021/jacs.6b03546
Return to citation in text: [1] [2] -
Liu, D.-N.; Tian, S.-K. Chem. – Eur. J. 2009, 15, 4538–4542. doi:10.1002/chem.200900177
Return to citation in text: [1] [2] -
Pan, G.-F.; Zhang, X.-L.; Zhu, X.-Q.; Guo, R.-L.; Wang, Y.-Q. iScience 2019, 20, 229–236. doi:10.1016/j.isci.2019.09.027
Return to citation in text: [1] [2] [3] -
Motika, S. E.; Wang, Q.; Ye, X.; Shi, X. Org. Lett. 2015, 17, 290–293. doi:10.1021/ol503393a
Return to citation in text: [1] [2] -
Xu, Y.-H.; Lu, J.; Loh, T.-P. J. Am. Chem. Soc. 2009, 131, 1372–1373. doi:10.1021/ja8084548
Return to citation in text: [1] [2] -
Yu, H.; Jin, W.; Sun, C.; Chen, J.; Du, W.; He, S.; Yu, Z. Angew. Chem., Int. Ed. 2010, 49, 5792–5797. doi:10.1002/anie.201002737
Return to citation in text: [1] [2] -
Goswami, P.; Das, B. Tetrahedron Lett. 2009, 50, 897–900. doi:10.1016/j.tetlet.2008.12.036
Return to citation in text: [1] -
Wood, J. L.; Thompson, B. D.; Yusuff, N.; Pflum, D. A.; Matthäus, M. S. P. J. Am. Chem. Soc. 2001, 123, 2097–2098. doi:10.1021/ja0057979
Return to citation in text: [1] -
Harned, A. M.; Volp, K. A. Nat. Prod. Rep. 2011, 28, 1790–1810. doi:10.1039/c1np00039j
Return to citation in text: [1] -
Wailes, P. C.; Weigold, H. J. Organomet. Chem. 1970, 24, 405–411. doi:10.1016/s0022-328x(00)80281-8
Return to citation in text: [1] -
Schwartz, J.; Labinger, J. A. Angew. Chem., Int. Ed. Engl. 1976, 15, 333–340. doi:10.1002/anie.197603331
Return to citation in text: [1] -
Buchwald, S. L.; LaMaire, S. J.; Nielsen, R. B.; Watson, B. T.; King, S. M. Org. Synth. 1993, 71, 77–82. doi:10.15227/orgsyn.071.0077
Return to citation in text: [1] -
Némethová, I.; Šebesta, R. Synthesis 2021, 53, 447–460. doi:10.1055/s-0040-1706055
Return to citation in text: [1] [2] -
Pinheiro, D. L. J.; de Castro, P. P.; Amarante, G. W. Eur. J. Org. Chem. 2018, 4828–4844. doi:10.1002/ejoc.201800852
Return to citation in text: [1] -
Więcław, M. M.; Stecko, S. Eur. J. Org. Chem. 2018, 6601–6623. doi:10.1002/ejoc.201701537
Return to citation in text: [1] -
Coelho, A.; Souvenir Zafindrajaona, M.-S.; Vallée, A.; Behr, J.-B.; Vasse, J.-L. Chem. – Eur. J. 2022, 28, e202103789. doi:10.1002/chem.202103789
Return to citation in text: [1] -
Yang, C.; Gao, Y.; Bai, S.; Jiang, C.; Qi, X. J. Am. Chem. Soc. 2020, 142, 11506–11513. doi:10.1021/jacs.0c03821
Return to citation in text: [1] -
Hostmann, T.; Neveselý, T.; Gilmour, R. Chem. Sci. 2021, 12, 10643–10648. doi:10.1039/d1sc02454j
Return to citation in text: [1] -
Gao, Y.; Yang, C.; Bai, S.; Liu, X.; Wu, Q.; Wang, J.; Jiang, C.; Qi, X. Chem 2020, 6, 675–688. doi:10.1016/j.chempr.2019.12.010
Return to citation in text: [1] -
Némethová, I.; Vargová, D.; Mudráková, B.; Filo, J.; Šebesta, R. J. Organomet. Chem. 2020, 908, 121099. doi:10.1016/j.jorganchem.2019.121099
Return to citation in text: [1] -
Ardkhean, R.; Roth, P. M. C.; Maksymowicz, R. M.; Curran, A.; Peng, Q.; Paton, R. S.; Fletcher, S. P. ACS Catal. 2017, 7, 6729–6737. doi:10.1021/acscatal.7b01453
Return to citation in text: [1] -
Rideau, E.; You, H.; Sidera, M.; Claridge, T. D. W.; Fletcher, S. P. J. Am. Chem. Soc. 2017, 139, 5614–5624. doi:10.1021/jacs.7b02440
Return to citation in text: [1] -
Gao, Z.; Fletcher, S. P. Chem. Sci. 2017, 8, 641–646. doi:10.1039/c6sc02811j
Return to citation in text: [1] -
Sidera, M.; Fletcher, S. P. Chem. Commun. 2015, 51, 5044–5047. doi:10.1039/c5cc00421g
Return to citation in text: [1] -
Maksymowicz, R. M.; Roth, P. M. C.; Thompson, A. L.; Fletcher, S. P. Chem. Commun. 2013, 49, 4211–4213. doi:10.1039/c2cc37155c
Return to citation in text: [1] -
Leichnitz, D.; Pflanze, S.; Beemelmanns, C. Org. Biomol. Chem. 2019, 17, 6964–6969. doi:10.1039/c9ob00990f
Return to citation in text: [1] -
Tu, W.; Ning, C.; Xu, J. Org. Chem. Front. 2018, 5, 29–31. doi:10.1039/c7qo00653e
Return to citation in text: [1] -
Galler, D. J.; Parker, K. A. Org. Lett. 2015, 17, 5544–5546. doi:10.1021/acs.orglett.5b02642
Return to citation in text: [1] -
Hu, T.; Panek, J. S. J. Am. Chem. Soc. 2002, 124, 11368–11378. doi:10.1021/ja0206700
Return to citation in text: [1] -
Wipf, P.; Xu, W. J. Org. Chem. 1996, 61, 6556–6562. doi:10.1021/jo960845m
Return to citation in text: [1] -
Mohan, P.; Koushik, K.; Fuertes, M. J. Tetrahedron Lett. 2015, 56, 61–65. doi:10.1016/j.tetlet.2014.09.128
Return to citation in text: [1] -
Ogawa, N.; Sone, S.; Hong, S.; Lu, Y.; Kobayashi, Y. Synlett 2020, 31, 1735–1739. doi:10.1055/s-0040-1706415
Return to citation in text: [1] -
Chandankar, S. S.; Raghavan, S. J. Org. Chem. 2019, 84, 9584–9602. doi:10.1021/acs.joc.9b01248
Return to citation in text: [1] -
Konstantinova, O. V.; Koskinen, A. M. P. Synthesis 2019, 51, 285–295. doi:10.1055/s-0037-1610387
Return to citation in text: [1] -
Schnabel, C.; Hiersemann, M. Org. Lett. 2009, 11, 2555–2558. doi:10.1021/ol900819u
Return to citation in text: [1] -
Arefolov, A.; Panek, J. S. J. Am. Chem. Soc. 2005, 127, 5596–5603. doi:10.1021/ja043168j
Return to citation in text: [1] -
Langille, N. F.; Panek, J. S. Org. Lett. 2004, 6, 3203–3206. doi:10.1021/ol048664r
Return to citation in text: [1] -
Ghasemi, H.; Antunes, L. M.; Organ, M. G. Org. Lett. 2004, 6, 2913–2916. doi:10.1021/ol0489853
Return to citation in text: [1] -
Sinast, M.; Claasen, B.; Stöckl, Y.; Greulich, A.; Zens, A.; Baro, A.; Laschat, S. J. Org. Chem. 2021, 86, 7537–7551. doi:10.1021/acs.joc.1c00580
Return to citation in text: [1] [2] [3] -
Wang, G.; Mohan, S.; Negishi, E.-i. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 11344–11349. doi:10.1073/pnas.1105155108
Return to citation in text: [1] [2] -
Crombie, L.; Hobbs, A. J. W.; Horsham, M. A.; Blade, R. J. Tetrahedron Lett. 1987, 28, 4875–4878. doi:10.1016/s0040-4039(00)96649-8
Return to citation in text: [1] -
Zeng, F.; Negishi, E.-i. Org. Lett. 2002, 4, 703–706. doi:10.1021/ol0102794
Return to citation in text: [1] [2] -
Dupont, J.; Donato, A. J. Tetrahedron: Asymmetry 1998, 9, 949–954. doi:10.1016/s0957-4166(98)00054-8
Return to citation in text: [1] [2] -
Maraval, A.; Igau, A.; Donnadieu, B.; Majoral, J.-P. Eur. J. Org. Chem. 2003, 385–394. doi:10.1002/ejoc.200390045
Return to citation in text: [1] -
Wan, S.; Green, M. E.; Park, J.-H.; Floreancig, P. E. Org. Lett. 2007, 9, 5385–5388. doi:10.1021/ol702184n
Return to citation in text: [1] -
Cox, R. J.; Evitt, A. S. Org. Biomol. Chem. 2007, 5, 229–232. doi:10.1039/b616582f
Return to citation in text: [1] [2] -
Loke, I.; Bentzinger, G.; Holz, J.; Raja, A.; Bhasin, A.; Sasse, F.; Köhn, A.; Schobert, R.; Laschat, S. Org. Biomol. Chem. 2016, 14, 884–894. doi:10.1039/c5ob01491c
Return to citation in text: [1] [2] -
Fernandes, R. A.; Gholap, S. P.; Chavan, V. P.; Saiyed, A. S.; Bhattacharyya, S. Org. Lett. 2020, 22, 3438–3443. doi:10.1021/acs.orglett.0c00901
Return to citation in text: [1] [2] -
Das, J. P.; Roy, S. J. Org. Chem. 2002, 67, 7861–7864. doi:10.1021/jo025868h
Return to citation in text: [1] -
Xu, S.; Zhang, Y.; Li, B.; Liu, S.-Y. J. Am. Chem. Soc. 2016, 138, 14566–14569. doi:10.1021/jacs.6b09759
Return to citation in text: [1]
| 63. | Fernandes, R. A.; Gholap, S. P.; Chavan, V. P.; Saiyed, A. S.; Bhattacharyya, S. Org. Lett. 2020, 22, 3438–3443. doi:10.1021/acs.orglett.0c00901 |
| 63. | Fernandes, R. A.; Gholap, S. P.; Chavan, V. P.; Saiyed, A. S.; Bhattacharyya, S. Org. Lett. 2020, 22, 3438–3443. doi:10.1021/acs.orglett.0c00901 |
| 1. | Jiao, Y.-J.; Liu, Y.; Wang, H.-X.; Zhu, D.-Y.; Shen, Y.-M.; Li, Y.-Y. J. Nat. Prod. 2020, 83, 2803–2808. doi:10.1021/acs.jnatprod.0c00900 |
| 5. | Trifonov, L. S.; Dreiding, A. S.; Hoesch, L.; Rast, D. M. Helv. Chim. Acta 1981, 64, 1843–1846. doi:10.1002/hlca.19810640616 |
| 24. | Harned, A. M.; Volp, K. A. Nat. Prod. Rep. 2011, 28, 1790–1810. doi:10.1039/c1np00039j |
| 4. | Sperry, S.; Samuels, G. J.; Crews, P. J. Org. Chem. 1998, 63, 10011–10014. doi:10.1021/jo9808122 |
| 25. | Wailes, P. C.; Weigold, H. J. Organomet. Chem. 1970, 24, 405–411. doi:10.1016/s0022-328x(00)80281-8 |
| 26. | Schwartz, J.; Labinger, J. A. Angew. Chem., Int. Ed. Engl. 1976, 15, 333–340. doi:10.1002/anie.197603331 |
| 27. | Buchwald, S. L.; LaMaire, S. J.; Nielsen, R. B.; Watson, B. T.; King, S. M. Org. Synth. 1993, 71, 77–82. doi:10.15227/orgsyn.071.0077 |
| 28. | Némethová, I.; Šebesta, R. Synthesis 2021, 53, 447–460. doi:10.1055/s-0040-1706055 |
| 29. | Pinheiro, D. L. J.; de Castro, P. P.; Amarante, G. W. Eur. J. Org. Chem. 2018, 4828–4844. doi:10.1002/ejoc.201800852 |
| 30. | Więcław, M. M.; Stecko, S. Eur. J. Org. Chem. 2018, 6601–6623. doi:10.1002/ejoc.201701537 |
| 18. | Pan, G.-F.; Zhang, X.-L.; Zhu, X.-Q.; Guo, R.-L.; Wang, Y.-Q. iScience 2019, 20, 229–236. doi:10.1016/j.isci.2019.09.027 |
| 22. | Goswami, P.; Das, B. Tetrahedron Lett. 2009, 50, 897–900. doi:10.1016/j.tetlet.2008.12.036 |
| 2. | Bell, P. J. L.; Karuso, P. J. Am. Chem. Soc. 2003, 125, 9304–9305. doi:10.1021/ja035496+ |
| 23. | Wood, J. L.; Thompson, B. D.; Yusuff, N.; Pflum, D. A.; Matthäus, M. S. P. J. Am. Chem. Soc. 2001, 123, 2097–2098. doi:10.1021/ja0057979 |
| 18. | Pan, G.-F.; Zhang, X.-L.; Zhu, X.-Q.; Guo, R.-L.; Wang, Y.-Q. iScience 2019, 20, 229–236. doi:10.1016/j.isci.2019.09.027 |
| 20. | Xu, Y.-H.; Lu, J.; Loh, T.-P. J. Am. Chem. Soc. 2009, 131, 1372–1373. doi:10.1021/ja8084548 |
| 21. | Yu, H.; Jin, W.; Sun, C.; Chen, J.; Du, W.; He, S.; Yu, Z. Angew. Chem., Int. Ed. 2010, 49, 5792–5797. doi:10.1002/anie.201002737 |
| 16. | Poulsen, P. H.; Vergura, S.; Monleón, A.; Jørgensen, D. K. B.; Jørgensen, K. A. J. Am. Chem. Soc. 2016, 138, 6412–6415. doi:10.1021/jacs.6b03546 |
| 17. | Liu, D.-N.; Tian, S.-K. Chem. – Eur. J. 2009, 15, 4538–4542. doi:10.1002/chem.200900177 |
| 6. | Crouch, I. T.; Dreier, T.; Frantz, D. E. Angew. Chem., Int. Ed. 2011, 50, 6128–6132. doi:10.1002/anie.201101820 |
| 7. | Li, C.; Li, M.; Zhong, W.; Jin, Y.; Li, J.; Wu, W.; Jiang, H. Org. Lett. 2019, 21, 872–875. doi:10.1021/acs.orglett.8b03606 |
| 8. | Yuan, F.-Q.; Han, F.-S. Org. Lett. 2012, 14, 1218–1221. doi:10.1021/ol203444g |
| 9. | He, Y.-H.; Hu, Y.; Guan, Z. Synth. Commun. 2011, 41, 1617–1628. doi:10.1080/00397911.2010.490626 |
| 10. | Liu, C.; Zhou, H.; Sheng, X. B.; Liu, X. H.; Chen, F. H. Bioorg. Chem. 2020, 102, 104077. doi:10.1016/j.bioorg.2020.104077 |
| 11. | Polaquini, C. R.; Torrezan, G. S.; Santos, V. R.; Nazaré, A. C.; Campos, D. L.; Almeida, L. A.; Silva, I. C.; Ferreira, H.; Pavan, F. R.; Duque, C.; Regasini, L. Molecules 2017, 22, 1685. doi:10.3390/molecules22101685 |
| 12. | Trost, B. M.; Biannic, B. Org. Lett. 2015, 17, 1433–1436. doi:10.1021/acs.orglett.5b00279 |
| 13. | Trost, B. M.; Kazmaier, U. J. Am. Chem. Soc. 1992, 114, 7933–7935. doi:10.1021/ja00046a062 |
| 14. | Trost, B. M.; Li, C.-J. J. Am. Chem. Soc. 1994, 116, 10819–10820. doi:10.1021/ja00102a071 |
| 15. | Kwong, C. K.-W.; Fu, M. Y.; Lam, C. S.-L.; Toy, P. H. Synthesis 2008, 2307–2317. doi:10.1055/s-2008-1067173 |
| 16. | Poulsen, P. H.; Vergura, S.; Monleón, A.; Jørgensen, D. K. B.; Jørgensen, K. A. J. Am. Chem. Soc. 2016, 138, 6412–6415. doi:10.1021/jacs.6b03546 |
| 17. | Liu, D.-N.; Tian, S.-K. Chem. – Eur. J. 2009, 15, 4538–4542. doi:10.1002/chem.200900177 |
| 18. | Pan, G.-F.; Zhang, X.-L.; Zhu, X.-Q.; Guo, R.-L.; Wang, Y.-Q. iScience 2019, 20, 229–236. doi:10.1016/j.isci.2019.09.027 |
| 19. | Motika, S. E.; Wang, Q.; Ye, X.; Shi, X. Org. Lett. 2015, 17, 290–293. doi:10.1021/ol503393a |
| 20. | Xu, Y.-H.; Lu, J.; Loh, T.-P. J. Am. Chem. Soc. 2009, 131, 1372–1373. doi:10.1021/ja8084548 |
| 21. | Yu, H.; Jin, W.; Sun, C.; Chen, J.; Du, W.; He, S.; Yu, Z. Angew. Chem., Int. Ed. 2010, 49, 5792–5797. doi:10.1002/anie.201002737 |
| 12. | Trost, B. M.; Biannic, B. Org. Lett. 2015, 17, 1433–1436. doi:10.1021/acs.orglett.5b00279 |
| 13. | Trost, B. M.; Kazmaier, U. J. Am. Chem. Soc. 1992, 114, 7933–7935. doi:10.1021/ja00046a062 |
| 14. | Trost, B. M.; Li, C.-J. J. Am. Chem. Soc. 1994, 116, 10819–10820. doi:10.1021/ja00102a071 |
| 15. | Kwong, C. K.-W.; Fu, M. Y.; Lam, C. S.-L.; Toy, P. H. Synthesis 2008, 2307–2317. doi:10.1055/s-2008-1067173 |
| 65. | Xu, S.; Zhang, Y.; Li, B.; Liu, S.-Y. J. Am. Chem. Soc. 2016, 138, 14566–14569. doi:10.1021/jacs.6b09759 |
| 6. | Crouch, I. T.; Dreier, T.; Frantz, D. E. Angew. Chem., Int. Ed. 2011, 50, 6128–6132. doi:10.1002/anie.201101820 |
| 7. | Li, C.; Li, M.; Zhong, W.; Jin, Y.; Li, J.; Wu, W.; Jiang, H. Org. Lett. 2019, 21, 872–875. doi:10.1021/acs.orglett.8b03606 |
| 8. | Yuan, F.-Q.; Han, F.-S. Org. Lett. 2012, 14, 1218–1221. doi:10.1021/ol203444g |
| 9. | He, Y.-H.; Hu, Y.; Guan, Z. Synth. Commun. 2011, 41, 1617–1628. doi:10.1080/00397911.2010.490626 |
| 10. | Liu, C.; Zhou, H.; Sheng, X. B.; Liu, X. H.; Chen, F. H. Bioorg. Chem. 2020, 102, 104077. doi:10.1016/j.bioorg.2020.104077 |
| 11. | Polaquini, C. R.; Torrezan, G. S.; Santos, V. R.; Nazaré, A. C.; Campos, D. L.; Almeida, L. A.; Silva, I. C.; Ferreira, H.; Pavan, F. R.; Duque, C.; Regasini, L. Molecules 2017, 22, 1685. doi:10.3390/molecules22101685 |
| 19. | Motika, S. E.; Wang, Q.; Ye, X.; Shi, X. Org. Lett. 2015, 17, 290–293. doi:10.1021/ol503393a |
| 47. | Ogawa, N.; Sone, S.; Hong, S.; Lu, Y.; Kobayashi, Y. Synlett 2020, 31, 1735–1739. doi:10.1055/s-0040-1706415 |
| 48. | Chandankar, S. S.; Raghavan, S. J. Org. Chem. 2019, 84, 9584–9602. doi:10.1021/acs.joc.9b01248 |
| 49. | Konstantinova, O. V.; Koskinen, A. M. P. Synthesis 2019, 51, 285–295. doi:10.1055/s-0037-1610387 |
| 50. | Schnabel, C.; Hiersemann, M. Org. Lett. 2009, 11, 2555–2558. doi:10.1021/ol900819u |
| 51. | Arefolov, A.; Panek, J. S. J. Am. Chem. Soc. 2005, 127, 5596–5603. doi:10.1021/ja043168j |
| 52. | Langille, N. F.; Panek, J. S. Org. Lett. 2004, 6, 3203–3206. doi:10.1021/ol048664r |
| 53. | Ghasemi, H.; Antunes, L. M.; Organ, M. G. Org. Lett. 2004, 6, 2913–2916. doi:10.1021/ol0489853 |
| 54. | Sinast, M.; Claasen, B.; Stöckl, Y.; Greulich, A.; Zens, A.; Baro, A.; Laschat, S. J. Org. Chem. 2021, 86, 7537–7551. doi:10.1021/acs.joc.1c00580 |
| 31. | Coelho, A.; Souvenir Zafindrajaona, M.-S.; Vallée, A.; Behr, J.-B.; Vasse, J.-L. Chem. – Eur. J. 2022, 28, e202103789. doi:10.1002/chem.202103789 |
| 32. | Yang, C.; Gao, Y.; Bai, S.; Jiang, C.; Qi, X. J. Am. Chem. Soc. 2020, 142, 11506–11513. doi:10.1021/jacs.0c03821 |
| 33. | Hostmann, T.; Neveselý, T.; Gilmour, R. Chem. Sci. 2021, 12, 10643–10648. doi:10.1039/d1sc02454j |
| 34. | Gao, Y.; Yang, C.; Bai, S.; Liu, X.; Wu, Q.; Wang, J.; Jiang, C.; Qi, X. Chem 2020, 6, 675–688. doi:10.1016/j.chempr.2019.12.010 |
| 35. | Némethová, I.; Vargová, D.; Mudráková, B.; Filo, J.; Šebesta, R. J. Organomet. Chem. 2020, 908, 121099. doi:10.1016/j.jorganchem.2019.121099 |
| 36. | Ardkhean, R.; Roth, P. M. C.; Maksymowicz, R. M.; Curran, A.; Peng, Q.; Paton, R. S.; Fletcher, S. P. ACS Catal. 2017, 7, 6729–6737. doi:10.1021/acscatal.7b01453 |
| 37. | Rideau, E.; You, H.; Sidera, M.; Claridge, T. D. W.; Fletcher, S. P. J. Am. Chem. Soc. 2017, 139, 5614–5624. doi:10.1021/jacs.7b02440 |
| 38. | Gao, Z.; Fletcher, S. P. Chem. Sci. 2017, 8, 641–646. doi:10.1039/c6sc02811j |
| 39. | Sidera, M.; Fletcher, S. P. Chem. Commun. 2015, 51, 5044–5047. doi:10.1039/c5cc00421g |
| 40. | Maksymowicz, R. M.; Roth, P. M. C.; Thompson, A. L.; Fletcher, S. P. Chem. Commun. 2013, 49, 4211–4213. doi:10.1039/c2cc37155c |
| 41. | Leichnitz, D.; Pflanze, S.; Beemelmanns, C. Org. Biomol. Chem. 2019, 17, 6964–6969. doi:10.1039/c9ob00990f |
| 42. | Tu, W.; Ning, C.; Xu, J. Org. Chem. Front. 2018, 5, 29–31. doi:10.1039/c7qo00653e |
| 43. | Galler, D. J.; Parker, K. A. Org. Lett. 2015, 17, 5544–5546. doi:10.1021/acs.orglett.5b02642 |
| 44. | Hu, T.; Panek, J. S. J. Am. Chem. Soc. 2002, 124, 11368–11378. doi:10.1021/ja0206700 |
| 45. | Wipf, P.; Xu, W. J. Org. Chem. 1996, 61, 6556–6562. doi:10.1021/jo960845m |
| 46. | Mohan, P.; Koushik, K.; Fuertes, M. J. Tetrahedron Lett. 2015, 56, 61–65. doi:10.1016/j.tetlet.2014.09.128 |
| 54. | Sinast, M.; Claasen, B.; Stöckl, Y.; Greulich, A.; Zens, A.; Baro, A.; Laschat, S. J. Org. Chem. 2021, 86, 7537–7551. doi:10.1021/acs.joc.1c00580 |
| 62. | Loke, I.; Bentzinger, G.; Holz, J.; Raja, A.; Bhasin, A.; Sasse, F.; Köhn, A.; Schobert, R.; Laschat, S. Org. Biomol. Chem. 2016, 14, 884–894. doi:10.1039/c5ob01491c |
| 28. | Némethová, I.; Šebesta, R. Synthesis 2021, 53, 447–460. doi:10.1055/s-0040-1706055 |
| 59. | Maraval, A.; Igau, A.; Donnadieu, B.; Majoral, J.-P. Eur. J. Org. Chem. 2003, 385–394. doi:10.1002/ejoc.200390045 |
| 60. | Wan, S.; Green, M. E.; Park, J.-H.; Floreancig, P. E. Org. Lett. 2007, 9, 5385–5388. doi:10.1021/ol702184n |
| 61. | Cox, R. J.; Evitt, A. S. Org. Biomol. Chem. 2007, 5, 229–232. doi:10.1039/b616582f |
| 58. | Dupont, J.; Donato, A. J. Tetrahedron: Asymmetry 1998, 9, 949–954. doi:10.1016/s0957-4166(98)00054-8 |
| 54. | Sinast, M.; Claasen, B.; Stöckl, Y.; Greulich, A.; Zens, A.; Baro, A.; Laschat, S. J. Org. Chem. 2021, 86, 7537–7551. doi:10.1021/acs.joc.1c00580 |
| 55. | Wang, G.; Mohan, S.; Negishi, E.-i. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 11344–11349. doi:10.1073/pnas.1105155108 |
| 57. | Zeng, F.; Negishi, E.-i. Org. Lett. 2002, 4, 703–706. doi:10.1021/ol0102794 |
| 58. | Dupont, J.; Donato, A. J. Tetrahedron: Asymmetry 1998, 9, 949–954. doi:10.1016/s0957-4166(98)00054-8 |
| 61. | Cox, R. J.; Evitt, A. S. Org. Biomol. Chem. 2007, 5, 229–232. doi:10.1039/b616582f |
| 62. | Loke, I.; Bentzinger, G.; Holz, J.; Raja, A.; Bhasin, A.; Sasse, F.; Köhn, A.; Schobert, R.; Laschat, S. Org. Biomol. Chem. 2016, 14, 884–894. doi:10.1039/c5ob01491c |
| 55. | Wang, G.; Mohan, S.; Negishi, E.-i. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 11344–11349. doi:10.1073/pnas.1105155108 |
| 56. | Crombie, L.; Hobbs, A. J. W.; Horsham, M. A.; Blade, R. J. Tetrahedron Lett. 1987, 28, 4875–4878. doi:10.1016/s0040-4039(00)96649-8 |
© 2023 Kolb et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.