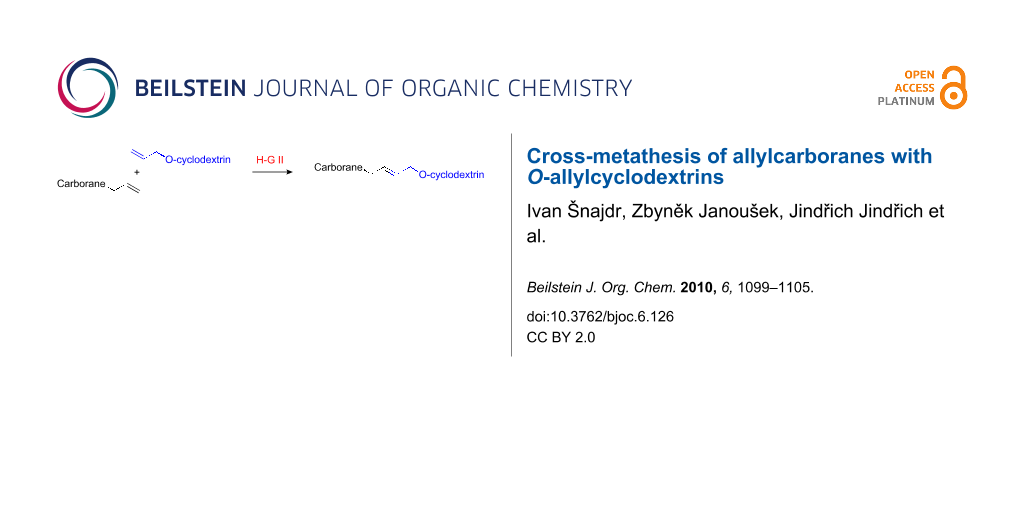
Abstract
Cross-metathesis between allylcarboranes and O-allylcyclodextrins was catalyzed by Hoveyda–Grubbs 2nd generation catalyst in toluene. The corresponding carboranyl-cyclodextrin conjugates were isolated in 15–25% yields.
Graphical Abstract
Introduction
Cross-metathesis of two different alkenes constitutes an efficient and powerful tool for synthesis of various unsymmetrically substituted alkenes. This procedure has found enormous application in organic synthesis of various types of molecules such as natural and biologically active compounds [1]. One of the key aspects of this methodology, which is responsible for a high cross-metathesis selectivity, is a proper choice of a suitable ruthenium catalyst [2,3]. Efficacy of the cross-metathesis procedure has prompted also us to investigate hitherto unexplored combinations of two different alkenes. Recently, we have shown that metathesis of various terminal alkenes with perfluoroalkylpropenes constitutes a simple and efficient approach for the synthesis of wide array of perfluoroalkylated compounds [4]. This methodology was then applied for the synthesis of perfluoroalkylated analogs of brassinosteroids [5], 17α-perfluoroalkylestradiols [6], perfluoroalkylcyclodextrins [7], and perfluoroalkylcarboranes [8]. Successful execution of these reactions prompted us to study also cross-metathesis of allylcarboranes with O-allylcyclodextrins as a route to carborane-cyclodextrin conjugates. Herein, we report our preliminary results.
Results and Discussion
Although studies on the inclusion of carboranes into cyclodextrins have previously been reported [9-13], a synthesis of cyclodextrin-carborane conjugates connected by a linker has not been, to the best of our knowledge, described. Since carboranes are of potential interest for various applications in medicine (e.g. boron neutron capture therapy for cancer, radionuclide diagnostics and therapy, and related fields [14-17], whilst some carboranes possess antiviral activity [18,19]), consequently, there is considerable interest in the synthesis of water soluble carborane derivatives. One strategy to access such compounds is based on the synthesis of carborane conjugates bearing a water-soluble moiety. With this in mind, we envisioned that this concept could be realized by the synthesis of carborane-cyclodextrin conjugates by the means of cross-metathesis between readily available allycarboranes and O-allylcyclodextrins (Figure 1).
Figure 1: Starting allylcarboranes 1a–1c and O-allylcyclodextrins 2a–2c.
Figure 1: Starting allylcarboranes 1a–1c and O-allylcyclodextrins 2a–2c.
The preparation of the starting allylcarboranes, i.e., 1-allyl-1,2-C2B10H11 1a [20], 8,8’-allyl-S-(C2B9H11)2Co 1b [21], and 8,8’-allyl-S2-(C2B9H11)2Co 1c [21], was carried out according to the previously reported procedures [8]. O-Allylcyclodextrins 2 were prepared by allylation of β-cyclodextrins under various reaction conditions (2I-O-allyl-β-cyclodextrin for 2a [22], 3I-O-allyl-β-cyclodextrin for 2b [23], and 6I-O-allyl-β-cyclodextrin for 2c) followed by peracetylation [7,24].
At the outset cross-metathesis of allylcarborane 1a with 2I-O-allylcyclodextrin 2a and various ruthenium-carbene complexes (10 mol %) in dichloromethane was carried out to assess the most suitable catalyst (for cross-metatheses involving carboranes, see: [25,26]). However, when the reaction was carried out in the presence of any of the following catalysts, Grubbs 1st, Grubbs 2nd, or Hoveyda–Grubbs 1st generation, no cross-metathesis products were obtained. Only catalysis by Hoveyda–Grubbs 2nd generation catalyst gave the desired product 3 in 14% yield. The suitability of Hoveyda–Grubbs 2nd generation catalyst for cross-metathesis reactions was consistent with the previously observed results [5-8]. Further tuning of the reaction conditions showed that the best yields were obtained by carrying out the reaction in toluene at 120 °C for 16 h (Scheme 1). Interestingly, when the reactions were carried out in CH2Cl2 (40 °C) the yields of the corresponding products were lower by 5–10%.
Scheme 1: Cross-metathesis of allylcarboranes 1 and O-allylcyclodextrins 2.
Scheme 1: Cross-metathesis of allylcarboranes 1 and O-allylcyclodextrins 2.
Cross-metatheses of various allylcarboranes 1 and O-allylcyclodextrins 2 were then carried out. In general, the reactions proceeded to give the expected products without any problems (Table 1). Thus, the cross-metathesis of 1a with 2a, 2b, and 2c furnished the corresponding carboranylcyclodextrins 3aa, 3ab, and 3ac in 24, 17, and 15% isolated yields, respectively. In an analogous manner the cross-metathesis reactions of 1b with 2a and 2c gave the carboranylcyclodextrins 3ba and 3bc in 20 and 19% yields, respectively. Finally, the reactions of the cyclodextrin derivatives 2a–2c with 1c afforded the corresponding carboranylcyclodextrins 3ca, 3cb, and 3cc in 18, 19 and 20% isolated yields, respectively. It is also of note to mention here the impressive E-selectivity of the cross-metathesis reactions, which has also been observed in other metathetical reactions with alkenylcyclodextrins derivatives [27] and can be explained by several factors [28]. Firstly, by a chelation of the intermediate Ru-carbene complex to the oxygen atoms of the cyclodextrin which results in a conformationally rigid intermediate and secondly, by a steric effect of the bulky carborane moiety. Although it may appear that the isolated yields are not high, conversions were in the range of ~50%. Isolation and purification of the products was a tedious task and the isolated yields we obtained represent the amounts of analytically pure compounds. It has been reported that low yields and conversions could be explained by isomerization of terminal to internal double bonds in both reactants (e.g., isomerization of allyl ethers to vinyl ethers [29-31] and allylcarboranes to propenylcarboranes [25]) and thus decreasing the reactant activity. However, NMR analysis of compounds isolated from the reaction mixtures revealed only the presence of the starting material and products, thus the low conversions could be attributed to deactivation of the catalysts by other routes. A similar effect has been also been observed in other cross-metathesis of various O-alkenylcyclodextrins which required the use of large amounts of catalyst [32,33]. Attempts to carry out the reaction with free (unprotected) O-allylcyclodextrins in dichloromethane or toluene has not so far resulted in the formation of any of the expected products, presumably because of their insolubility in the aforementioned solvents. To overcome the problem of the solubility of free O-allylcyclodextrins, the reaction was carried out in water in the presence of surfactant (SDS – sodium dodecyl sulfate), however, cross-metathesis did not occur.
Conclusion
The results described above clearly indicate that the cross-metathesis of allylcarboranes and O-allylcyclodextrins catalyzed by Hoveyda–Grubbs 2nd generation catalyst provides a simple and straightforward method for the synthesis of cyclodextrin-carborane conjugates. The high boron content and the presence of a water soluble moiety (after removal of the protecting groups) suggest that the compounds may have potential for use in medical applications.
Experimental
General procedure for metathesis of allylcyclodextrins with allylcarboranes. The Hoveyda–Grubbs 2nd generation catalyst (3.13 mg, 0.005 mmol) was added under an argon atmosphere to a mixture of an allylcyclodextrin (0.07 mmol) and an allylcarborane (0.05 mmol) in toluene (5 mL). The resulting solution was stirred at 110 °C overnight. Removal of the solvent under reduced pressure gave a brown residue that was purified by column chromatography (85/15 MeOH/H2O) on C18-reversed phase.
Per-O-acetyl-2I-O-[4-(1,2-dicarbadodecaboran-1-yl)-but-2-en-1-yl]-β-cyclodextrin (3aa). The compound was prepared from 2a (0.15 g, 0.07 mmol) and 1a (15 mg, 0.05 mmol). Column chromatography gave the title compound, 0.041 g (24%), as a white powder: m. p. 188–190 °C; IR (KBr) = 2591, 1747, 1371, 1236, 1044 cm−1; 1H NMR (300 MHz, CDCl3): δ = 6.08–6.02 (m, 1 H, H-2’), 5.66–5.58 (m, 1 H, H-3’), 5.46–5.15 (m, 7 H, 7 × H-3), 5.13–4.92 (m, 7 H, 7 × H-1), 4.86–4.70 (m, 6 H, 6 × H-2), 4.61–4.43 (m, 6 H, 6 × H-6), 4.39–3.82 (m, 17 H, 8 × H-6, 7 × H-5, 2 × H-1’), 3.78–3.53 (m, 9 H, 7 × H-4, 2 × H-4’), 3.34 (d, J = 7.0 Hz, 1 H, H-2I), 2.97 (s, 1 H, Ccarb-H), 2.11–1.97 (m, 60 H, 20 × CH3); 13C NMR (100 MHz, CDCl3): δ = 171.15–169.71 (20 × C=O), 132.28 (C-2’), 126.69 (C-3’), 98.35–97.06 (7 × C-1), 78.05–76.42 (7 × C-4), 74.19 (C-1’), 72.29–69.66 (7 × C-2, 7 × C-3, 7 × C-5), 63.10–62.85 (7 × C-6), 60.34 (2 × Ccarb), 40.54 (C-4’), 21.56-21.02 (20 × CH3); 11B NMR (128 MHz, CDCl3): δ = −3.29 (s, 1 B, B-9), −6.45 (s, 1 B, B-12), −10.14 (s, 2 B, B-8, B-10), −12.21 (s, 2 B, B-4, B-5), −13.47 (s, 4 B, B-3, B-6, B-7, B-11); MS (EI, m/z (rel.%)) 1108.7 (80), 1010.5 (19), 799.0 (12), 516.9 (14), 456.8 (25), 374.0 (29), 242.3 (100), 228.6 (56), 168.9 (41); HR-MS (ESI) calcd. for C88H126O55B10: 1108.3925, found 1108.3954 (C88H126O5510B2B8Na2).
Per-O-acetyl-3I-O-[4-(1,2-dicarbadodecaboran-1-yl)-but-2-en-1-yl]-β-cyclodextrin (3ab). The compound was prepared from 2b (0.15 g, 0.07 mmol) and 1a (15 mg, 0.05 mmol). Column chromatography gave the title compound, 0.029 g (17%), as a white powder: m. p. 198–201 °C; IR (KBr): = 2593, 1747, 1369, 1237, 1043 cm−1; 1H NMR (400 MHz, CDCl3): δ = 5.91–5.70 (m, 1 H, H-2’), 5.60–5.50 (m, 1 H, H-3’), 5.49–5.28 (m, 6 H, H-3), 5.24–5.05 (m, 7 H, H-1), 4.89–4.66 (m, 8 H, 7 × H-4, 1 × H-1’), 4.66–3.64 (m, 31 H, 14 × H-6, 1 × H1’, 2 × H-4’, 7 × H-5, 7 × H-4), 3.23–3.13 (m, 1 H, H-3I), 2.84 (bs, 1 H, Ccarb-H), 2.20–1.95 (m, 60 H, 20 × CH3); 13C NMR (100 MHz, CDCl3): δ = 170.76–170.04 (20 × C=O), 133.74 (C-2’), 126.51 (C-3’), 97.41–96.70 (7 × C-1), 77.68–75.86 (7 × C-4), 72.42–69.46 (7 × C-2, 7 × C-3, 7 × C-5), 62.91–62.18 (7 × C-6), 60.57 (2 × Ccarb), 40.31(C-4’), 21.14–20.87 (20 × CH3); 11B NMR (128 MHz, CDCl3): δ = −3.32 (s, 1 B, B-9), −6.47 (s, 1 B, B-12), −10.12 (s, 2 B, B-8, B-10), −12.02 (s, 2 B, B-4, B-5), −13.99 (s, 4 B, B-3, B-6, B-7, B-11); MS (EI, m/z (rel.%)): 1108.6 (100), 917.4 (19), 802.8 (20), 690.9 (18), 469.4 (42), 413.2 (65), 370.1 (96), 301.1 (75); HR-MS (ESI) calcd. for C88H126O55B10 1108.3925, found 1108.3957 (C88H126O5510B2B8Na2).
Per-O-acetyl-6I-O-[4-(1,2-dicarbadodecaboran-1-yl)-but-2-en-1-yl]-β-cyclodextrin (3ac). The compound was prepared from 2c (0.15 g, 0.07 mmol) and 1a (15 mg, 0.05 mmol). Column chromatography gave the title compound,0.026 g (15%), as a white powder: m. p. 194–197 °C; IR (KBr): = 2917, 2848, 1747, 1370, 1235, 1044 cm−1; 1H NMR (400 MHz, CDCl3): δ = 5.91–5.82 (m, 1 H, H-2’), 5.75–5.65 (m, 1 H, H-3’), 5.42–5.00 (m, 14 H, 7 × H-3, 7 × H-1), 4.91–4.68 (m, 7 H, 7 × H-2), 4.64–4.42 (m, 5 H, 5 × H-6), 4.40–3.94 (m, 17 H, 2 × H-1’, 5 × H-5, 8 × H-6, 2 × H-4’), 3.90–3.82 (m, 2 H, 2 × H-5), 3.80–3.56 (m, 8 H, 1 × H-6I, 7 × H-4), 2.96 (bs, 1 H, Ccarb-H), 2.17–1.97 (m, 60 H, 20 × CH3); 13C NMR (100 MHz, CDCl3): δ = 171.10–169.81 (20 × C=O), 134.05 (C-2’), 126.31 (C-3’), 97.45–96.64 (7 × C-1), 77.69–76.30 (7 × C-4), 71.94–69.79 (7 × C-2, 7 × C-3, 7 × C-5, C-1’), 68.12 (C-6I), 63.02–62.71 (6 × C-6), 60.18 (2 × Ccarb), 40.08 (C-4’), 21.87–21.16 (20 × CH3); 11B NMR (128 MHz, CDCl3): δ = −3.27 (s, 1 B, B-9), −6.69 (s, 1 B, B-12), −10.12 (s, 2 B, B-8, B-10), −12.04 (s, 2 B, B-4, B-5), −13.94 (s, 4 B, B-3, B-6, B-7, B-11); MS (EI, m/z (rel.%)): 1108.5 (52), 1010.1 (62), 928.6 (51), 909.0 (60), 667.1 (31), 351.3 (57), 307.3 (100); HR-MS (ESI) calcd. for C88H126O55B10: 1108.3925, found 1108.3955 (C88H126O5510B2B8Na2).
Per-O-acetyl-2I-O-{4-{8,8’-μ-(sulfido)-[3,3’-commo-cobalt(III)-bis-(1,2-dicarbaundecaborate)]-8-yl}but-2-en-1-yl}-β-cyclodextrin (3ba). The compound was prepared from 2a (0.15 g, 0.07 mmol) and 1b (20 mg, 0.05 mmol). Column chromatography gave the title compound, 0.023 g (18%), as an orange powder: m. p. 207–209 °C; IR (KBr): = 2575, 1746, 1371, 1236, 1044 cm−1. 1H NMR (600 MHz, CDCl3): δ = 5.75 (dt, J = 20.4, 5.4 Hz, 1 H, H-2’), 5.65–5.58 (m, 1 H, H-3’), 5.43–5.13 (m, 7 H, 7 × H-3), 5.12–4.91 (m, 6 H, 6 × H-1), 4.86 (d, J = 3.6 Hz, 1 H, H-1I), 4.98–4.62 (m, 6 H, 6 × H-2), 4.56–4.38 (m, 6 H, 6 × H-6), 4.30–3.87 (m, 17 H, 8 × H-6, 7 × H-5, 2 × H-1’), 3.72–3.50 (m, 9 H, 7 × H-4, 2 × H-4’), 3.46–3.34 (m, 4 H, Ccarb-H), 3.24 (dd, J = 3.0, 9.6 Hz, 1 H, H-2I), 2.09–1.97 (m, 60 H, 20 × CH3); 13C NMR (150 MHz, CDCl3): δ = 170.81–169.37 (20 × C=O), 134.55 (C-2’), 122.80 (C-3’), 98.16–96.58 (7 × C-1), 78.05–76.17 (7 × C-4), 71.03–69.54 (7 × C-2, 7 × C-3, 7 × C-5, C-1’), 62.85–62.41 (7 × C-6), 49.33–48.66 (4 × Ccarb), 42.02 (C-4’), 20.99–20.22 (20 × CH3); 11B NMR (128 MHz, CDCl3): δ = 1.26 (bs, 2 B, B-8, B-8’), −4.70 (bs, 2 B, B-10, B-10’), −(6.00–11.72) (m, 12 B, B-4, B-4’, B-5, B-5’, B-7, B-7’, B-9, B-9’, B-11, B-11’, B-12, B-12’), −14.68 (bs, 2 B, B-6, B-6’); MS (EI, m/z (rel.%)): 1214.4 (100), 1010.3 (10), 413.3 (68), 391.3 (10), 307.2 (52); HR-MS (ESI) calcd. for C90H135O5510B3B15CoNa2S: 1213.9194, found 1213.9233.
Per-O-acetyl-6I-O-{4-{8,8’-μ-(sulfido)-[3,3’-commo-cobalt(III)-bis-(1,2-dicarbaundecaborate)]-8-yl}but-2-en-1-yl}-β-cyclodextrin (3bc). The compound was prepared from 2c (0.15 g, 0.07 mmol) and 1b (20 mg, 0.05 mmol). Column chromatography gave the title compound, 0.028 g (22%), as an orange powder: m. p. 188–191 °C; IR (KBr): = 2955, 2575, 1747, 1370, 1237, 1046 cm−1; 1H NMR (600 MHz, CDCl3): δ = 5.90 (dt, J = 15.1, 5.1 Hz, 1 H, H-2’), 5.78–5.68 (m, 1 H, H-3’), 5.40–5.19 (m, 7 H, 7 × H-3), 5.17–5.02 (m, 7 H, 7 × H-1), 4.86–4.69 (m, 7 H, 7 × H-2), 4.63–4.42 (m, 5 H, 5 × H-6), 4.36–3.96 (m, 17 H, 2 × H-1’, 2 × H-4’, 5 × H-5, 8 × H-6), 3.90–3.85 (m, 2 H, 2 × H-5), 3.78–3.65 (m, 10 H, 1 × H-6I, 7 × H-4, 2 × Ccarb-H), 3.48 (bs, 1 H, Ccarb-H), 3.42 (bs, 1 H, Ccarb-H), 2.16–1.98 (m, 60 H, 20 × CH3); 13C NMR (100 MHz, CDCl3): δ = 171.10–169.80 (20 × C=O), 134.85 (C-2’), 122.80 (C-3’), 97.46–96.77 (7 × C-1), 77.69–76.21 (7 × C-4), 72.02–69.63 (7 × C-2, 7 × C-3, 7 × C-5, C-1’), 68.28 (C-6I), 62.99–62.77 (6 × C-6), 49.93–49.27 (4 × Ccarb), 42.71 (C-4’), 21.15–20.58 (20 × CH3); 11B NMR (128 MHz, CDCl3): δ = 1.39 (bs, 2 B, B-8, B-8’), −4.58 (bs, 2 B, B-10, B-10’), −(5.84–11.21) (m, 12 B, B-4, B-4’, B-5, B-5’, B-7, B-7’, B-9, B-9’, B-11, B-11’, B-12, B-12’), −14.92 (bs, 2 B, B-6, B-6’); MS (EI, m/z (rel.%)): 1214.1 (100), 1010.3 (10), 414.3 (52), 360.3 (10), 307.2 (96); HR-MS (ESI) calcd. for C90H135O5510B3B15CoNa2S: 1213.9194, found 1213.9234.
Per-O-acetyl-2I-O-{4-{8,8’-μ-(disulfido)-[3,3’-commo-cobalt(III)-bis-(1,2-dicarbaundecaborate)]-8-yl}but-2-en-1-yl}-β-cyclodextrin (3ca). The compound was prepared from 2a (0.15 g, 0.07 mmol) and 1c (20 mg, 0.05 mmol). Column chromatography gave the title compound, 0.026 g (20%), as a red powder: m. p. 188–191 °C; IR (KBr): = 2581, 1746, 1371, 1236, 1043 cm−1; 1H NMR (600 MHz, CDCl3): δ = 6.04–5.95 (m, 1 H, H-2’), 5.72–5.63 (m, 1 H, H-3’), 5.34–5.16 (m, 7 H, 7 × H-3), 5.10–4.97 (m, 6 H, 6 × H-1), 4.96 (t, J = 3.6 Hz, 1 H, H-1I), 4.84–4.76 (m, 6 H, 6 × H-2), 4.58–4.47 (m, 7 H, 7 × H-6), 4.37–4.00 (m, 18 H, 2 × H-1’, 2 × H-4’, 7 × H-5, 7 × H-6), 3.74–3.55 (m, 8 H, 6 × H-4, 2 × Ccarb-H), 3.51–3.45 (m, 1 H, Ccarb-H), 3.37–3.31 (m, 2 H, 1 × H-2I, 1 × Ccarb-H), 2.12–1.95 (m, 60 H, 20 × CH3); 13C NMR (150 MHz, CDCl3): δ = 171.11–169.56 (20 × C=O), 138.15 (C-2’), 121.0 (C-3’), 98.27–96.81 (7 × C-1), 77.97–76.38 (7 × C-4), 72.24–69.43 (7 × C-2, 7 × C-3, 7 × C-5, C-1’), 63.06–62.62 (7 × C-6), 51.99 (Ccarb), 50.92 (Ccarb), 50.85 (Ccarb), 49.36–49.08 (C-4’, Ccarb), 21.24–21.01 (20 × CH3); 11B NMR (128 MHz, CDCl3): δ = 21.13 (bs, 2 B, B-8, B-8’), 0.56 (bs, 2 B, B-10, B-10’), −(1.91–10.86) (m, 12 B, B-4, B-4’, B-5, B-5’, B-7, B-7’, B-9, B-9’, B-11, B-11’, B-12, B-12’), −15.30 (bs, 2 B, B-6, B-6’); MS (EI, m/z (rel.%)): 2436.4 (60), 2037.3 (10), 1272.7 (10), 1230.2 (100), 1030.6 (16), 1010.1 (10), 307.1 (61); HR-MS (ESI) calcd. for C90H135O5510B6B12CoNa2S2: 1228.4109, found 1228.4128.
Per-O-acetyl-3I-O-{4-{8,8’-μ-(disulfido)-[3,3’-commo-cobalt(III)-bis-(1,2-dicarbaundecaborate)]-8-yl}but-2-en-1-yl}-β-cyclodextrin (3cb). The compound was prepared from 2b (0.15 g, 0.07 mmol) and 1c (20 mg, 0.05 mmol). Column chromatography gave the title compound, 0.029 g (25%), as a red powder: m. p. 201–203 °C; IR (KBr): = 2955, 2582, 1747, 1368, 1236, 1042 cm−1; 1H NMR (600 MHz, CDCl3): δ = 6.15–6.06 (m, 1 H, H-2’), 5.74–5.65 (m, 1 H, H-3’), 5.50–5.22 (m, 7 H, 7 × H-3), 5.15–4.99 (m, 6 H, 6 × H-1), 4.85–4.61 (m, 8 H, 7 × H-4, 1 × H-1’), 4.60–3.85 (m, 25 H, 14 × H-6, 2 × H1’, 2 × H-4’, 7 × H-5), 3.84–3.49 (m, 9 H, 7 × H-4, 2 × Ccarb-H), 3.37 (bs, 1 H, Ccarb-H), 3.34 (bs, 1 H, Ccarb-H), 2.12–1.93 (m, 60 H, 20 × CH3); 13C NMR (150 MHz, CDCl3): δ = 170.86–169.86 (20 × C=O), 139.90 (C-2’), 119.35 (C-3’), 97.80–96.37 (7 × C-1), 77.89–75.67 (7 × C-4), 73.50 (C-1’), 72.23–69.05 (7 × C-2, 7 × C-3, 7 × C-5), 62.94–62.28 (7xC-6), 51.82 (Ccarb), 51.07 (Ccarb), 50.83 (Ccarb), 49.63–49.42 (C-4’, Ccarb), 21.09–21.00 (20 × CH3); 11B NMR (128 MHz, CDCl3): δ = 20.96 (bs, 2 B, B-8, B-8’), 0.54 (bs, 2 B, B-10, B-10’), −(1.99–10.87) (m, 12 B, B-4, B-4’, B-5, B-5’, B-7, B-7’, B-9, B-9’, B-11, B-11’, B-12, B-12’), −15.42 (bs, 2 B, B-6, B-6’); MS (EI, m/z (rel.%)): 2438.8 (41), 2250.9 (10), 2037.7 (15), 1229.9 (50), 1030.8 (33), 307.2 (100); HR-MS (ESI) calcd. for C90H135O5510B6B12CoNa2S2: 1228.4109, found 1228.4127.
Per-O-acetyl-6I-O-{4-{8,8’-μ-(disulfido)-[3,3’-commo-cobalt(III)-bis-(1,2-dicarbaundecaborate)]-8-yl}but-2-en-1-yl}-β-cyclodextrin (3cc). The compound was prepared from 2c (0.15 g, 0.07 mmol) and 1c (20 mg, 0.05 mmol). Column chromatography gave the title compound, 0.022 g (19%), as a red powder: m. p. 198–200 °C; IR (KBr): = 2578, 1747, 1370, 1236, 1045 cm−1; 1H NMR (600 MHz, CDCl3): δ = 6.07 (dt, J = 15.0, 5.0 Hz, 1 H, H-2’), 5.77–5.70 (m, 1 H, H-3’), 5.40–5.21 (m, 7 H, 7 × H-3), 5.16–5.05 (m, 7 H, 7 × H-1), 4.86–4.69 (m, 7 H, 7 × H-2), 4.63–4.52 (m, 4 H, 4 × H-6), 4.46 (dd, J = 10.8, 3.5 Hz, 1 H, 1 × H-6), 4.41–4.03 (m, 17 H, 2 × H-1’, 2 × H-4’, 5 × H-5, 8 × H-6), 3.93–3.89 (m, 2 H, 2 × H-5), 3.87 (m, 2 H, 2 × H-4), 3.75–3.62 (m, 8 H, 1 × H-6I, 5 × H-4, 2 × Ccarb-H), 3.49 (bs, 1 H, Ccarb-H), 3.34 (bs, 1 H, Ccarb-H), 2.16–2.00 (m, 60 H, 20 × CH3); 13C NMR (150 MHz, CDCl3): δ = 171.13–169.51 (20 × C=O), 137.95 (C-2’), 120.88 (C-3’), 97.38–96.55 (7 × C-1), 77.82–76.21 (7 × C-4), 71.86–68.37 (7 × C-2, 7 × C-3, 7 × C-5, C-1’), 68.37 (C-6I), 62.93–62.65 (6 × C-6), 52.23 (Ccarb), 51.68 (Ccarb), 50.99 (Ccarb), 49.64–49.21 (C-4’, Ccarb), 21.07–20.98 (20 × CH3); 11B NMR (128 MHz, CDCl3) δ 21.66 (bs, 2 B, B-8, B-8’), 0.18 (s, 1 B, B-10 or B-10’), −1.02 (s, 1 B, B-10 or B-10’), −(1.93-10.95) (m, 12 B, B-4, B-4’, B-5, B-5’, B-7, B-7’, B-9, B-9’, B-11, B-11’, B-12, B-12’), −15.63 (s, 2 B, B-6, B-6’); MS (EI, m/z (rel.%)): 2437.8 (23), 2085.6 (11), 1229.9 (20), 1010.1 (15), 528.1 (10), 307.2 (100); HR-MS (ESI) calcd. for C90H135O5510B6B12CoNa2S2: 1228.4109, found 1228.4119.
Supporting Information
| Supporting Information File 1: Experimental details and characterisation data of peracetylated cyclodextrins 2a, 2b and 2c. | ||
| Format: PDF | Size: 176.6 KB | Download |
References
-
Connon, S. J.; Blechert, S. Angew. Chem., Int. Ed. 2003, 42, 1900–1923. doi:10.1002/anie.200200556
Return to citation in text: [1] -
Bieniek, M.; Michrowska, A.; Usanov, D. L.; Grela, K. Chem.–Eur. J. 2008, 14, 806–818. doi:10.1002/chem.200701340
Return to citation in text: [1] -
Vougioukalakis, G. C.; Grubbs, R. H. Chem. Rev. 2010, 110, 1746–1787. doi:10.1021/cr9002424
Return to citation in text: [1] -
Eignerová, B.; Dračínský, M.; Kotora, M. Eur. J. Org. Chem. 2008, 4493–4499. doi:10.1002/ejoc.200800230
Return to citation in text: [1] -
Eignerová, B.; Slavíková, B.; Buděšínský, M.; Dračínský, M.; Klepetářová, B.; Šťastná, E.; Kotora, M. J. Med. Chem. 2009, 52, 5753–5757. doi:10.1021/jm900495f
Return to citation in text: [1] [2] -
Eignerová, B.; Sedlák, D.; Dračínský, M.; Bartůněk, P.; Kotora, M. J. Med. Chem. 2010, 53, 6947–6953. doi:10.1021/jm100563h
Return to citation in text: [1] [2] -
Řezanka, M.; Eignerová, B.; Jindřich, J.; Kotora, M. Eur. J. Org. Chem. 2010, 6256–6262. doi:10.1002/ejoc.201000807
Return to citation in text: [1] [2] [3] -
Eignerová, B.; Janoušek, Z.; Dračínský, M.; Kotora, M. Synlett 2010, 885–887.
Return to citation in text: [1] [2] [3] -
Grüner, B.; Holub, J.; Plešek, J.; Vaněk, T.; Votavová, H. J. Chromatogr., A 1998, 793, 249–256. doi:10.1016/S0021-9673(97)00904-7
Return to citation in text: [1] -
Frixa, C.; Scobie, M.; Black, S. J.; Thompson, A. S.; Threadgill, M. D. Chem. Commun. 2002, 2876–2877. doi:10.1039/b209339a
Return to citation in text: [1] -
Ohta, K.; Konno, S.; Endo, Y. Tetrahedron Lett. 2008, 49, 6525–6528. doi:10.1016/j.tetlet.2008.08.107
Return to citation in text: [1] -
Ohta, K.; Konno, S.; Endo, Y. Chem. Pharm. Bull. 2009, 57, 307–310. doi:10.1248/cpb.57.307
Return to citation in text: [1] -
Uchman, M.; Jurkiewicz, P.; Cígler, P.; Grüner, B.; Hof, M.; Procházka, K.; Matějíček, P. Langmuir 2010, 26, 6268–6275. doi:10.1021/la904047k
Return to citation in text: [1] -
Plešek, J. Chem. Rev. 1992, 92, 269–278. doi:10.1021/cr00010a005
Return to citation in text: [1] -
Soloway, A. H.; Tjarks, W.; Barnum, B. A.; Rong, F.-G.; Barth, R. F.; Codogni, I. M.; Wilson, J. G. Chem. Rev. 1998, 98, 1515–1562. doi:10.1021/cr941195u
Return to citation in text: [1] -
Hawthorne, M. F.; Maderna, A. Chem. Rev. 1999, 99, 3421–3434. doi:10.1021/cr980442h
Return to citation in text: [1] -
Sivaev, I. B.; Bregadze, V. V. Eur. J. Inorg. Chem. 2009, 1433–1450. doi:10.1002/ejic.200900003
Return to citation in text: [1] -
Cígler, P.; Kožíšek, M.; Řezáčová, P.; Brynda, J.; Otwinowski, Z.; Pokorná, J.; Plešek, J.; Grüner, B.; Dolečková-Marešová, L.; Máša, M.; Sedláček, J.; Bodem, J.; Kräusslich, H.-G.; Král, V.; Konvalinka, J. Proc. Natl. Acad. Sci. U. S. A. 2005, 102, 15394–15399. doi:10.1073/pnas.0507577102
Return to citation in text: [1] -
Řezáčová, P.; Pokorná, J.; Brynda, J.; Kožíšek, M.; Cígler, P.; Lepšík, M.; Fanfrlík, J.; Řezáč, J.; Šašková, K. G.; Sieglová, I.; Plešek, J.; Šícha, V.; Grüner, B.; Oberwinkler, H.; Sedláček, J.; Kräusslich, H.-G.; Hobza, P.; Král, V.; Konvalinka, J. J. Med. Chem. 2009, 52, 7132–7141. doi:10.1021/jm9011388
Return to citation in text: [1] -
Plešek, J.; Štíbr, B.; Drdácká, E.; Plzák, Z.; Heřmánek, S. Chem. Ind. 1982, 778–779.
Return to citation in text: [1] -
Janoušek, Z.; Plešek, J.; Heřmánek, S.; Baše, K.; Todd, L. J.; Wright, W. F. Collect. Czech. Chem. Commun. 1981, 46, 2818–2833.
Return to citation in text: [1] [2] -
Jindrich, J.; Pitha, J.; Lindberg, B.; Seffers, P.; Harata, K. Carbohydr. Res. 1995, 266, 75–80. doi:10.1016/0008-6215(94)00251-A
Return to citation in text: [1] -
Masurier, N.; Estour, F.; Lefèvre, B.; Brasme, B.; Masson, P.; Lafont, O. Carbohydr. Res. 2006, 341, 935–940. doi:10.1016/j.carres.2006.02.012
Return to citation in text: [1] -
Jindřich, J.; Tišlerová, I. J. Org. Chem. 2005, 70, 9054–9055. doi:10.1021/jo051339c
Return to citation in text: [1] -
Wei, X.; Carroll, P. J.; Sneddon, L. G. Organometallics 2006, 25, 609–621. doi:10.1021/om050851l
Return to citation in text: [1] [2] -
Simon, Y. C.; Ohm, C.; Zimny, M. J.; Coughlin, E. B. Macromolecules 2007, 40, 5628–5630. doi:10.1021/ma0709093
Return to citation in text: [1] -
Ghera, B. B.; Fache, F.; Parrot-Lopez, H. Tetrahedron 2006, 62, 4807–4813. doi:10.1016/j.tet.2006.03.010
Return to citation in text: [1] -
Connon, S. J.; Blechert, S. Angew. Chem., Int. Ed. 2003, 42, 1900–1923. doi:10.1002/anie.200200556
Return to citation in text: [1] -
Schmidt, B. Eur. J. Org. Chem. 2003, 816–819. doi:10.1002/ejoc.200390124
Return to citation in text: [1] -
Hong, S. H.; Sanders, D. P.; Lee, C. W.; Grubbs, R. H. J. Am. Chem. Soc. 2005, 127, 17160–17161. doi:10.1021/ja052939w
Return to citation in text: [1] -
Finnegan, D.; Seigal, B. A.; Snapper, M. L. Org. Lett. 2006, 8, 2603–2606. doi:10.1021/ol060918g
Return to citation in text: [1] -
Chiu, S.-H.; Myles, D. C.; Garrell, R. L.; Stoddart, J. F. J. Org. Chem. 2000, 62, 2792–2796. doi:10.1021/jo9910381
Return to citation in text: [1] -
Aime, S.; Gianolio, E.; Palmisano, G.; Robaldo, B.; Barge, A.; Boffa, L.; Cravotto, G. Org. Biomol. Chem. 2006, 4, 1124–1130. doi:10.1039/b517068k
Return to citation in text: [1]
| 5. | Eignerová, B.; Slavíková, B.; Buděšínský, M.; Dračínský, M.; Klepetářová, B.; Šťastná, E.; Kotora, M. J. Med. Chem. 2009, 52, 5753–5757. doi:10.1021/jm900495f |
| 6. | Eignerová, B.; Sedlák, D.; Dračínský, M.; Bartůněk, P.; Kotora, M. J. Med. Chem. 2010, 53, 6947–6953. doi:10.1021/jm100563h |
| 7. | Řezanka, M.; Eignerová, B.; Jindřich, J.; Kotora, M. Eur. J. Org. Chem. 2010, 6256–6262. doi:10.1002/ejoc.201000807 |
| 8. | Eignerová, B.; Janoušek, Z.; Dračínský, M.; Kotora, M. Synlett 2010, 885–887. |
| 7. | Řezanka, M.; Eignerová, B.; Jindřich, J.; Kotora, M. Eur. J. Org. Chem. 2010, 6256–6262. doi:10.1002/ejoc.201000807 |
| 24. | Jindřich, J.; Tišlerová, I. J. Org. Chem. 2005, 70, 9054–9055. doi:10.1021/jo051339c |
| 25. | Wei, X.; Carroll, P. J.; Sneddon, L. G. Organometallics 2006, 25, 609–621. doi:10.1021/om050851l |
| 26. | Simon, Y. C.; Ohm, C.; Zimny, M. J.; Coughlin, E. B. Macromolecules 2007, 40, 5628–5630. doi:10.1021/ma0709093 |
| 1. | Connon, S. J.; Blechert, S. Angew. Chem., Int. Ed. 2003, 42, 1900–1923. doi:10.1002/anie.200200556 |
| 6. | Eignerová, B.; Sedlák, D.; Dračínský, M.; Bartůněk, P.; Kotora, M. J. Med. Chem. 2010, 53, 6947–6953. doi:10.1021/jm100563h |
| 22. | Jindrich, J.; Pitha, J.; Lindberg, B.; Seffers, P.; Harata, K. Carbohydr. Res. 1995, 266, 75–80. doi:10.1016/0008-6215(94)00251-A |
| 5. | Eignerová, B.; Slavíková, B.; Buděšínský, M.; Dračínský, M.; Klepetářová, B.; Šťastná, E.; Kotora, M. J. Med. Chem. 2009, 52, 5753–5757. doi:10.1021/jm900495f |
| 23. | Masurier, N.; Estour, F.; Lefèvre, B.; Brasme, B.; Masson, P.; Lafont, O. Carbohydr. Res. 2006, 341, 935–940. doi:10.1016/j.carres.2006.02.012 |
| 4. | Eignerová, B.; Dračínský, M.; Kotora, M. Eur. J. Org. Chem. 2008, 4493–4499. doi:10.1002/ejoc.200800230 |
| 21. | Janoušek, Z.; Plešek, J.; Heřmánek, S.; Baše, K.; Todd, L. J.; Wright, W. F. Collect. Czech. Chem. Commun. 1981, 46, 2818–2833. |
| 32. | Chiu, S.-H.; Myles, D. C.; Garrell, R. L.; Stoddart, J. F. J. Org. Chem. 2000, 62, 2792–2796. doi:10.1021/jo9910381 |
| 33. | Aime, S.; Gianolio, E.; Palmisano, G.; Robaldo, B.; Barge, A.; Boffa, L.; Cravotto, G. Org. Biomol. Chem. 2006, 4, 1124–1130. doi:10.1039/b517068k |
| 2. | Bieniek, M.; Michrowska, A.; Usanov, D. L.; Grela, K. Chem.–Eur. J. 2008, 14, 806–818. doi:10.1002/chem.200701340 |
| 3. | Vougioukalakis, G. C.; Grubbs, R. H. Chem. Rev. 2010, 110, 1746–1787. doi:10.1021/cr9002424 |
| 14. | Plešek, J. Chem. Rev. 1992, 92, 269–278. doi:10.1021/cr00010a005 |
| 15. | Soloway, A. H.; Tjarks, W.; Barnum, B. A.; Rong, F.-G.; Barth, R. F.; Codogni, I. M.; Wilson, J. G. Chem. Rev. 1998, 98, 1515–1562. doi:10.1021/cr941195u |
| 16. | Hawthorne, M. F.; Maderna, A. Chem. Rev. 1999, 99, 3421–3434. doi:10.1021/cr980442h |
| 17. | Sivaev, I. B.; Bregadze, V. V. Eur. J. Inorg. Chem. 2009, 1433–1450. doi:10.1002/ejic.200900003 |
| 20. | Plešek, J.; Štíbr, B.; Drdácká, E.; Plzák, Z.; Heřmánek, S. Chem. Ind. 1982, 778–779. |
| 29. | Schmidt, B. Eur. J. Org. Chem. 2003, 816–819. doi:10.1002/ejoc.200390124 |
| 30. | Hong, S. H.; Sanders, D. P.; Lee, C. W.; Grubbs, R. H. J. Am. Chem. Soc. 2005, 127, 17160–17161. doi:10.1021/ja052939w |
| 31. | Finnegan, D.; Seigal, B. A.; Snapper, M. L. Org. Lett. 2006, 8, 2603–2606. doi:10.1021/ol060918g |
| 9. | Grüner, B.; Holub, J.; Plešek, J.; Vaněk, T.; Votavová, H. J. Chromatogr., A 1998, 793, 249–256. doi:10.1016/S0021-9673(97)00904-7 |
| 10. | Frixa, C.; Scobie, M.; Black, S. J.; Thompson, A. S.; Threadgill, M. D. Chem. Commun. 2002, 2876–2877. doi:10.1039/b209339a |
| 11. | Ohta, K.; Konno, S.; Endo, Y. Tetrahedron Lett. 2008, 49, 6525–6528. doi:10.1016/j.tetlet.2008.08.107 |
| 12. | Ohta, K.; Konno, S.; Endo, Y. Chem. Pharm. Bull. 2009, 57, 307–310. doi:10.1248/cpb.57.307 |
| 13. | Uchman, M.; Jurkiewicz, P.; Cígler, P.; Grüner, B.; Hof, M.; Procházka, K.; Matějíček, P. Langmuir 2010, 26, 6268–6275. doi:10.1021/la904047k |
| 21. | Janoušek, Z.; Plešek, J.; Heřmánek, S.; Baše, K.; Todd, L. J.; Wright, W. F. Collect. Czech. Chem. Commun. 1981, 46, 2818–2833. |
| 25. | Wei, X.; Carroll, P. J.; Sneddon, L. G. Organometallics 2006, 25, 609–621. doi:10.1021/om050851l |
| 27. | Ghera, B. B.; Fache, F.; Parrot-Lopez, H. Tetrahedron 2006, 62, 4807–4813. doi:10.1016/j.tet.2006.03.010 |
| 7. | Řezanka, M.; Eignerová, B.; Jindřich, J.; Kotora, M. Eur. J. Org. Chem. 2010, 6256–6262. doi:10.1002/ejoc.201000807 |
| 18. | Cígler, P.; Kožíšek, M.; Řezáčová, P.; Brynda, J.; Otwinowski, Z.; Pokorná, J.; Plešek, J.; Grüner, B.; Dolečková-Marešová, L.; Máša, M.; Sedláček, J.; Bodem, J.; Kräusslich, H.-G.; Král, V.; Konvalinka, J. Proc. Natl. Acad. Sci. U. S. A. 2005, 102, 15394–15399. doi:10.1073/pnas.0507577102 |
| 19. | Řezáčová, P.; Pokorná, J.; Brynda, J.; Kožíšek, M.; Cígler, P.; Lepšík, M.; Fanfrlík, J.; Řezáč, J.; Šašková, K. G.; Sieglová, I.; Plešek, J.; Šícha, V.; Grüner, B.; Oberwinkler, H.; Sedláček, J.; Kräusslich, H.-G.; Hobza, P.; Král, V.; Konvalinka, J. J. Med. Chem. 2009, 52, 7132–7141. doi:10.1021/jm9011388 |
| 28. | Connon, S. J.; Blechert, S. Angew. Chem., Int. Ed. 2003, 42, 1900–1923. doi:10.1002/anie.200200556 |
© 2010 Šnajdr et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)