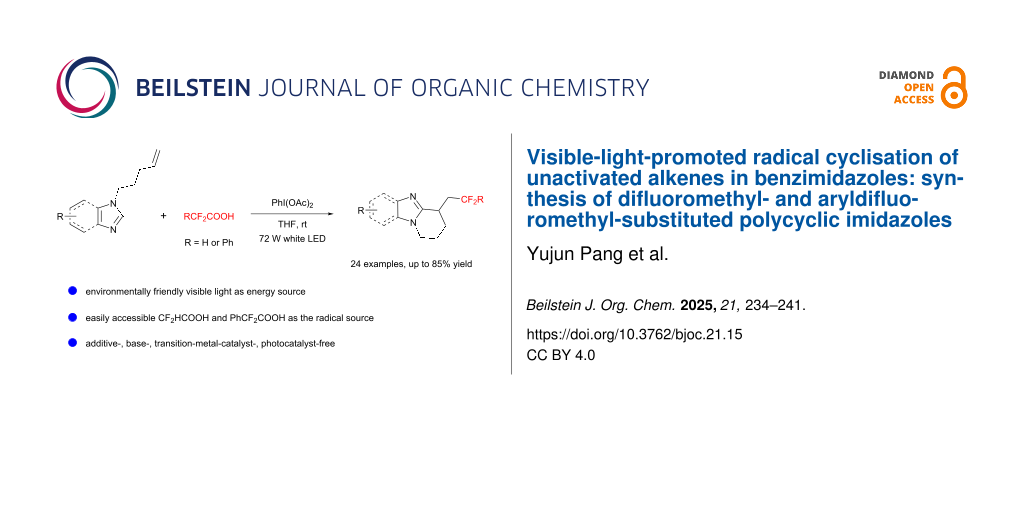
Abstract
An efficient and eco-friendly approach for synthesizing difluoromethyl- and aryldifluoromethyl-substituted polycyclic imidazoles was established via a visible-light-promoted radical cyclization reaction. This method employed the readily accessible and inexpensive CF2HCO2H or PhCF2COOH, along with benzimidazoles bearing unactivated alkenes and PhI(OAc)2 as substrates, and proceeded without the need of any base, metal catalyst, photocatalyst or additive. In total, 24 examples were examined, and all of them successfully underwent cyclization reaction to produce the target products in good to excellent yields. Mechanistic studies revealed that the reaction proceeds via a radical pathway.
Graphical Abstract
Introduction
Organofluorine compounds continue to play important roles in pharmaceuticals and agrochemicals nowadays, largely due to the unique ability of fluorinated groups to influence the physicochemical and biochemical properties of molecules [1-3]. Among the various fluorinated functionalities, the difluoromethyl (CF2H) group and its aryl-substituted derivative, the benzylic difluoromethylene (PhCF2) group, stand out as particularly valuable in drug design. The CF2H group can serve as a lipophilic isostere for hydroxy (OH), amino (NH2), and thiol (SH) groups, thereby enhancing the efficacy and selectivity of therapeutic agents [4-6]. Similarly, the PhCF2 group offers unique properties that can modify the activity and pharmacokinetic profiles of drugs [7]. Prominent examples include pantoprazole, a widely used proton-pump inhibitor (PPI) featuring a CF2H group; deracoxib, another drug that also incorporates a CF2H moiety in its structure; and a MET inhibitor specifically designed with a PhCF2 group (Figure 1a) [8-10]. As a result, there is a pressing need for the development of efficient methods for incorporating both the CF2H and PhCF2 groups into diverse molecular frameworks, particularly those with bioactivity properties.
Figure 1: Selected examples containing tricyclic imidazole, CF2H or PhCF2 group.
Figure 1: Selected examples containing tricyclic imidazole, CF2H or PhCF2 group.
The benzimidazole core is widely recognized as a vital pharmacophore in medicinal chemistry due to its special biological activity [11-13]. In particular, the tricyclic benzimidazole skeleton is ubiquitous in many bioactive compounds and therapeutic agents (Figure 1b) [14-16]. Recent studies have shown that fluorinated benzimidazole derivatives exhibit improved pharmacokinetic properties [17], which has further sparked interest in their development. Consequently, constructing benzimidazoles bearing the CF2H and PhCF2 groups has garnered significant attention. However, despite this growing interest, only a limited number of research groups have reported the direct difluoromethylation/cyclization reaction of benzimidazoles with alkenes for the syntheses of difluoromethylated tricyclic benzimidazoles to date. For example, in 2023, Chen and co-workers pioneered an electrochemical approach for the difluoromethylation and cyclization reaction of unactivated alkenes within benzimidazole molecules using CF2HSO2Na [18]. Subsequently, in 2024, Jin [19] and Yang [20] developed visible light-induced difluoromethylation strategies for unactivated alkenes within benzimidazoles using different CF2H sources (CF2HSO2Na and ([Ph3PCF2H]+Br−), respectively (Scheme 1a). Despite these advances, the above methods still suffer from several limitations, including a narrow substrate scope, the reliance on expensive metal catalysts and excess additives, and the need for multistep synthesis of difluoromethylating reagents. These drawbacks restrict their broader applicability in drug design to some extent. Besides, the incorporation of the PhCF2 group into tricyclic imidazoles has never been reported according to our best knowledge. Therefore, it is essential to explore environmentally friendly, cost-effective synthetic approaches for the construction of both difluoromethylated and aryldifluoromethylated benzimidazoles.
Scheme 1: Strategies for the synthesis of difluoromethylated and difluoroarylmethylated tricyclic imidazoles.
Scheme 1: Strategies for the synthesis of difluoromethylated and difluoroarylmethylated tricyclic imidazoles.
Inspired by previous work in radical chemistry, we turned our attention to difluoroacetic acid (CF2HCOOH) and α,α-difluorobenzeneacetic acid (PhCF2COOH), both of which are inexpensive and readily available industrial raw materials. In 2019, Gouverneur and co-workers reported a hydrodifluoromethylation of unactivated alkenes, wherein a CF2H radical was generated from CF2HCOOH using (diacetoxyiodo)benzene (PIDA) and light [21]. This CF2H radical then added to the double bond to form a new alkyl radical, which underwent hydrogen atom abstraction to yield the hydrodifluoromethylation product. Building upon this work, we hypothesized that if the newly formed alkyl radical could undergo intramolecular cyclization with an aromatic ring, instead of hydrogen abstraction, it could enable the construction of polycyclic structures. Thus, as part of our ongoing interest in radical cyclization reactions [22-26], we report here a sustainable and efficient protocol for synthesizing difluoromethylated and aryldifluoromethylated polycyclic imidazoles via visible-light-promoted cyclization of unactivated alkene-containing imidazoles with CF2HCOOH or PhCF2COOH, and PIDA under additive-, base-, and metal catalyst-free conditions (Scheme 1b).
Results and Discussion
Initially, 1-(pent-4-en-1-yl)-1H-benzo[d]imidazole (1a), CF2HCOOH, and PIDA were chosen as the template substrates for this radical difluoromethylation and cyclization reaction (Table 1). Employing PIDA as the promoter, THF as the solvent, and 72 W white LED as the light source, the desired product 3a formed in 85% isolated yield at room temperature (Table 1, entry 1). We found that the hypervalent iodine reagent was of significant importance for the present transformation (Table 1, entries 2 and 3), and PIDA was the most efficient promoter. Changing THF to other solvents, such as DCM, EtOH, DMF, CH3CN, EtOAc, or DMSO, resulted in a lower yield (Table 1, entries 4–9). Furthermore, variations in the amounts of PIDA or CF2HCOOH led to diminished yields (Table 1, entries 10–13), and conducting the reaction under air instead of nitrogen significantly lowered the yield (Table 1, entry 14). Control experiments showed that the absence of PIDA resulted in no reaction (Table 1, entry 15), while the use of a 40 W light source or the absence of visible light also reduced the product yield (Table 1, entries 16 and 17).
Table 1: Optimization of reaction conditions.a
|
|
||
| Entry | Variation from the standard conditions | Yield (%)b |
| 1 | none | 85 |
| 2 | PIFA | 37 |
| 3 | 4-Br-PIDA | trace |
| 4 | DCM instead of THF | trace |
| 5 | EtOH instead of THF | NR |
| 6 | DMF instead of THF | 13 |
| 7 | CH3CN instead of THF | 17 |
| 8 | EtOAc instead of THF | 12 |
| 9 | DMSO instead of THF | 12 |
| 10 | PIDA (3.0 equiv) instead of PIDA (4.0 equiv) | 62 |
| 11 | PIDA (5.0 equiv) instead of PIDA (4.0 equiv) | 80 |
| 12 | 2 (6 equiv) instead of 2 (7.0 equiv) | 78 |
| 13 | 2 (8 equiv) instead of 2 (7.0 equiv) | 83 |
| 14 | air instead of N2 | 55 |
| 15 | without PIDA | NR |
| 16 | 40 W white LED instead of 72 W white LED | 42 |
| 17 | dark | 40 |
aReaction conditions: 1a (0.2 mmol), 2 (1.4 mmol), and PIDA (0.8 mmol) in solvent (2 mL) irradiated with 72 W white LEDs at room temperature for 12 h under a N2 atmosphere. NR no reaction. bIsolated yield.
With the optimized conditions in hand (Table 1, entry 1), the generality of the visible-light-promoted radical difluoromethlation/cyclization reaction was first investigated (Scheme 2). We were delighted to observe that the benzimidazole ring exhibited good tolerance for both electron-withdrawing groups such as fluorine (–F), bromine (–Br), and chlorine (–Cl), as well as electron-donating substituents like methoxy (–OMe) and methyl (–Me), yielding the corresponding 6-membered tricyclic imidazoles in moderate to good yields (3b–h). Benzene rings substituted with halogen atoms (–F, –Cl, –Br) were also suitable for this transformation, efficiently giving the desired products in yields of 65–80% (3b, 3e–g), thus facilitating further functionalization possibilities. Notably, substrates with substituents at the sterically hindered 7-position of the benzimidazole ring also successfully underwent smooth cyclization, leading to the formation of products 3c and 3d. Furthermore, the methodology was compatible with 5,6-disubstituted N-alkenylbenzimidazoles, including those with -difluoro, -dichloro, -dibromo, and -dimethyl substitutions, resulting in the production of the anticipated products in yields ranging from moderate to good (3e–h). Afterwards, we shifted our focus to substrates containing a single imidazole ring and discovered that the radical difluoromethylation and subsequent cyclization of unactivated olefin-containing imidazoles proceeded efficiently, generating the CF2H-substituted bicyclic imidazoles with yields ranging from moderate to high (specifically, 3i yielded 42%, 3j yielded 70%, and 3k yielded 80%). The relatively lower yield of 3i can be attributed to the formation of side products due to the presence of the phenyl ring. Furthermore, terminal olefins with varying chain lengths also reacted successfully, resulting in 5-membered and 7-membered cyclized products (3l–p) with yields between 44% and 66%. The lower yields in these cases might be due to the low reactivity of the intermediate C (Scheme 3), which may have made it less likely to undergo the desired transformation. To broaden application of this strategy, we tested other substrates as well. For instance, we successfully converted the N-alkenyl 2-arylbenzimidazole substrate into the desired product (3q). Finally, we examined the substrates for the radical aryldifluoromethylation/cyclization reaction (for details about optimization conditions, please see Supporting Information File 1). We were delighted to find that when 2-fluorophenylacetic acid was employed as the fluorine source, a wide range of benzimidazole substrates were also compatible with this reaction. For example, substrates with a bromine atom occupying the 4-position and a methoxy group at the 7-position could be successfully converted into the target products (3s and 3t). In addition, doubly substituted benzimidazoles (3u–w), as well as the single imidazole (3x), were also found to be applicable. This demonstrates the versatility of our methodology and its potential for further exploration in diverse chemical spaces.
Scheme 2: Substrate scope of the protocol. Reaction conditions: 1 (0.2 mmol), 2 (1.4 mmol), and PIDA (0.8 mmol) in solvent (2 mL) irradiated with 72 W white LEDs at room temperature for 12 h under a N2 atmosphere. Yields refer to isolated yield. aα,α-Difluorobenzeneacetic acid (2 equiv) was used.
Scheme 2: Substrate scope of the protocol. Reaction conditions: 1 (0.2 mmol), 2 (1.4 mmol), and PIDA (0.8 mmo...
To gain a deeper understanding of the mechanism behind the observed reaction, we conducted a series of control experiments as outlined in Scheme 3a. Initially, we performed the model reaction with 1a and PhI(OCOCF2H)2, which resulted in the formation of product 3a with an 85% yield. This finding indicated that PhI(OCOCF2H)2 played a crucial role as an intermediate in the reaction. Subsequently, we introduced 3 equivalents of a radical scavenger (either TEMPO or BHT) into the reaction mixture, which significantly impeded the progress of the desired reaction. Therefore, on the basis of the above experimental results and previous reports [21,27-30], we proposed a possible reaction mechanism (Scheme 3b), taking CF2HCOOH as the illustrative example. Initially, a double ligand exchange between PIDA and CF2HCOOH would generate PhI(OCOCF2H)2 A. Homolysis of A under visible light (72 W white light) produced an iodanyl radical B and a CF2H radical. The CF2H radical regioselectively added to 1a to form intermediate C. Subsequently, intermediate C could be converted into the radical intermediate D via intramolecular radical cyclization. A single-electron-transfer (SET) process then occurred between the radical B and the radical D, resulting in the generation of cationic intermediate E, difluoroacetate anion and PhI. Finally, the product 3a was obtained after the deprotonation by difluoroacetate anion.
Scheme 3: Control experiments and plausible mechanism.
Scheme 3: Control experiments and plausible mechanism.
Conclusion
In summary, we have successfully developed a sustainable and efficient method for synthesizing difluoromethylated and aryldifluoromethylated polycyclic imidazoles through visible-light-promoted radical reactions. In contrast to previous reports, we achieved high yields of tricyclic and bicyclic imidazoles under additive-, base-, and metal catalyst-free conditions utilizing difluoroacetic acid and α,α-difluorobenzeneacetic acid as the readily available fluorine sources. The significant advantages of this approach, including its environmental friendliness and cost-effectiveness, position it as a valuable strategy in drug design and the synthesis of fluorinated compounds.
Supporting Information
| Supporting Information File 1: Experimental procedures, product characterization, and copies of NMR spectra. | ||
| Format: PDF | Size: 4.7 MB | Download |
Funding
We are grateful to the Scientific Research Project of Hubei Education Department (T2020023 and Q20211402) and the open project of Hubei Provincial Key Laboratory of Green Materials for Light Industry (202107B06) and the Doctoral Research Start-up Fund of Hubei University of Technology (XJKY20220023) for financial support.
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information of this article.
References
-
Isanbor, C.; O’Hagan, D. J. Fluorine Chem. 2006, 127, 303–319. doi:10.1016/j.jfluchem.2006.01.011
Return to citation in text: [1] -
Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320–330. doi:10.1039/b610213c
Return to citation in text: [1] -
Meanwell, N. A. J. Med. Chem. 2011, 54, 2529–2591. doi:10.1021/jm1013693
Return to citation in text: [1] -
Zafrani, Y.; Sod-Moriah, G.; Yeffet, D.; Berliner, A.; Amir, D.; Marciano, D.; Elias, S.; Katalan, S.; Ashkenazi, N.; Madmon, M.; Gershonov, E.; Saphier, S. J. Med. Chem. 2019, 62, 5628–5637. doi:10.1021/acs.jmedchem.9b00604
Return to citation in text: [1] -
Sessler, C. D.; Rahm, M.; Becker, S.; Goldberg, J. M.; Wang, F.; Lippard, S. J. J. Am. Chem. Soc. 2017, 139, 9325–9332. doi:10.1021/jacs.7b04457
Return to citation in text: [1] -
Zafrani, Y.; Yeffet, D.; Sod-Moriah, G.; Berliner, A.; Amir, D.; Marciano, D.; Gershonov, E.; Saphier, S. J. Med. Chem. 2017, 60, 797–804. doi:10.1021/acs.jmedchem.6b01691
Return to citation in text: [1] -
Geri, J. B.; Wade Wolfe, M. M.; Szymczak, N. K. J. Am. Chem. Soc. 2018, 140, 9404–9408. doi:10.1021/jacs.8b06093
Return to citation in text: [1] -
Jungnickel, P. W. Clin. Ther. 2000, 22, 1268–1293. doi:10.1016/s0149-2918(00)83025-8
Return to citation in text: [1] -
Bienhoff, S. E.; Smith, E. S.; Roycroft, L. M.; Roberts, E. S.; Baker, L. D. Int. Scholarly Res. Not. 2011, 593015. doi:10.5402/2011/593015
Return to citation in text: [1] -
Wade Wolfe, M. M.; Guo, S.; Yu, L. S.; Vogel, T. R.; Tucker, J. W.; Szymczak, N. K. Chem. Commun. 2022, 58, 11705–11708. doi:10.1039/d2cc01938h
Return to citation in text: [1] -
Venugopal, S.; Kaur, B.; Verma, A.; Wadhwa, P.; Magan, M.; Hudda, S.; Kakoty, V. Chem. Biol. Drug Des. 2023, 102, 357–376. doi:10.1111/cbdd.14236
Return to citation in text: [1] -
Guo, Y.; Hou, X.; Fang, H. Mini-Rev. Med. Chem. 2021, 21, 1367–1379. doi:10.2174/1389557520666200804124924
Return to citation in text: [1] -
Beltran-Hortelano, I.; Alcolea, V.; Font, M.; Pérez-Silanes, S. Eur. J. Med. Chem. 2020, 206, 112692. doi:10.1016/j.ejmech.2020.112692
Return to citation in text: [1] -
Kojima, T.; Mochizuki, M.; Takai, T.; Hoashi, Y.; Morimoto, S.; Seto, M.; Nakamura, M.; Kobayashi, K.; Sako, Y.; Tanaka, M.; Kanzaki, N.; Kosugi, Y.; Yano, T.; Aso, K. Bioorg. Med. Chem. 2018, 26, 2229–2250. doi:10.1016/j.bmc.2018.01.020
Return to citation in text: [1] -
Edmondson, S. D.; Mastracchio, A.; Cox, J. M.; Eiermann, G. J.; He, H.; Lyons, K. A.; Patel, R. A.; Patel, S. B.; Petrov, A.; Scapin, G.; Wu, J. K.; Xu, S.; Zhu, B.; Thornberry, N. A.; Roy, R. S.; Weber, A. E. Bioorg. Med. Chem. Lett. 2009, 19, 4097–4101. doi:10.1016/j.bmcl.2009.06.011
Return to citation in text: [1] -
Guo, Z.; Chen, X.; Xue, Y.; Li, J.; Zou, D.; Wu, Y.; Wu, Y. Adv. Synth. Catal. 2022, 364, 4231–4236. doi:10.1002/adsc.202200854
Return to citation in text: [1] -
Randolph, J. T.; Flentge, C. A.; Donner, P.; Rockway, T. W.; Patel, S. V.; Nelson, L.; Hutchinson, D. K.; Mondal, R.; Mistry, N.; Reisch, T.; Dekhtyar, T.; Krishnan, P.; Pilot-Matias, T.; Stolarik, D. F.; Beno, D. W. A.; Wagner, R.; Maring, C.; Kati, W. M. Bioorg. Med. Chem. Lett. 2016, 26, 5462–5467. doi:10.1016/j.bmcl.2016.10.030
Return to citation in text: [1] -
Chen, Z.; Li, Z.; Li, S.; Qian, G.; Sun, Y. New J. Chem. 2023, 47, 11465–11469. doi:10.1039/d3nj01759a
Return to citation in text: [1] -
Huang, P.; Lv, C.; Song, H.; Wang, C.; Du, J.; Li, J.; Sun, B.; Jin, C. Green Chem. 2024, 26, 7198–7205. doi:10.1039/d4gc00728j
Return to citation in text: [1] -
Lin, S.-N.; Deng, Y.; Zhong, H.; Mao, L.-L.; Ji, C.-B.; Zhu, X.-H.; Zhang, X.; Yang, B.-M. ACS Omega 2024, 9, 28129–28143. doi:10.1021/acsomega.4c01177
Return to citation in text: [1] -
Meyer, C. F.; Hell, S. M.; Misale, A.; Trabanco, A. A.; Gouverneur, V. Angew. Chem., Int. Ed. 2019, 58, 8829–8833. doi:10.1002/anie.201903801
Return to citation in text: [1] [2] -
Xie, L.; Cao, R.; Huang, Y.; Zhang, Q.; Fang, Z.; Li, D. Org. Chem. Front. 2022, 9, 5929–5934. doi:10.1039/d2qo01175a
Return to citation in text: [1] -
Fang, Z.; Liu, W.; Al-Maharik, N.; Cao, R.; Huang, Y.; Yuan, Y.; Zhang, Q.; Li, D. J. Org. Chem. 2023, 88, 15428–15436. doi:10.1021/acs.joc.3c01964
Return to citation in text: [1] -
Fang, Z.; Xie, L.; Wang, L.; Zhang, Q.; Li, D. RSC Adv. 2022, 12, 26776–26780. doi:10.1039/d2ra05283k
Return to citation in text: [1] -
Liu, W.; Wang, L.; Mu, H.; Zhang, Q.; Fang, Z.; Li, D. Org. Biomol. Chem. 2023, 21, 1168–1171. doi:10.1039/d2ob02086f
Return to citation in text: [1] -
Wang, L.; Xie, L.; Fang, Z.; Zhang, Q.; Li, D. Org. Chem. Front. 2022, 9, 3061–3067. doi:10.1039/d2qo00207h
Return to citation in text: [1] -
Sakamoto, R.; Kashiwagi, H.; Maruoka, K. Org. Lett. 2017, 19, 5126–5129. doi:10.1021/acs.orglett.7b02416
Return to citation in text: [1] -
Mei, Y.; Zhao, L.; Liu, Q.; Ruan, S.; Wang, L.; Li, P. Green Chem. 2020, 22, 2270–2278. doi:10.1039/d0gc00009d
Return to citation in text: [1] -
Yoshimura, A.; Zhdankin, V. V. Chem. Rev. 2024, 124, 11108–11186. doi:10.1021/acs.chemrev.4c00303
Return to citation in text: [1] -
Gui, Q.-W.; Teng, F.; Li, Z.-C.; Xiong, Z.-Y.; Jin, X.-F.; Lin, Y.-W.; Cao, Z.; He, W.-M. Chin. Chem. Lett. 2021, 32, 1907–1910. doi:10.1016/j.cclet.2021.01.021
Return to citation in text: [1]
| 1. | Isanbor, C.; O’Hagan, D. J. Fluorine Chem. 2006, 127, 303–319. doi:10.1016/j.jfluchem.2006.01.011 |
| 2. | Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320–330. doi:10.1039/b610213c |
| 3. | Meanwell, N. A. J. Med. Chem. 2011, 54, 2529–2591. doi:10.1021/jm1013693 |
| 11. | Venugopal, S.; Kaur, B.; Verma, A.; Wadhwa, P.; Magan, M.; Hudda, S.; Kakoty, V. Chem. Biol. Drug Des. 2023, 102, 357–376. doi:10.1111/cbdd.14236 |
| 12. | Guo, Y.; Hou, X.; Fang, H. Mini-Rev. Med. Chem. 2021, 21, 1367–1379. doi:10.2174/1389557520666200804124924 |
| 13. | Beltran-Hortelano, I.; Alcolea, V.; Font, M.; Pérez-Silanes, S. Eur. J. Med. Chem. 2020, 206, 112692. doi:10.1016/j.ejmech.2020.112692 |
| 8. | Jungnickel, P. W. Clin. Ther. 2000, 22, 1268–1293. doi:10.1016/s0149-2918(00)83025-8 |
| 9. | Bienhoff, S. E.; Smith, E. S.; Roycroft, L. M.; Roberts, E. S.; Baker, L. D. Int. Scholarly Res. Not. 2011, 593015. doi:10.5402/2011/593015 |
| 10. | Wade Wolfe, M. M.; Guo, S.; Yu, L. S.; Vogel, T. R.; Tucker, J. W.; Szymczak, N. K. Chem. Commun. 2022, 58, 11705–11708. doi:10.1039/d2cc01938h |
| 7. | Geri, J. B.; Wade Wolfe, M. M.; Szymczak, N. K. J. Am. Chem. Soc. 2018, 140, 9404–9408. doi:10.1021/jacs.8b06093 |
| 21. | Meyer, C. F.; Hell, S. M.; Misale, A.; Trabanco, A. A.; Gouverneur, V. Angew. Chem., Int. Ed. 2019, 58, 8829–8833. doi:10.1002/anie.201903801 |
| 27. | Sakamoto, R.; Kashiwagi, H.; Maruoka, K. Org. Lett. 2017, 19, 5126–5129. doi:10.1021/acs.orglett.7b02416 |
| 28. | Mei, Y.; Zhao, L.; Liu, Q.; Ruan, S.; Wang, L.; Li, P. Green Chem. 2020, 22, 2270–2278. doi:10.1039/d0gc00009d |
| 29. | Yoshimura, A.; Zhdankin, V. V. Chem. Rev. 2024, 124, 11108–11186. doi:10.1021/acs.chemrev.4c00303 |
| 30. | Gui, Q.-W.; Teng, F.; Li, Z.-C.; Xiong, Z.-Y.; Jin, X.-F.; Lin, Y.-W.; Cao, Z.; He, W.-M. Chin. Chem. Lett. 2021, 32, 1907–1910. doi:10.1016/j.cclet.2021.01.021 |
| 4. | Zafrani, Y.; Sod-Moriah, G.; Yeffet, D.; Berliner, A.; Amir, D.; Marciano, D.; Elias, S.; Katalan, S.; Ashkenazi, N.; Madmon, M.; Gershonov, E.; Saphier, S. J. Med. Chem. 2019, 62, 5628–5637. doi:10.1021/acs.jmedchem.9b00604 |
| 5. | Sessler, C. D.; Rahm, M.; Becker, S.; Goldberg, J. M.; Wang, F.; Lippard, S. J. J. Am. Chem. Soc. 2017, 139, 9325–9332. doi:10.1021/jacs.7b04457 |
| 6. | Zafrani, Y.; Yeffet, D.; Sod-Moriah, G.; Berliner, A.; Amir, D.; Marciano, D.; Gershonov, E.; Saphier, S. J. Med. Chem. 2017, 60, 797–804. doi:10.1021/acs.jmedchem.6b01691 |
| 19. | Huang, P.; Lv, C.; Song, H.; Wang, C.; Du, J.; Li, J.; Sun, B.; Jin, C. Green Chem. 2024, 26, 7198–7205. doi:10.1039/d4gc00728j |
| 21. | Meyer, C. F.; Hell, S. M.; Misale, A.; Trabanco, A. A.; Gouverneur, V. Angew. Chem., Int. Ed. 2019, 58, 8829–8833. doi:10.1002/anie.201903801 |
| 18. | Chen, Z.; Li, Z.; Li, S.; Qian, G.; Sun, Y. New J. Chem. 2023, 47, 11465–11469. doi:10.1039/d3nj01759a |
| 22. | Xie, L.; Cao, R.; Huang, Y.; Zhang, Q.; Fang, Z.; Li, D. Org. Chem. Front. 2022, 9, 5929–5934. doi:10.1039/d2qo01175a |
| 23. | Fang, Z.; Liu, W.; Al-Maharik, N.; Cao, R.; Huang, Y.; Yuan, Y.; Zhang, Q.; Li, D. J. Org. Chem. 2023, 88, 15428–15436. doi:10.1021/acs.joc.3c01964 |
| 24. | Fang, Z.; Xie, L.; Wang, L.; Zhang, Q.; Li, D. RSC Adv. 2022, 12, 26776–26780. doi:10.1039/d2ra05283k |
| 25. | Liu, W.; Wang, L.; Mu, H.; Zhang, Q.; Fang, Z.; Li, D. Org. Biomol. Chem. 2023, 21, 1168–1171. doi:10.1039/d2ob02086f |
| 26. | Wang, L.; Xie, L.; Fang, Z.; Zhang, Q.; Li, D. Org. Chem. Front. 2022, 9, 3061–3067. doi:10.1039/d2qo00207h |
| 17. | Randolph, J. T.; Flentge, C. A.; Donner, P.; Rockway, T. W.; Patel, S. V.; Nelson, L.; Hutchinson, D. K.; Mondal, R.; Mistry, N.; Reisch, T.; Dekhtyar, T.; Krishnan, P.; Pilot-Matias, T.; Stolarik, D. F.; Beno, D. W. A.; Wagner, R.; Maring, C.; Kati, W. M. Bioorg. Med. Chem. Lett. 2016, 26, 5462–5467. doi:10.1016/j.bmcl.2016.10.030 |
| 14. | Kojima, T.; Mochizuki, M.; Takai, T.; Hoashi, Y.; Morimoto, S.; Seto, M.; Nakamura, M.; Kobayashi, K.; Sako, Y.; Tanaka, M.; Kanzaki, N.; Kosugi, Y.; Yano, T.; Aso, K. Bioorg. Med. Chem. 2018, 26, 2229–2250. doi:10.1016/j.bmc.2018.01.020 |
| 15. | Edmondson, S. D.; Mastracchio, A.; Cox, J. M.; Eiermann, G. J.; He, H.; Lyons, K. A.; Patel, R. A.; Patel, S. B.; Petrov, A.; Scapin, G.; Wu, J. K.; Xu, S.; Zhu, B.; Thornberry, N. A.; Roy, R. S.; Weber, A. E. Bioorg. Med. Chem. Lett. 2009, 19, 4097–4101. doi:10.1016/j.bmcl.2009.06.011 |
| 16. | Guo, Z.; Chen, X.; Xue, Y.; Li, J.; Zou, D.; Wu, Y.; Wu, Y. Adv. Synth. Catal. 2022, 364, 4231–4236. doi:10.1002/adsc.202200854 |
| 20. | Lin, S.-N.; Deng, Y.; Zhong, H.; Mao, L.-L.; Ji, C.-B.; Zhu, X.-H.; Zhang, X.; Yang, B.-M. ACS Omega 2024, 9, 28129–28143. doi:10.1021/acsomega.4c01177 |
© 2025 Pang et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.