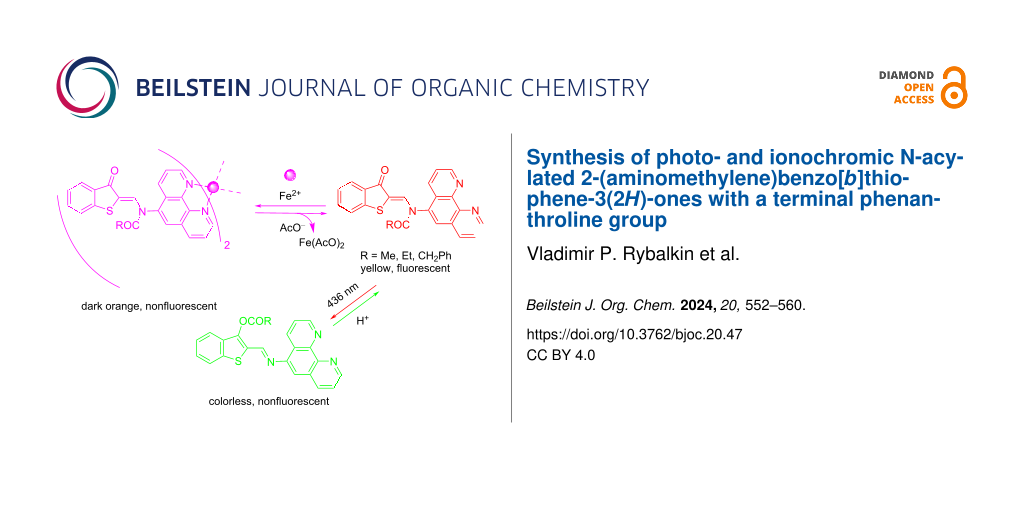
Abstract
A series of novel photo- and ionochromic N-acylated 2-(aminomethylene)benzo[b]thiophene-3(2Н)-ones with a terminal phenanthroline receptor substituent was synthesized. Upon irradiation in acetonitrile or DMSO with light of 436 nm, they underwent Z–E isomerization of the C=C bond, followed by very fast N→O migration of the acyl group and the formation of nonemissive O-acylated isomers. These isomers were isolated preparatively and fully characterized by IR, 1H, and 13C NMR spectroscopy as well as HRMS and XRD methods. The reverse thermal reaction was catalyzed by protonic acids. N-Acylated compounds exclusively with Fe2+ formed nonfluorescent complexes with a contrast naked-eye effect: a color change of the solutions from yellow to dark orange. Subsequent selective interaction with AcO− led to the restoration of the initial absorption and emission properties. Thus, the obtained compounds represent dual-mode “on–off–on” switches of optical and fluorescent properties under sequential exposure to light and H+ or sequential addition of Fe2+ and AcO− ions.
Graphical Abstract
Introduction
Photochromism is defined as the reversible transformation of a molecular entity between different forms, having different absorption spectra, induced in one or both directions by absorption of electromagnetic radiation [1-4]. Due to the different structures and the different optical and emission properties of these forms, photochromic compounds are used in 3D optical memory devices, photoswitches of different types, molecular logic gates, photopharmacology, bioimaging and chemosensorics [5-11]. For most photochromic compounds, irradiation of the solution or solid results in a deepening of the color (positive photochromism). Less studied are those characterized by photoinduced bleaching (negative or inverse photochromism), such as merocyanine forms of spiropyrans and spirooxazines, azomethine imines, thioindigoid dyes and N→O acylotropic systems [12-15]. Recently, they have been actively used to create next-generation molecular switches, materials with new properties (in particular, color change depending on the intensity of sunlight), photochromic tags for biological research and optical sensors [15-21].
To develop new dual-mode molecular switches capable of efficient modulation of optical and fluorescent properties, both upon irradiation with visible light and upon sequential addition of Fe2+ and AcO−, we synthesized N-acylated 2-(aminomethylene)benzo[b]thiophene-3(2Н)-ones with a terminal phenanthroline substituent and studied the spectral-luminescent, photochromic and ionochromic properties. The phenanthroline moiety was incorporated into the molecule due to the known ability to coordinate with metal cations [22,23]. Dual-mode naked-eye molecular switches controlled by both light and changes in the ionic composition of the medium can be used as elements of electronic devices, for optical recording of information as well as in photopharmacology, light-gated catalysis and bioimaging [24-26]. In addition, such systems provide a convenient platform for creating chemo- and biosensors for rapid analysis of the ionic composition of the environment [27,28].
Results and Discussion
The starting compound for the synthesis of N-acylated 2-(aminomethylene)benzo[b]thiophene-3(2Н)-ones 2a–c with a terminal phenanthroline substituent was (E)-2-(((1,10-phenanthrolin-5-yl)amino)methylene)benzo[b]thiophen-3(2H)-one (1), obtained by condensation of 3-hydroxybenzo[b]thiophene-2-carbaldehyde with 5-aminophenanthroline in acetonitrile (Scheme 1). (Z)-N-((3-Oxobenzo[b]thiophen-2(3H)-ylidene)methyl)-N-(1,10-phenanthrolin-5-yl)acetamide (2a) and (Z)-N-((3-oxobenzo[b]thiophen-2(3H)-ylidene)methyl)-N-(1,10-phenanthrolin-5-yl)propionamide (2b) were prepared by short-term boiling of 1 in acetic or propionic anhydride, respectively. To obtain (Z)-N-((3-oxobenzo[b]thiophen-2(3H)-ylidene)methyl)-N-(1,10-phenanthrolin-5-yl)-2-phenylacetamide (2c), a suspension of 1 in acetonitrile was boiled with phenylacetyl chloride until completely dissolved (Scheme 1 and Supporting Information File 1).
Scheme 1: Synthesis of compound 1 and N-acylated compounds 2a–c.
Scheme 1: Synthesis of compound 1 and N-acylated compounds 2a–c.
The obtained compounds 2a–c existed as an N-acylated keto form. In the IR spectra, stretching vibrations of the thiophene and amide carbonyl groups were observed at 1663–1678 and 1705–1713 cm−1, respectively. The 1H NMR spectra contained signals of methine protons (=CH–) in the region 7.92–9.02 ppm, which corresponded to the Z-configuration of the C=C bond. According to data previously obtained [14], the signals of methine protons of E-isomers should be in the region of approximately 5.90 ppm [14]. Other IR, 1Н and 13С NMR spectroscopy and HRMS data confirming the structure of the synthesized compounds 1 and 2a–c are presented in Supporting Information File 2.
Nonacylated compound 1 showed long-wavelength absorption at 458 nm, while acylation led to a hypsochromic shift of the maximum in compounds 2a–c to 423–426 nm (Table 1). The intensity of this absorption band decreased with increasing steric hindrance in the order R = acetyl (i.e., 2a) > propionyl (i.e., 2b) > phenylacetyl (i.e., 2c).
Table 1: Absorption and fluorescence spectra of compounds 1, 2a and 2b in acetonitrile and compound 2c in DMSO, and quantum yields of the 2a–c→3a–c rearrangementa.
| compound |
λabs, nm
(ε, L⋅mol−1⋅cm−1) |
λfl, nm (φfl) | φ2→3 |
| 1 | 344 (12800), 458 (20000) | 506 (0.12) | — |
| 2a | 303 (25000), 423 (14000) | 468 (0.16) | 0.34 |
| 2b | 300 (18800), 426 (9200) | 465 (0.15) | 0.35 |
| 2c | 309 (24600), 425 (6800) | 467 (0.13) | 0.40 |
aλabs and λfl: maxima of the absorption and fluorescence bands, respectively. φfl: quantum yield of fluorescence. φ2→3: overall quantum yield for stepwise N→O rearrangement. c 5.0 × 10−5 mol⋅L−1. λex 420 nm (455 nm for 1). PMT voltage 800 V.
N-Acylated compounds 2a–c in solutions exhibited fluorescence in the region of 465–468 nm, and the excitation emission spectra agreed well with the absorption spectra (Figure 1).
![[1860-5397-20-47-1]](/bjoc/content/figures/1860-5397-20-47-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: Absorption (1), fluorescence (2, λex = 410 nm) and fluorescence excitation (3, λfl = 465 nm) spectra of compound 2a in acetonitrile (c 5.0 × 10−5 mol·L−1).
Figure 1: Absorption (1), fluorescence (2, λex = 410 nm) and fluorescence excitation (3, λfl = 465 nm) spectr...
Compounds 2a–c in solutions demonstrated a typical negative photochromism [1,16] when irradiated with visible light of 436 nm (Figure 2). A decrease in the long-wavelength absorption was accompanied by a simultaneous increase in the shorter-wavelength absorption in the spectral region around 370 nm. During this process, a gradual diminishment of the initial fluorescence intensity at 465–468 nm up to zero was observed. The resulting O-acylated isomers 3a–c were nonemissive. According to previous findings, the 2a–c→3a–c transformation is a two-step process: 1) Z–E photoisomerization and 2) extremely fast nonadiabatic N→O acyl group transfer (Scheme 2) [14,16]. These two stages occur almost simultaneously, and hence the term “overall quantum yield” is used in Table 1 [29]. The formation of the short-lived E-isomer was earlier observed only in vitrified solvents at 77 K or under flash photolysis conditions [14]. As such, the parameters of the thermal acyl migration under steady-state irradiation conditions were not determined.
![[1860-5397-20-47-2]](/bjoc/content/figures/1860-5397-20-47-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: Electronic absorption spectra of compound 2b in acetonitrile before (1) and after 15 s (2), 35 s (3), 75 s (4), 2.5 min (5) and 5 min (6) of irradiation with light of 436 nm (c 5.0 × 10−5 mol·L−1).
Figure 2: Electronic absorption spectra of compound 2b in acetonitrile before (1) and after 15 s (2), 35 s (3...
Scheme 2: Photoisomerization of N-acylated ketoenamines 2a–c.
Scheme 2: Photoisomerization of N-acylated ketoenamines 2a–c.
The reverse reaction 3a–c→2a–c with full restoration of the initial absorption and fluorescence properties was characterized by a high activation barrier and could be accomplished by heating a solution of 3a–c in o-dichlorobenzene at 423 K or by passing dry hydrogen chloride through a solution of 3a–c in acetonitrile. However, the simplest and most effective method was the addition of a catalytic amount of HClO4 [14].
Although studies on photoacylotropic systems have been carried out for some time [14,16], only within the framework of this work, a method for the preparative synthesis of photoproducts 3a–c under the influence of irradiation was developed. We applied a modified procedure using a Sweko IP65 LED emitter that had been previously developed in our studies for similar tasks [30]. For this purpose, a suspension of a yellow solid 2a–c in acetonitrile was boiled for 10–15 s and then irradiated with an emitter for 3–5 min. This process was repeated up to 10 times until complete dissolution had occurred. The colorless solids 3a (80%), 3b (75%) and 3c (85%), respectively, gradually precipitated. For the first time in the course of studying N→O acylotropic migrations, photorearrangement products 3a–c were comprehensively characterized by IR, 1Н and 13C NMR spectroscopy, HRMS (Supporting Information File 2) as well as by X-ray diffraction analysis.
The molecular structure of 3b is shown in Figure 3. The crystal data, details of the data collection and refinements for 3b as well as complete lists of bond lengths and bond angles are given in Tables S1–S4, Supporting Information File 3.
![[1860-5397-20-47-3]](/bjoc/content/figures/1860-5397-20-47-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: Molecular structure of O-acylated isomer 3b. Thermal ellipsoids are drawn at the 50% probability level.
Figure 3: Molecular structure of O-acylated isomer 3b. Thermal ellipsoids are drawn at the 50% probability le...
Compound 3b had the structure of an O-acylated isomer and possessed an E-s-cis(S,N) conformation relative to the C(l)–C(9) bond (Figure 3). The benzo[b]thiophene fragment was planar, whereas the propionyl group COCH2CH3 was not coplanar with this plane. This was due to a torsional rotation around the O(l)–C(2) bond by the C(22)–O(1)–C(2)–C(1) fragment with an angle of 105.89°. The phenanthroline moiety was planar and rotated relative to the benzo[b]thiophene part of the molecule (plane twist angle 122.73°, fold angle 4.38°).
The molecular packing of compound 3b was characterized by the presence of numerous π–π interactions (Figure 4).
![[1860-5397-20-47-4]](/bjoc/content/figures/1860-5397-20-47-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: Fragment of the molecular packing of compound 3b, showing π–π interactions in the crystalline state. The main centroid–centroid distances are indicated in Å.
Figure 4: Fragment of the molecular packing of compound 3b, showing π–π interactions in the crystalline state...
Intermolecular interactions in the benzo[b]thiophene fragment (red planes in Figure 4) were characterized by the following parameters: plane centroid–plane centroid distance 3.6153(10) Å (shift 1.6063(18) Å, twist and fold angles 0.0°). The closest contact in the phenanthroline fragment (blue plane–blue plane, top fragment in Figure 4) had a plane centroid–plane centroid distance of 3.5398(9) Å (shift 0.4273(18) Å, twist and fold angles 0.00°). One pyridine cycle of the phenanthroline unit was also involved in a π–π-stacking interaction (blue plane–green plane in Figure 4), with the plane centroid–plane centroid distance being 3.6998(8) Å (plane shift 1.4919(17) Å, twist and fold angles 1.54° and 1.92°, respectively).
Cation-induced transformations of the absorption and fluorescence spectra of 2a–c were studied by the action of d-metal perchlorates (Zn2+, Hg2+, Cu2+, Cd2+, Ni2+, Co2+ and Fe2+) in acetonitrile (2a and 2b) and DMSO (2c). Exclusively Fe2+ caused an appearance of new broad long-wavelength absorption bands at 480–530 nm with a contrast naked-eye effect: a visually distinguishable color change of the solutions from yellow to dark orange (Figure 5). Other cations did not demonstrate a measurable effect (Figure 6). Complexes 2a–c with Fe2+ in acetonitrile and DMSO were nonfluorescent.
![[1860-5397-20-47-5]](/bjoc/content/figures/1860-5397-20-47-5.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: Absorption spectra of compound 2a in acetonitrile before (1) and after (2) the addition of Fe2+ (c2a 5.0 × 10−5 mol·L−1, cFe2+ 1.0 × 10−4 mol·L−1). Inset: fluorescence spectra before (1’) and after (2’) the addition of Fe2+ (λex = 422 nm). The photographs show absorption (bottom right) and fluorescence (upon irradiation with light of 436 nm, top right) before and after the addition of Fe2+.
Figure 5: Absorption spectra of compound 2a in acetonitrile before (1) and after (2) the addition of Fe2+ (c2a...
![[1860-5397-20-47-6]](/bjoc/content/figures/1860-5397-20-47-6.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 6: Changes in the absorption intensity of compound 2a in acetonitrile at 520 nm after the addition of metal perchlorates (с2a 5 × 10−5 mol·L−1, сM2+ 1.0 × 10−4 mol·L−1).
Figure 6: Changes in the absorption intensity of compound 2a in acetonitrile at 520 nm after the addition of ...
According to spectrophotometric titration data and the isomolar series method, compounds 2a–c formed the 2:1 complexes 4a–c with Fe2+ (Scheme 3 and Figure 7).
Scheme 3: Sequential interaction of compounds 2a–c with Fe2+ and AcO−.
Scheme 3: Sequential interaction of compounds 2a–c with Fe2+ and AcO−.
![[1860-5397-20-47-7]](/bjoc/content/figures/1860-5397-20-47-7.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 7: Job’s plot at the wavelength 429 nm, reflecting the interaction of compound 2a with Fe2+ in acetonitrile. The total concentration c2a + cFe2+ was 1.5 × 10−4 mol·L−1.
Figure 7: Job’s plot at the wavelength 429 nm, reflecting the interaction of compound 2a with Fe2+ in acetoni...
It was found that selective interaction of the resulting in situ complex 4a with AcO− led to restoration of the initial absorption and emission properties [31,32]. Other tetra-n-butylammonium salts (TBAX, X = F, Cl, Br, I, CN, SCN, NO3) did not cause similar changes in the absorption spectra. This process could be carried out at least 4 or 5 times, which allows modulating optical and fluorescent properties by sequentially adding Fe2+ and AcO− to the acetonitrile solution of compound 2a (Figure 8).
![[1860-5397-20-47-8]](/bjoc/content/figures/1860-5397-20-47-8.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 8: Fluorescence intensity of compound 2a upon alternate addition of Fe2+ and AcO−.
Figure 8: Fluorescence intensity of compound 2a upon alternate addition of Fe2+ and AcO−.
Conclusion
A series of novel photo- and ionochromic N-acylated 2-(aminomethylene)benzo[b]thiophene-3(2Н)-ones with a terminal phenanthroline receptor substituent was synthesized. Upon irradiation with light of 436 nm, the resulting compounds in solutions exhibited negative photochromism due to Z–E photoisomerization of the C=C bond, followed by very fast thermal N→O migration of the acyl group and the formation of O-acylated isomers. This rearrangement was accompanied by a decrease of the initial fluorescence intensity at 465–468 nm up to zero, since the resulting OAc− form was nonemissive. The reverse reaction occurred catalytically in the presence of HClO4.
A special technique for the preparative synthesis of photoproducts was developed. For the first time in the course of studying N→O acylotropic migrations, O-acylated photoproducts were comprehensively characterized by IR, 1H and 13C NMR spectroscopy, HRMS and X-ray diffraction analysis. Selectively, Fe2+ caused an appearance of new broad long-wavelength absorption bands at 480−530 nm with a contrast naked-eye effect: a visually distinguishable color change of the solutions from yellow to dark orange. The obtained complexes with Fe2+ in acetonitrile and DMSO were nonfluorescent. They selectively interacted with AcO−, which led to the restoration of the initial absorption and emission properties. Thus, the obtained compounds were dual-mode “on−off−on” switches of the fluorescent properties upon sequential exposure to light and H+ as well as sequential addition of Fe2+ and AcO−.
Experimental
General
The 1H and 13C NMR spectra were recorded on an integrated analytical LC–SPE–NMR–MS system AVANCE-600 (Bruker, 600 MHz for 1H and 150.96 MHz for 13C) in CDCl3. The signals were referred to with respect to the signals of residual protons of deuterated solvent (7.24 ppm). IR spectra were recorded on an FT/IR-6800 FTIR spectrometer (JASCO). The IR and NMR spectra were recorded using equipment from the Shared Use Centre “Molecular spectroscopy” of the Southern Federal University. Electronic absorption spectra were obtained on a Varian Cary 100 spectrophotometer. Electronic emission spectra were recorded on a Varian Cary Eclipse spectrofluorimeter. Acetonitrile and DMSO (spectral pure grade), Cd2+, Hg2+, Cu2+, Zn2+, Ni2+, Co2+ and Fe2+ perchlorates as well as TBAX (X = F, Cl, Br, I, CN, SCN, NO3) salts (Aldrich) were used to prepare the solutions. The solutions (in a quartz cell, l = 1 cm) were irradiated with filtered light from a high-pressure Hg lamp on a Newport 66941 equipment supplied with a set of interference light filters. The light intensity was 6.4 × 1016 photons⋅s−1 for the 436 nm spectral line. (Z)-N-((3-Oxobenzo[b]thiophen-2(3H)-ylidene)methyl)-N-phenylacetamide was used as an actinometer for the quantum yield calculations (φfl = 0.60 ± 0.005) [14,33]. For preparative purposes, a Sweko IP65 led emitter (SUL-S1-20W-230-4000K-WH) was used. Spectral-fluorescent experiments were performed using solutions in acetonitrile or DMSO in quartz cells (l = 1 cm, V = 2 mL). Fluorescence quantum yields were determined relatively to (Z)-N-((3-oxobenzo[b]thiophen-2(3H)-ylidene)methyl)-N-phenylacetamide as a standard (φfl = 0.16 ± 0.005) [14,33]. Stock solutions of compounds 2a–c (c 1.0 × 10–4 mol⋅L−1) and metal perchlorates (c 2.0 × 10–4 mol⋅L−1) were used. 1 mL of a 2a–c solution and 1 mL of a perchlorate solution were mixed directly in the cuvette and thoroughly stirred. Hence, the working concentration of the compounds 2–c and the cations was 5.0 × 10–5 mol⋅L−1 and 1.0 × 10−5 mol⋅L−1. HRMS analysis was performed on a Bruker UHR-TOF Maxis™ Impact instrument (electrospray ionization). Melting points were determined on a Fisher–Johns melting point apparatus.
X-ray diffraction study
The X-ray diffraction dataset of compound 3b was recorded on an Agilent SuperNova diffractometer using a microfocus X-ray radiation source with copper anode and Atlas S2 two-dimensional CCD detector. Crystal data for C24H17N3O2S (M = 411.46 g⋅mol−1): triclinic, space group P−1 (no. 2), a = 7.43030(10) Å, b = 9.6398(2) Å, c = 14.3294(3) Å, α = 75.731(2)°, β = 82.686(2)°, γ = 78.664(2)°, V = 971.93(3) Å3, Z = 2, T = 293(2) K, μ (Cu Kα) = 1.701 mm−1, Dcalc = 1.406 g/cm3, 17777 reflections measured (9.61° ≤ 2Θ ≤ 152.768°), 4053 unique (Rint = 0.0201, Rσ = 0.0153) that were used in all calculations. The final R1 was 0.0307 (I ≥ 2σ(I)) and wR2 was 0.0813 (all data).
Reflections were recorded and unit cell parameters were determined and refined using the dedicated CrysAlisPro software suite [34]. The structure was solved with the ShelXT program [35] and refined with the ShelXL program [36], and the graphics were rendered using the Olex2 software suite [37]. The complete X-ray structural dataset for compound 2a was deposited with the Cambridge Crystallographic Data Centre (CCDC 2299603). The data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
Supporting Information
| Supporting Information File 1: Experimental procedures and characterization data for all new compounds 1, 2a–c and 3a–c. | ||
| Format: PDF | Size: 199.2 KB | Download |
| Supporting Information File 2: 1H and 13C NMR, IR and HRMS spectra of all novel compounds. | ||
| Format: PDF | Size: 2.0 MB | Download |
| Supporting Information File 3: X-ray analysis data of 3b. | ||
| Format: PDF | Size: 272.5 KB | Download |
Funding
This study was financially supported by the Ministry of Science and Higher Education of the Russian Federation in the scope of the State Task in the Field of Science (No. FENW_2023-0020) and in the scope of the State Task to the Southern Scientific Centre of the Russian Academy of Sciences (No. 122020100282-6, V. P. Rybalkin and A. D. Dubonosov).
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information to this article.
References
-
Braslavsky, S. E. Pure Appl. Chem. 2007, 79, 293–465. doi:10.1351/pac200779030293
Return to citation in text: [1] [2] -
Pianowski, Z. L., Ed. Molecular Photoswitches: Chemistry, Properties, and Applications; Wiley-VCH: Weinheim, Germany, 2022. doi:10.1002/9783527827626
Return to citation in text: [1] -
Tian, H.; Zhang, J., Eds. Photochromic Materials: Preparation, Properties and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2016. doi:10.1002/9783527683734
Return to citation in text: [1] -
Duerr, H.; Bouas-Laurent, H., Eds. Photochromism: Molecules and Systems, revised edition; Elsevier: Amsterdam, Netherlands, 2003.
Return to citation in text: [1] -
Crano, J. C.; Guglielmetti, R. J., Eds. Organic Photochromic and Thermochromic Compounds; Springer: New York, NY, USA, 2002. doi:10.1007/b114211
Return to citation in text: [1] -
Fitzmaurice, O.; Bartkowski, M.; Giordani, S. Front. Chem. (Lausanne, Switz.) 2022, 10, 859450. doi:10.3389/fchem.2022.859450
Return to citation in text: [1] -
Magri, D. C. Coord. Chem. Rev. 2021, 426, 213598. doi:10.1016/j.ccr.2020.213598
Return to citation in text: [1] -
Andréasson, J.; Pischel, U. Coord. Chem. Rev. 2021, 429, 213695. doi:10.1016/j.ccr.2020.213695
Return to citation in text: [1] -
Welleman, I. M.; Hoorens, M. W. H.; Feringa, B. L.; Boersma, H. H.; Szymański, W. Chem. Sci. 2020, 11, 11672–11691. doi:10.1039/d0sc04187d
Return to citation in text: [1] -
Krämer, J.; Kang, R.; Grimm, L. M.; De Cola, L.; Picchetti, P.; Biedermann, F. Chem. Rev. 2022, 122, 3459–3636. doi:10.1021/acs.chemrev.1c00746
Return to citation in text: [1] -
Li, M.; Zhao, J.; Chu, H.; Mi, Y.; Zhou, Z.; Di, Z.; Zhao, M.; Li, L. Adv. Mater. (Weinheim, Ger.) 2019, 31, 1804745. doi:10.1002/adma.201804745
Return to citation in text: [1] -
Metelitsa, A.; Chernyshev, A.; Voloshin, N.; Solov'eva, E.; Rostovtseva, I.; Dorogan, I.; Gaeva, E.; Guseva, A. Dyes Pigm. 2021, 186, 109070. doi:10.1016/j.dyepig.2020.109070
Return to citation in text: [1] -
Bren, V. A.; Dubonosov, A. D.; Popova, O. S.; Revinskii, Y. V.; Tikhomirova, K. S.; Minkin, V. I. Int. J. Photoenergy 2018, 9746534. doi:10.1155/2018/9746534
Return to citation in text: [1] -
Minkin, V. I.; Bren’, V. A.; Dubonosov, A. D.; Tsukanov, A. V. Chem. Heterocycl. Compd. 2012, 48, 107–116. doi:10.1007/s10593-012-0974-6
Return to citation in text: [1] [2] [3] [4] [5] [6] [7] [8] [9] -
Crespi, S.; Simeth, N. A.; Di Donato, M.; Doria, S.; Stindt, C. N.; Hilbers, M. F.; Kiss, F. L.; Toyoda, R.; Wesseling, S.; Buma, W. J.; Feringa, B. L.; Szymański, W. Angew. Chem., Int. Ed. 2021, 60, 25290–25295. doi:10.1002/anie.202111748
Return to citation in text: [1] [2] -
Aiken, S.; Edgar, R. J. L.; Gabbutt, C. D.; Heron, B. M.; Hobson, P. A. Dyes Pigm. 2018, 149, 92–121. doi:10.1016/j.dyepig.2017.09.057
Return to citation in text: [1] [2] [3] [4] -
Kobauri, P.; Dekker, F. J.; Szymanski, W.; Feringa, B. L. Angew. Chem., Int. Ed. 2023, 62, e202300681. doi:10.1002/anie.202300681
Return to citation in text: [1] -
Kuntze, K.; Pooler, D. R. S.; Di Donato, M.; Hilbers, M. F.; van der Meulen, P.; Buma, W. J.; Priimagi, A.; Feringa, B. L.; Crespi, S. Chem. Sci. 2023, 14, 8458–8465. doi:10.1039/d3sc03090c
Return to citation in text: [1] -
Sacherer, M.; Hampel, F.; Dube, H. Nat. Commun. 2023, 14, 4382. doi:10.1038/s41467-023-39944-x
Return to citation in text: [1] -
Huang, C.-Y.; Hecht, S. Chem. – Eur. J. 2023, 29, e202300981. doi:10.1002/chem.202300981
Return to citation in text: [1] -
Popova, O. S.; Podshibyakin, V. А.; Shepelenko, Е. N.; Kuzmina, L. G.; Zaitsev, S. A.; Dubonosov, A. D.; Bren, V. A.; Minkin, V. I. J. Mol. Struct. 2023, 1272, 134211. doi:10.1016/j.molstruc.2022.134211
Return to citation in text: [1] -
Alreja, P.; Kaur, N. RSC Adv. 2016, 6, 23169–23217. doi:10.1039/c6ra00150e
Return to citation in text: [1] -
Bencini, A.; Lippolis, V. Coord. Chem. Rev. 2010, 254, 2096–2180. doi:10.1016/j.ccr.2010.04.008
Return to citation in text: [1] -
Huang, X.; Li, T. J. Mater. Chem. C 2020, 8, 821–848. doi:10.1039/c9tc06054e
Return to citation in text: [1] -
Pischel, U. Chimia 2014, 68, 505–511. doi:10.2533/chimia.2014.505
Return to citation in text: [1] -
Wang, G.; Zhang, J. J. Photochem. Photobiol., C 2012, 13, 299–309. doi:10.1016/j.jphotochemrev.2012.06.002
Return to citation in text: [1] -
Wu, J.; Kwon, B.; Liu, W.; Anslyn, E. V.; Wang, P.; Kim, J. S. Chem. Rev. 2015, 115, 7893–7943. doi:10.1021/cr500553d
Return to citation in text: [1] -
Lee, M. H.; Kim, J. S.; Sessler, J. L. Chem. Soc. Rev. 2015, 44, 4185–4191. doi:10.1039/c4cs00280f
Return to citation in text: [1] -
Bren, V. A.; Dubonosov, A. D.; Minkin, V. I.; Tsukanov, A. V.; Gribanova, T. N.; Shepelenko, E. N.; Revinsky, Y. V.; Rybalkin, V. P. J. Phys. Org. Chem. 2007, 20, 917–928. doi:10.1002/poc.1264
Return to citation in text: [1] -
Rybalkin, V. P.; Zmeeva, S. Y.; Popova, L. L.; Tkachev, V. V.; Utenyshev, A. N.; Karlutova, O. Y.; Dubonosov, A. D.; Bren, V. A.; Aldoshin, S. M.; Minkin, V. I. Beilstein J. Org. Chem. 2020, 16, 1820–1829. doi:10.3762/bjoc.16.149
Return to citation in text: [1] -
Suganya, S.; Naha, S.; Velmathi, S. ChemistrySelect 2018, 3, 7231–7268. doi:10.1002/slct.201801222
Return to citation in text: [1] -
Mukherjee, S.; Talukder, S. J. Fluoresc. 2017, 27, 1567–1572. doi:10.1007/s10895-016-1974-1
Return to citation in text: [1] -
Bren, V. A.; Dubonosov, A. D.; Popova, L. L.; Rybalkin, V. P.; Sadekov, I. D.; Shepelenko, E. N.; Tsukanov, A. V. ARKIVOC (Gainesville, FL, U. S.) 2005, No. vii, 60–66. doi:10.3998/ark.5550190.0006.707
Return to citation in text: [1] [2] -
CrysAlisPro 1.171.38.41. Rigaku, 2015; https://www.rigaku.com/en/products/smc/chrysalis (accessed Nov 11, 2023).
Return to citation in text: [1] -
Sheldrick, G. M. Acta Crystallogr., Sect. C: Struct. Chem. 2015, 71, 3–8. doi:10.1107/s2053229614024218
Return to citation in text: [1] -
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Adv. 2015, 71, 3–8. doi:10.1107/s2053273314026370
Return to citation in text: [1] -
Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. J. Appl. Crystallogr. 2009, 42, 339–341. doi:10.1107/s0021889808042726
Return to citation in text: [1]
| 14. | Minkin, V. I.; Bren’, V. A.; Dubonosov, A. D.; Tsukanov, A. V. Chem. Heterocycl. Compd. 2012, 48, 107–116. doi:10.1007/s10593-012-0974-6 |
| 33. | Bren, V. A.; Dubonosov, A. D.; Popova, L. L.; Rybalkin, V. P.; Sadekov, I. D.; Shepelenko, E. N.; Tsukanov, A. V. ARKIVOC (Gainesville, FL, U. S.) 2005, No. vii, 60–66. doi:10.3998/ark.5550190.0006.707 |
| 31. | Suganya, S.; Naha, S.; Velmathi, S. ChemistrySelect 2018, 3, 7231–7268. doi:10.1002/slct.201801222 |
| 32. | Mukherjee, S.; Talukder, S. J. Fluoresc. 2017, 27, 1567–1572. doi:10.1007/s10895-016-1974-1 |
| 14. | Minkin, V. I.; Bren’, V. A.; Dubonosov, A. D.; Tsukanov, A. V. Chem. Heterocycl. Compd. 2012, 48, 107–116. doi:10.1007/s10593-012-0974-6 |
| 33. | Bren, V. A.; Dubonosov, A. D.; Popova, L. L.; Rybalkin, V. P.; Sadekov, I. D.; Shepelenko, E. N.; Tsukanov, A. V. ARKIVOC (Gainesville, FL, U. S.) 2005, No. vii, 60–66. doi:10.3998/ark.5550190.0006.707 |
| 1. | Braslavsky, S. E. Pure Appl. Chem. 2007, 79, 293–465. doi:10.1351/pac200779030293 |
| 2. | Pianowski, Z. L., Ed. Molecular Photoswitches: Chemistry, Properties, and Applications; Wiley-VCH: Weinheim, Germany, 2022. doi:10.1002/9783527827626 |
| 3. | Tian, H.; Zhang, J., Eds. Photochromic Materials: Preparation, Properties and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2016. doi:10.1002/9783527683734 |
| 4. | Duerr, H.; Bouas-Laurent, H., Eds. Photochromism: Molecules and Systems, revised edition; Elsevier: Amsterdam, Netherlands, 2003. |
| 22. | Alreja, P.; Kaur, N. RSC Adv. 2016, 6, 23169–23217. doi:10.1039/c6ra00150e |
| 23. | Bencini, A.; Lippolis, V. Coord. Chem. Rev. 2010, 254, 2096–2180. doi:10.1016/j.ccr.2010.04.008 |
| 14. | Minkin, V. I.; Bren’, V. A.; Dubonosov, A. D.; Tsukanov, A. V. Chem. Heterocycl. Compd. 2012, 48, 107–116. doi:10.1007/s10593-012-0974-6 |
| 16. | Aiken, S.; Edgar, R. J. L.; Gabbutt, C. D.; Heron, B. M.; Hobson, P. A. Dyes Pigm. 2018, 149, 92–121. doi:10.1016/j.dyepig.2017.09.057 |
| 15. | Crespi, S.; Simeth, N. A.; Di Donato, M.; Doria, S.; Stindt, C. N.; Hilbers, M. F.; Kiss, F. L.; Toyoda, R.; Wesseling, S.; Buma, W. J.; Feringa, B. L.; Szymański, W. Angew. Chem., Int. Ed. 2021, 60, 25290–25295. doi:10.1002/anie.202111748 |
| 16. | Aiken, S.; Edgar, R. J. L.; Gabbutt, C. D.; Heron, B. M.; Hobson, P. A. Dyes Pigm. 2018, 149, 92–121. doi:10.1016/j.dyepig.2017.09.057 |
| 17. | Kobauri, P.; Dekker, F. J.; Szymanski, W.; Feringa, B. L. Angew. Chem., Int. Ed. 2023, 62, e202300681. doi:10.1002/anie.202300681 |
| 18. | Kuntze, K.; Pooler, D. R. S.; Di Donato, M.; Hilbers, M. F.; van der Meulen, P.; Buma, W. J.; Priimagi, A.; Feringa, B. L.; Crespi, S. Chem. Sci. 2023, 14, 8458–8465. doi:10.1039/d3sc03090c |
| 19. | Sacherer, M.; Hampel, F.; Dube, H. Nat. Commun. 2023, 14, 4382. doi:10.1038/s41467-023-39944-x |
| 20. | Huang, C.-Y.; Hecht, S. Chem. – Eur. J. 2023, 29, e202300981. doi:10.1002/chem.202300981 |
| 21. | Popova, O. S.; Podshibyakin, V. А.; Shepelenko, Е. N.; Kuzmina, L. G.; Zaitsev, S. A.; Dubonosov, A. D.; Bren, V. A.; Minkin, V. I. J. Mol. Struct. 2023, 1272, 134211. doi:10.1016/j.molstruc.2022.134211 |
| 30. | Rybalkin, V. P.; Zmeeva, S. Y.; Popova, L. L.; Tkachev, V. V.; Utenyshev, A. N.; Karlutova, O. Y.; Dubonosov, A. D.; Bren, V. A.; Aldoshin, S. M.; Minkin, V. I. Beilstein J. Org. Chem. 2020, 16, 1820–1829. doi:10.3762/bjoc.16.149 |
| 12. | Metelitsa, A.; Chernyshev, A.; Voloshin, N.; Solov'eva, E.; Rostovtseva, I.; Dorogan, I.; Gaeva, E.; Guseva, A. Dyes Pigm. 2021, 186, 109070. doi:10.1016/j.dyepig.2020.109070 |
| 13. | Bren, V. A.; Dubonosov, A. D.; Popova, O. S.; Revinskii, Y. V.; Tikhomirova, K. S.; Minkin, V. I. Int. J. Photoenergy 2018, 9746534. doi:10.1155/2018/9746534 |
| 14. | Minkin, V. I.; Bren’, V. A.; Dubonosov, A. D.; Tsukanov, A. V. Chem. Heterocycl. Compd. 2012, 48, 107–116. doi:10.1007/s10593-012-0974-6 |
| 15. | Crespi, S.; Simeth, N. A.; Di Donato, M.; Doria, S.; Stindt, C. N.; Hilbers, M. F.; Kiss, F. L.; Toyoda, R.; Wesseling, S.; Buma, W. J.; Feringa, B. L.; Szymański, W. Angew. Chem., Int. Ed. 2021, 60, 25290–25295. doi:10.1002/anie.202111748 |
| 14. | Minkin, V. I.; Bren’, V. A.; Dubonosov, A. D.; Tsukanov, A. V. Chem. Heterocycl. Compd. 2012, 48, 107–116. doi:10.1007/s10593-012-0974-6 |
| 5. | Crano, J. C.; Guglielmetti, R. J., Eds. Organic Photochromic and Thermochromic Compounds; Springer: New York, NY, USA, 2002. doi:10.1007/b114211 |
| 6. | Fitzmaurice, O.; Bartkowski, M.; Giordani, S. Front. Chem. (Lausanne, Switz.) 2022, 10, 859450. doi:10.3389/fchem.2022.859450 |
| 7. | Magri, D. C. Coord. Chem. Rev. 2021, 426, 213598. doi:10.1016/j.ccr.2020.213598 |
| 8. | Andréasson, J.; Pischel, U. Coord. Chem. Rev. 2021, 429, 213695. doi:10.1016/j.ccr.2020.213695 |
| 9. | Welleman, I. M.; Hoorens, M. W. H.; Feringa, B. L.; Boersma, H. H.; Szymański, W. Chem. Sci. 2020, 11, 11672–11691. doi:10.1039/d0sc04187d |
| 10. | Krämer, J.; Kang, R.; Grimm, L. M.; De Cola, L.; Picchetti, P.; Biedermann, F. Chem. Rev. 2022, 122, 3459–3636. doi:10.1021/acs.chemrev.1c00746 |
| 11. | Li, M.; Zhao, J.; Chu, H.; Mi, Y.; Zhou, Z.; Di, Z.; Zhao, M.; Li, L. Adv. Mater. (Weinheim, Ger.) 2019, 31, 1804745. doi:10.1002/adma.201804745 |
| 14. | Minkin, V. I.; Bren’, V. A.; Dubonosov, A. D.; Tsukanov, A. V. Chem. Heterocycl. Compd. 2012, 48, 107–116. doi:10.1007/s10593-012-0974-6 |
| 14. | Minkin, V. I.; Bren’, V. A.; Dubonosov, A. D.; Tsukanov, A. V. Chem. Heterocycl. Compd. 2012, 48, 107–116. doi:10.1007/s10593-012-0974-6 |
| 14. | Minkin, V. I.; Bren’, V. A.; Dubonosov, A. D.; Tsukanov, A. V. Chem. Heterocycl. Compd. 2012, 48, 107–116. doi:10.1007/s10593-012-0974-6 |
| 16. | Aiken, S.; Edgar, R. J. L.; Gabbutt, C. D.; Heron, B. M.; Hobson, P. A. Dyes Pigm. 2018, 149, 92–121. doi:10.1016/j.dyepig.2017.09.057 |
| 36. | Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Adv. 2015, 71, 3–8. doi:10.1107/s2053273314026370 |
| 14. | Minkin, V. I.; Bren’, V. A.; Dubonosov, A. D.; Tsukanov, A. V. Chem. Heterocycl. Compd. 2012, 48, 107–116. doi:10.1007/s10593-012-0974-6 |
| 29. | Bren, V. A.; Dubonosov, A. D.; Minkin, V. I.; Tsukanov, A. V.; Gribanova, T. N.; Shepelenko, E. N.; Revinsky, Y. V.; Rybalkin, V. P. J. Phys. Org. Chem. 2007, 20, 917–928. doi:10.1002/poc.1264 |
| 37. | Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. J. Appl. Crystallogr. 2009, 42, 339–341. doi:10.1107/s0021889808042726 |
| 27. | Wu, J.; Kwon, B.; Liu, W.; Anslyn, E. V.; Wang, P.; Kim, J. S. Chem. Rev. 2015, 115, 7893–7943. doi:10.1021/cr500553d |
| 28. | Lee, M. H.; Kim, J. S.; Sessler, J. L. Chem. Soc. Rev. 2015, 44, 4185–4191. doi:10.1039/c4cs00280f |
| 34. | CrysAlisPro 1.171.38.41. Rigaku, 2015; https://www.rigaku.com/en/products/smc/chrysalis (accessed Nov 11, 2023). |
| 24. | Huang, X.; Li, T. J. Mater. Chem. C 2020, 8, 821–848. doi:10.1039/c9tc06054e |
| 25. | Pischel, U. Chimia 2014, 68, 505–511. doi:10.2533/chimia.2014.505 |
| 26. | Wang, G.; Zhang, J. J. Photochem. Photobiol., C 2012, 13, 299–309. doi:10.1016/j.jphotochemrev.2012.06.002 |
| 1. | Braslavsky, S. E. Pure Appl. Chem. 2007, 79, 293–465. doi:10.1351/pac200779030293 |
| 16. | Aiken, S.; Edgar, R. J. L.; Gabbutt, C. D.; Heron, B. M.; Hobson, P. A. Dyes Pigm. 2018, 149, 92–121. doi:10.1016/j.dyepig.2017.09.057 |
| 35. | Sheldrick, G. M. Acta Crystallogr., Sect. C: Struct. Chem. 2015, 71, 3–8. doi:10.1107/s2053229614024218 |
© 2024 Rybalkin et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.