Abstract
A new class of aminocyclitol derivatives with the bicyclo[4.2.0]octane skeleton was synthesized starting from cyclooctatetraene. Photooxygenation of trans-7,8-diacetoxy- and cis-7,8-dichlorobicyclo[4.2.0]octa-2,4-diene afforded the bicyclic endoperoxides. Reduction of the latter with thiourea followed by a Pd(0) catalyzed ionization/cyclization reaction gave the corresponding oxazolidinone derivatives. Oxidation of the double bond with KMnO4 or OsO4 followed by acetylation gave the acetate derivatives, the exact configuration of which was determined by spectroscopic methods. Hydrolysis of the oxazolidinone rings and removal of the acetate groups furnished the desired aminocyclitols.
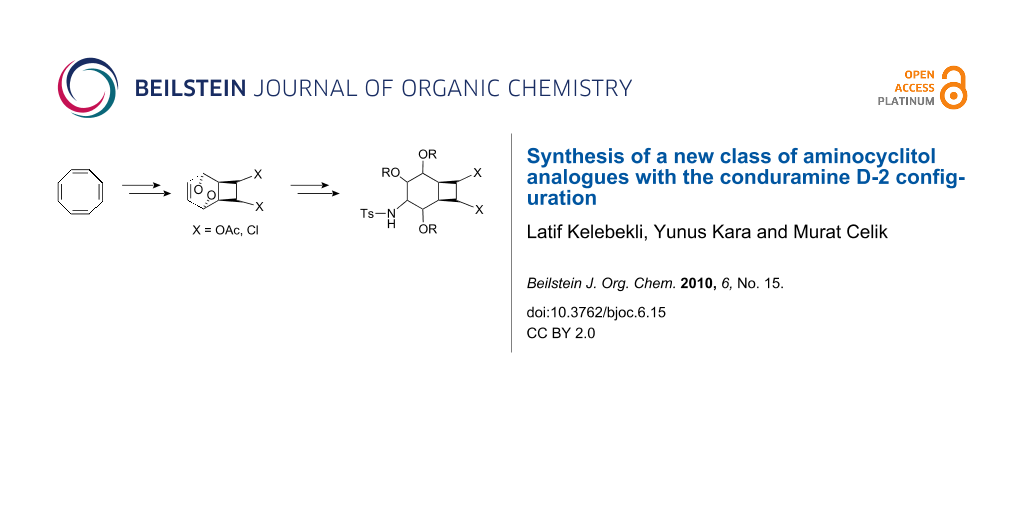
Graphical Abstract
Introduction
Among the myriad of naturally occurring compounds are the aminocyclitol-containing natural products, which represent a large family of sugar derived microbial secondary metabolites and include the clinically active aminoglycoside inhibitors [1-11], many of which are widely used for the treatment of diseases in humans, animals and plants [1-15].
Glycosidase and related enzymes are involved in the biosynthesis of the oligosaccharide chains [1-15]. Carba analogues of oligosaccharides (carbasugars), generated by replacing the endocyclic O-atom in a monosaccharide [1-11], are thought to be better drug candidates than natural sugars, since they are hydrolytically stable. Spurred on by the heightened interest in the design of carbohydrate mimetics, which can be potent inhibitors of glycosidase (1–4) [11-16], we have developed a method for rapid entry to these compounds.
Antibiotics containing an aminocyclitol unit have stimulated the development of synthetic methodologies [16] in the search for analogues with enhanced pharmacological profiles [6]. Balci and Kara [20-22] have synthesized the polyhydroxylated bicyclic molecule 5 having the bicyclo[4.2.0]octane skeleton bis-homoinositol. Furthermore, Trost et al. [23,24] have reported a regio- and stereoselective Pd(0) catalyzed reaction of diols in the presence of p-toluenesulfonyl isocyanate for the introduction of the amino alcohol functionality.
We are currently interested in the synthesis of cyclitols and their derivatives [25]. As a part of our program directed towards the synthesis of potential glycosidase inhibitors we used a bicyclo[4.2.0]octane framework for OH, chlorine and NH2 groups as an intriguing carbohydrate alternative [26-30].
Herein, we report the synthesis of the new aminocyclitol analogues 6 and 7 from cyclooctatetraene.
Results and Discussion
Diacetoxydiene 9 was synthesized in 99% yield from cyclooctatetraene (8) by the addition of mercury(II) acetate [31]. Tetraphenylporphyrin sensitized photooxygenation of diacetoxydiene 9 with singlet oxygen gave the expected endoperoxide 10. Reduction of the peroxide bond in 10 was performed with thiourea under very mild conditions to give the cis-diol 11 in 99% yield. The introduction of the amino alcohol functionality was achieved by a regio- and stereoselective Pd(0) catalyzed reaction of diol 11 and TsNCO [32]. Thus treatment of the cis-diol 11 in THF with 2 equiv of p-toluenesulfonyl isocyanate gave the corresponding bis-carbamate 12 which was subsequently added to a solution of 5 mol % of the catalyst, prepared by stirring a mixture of ligand (triisopropylphosphite) and tris(dibenzylideneacetone)–dipalladium-chloroform complex in THF. Subsequent purification by column chromatography gave oxazolidinone 13 in 48% yield (Scheme 1). The structure of 13 was assigned by 1H and 13C NMR and later by X-ray analysis of product 15.
Scheme 1: Synthesis of bis-carbamate 12 and oxazolidinone 13.
Scheme 1: Synthesis of bis-carbamate 12 and oxazolidinone 13.
The observed regio- and stereoselectivity was remarkable since the leaving groups are diastereotopic.
The metal–olefin complexation is a likely source of the stereoselectivity. Mechanistically, only palladium–olefin complexation anti to the leaving group will lead to the product 13 [33,34], which is inconsistent with a steric preference for the metal approaching the double bond in 12 from the side of the four-membered ring to form complex 14 (Scheme 2).
Scheme 2: Mechanism of the palladium-catalyzed ionization/cyclization reaction.
Scheme 2: Mechanism of the palladium-catalyzed ionization/cyclization reaction.
Since the double bond is not symmetrically disubstituted, palladium can theoretically form two complexes 15 and 16 after ionization. We assume that the formation of complex 15 is hindered due to the presence of an acetate group in the endo position.
cis-Dihydroxylation of 13 with KMnO4 at −15 °C gave a single diol 17, which was converted into the tetraacetate by treatment with acetic anhydride/CH3COONa [35] (Scheme 3). Careful examination of the reaction mixture did not reveal the formation of the other isomer. The stereochemical course of the hydroxylation may be syn or anti with respect to the oxazolidinone and cyclobutane rings. NMR spectroscopic studies did not allow the assignment of the exact orientation of the hydroxyl groups. X-ray analysis of 18 (Figure 1) revealed the exact configuration of the compound. This also confirms the configurations of endoperoxide 10, oxazolidinone 13 and cis-hydroxylation product 17.
Scheme 3: Synthesis of aminocyclitol analogue 6.
Scheme 3: Synthesis of aminocyclitol analogue 6.
The all cis-configuration of the four acetate and amino groups in aminocyclitol 18 [25] attached to the six-membered ring resembles the configuration of conduramine D-2 [36-38]. Hydrolysis of the acetate groups with H2SO4 proceeded smoothly to deliver aminocyclitol 6 in 84% yield.
Figure 1: The thermal ellipsoid plot of the single crystal X-ray crystallographic structure of 18.
Figure 1: The thermal ellipsoid plot of the single crystal X-ray crystallographic structure of 18.
For the synthesis of dichloro derivative, we replaced the acetoxy groups in 9 with cis- configured chlorine atoms. This provides a route for the synthesis of other haloaminocyclitol derivatives.
cis-Dichlorobicyclooctadiene 19 was synthesized from cyclooctatetraene 8 by the addition of chlorine following the literature procedure [39]. Photooxygenation of cis-dichlorobicylooctadiene 19 with singlet oxygen gave the expected endoperoxide 20 [19-21] (Scheme 4). Since the dichlorobicyclooctadiene 19 has no plane of symmetry, singlet oxygen approaches the diene unit exclusively from the less crowded side of the molecule in accord with previous reports [20,21]. Reduction of the peroxide bond in 20 with thiourea under very mild conditions gave the cis-diol 21 in 95% yield. Diol 21 in THF was treated with 2 equiv of p-toluenesulfonyl isocyanate to give the intermediate bis-carbamate 22 which was then treated as described above for 12 with the same Pd(0) catalyst to afford, after chromatography on a silica gel with hexane/ethyl acetate (3:1) as eluant, the oxazolidinone 23 in 61% yield.
The structure of 23 was assigned by 1H NMR and 13C NMR spectroscopy. The double bond in 22 is symmetrically disubstituted, and therefore palladium can form only one complex 25 after ionization (Scheme 5).
Scheme 5: Mechanism of the palladium-catalyzed ionization/cyclization reaction in dichloro biscarbamate 22.
Scheme 5: Mechanism of the palladium-catalyzed ionization/cyclization reaction in dichloro biscarbamate 22.
Hydrolysis of oxazolidinone 23 with K2CO3 gave alcohol 26, which was subsequently converted into acetate 27 by treatment with Ac2O/NaOAc [35] (Scheme 6).
Scheme 6: Synthesis of dichloroaminocyclitol 7.
Scheme 6: Synthesis of dichloroaminocyclitol 7.
cis-Hydroxylation of 27 with OsO4 at 0°C gave the corresponding diol 28, which was further converted into triacetate 7 with Ac2O/NaOAc (Scheme 6). The exact configuration of triacetate 7 was confirmed by differential 1H NMR NOE measurements (Figure 2). Irradiation of H3 (H3–CNHTs) at δ 3.85 caused signal enhancements of H1, H2, and H4 at δ = 5.45, 5.28 and 5.15, respectively. This result is consistent with a cis-relationship between H1, H2, H3 and H4 protons.
We assume that the stereochemical course of the hydroxylation of 27 proceeds through syn addition as previously observed in the hydroxylation of 13, a quite similar structure to 27.
Figure 2: 1H NMR NOE spectrum of compound 7.
Figure 2: 1H NMR NOE spectrum of compound 7.
The all cis-configuration of the acetate and amino groups attached to the six-membered ring resembles the configuration of conduramine D-2 [31,40,41]. The cyclic polyhdroxylated amines, also known as aminocyclitols, possess a wide variety of biological activities [42-45]. In conclusion, we have outlined the synthesis of a new family of aminocyclitols analogues 6 and 7 based on the bicyclo[4.2.0]octane frame work, with stereocontrol during the formation of all the stereogenic centres.
Experimental
Melting points were determined on a Büchi 539 capillary melting apparatus and are uncorrected. Infrared spectra were obtained from KBr or film on a Mattson 1000 FT-IR spectrophotometer. The 1H and 13C NMR spectra were recorded on 200 (50) and 400 (100) MHz Varian spectrometer and are reported in δ units with SiMe4 as internal standard. Thin layer chromatography (TLC) was performed on E. Merck Silica Gel 60 F254 plate (0.2 mm). All column chromatography was performed on silica gel (60 mesh, Merck). Elemental analyses were carried out on a Carlo Erba 1108 model CHNS-O analyzer.
Supporting Information
| Supporting Information File 1:
Experimental Section
The experimental section describes the synthesis, purification and characterization data of all substances given in this article. |
||
| Format: PDF | Size: 122.6 KB | Download |
References
-
Posternak, T. The Cyclitols; Holden Day: San Francisco, CA, 1962.
Return to citation in text: [1] [2] [3] [4] -
Anderson, L. The Cyclitols. In The Carbohydrates, 2nd ed.; Pigman, W.; Horton, D., Eds.; Academic Press: New York, 1972; Vol. 1A, pp 519–579.
Return to citation in text: [1] [2] [3] [4] -
Arjona, O.; Gómez, A. M.; López, C.; Plumet, J. Chem. Rev. 2007, 107, 1919. doi:10.1021/cr0203701
Return to citation in text: [1] [2] [3] [4] -
Asano, N. Glycobiology 2003, 13, 93R. doi:10.1093/glycob/cwg090
Return to citation in text: [1] [2] [3] [4] -
Heightman, T. D.; Vasella, A. T. Angew. Chem., Int. Ed. 1999, 38, 750. doi:10.1002/(SICI)1521-3773(19990315)38:6<750::AID-ANIE750>3.0.CO;2-6
Return to citation in text: [1] [2] [3] [4] -
Shan, M.; O’Doherty, G. A. Org. Lett. 2008, 10, 3381. doi:10.1021/ol801106r
Return to citation in text: [1] [2] [3] [4] [5] -
Shan, M.; O’Doherty, G. A. Synthesis 2008, 19, 3171. doi:10.1055/s-2008-1067262
Return to citation in text: [1] [2] [3] [4] -
Chakraborty, C.; Vyavahare, V. P.; Dhavale, D. D. Tetrahedron 2007, 63, 11984. doi:10.1016/j.tet.2007.09.011
Return to citation in text: [1] [2] [3] [4] -
Frigell, J.; Cumpstey, I. Tetrahedron Lett. 2007, 48, 9073. doi:10.1016/j.tetlet.2007.10.138
Return to citation in text: [1] [2] [3] [4] -
Sardinha, J.; Guieu, S.; Deleuze, A.; Fernández-Alonso, M. C.; Rauter, A. P.; Sinaÿ, P.; Marrot, J.; Jiménez-Babero, J.; Sollogoub, M. Carbohydr. Res. 2007, 342, 1689. doi:10.1016/j.carres.2007.05.021
Return to citation in text: [1] [2] [3] [4] -
Plumet, J.; Gomez, A. M.; Lopez, J. C. Mini-Rev. Org. Chem. 2007, 4, 201. doi:10.2174/157019307781369931
Return to citation in text: [1] [2] [3] [4] [5] -
Chiasson, J.-L.; Josse, R. G.; Hunt, J. A.; Palmason, C.; Roger, N. W.; Ross, S. A.; Ryan, E. A.; Tan, M. H.; Wolever, T. M. S. Ann. Intern. Med. 1994, 121, 928.
Return to citation in text: [1] [2] [3] -
Balci, M. Pure Appl. Chem. 1997, 69, 97. doi:10.1351/pac199769010097
Return to citation in text: [1] [2] [3] -
Hudlicky, T.; Reed, J. W. Advances in Asymmetric Synthesis; JAI Press: Greenwich, 1995; Vol. 1, p 271.
Return to citation in text: [1] [2] [3] -
Carless, H. A. J. Tetrahedron: Asymmetry 1992, 3, 795. doi:10.1016/S0957-4166(00)82174-6
Return to citation in text: [1] [2] [3] -
Gultekin, M. S.; Celik, M.; Balci, M. Curr. Org. Chem. 2004, 8, 1159. doi:10.2174/1385272043370069
Return to citation in text: [1] [2] -
El Ashry, E. S. H.; Rashed, N.; Shobier, A. H. S. Pharmazie 2000, 55, 251.
-
El Ashry, E. S. H.; Rashed, N.; Shobier, A. H. S. Pharmazie 2000, 55, 321.
-
El Ashry, E. S. H.; Rashed, N.; Shobier, A. H. S. Pharmazie 2000, 55, 403.
Return to citation in text: [1] -
Kelebekli, L.; Kara, Y.; Balci, M. Carbohydr. Res. 2005, 340, 1940. doi:10.1016/j.carres.2005.05.021
Return to citation in text: [1] [2] [3] -
Kara, Y.; Balci, M. Tetrahedron 2003, 59, 2063. doi:10.1016/S0040-4020(03)00209-6
Return to citation in text: [1] [2] [3] -
Sahin, E.; Kelebekli, L.; Kara, Y.; Celik, M.; Balci, M. Acta Crystallogr. 2007, E63, o2464. doi:10.1107/S1600536807017011
Return to citation in text: [1] -
Trost, B. M.; Van Vranken, D. L.; Birgel, C. J. Am. Chem. Soc. 1992, 114, 9327. doi:10.1021/ja00050a013
Return to citation in text: [1] -
Trost, B. M.; Murphy, D. J. Organometallics 1985, 4, 1143. doi:10.1021/om00125a039
Return to citation in text: [1] -
Kelebekli, L.; Celik, M.; Kara, Y.; Balci, M. Tetrahedron Lett. 2006, 47, 7031. doi:10.1016/j.tetlet.2006.07.108
Return to citation in text: [1] [2] -
Curti, C.; Zanardi, F.; Battistini, L.; Sartori, A.; Rassu, G.; Auzzas, L.; Roggio, A.; Pinna, L.; Casiraghi, G. J. Org. Chem. 2006, 71, 225. doi:10.1021/jo0520137
Return to citation in text: [1] -
Contelles, Y.; de Opaza, E. J. Org. Chem. 2003, 67, 3705. doi:10.1021/jo0111107
Return to citation in text: [1] -
Blériot, Y.; Giroult, A.; Mallet, J.-M.; Rodriguez, E.; Vogel, P.; Sinaÿ, P. Tetrahedron: Asymmetry 2002, 13, 2553. doi:10.1016/S0957-4166(02)00654-7
Return to citation in text: [1] -
Honda, T.; Kimura, N. Org. Lett. 2002, 4, 4567. doi:10.1021/ol027192i
Return to citation in text: [1] -
Boyer, F. D. J.; Lallemand, J.-Y. Synlett 1992, 969. doi:10.1055/s-1992-21548
Return to citation in text: [1] -
Cope, A. C.; Nelson, N. A.; Smith, D. S. J. Am. Chem. Soc. 1954, 76, 1100. doi:10.1021/ja01633a049
Return to citation in text: [1] [2] -
Andriuzzi, O.; Gravier-Pelletier, C.; Vogel, P.; Le Merrer, Y. Tetrahedron 2005, 61, 7094. doi:10.1016/j.tet.2005.05.066
Return to citation in text: [1] -
Trost, B. M.; Van Vranken, D. L. J. Am. Chem. Soc. 1993, 115, 444. doi:10.1021/ja00055a013
Return to citation in text: [1] -
Trost, B. M.; Van Vranken, D. L. Chem. Rev. 1996, 96, 395. doi:10.1021/cr9409804
Return to citation in text: [1] -
Celik, M.; Balci, M. ARKIVOC 2007, No. viii, 150.
Return to citation in text: [1] [2] -
Leung-Toung, R.; Liu, Y.; Muchowski, J. M.; Wu, Y.-L. Tetrahedron Lett. 1994, 35, 1639. doi:10.1016/0040-4039(94)88307-6
Return to citation in text: [1] -
Leung-Toung, R. Y.; Liu, J.; Muchowski, J. M.; Wu, Y.-L. J. Org. Chem. 1998, 63, 3235. doi:10.1021/jo971907r
Return to citation in text: [1] -
Supplementary data in the form of CIFs have been deposited with the Cambridge Crystallographic Data Centre (CCDC 299509). Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB2 IEZ, UK [Fax: +44(0)-1223-336033 or e-mail: deposit@ccdc.cam.ac.uk]. Selected X-ray crystallographic data for 18 (C24H27NO12S): Space group: Orthorhombic, Pbn21; a = 11.6906(4) Å, b = 20.8899(10) Å, c = 22.1919(9) Å, V = 5419 Å3, Z = 8, F(000) = 2320, Dcalc = 1.36 g cm−3, MoKα = 0.71073 Å, independent reflections 7705 (Rint = 0.0421), λ radiation observed reflections 7093 (I > 2rI), refinement method; full-matrix least-squares on F2, data/restraints/parameters 7093/1/695, R1 = 0.0557, Rw = 0.1186, goodness-of-fit on F2 = 1.21.
Return to citation in text: [1] -
Reppe, M.; Schlichting, O.; Klager, K.; Topel, T. Justus Liebigs Ann. Chem. 1948, 560, 1–92. doi:10.1002/jlac.19485600102
Return to citation in text: [1] -
Mehta, G.; Ramesh, S. S. Can. J. Chem. 2005, 83, 581. doi:10.1139/v05-032
Return to citation in text: [1] -
Mehta, G.; Pavolli, K. Chem. Commun. 2002, 23, 2828. doi:10.1039/b208918a
Return to citation in text: [1] -
Lysek, R.; Vogel, P. Tetrahedron 2006, 62, 2733. doi:10.1016/j.tet.2005.11.046
Return to citation in text: [1] -
Miyabe, H.; Nishiki, A.; Naito, T. Chem. Pharm. Bull. 2003, 51, 100. doi:10.1248/cpb.51.100
Return to citation in text: [1] -
McIntosh, M. C.; Weinreb, S. M. J. Org. Chem. 1993, 58, 4823. doi:10.1021/jo00070a016
Return to citation in text: [1] -
Secen, H.; Sütbeyaz, Y.; Balci, M. Tetrahedron 1990, 46, 3715. doi:10.1016/S0040-4020(01)90509-5
Return to citation in text: [1]
| 19. | El Ashry, E. S. H.; Rashed, N.; Shobier, A. H. S. Pharmazie 2000, 55, 403. |
| 20. | Kelebekli, L.; Kara, Y.; Balci, M. Carbohydr. Res. 2005, 340, 1940. doi:10.1016/j.carres.2005.05.021 |
| 21. | Kara, Y.; Balci, M. Tetrahedron 2003, 59, 2063. doi:10.1016/S0040-4020(03)00209-6 |
| 36. | Leung-Toung, R.; Liu, Y.; Muchowski, J. M.; Wu, Y.-L. Tetrahedron Lett. 1994, 35, 1639. doi:10.1016/0040-4039(94)88307-6 |
| 37. | Leung-Toung, R. Y.; Liu, J.; Muchowski, J. M.; Wu, Y.-L. J. Org. Chem. 1998, 63, 3235. doi:10.1021/jo971907r |
| 38. | Supplementary data in the form of CIFs have been deposited with the Cambridge Crystallographic Data Centre (CCDC 299509). Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB2 IEZ, UK [Fax: +44(0)-1223-336033 or e-mail: deposit@ccdc.cam.ac.uk]. Selected X-ray crystallographic data for 18 (C24H27NO12S): Space group: Orthorhombic, Pbn21; a = 11.6906(4) Å, b = 20.8899(10) Å, c = 22.1919(9) Å, V = 5419 Å3, Z = 8, F(000) = 2320, Dcalc = 1.36 g cm−3, MoKα = 0.71073 Å, independent reflections 7705 (Rint = 0.0421), λ radiation observed reflections 7093 (I > 2rI), refinement method; full-matrix least-squares on F2, data/restraints/parameters 7093/1/695, R1 = 0.0557, Rw = 0.1186, goodness-of-fit on F2 = 1.21. |
| 39. | Reppe, M.; Schlichting, O.; Klager, K.; Topel, T. Justus Liebigs Ann. Chem. 1948, 560, 1–92. doi:10.1002/jlac.19485600102 |
| 1. | Posternak, T. The Cyclitols; Holden Day: San Francisco, CA, 1962. |
| 2. | Anderson, L. The Cyclitols. In The Carbohydrates, 2nd ed.; Pigman, W.; Horton, D., Eds.; Academic Press: New York, 1972; Vol. 1A, pp 519–579. |
| 3. | Arjona, O.; Gómez, A. M.; López, C.; Plumet, J. Chem. Rev. 2007, 107, 1919. doi:10.1021/cr0203701 |
| 4. | Asano, N. Glycobiology 2003, 13, 93R. doi:10.1093/glycob/cwg090 |
| 5. | Heightman, T. D.; Vasella, A. T. Angew. Chem., Int. Ed. 1999, 38, 750. doi:10.1002/(SICI)1521-3773(19990315)38:6<750::AID-ANIE750>3.0.CO;2-6 |
| 6. | Shan, M.; O’Doherty, G. A. Org. Lett. 2008, 10, 3381. doi:10.1021/ol801106r |
| 7. | Shan, M.; O’Doherty, G. A. Synthesis 2008, 19, 3171. doi:10.1055/s-2008-1067262 |
| 8. | Chakraborty, C.; Vyavahare, V. P.; Dhavale, D. D. Tetrahedron 2007, 63, 11984. doi:10.1016/j.tet.2007.09.011 |
| 9. | Frigell, J.; Cumpstey, I. Tetrahedron Lett. 2007, 48, 9073. doi:10.1016/j.tetlet.2007.10.138 |
| 10. | Sardinha, J.; Guieu, S.; Deleuze, A.; Fernández-Alonso, M. C.; Rauter, A. P.; Sinaÿ, P.; Marrot, J.; Jiménez-Babero, J.; Sollogoub, M. Carbohydr. Res. 2007, 342, 1689. doi:10.1016/j.carres.2007.05.021 |
| 11. | Plumet, J.; Gomez, A. M.; Lopez, J. C. Mini-Rev. Org. Chem. 2007, 4, 201. doi:10.2174/157019307781369931 |
| 11. | Plumet, J.; Gomez, A. M.; Lopez, J. C. Mini-Rev. Org. Chem. 2007, 4, 201. doi:10.2174/157019307781369931 |
| 12. | Chiasson, J.-L.; Josse, R. G.; Hunt, J. A.; Palmason, C.; Roger, N. W.; Ross, S. A.; Ryan, E. A.; Tan, M. H.; Wolever, T. M. S. Ann. Intern. Med. 1994, 121, 928. |
| 13. | Balci, M. Pure Appl. Chem. 1997, 69, 97. doi:10.1351/pac199769010097 |
| 14. | Hudlicky, T.; Reed, J. W. Advances in Asymmetric Synthesis; JAI Press: Greenwich, 1995; Vol. 1, p 271. |
| 15. | Carless, H. A. J. Tetrahedron: Asymmetry 1992, 3, 795. doi:10.1016/S0957-4166(00)82174-6 |
| 16. | Gultekin, M. S.; Celik, M.; Balci, M. Curr. Org. Chem. 2004, 8, 1159. doi:10.2174/1385272043370069 |
| 1. | Posternak, T. The Cyclitols; Holden Day: San Francisco, CA, 1962. |
| 2. | Anderson, L. The Cyclitols. In The Carbohydrates, 2nd ed.; Pigman, W.; Horton, D., Eds.; Academic Press: New York, 1972; Vol. 1A, pp 519–579. |
| 3. | Arjona, O.; Gómez, A. M.; López, C.; Plumet, J. Chem. Rev. 2007, 107, 1919. doi:10.1021/cr0203701 |
| 4. | Asano, N. Glycobiology 2003, 13, 93R. doi:10.1093/glycob/cwg090 |
| 5. | Heightman, T. D.; Vasella, A. T. Angew. Chem., Int. Ed. 1999, 38, 750. doi:10.1002/(SICI)1521-3773(19990315)38:6<750::AID-ANIE750>3.0.CO;2-6 |
| 6. | Shan, M.; O’Doherty, G. A. Org. Lett. 2008, 10, 3381. doi:10.1021/ol801106r |
| 7. | Shan, M.; O’Doherty, G. A. Synthesis 2008, 19, 3171. doi:10.1055/s-2008-1067262 |
| 8. | Chakraborty, C.; Vyavahare, V. P.; Dhavale, D. D. Tetrahedron 2007, 63, 11984. doi:10.1016/j.tet.2007.09.011 |
| 9. | Frigell, J.; Cumpstey, I. Tetrahedron Lett. 2007, 48, 9073. doi:10.1016/j.tetlet.2007.10.138 |
| 10. | Sardinha, J.; Guieu, S.; Deleuze, A.; Fernández-Alonso, M. C.; Rauter, A. P.; Sinaÿ, P.; Marrot, J.; Jiménez-Babero, J.; Sollogoub, M. Carbohydr. Res. 2007, 342, 1689. doi:10.1016/j.carres.2007.05.021 |
| 11. | Plumet, J.; Gomez, A. M.; Lopez, J. C. Mini-Rev. Org. Chem. 2007, 4, 201. doi:10.2174/157019307781369931 |
| 25. | Kelebekli, L.; Celik, M.; Kara, Y.; Balci, M. Tetrahedron Lett. 2006, 47, 7031. doi:10.1016/j.tetlet.2006.07.108 |
| 1. | Posternak, T. The Cyclitols; Holden Day: San Francisco, CA, 1962. |
| 2. | Anderson, L. The Cyclitols. In The Carbohydrates, 2nd ed.; Pigman, W.; Horton, D., Eds.; Academic Press: New York, 1972; Vol. 1A, pp 519–579. |
| 3. | Arjona, O.; Gómez, A. M.; López, C.; Plumet, J. Chem. Rev. 2007, 107, 1919. doi:10.1021/cr0203701 |
| 4. | Asano, N. Glycobiology 2003, 13, 93R. doi:10.1093/glycob/cwg090 |
| 5. | Heightman, T. D.; Vasella, A. T. Angew. Chem., Int. Ed. 1999, 38, 750. doi:10.1002/(SICI)1521-3773(19990315)38:6<750::AID-ANIE750>3.0.CO;2-6 |
| 6. | Shan, M.; O’Doherty, G. A. Org. Lett. 2008, 10, 3381. doi:10.1021/ol801106r |
| 7. | Shan, M.; O’Doherty, G. A. Synthesis 2008, 19, 3171. doi:10.1055/s-2008-1067262 |
| 8. | Chakraborty, C.; Vyavahare, V. P.; Dhavale, D. D. Tetrahedron 2007, 63, 11984. doi:10.1016/j.tet.2007.09.011 |
| 9. | Frigell, J.; Cumpstey, I. Tetrahedron Lett. 2007, 48, 9073. doi:10.1016/j.tetlet.2007.10.138 |
| 10. | Sardinha, J.; Guieu, S.; Deleuze, A.; Fernández-Alonso, M. C.; Rauter, A. P.; Sinaÿ, P.; Marrot, J.; Jiménez-Babero, J.; Sollogoub, M. Carbohydr. Res. 2007, 342, 1689. doi:10.1016/j.carres.2007.05.021 |
| 11. | Plumet, J.; Gomez, A. M.; Lopez, J. C. Mini-Rev. Org. Chem. 2007, 4, 201. doi:10.2174/157019307781369931 |
| 12. | Chiasson, J.-L.; Josse, R. G.; Hunt, J. A.; Palmason, C.; Roger, N. W.; Ross, S. A.; Ryan, E. A.; Tan, M. H.; Wolever, T. M. S. Ann. Intern. Med. 1994, 121, 928. |
| 13. | Balci, M. Pure Appl. Chem. 1997, 69, 97. doi:10.1351/pac199769010097 |
| 14. | Hudlicky, T.; Reed, J. W. Advances in Asymmetric Synthesis; JAI Press: Greenwich, 1995; Vol. 1, p 271. |
| 15. | Carless, H. A. J. Tetrahedron: Asymmetry 1992, 3, 795. doi:10.1016/S0957-4166(00)82174-6 |
| 32. | Andriuzzi, O.; Gravier-Pelletier, C.; Vogel, P.; Le Merrer, Y. Tetrahedron 2005, 61, 7094. doi:10.1016/j.tet.2005.05.066 |
| 1. | Posternak, T. The Cyclitols; Holden Day: San Francisco, CA, 1962. |
| 2. | Anderson, L. The Cyclitols. In The Carbohydrates, 2nd ed.; Pigman, W.; Horton, D., Eds.; Academic Press: New York, 1972; Vol. 1A, pp 519–579. |
| 3. | Arjona, O.; Gómez, A. M.; López, C.; Plumet, J. Chem. Rev. 2007, 107, 1919. doi:10.1021/cr0203701 |
| 4. | Asano, N. Glycobiology 2003, 13, 93R. doi:10.1093/glycob/cwg090 |
| 5. | Heightman, T. D.; Vasella, A. T. Angew. Chem., Int. Ed. 1999, 38, 750. doi:10.1002/(SICI)1521-3773(19990315)38:6<750::AID-ANIE750>3.0.CO;2-6 |
| 6. | Shan, M.; O’Doherty, G. A. Org. Lett. 2008, 10, 3381. doi:10.1021/ol801106r |
| 7. | Shan, M.; O’Doherty, G. A. Synthesis 2008, 19, 3171. doi:10.1055/s-2008-1067262 |
| 8. | Chakraborty, C.; Vyavahare, V. P.; Dhavale, D. D. Tetrahedron 2007, 63, 11984. doi:10.1016/j.tet.2007.09.011 |
| 9. | Frigell, J.; Cumpstey, I. Tetrahedron Lett. 2007, 48, 9073. doi:10.1016/j.tetlet.2007.10.138 |
| 10. | Sardinha, J.; Guieu, S.; Deleuze, A.; Fernández-Alonso, M. C.; Rauter, A. P.; Sinaÿ, P.; Marrot, J.; Jiménez-Babero, J.; Sollogoub, M. Carbohydr. Res. 2007, 342, 1689. doi:10.1016/j.carres.2007.05.021 |
| 11. | Plumet, J.; Gomez, A. M.; Lopez, J. C. Mini-Rev. Org. Chem. 2007, 4, 201. doi:10.2174/157019307781369931 |
| 12. | Chiasson, J.-L.; Josse, R. G.; Hunt, J. A.; Palmason, C.; Roger, N. W.; Ross, S. A.; Ryan, E. A.; Tan, M. H.; Wolever, T. M. S. Ann. Intern. Med. 1994, 121, 928. |
| 13. | Balci, M. Pure Appl. Chem. 1997, 69, 97. doi:10.1351/pac199769010097 |
| 14. | Hudlicky, T.; Reed, J. W. Advances in Asymmetric Synthesis; JAI Press: Greenwich, 1995; Vol. 1, p 271. |
| 15. | Carless, H. A. J. Tetrahedron: Asymmetry 1992, 3, 795. doi:10.1016/S0957-4166(00)82174-6 |
| 33. | Trost, B. M.; Van Vranken, D. L. J. Am. Chem. Soc. 1993, 115, 444. doi:10.1021/ja00055a013 |
| 34. | Trost, B. M.; Van Vranken, D. L. Chem. Rev. 1996, 96, 395. doi:10.1021/cr9409804 |
| 23. | Trost, B. M.; Van Vranken, D. L.; Birgel, C. J. Am. Chem. Soc. 1992, 114, 9327. doi:10.1021/ja00050a013 |
| 24. | Trost, B. M.; Murphy, D. J. Organometallics 1985, 4, 1143. doi:10.1021/om00125a039 |
| 26. | Curti, C.; Zanardi, F.; Battistini, L.; Sartori, A.; Rassu, G.; Auzzas, L.; Roggio, A.; Pinna, L.; Casiraghi, G. J. Org. Chem. 2006, 71, 225. doi:10.1021/jo0520137 |
| 27. | Contelles, Y.; de Opaza, E. J. Org. Chem. 2003, 67, 3705. doi:10.1021/jo0111107 |
| 28. | Blériot, Y.; Giroult, A.; Mallet, J.-M.; Rodriguez, E.; Vogel, P.; Sinaÿ, P. Tetrahedron: Asymmetry 2002, 13, 2553. doi:10.1016/S0957-4166(02)00654-7 |
| 29. | Honda, T.; Kimura, N. Org. Lett. 2002, 4, 4567. doi:10.1021/ol027192i |
| 30. | Boyer, F. D. J.; Lallemand, J.-Y. Synlett 1992, 969. doi:10.1055/s-1992-21548 |
| 31. | Cope, A. C.; Nelson, N. A.; Smith, D. S. J. Am. Chem. Soc. 1954, 76, 1100. doi:10.1021/ja01633a049 |
| 40. | Mehta, G.; Ramesh, S. S. Can. J. Chem. 2005, 83, 581. doi:10.1139/v05-032 |
| 41. | Mehta, G.; Pavolli, K. Chem. Commun. 2002, 23, 2828. doi:10.1039/b208918a |
| 20. | Kelebekli, L.; Kara, Y.; Balci, M. Carbohydr. Res. 2005, 340, 1940. doi:10.1016/j.carres.2005.05.021 |
| 21. | Kara, Y.; Balci, M. Tetrahedron 2003, 59, 2063. doi:10.1016/S0040-4020(03)00209-6 |
| 22. | Sahin, E.; Kelebekli, L.; Kara, Y.; Celik, M.; Balci, M. Acta Crystallogr. 2007, E63, o2464. doi:10.1107/S1600536807017011 |
| 31. | Cope, A. C.; Nelson, N. A.; Smith, D. S. J. Am. Chem. Soc. 1954, 76, 1100. doi:10.1021/ja01633a049 |
| 42. | Lysek, R.; Vogel, P. Tetrahedron 2006, 62, 2733. doi:10.1016/j.tet.2005.11.046 |
| 43. | Miyabe, H.; Nishiki, A.; Naito, T. Chem. Pharm. Bull. 2003, 51, 100. doi:10.1248/cpb.51.100 |
| 44. | McIntosh, M. C.; Weinreb, S. M. J. Org. Chem. 1993, 58, 4823. doi:10.1021/jo00070a016 |
| 45. | Secen, H.; Sütbeyaz, Y.; Balci, M. Tetrahedron 1990, 46, 3715. doi:10.1016/S0040-4020(01)90509-5 |
| 20. | Kelebekli, L.; Kara, Y.; Balci, M. Carbohydr. Res. 2005, 340, 1940. doi:10.1016/j.carres.2005.05.021 |
| 21. | Kara, Y.; Balci, M. Tetrahedron 2003, 59, 2063. doi:10.1016/S0040-4020(03)00209-6 |
| 16. | Gultekin, M. S.; Celik, M.; Balci, M. Curr. Org. Chem. 2004, 8, 1159. doi:10.2174/1385272043370069 |
| 25. | Kelebekli, L.; Celik, M.; Kara, Y.; Balci, M. Tetrahedron Lett. 2006, 47, 7031. doi:10.1016/j.tetlet.2006.07.108 |
© 2010 Kelebekli et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)