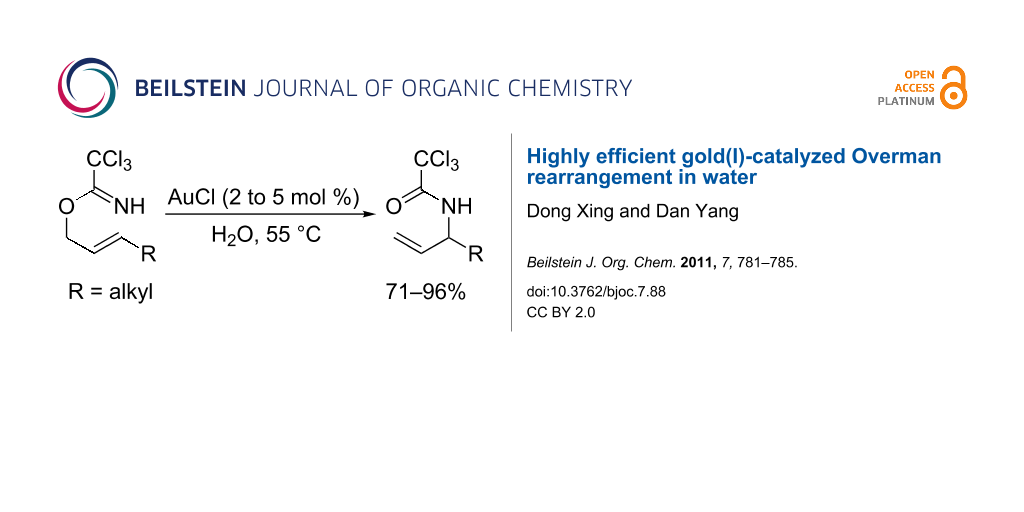
Abstract
A highly efficient gold(I)-catalyzed Overman rearrangement of allylic trichloroacetimidates to allylic trichloroacetamides in water is reported. With this environmentally benign and scalable protocol, a series of C3-alkyl substituted allylic trichloroacetamides were synthesized in good to high yields.
Graphical Abstract
Introduction
The aza-Claisen rearrangement of allylic trichloroacetimidates to allylic trichloroacetamides (Overman rearrangement) is a powerful and attractive strategy for the synthesis of allylic amines from readily available allylic alcohols [1,2]. This transformation can be conducted thermally at high temperatures or by transition metal catalysis under very mild conditions [3-7]. Asymmetric induction has been achieved with certain types of transition metal catalysts (e.g., palladium complexes) in combination with chiral ancillary ligands [8-13]. However, although a large number of late transition metal catalysts have been used for different types of [3,3]-sigmatropic rearrangements [14,15], only Pd(II) and Hg(II) salts have found wide application in Overman rearrangements. In recent years, gold catalysts have been successfully applied to a series of [3,3]-sigmatropic rearrangements, such as the rearrangement of propargylic esters to allenyl esters [16-21], allenyl carbinol esters to 1,3-butadien-2-ol esters [22] and the isomerization of allylic acetates [23,24]. However, when they were used as catalysts for the Overman rearrangement, the substrate scope was limited and only poor to moderate yields were achieved [25-28]. Very recently, our group developed an efficient gold(I)-catalyzed decarboxylative aza-Claisen rearrangement of allylic N-tosylcarbamates for the synthesis of N-tosyl allylic amines [29]. This reaction was performed in water and therefore represented an environmentally benign protocol [30-34]. We decided to apply this extremely mild catalytic system to the Overman rearrangement of allylic trichloroacetimidates.
Results and Discussion
Trichloroacetimidate 1a was prepared by the DBU-catalyzed addition of trans-2-penten-1-ol to trichloroacetonitrile [10,35]. With this substrate, the catalytic activities of different gold(I) complexes in H2O were examined. When 1a was subjected to the optimal catalytic conditions previously reported by our group (5 mol % AuCl/AgOTf at 75 °C) [29], the desired allylic trichloroacetamide 2a was obtained in 91% yield in a reaction time of 1 h (Table 1, entry 1). Gold(I) complexes with phosphine ligands, Au(PPh3)Cl or Au[P(t-Bu)2(o-Ph)Ph]Cl, in place of AuCl gave none of the desired product (Table 1, entries 2 and 3). Further screening revealed that AuCl alone could catalyze this reaction with high efficiency to give 2a in 92% yield in 2 h (Table 1, entry 4). On the other hand, with only AgOTf as the catalyst, the formation of 2a was not observed and substrate 1a decomposed completely (Table 1, entry 5). In the absence of AuCl, substrate 1a remained unreacted, even when the temperature was increased to 100 °C for 3 h (Table 1, entry 6), indicating that the gold(I) catalyst is indispensible for this transformation. This gold(I)-catalyzed reaction could be performed at room temperature, albeit with a prolonged reaction time (Table 1, entry 7). When the temperature was raised to 55 °C the reaction was complete within 2 h and in excellent yield (94%; Table 1, entry 8).
Table 1: Optimization of reaction conditions.a
|
|
||||
| Entry | Catalyst (mol %) | Temp (°C) | Time (h) | Yield (%)b |
|---|---|---|---|---|
| 1 | AuCl/AgOTf | 75 | 2 | 91 |
| 2 | AuPPh3Cl/AgOTf | 75 | 12 | <5 |
| 3 | Au[P(t-Bu)2(o-Ph)Ph]Cl/AgOTf | 75 | 12 | <5 |
| 4 | AuCl | 75 | 2 | 92 |
| 5 | AgOTf | 75 | 12 | <5 |
| 6 | – | 100 | 3 | <5 |
| 7 | AuCl | rt | 12 | 90 |
| 8 | AuCl | 55 | 2 | 94 |
aReaction conditions: 0.3 mmol of substrate, 5 mol % of the catalyst, 3 mL H2O; byield determined by 1H NMR with nitrobenzene as internal standard.
With the optimized reaction conditions in hand, the substrate scope of this gold(I)-catalyzed Overman rearrangement was surveyed. Different alkyl substituents at the C1 position of allylic trichloroacetimidates, including methyl (1b), ethyl (1a), n-propyl (1c) and phenethyl (1d) groups, underwent the desired transformation smoothly to afford the corresponding C3-alkyl substituted allylic trichloroacetamides in high yields (Table 2, entries 1–4). The C1-diethylmethyl substituted substrate (1e) also underwent the desired rearrangement, affording the desired product (2e) in 67% isolated yield (Table 2, entry 5). However, neither the substrates with phenyl (1f) nor dimethyl (1g) substituents at the C1 position gave the rearranged product (Table 2, entries 6 and 7), indicating that both electronic and steric effects at the C1 position play roles in the rearrangement. Although the substrate scope is currently limited to C1-alkyl substituted trichloroacetimidates, this method is still very convenient and attractive for the preparation of synthetically useful allylic amines. For example, substrates containing either TBDMS- or THP-protected hydroxy groups (1h and 1i) efficiently underwent the desired rearrangement to afford the corresponding products, which are precursors for the synthesis of a variety of β-substituted β-amino alcohols (Table 2, entries 8 and 9). Compound 2j was also obtained in 71% yield under the reaction conditions from the corresponding trichloroacetimidate 1j (Table 2, entry 10). Trichloroacetamide 2j could be transformed to vigabatrin, a GABA aminotransaminase inhibitor [36], in one single step [37].
Table 2: Gold(I)-catalyzed Overman rearrangement in H2O.a
|
|
||||
| Entry | Substrate | Product | Time (h) | Yield (%)b |
|---|---|---|---|---|
| 1 |
1a |
2a |
2 | 92 |
| 2 |
1b |
2b |
2 | 96 |
| 3 |
1c |
2c |
2 | 95 |
| 4 |
1d |
2d |
2 | 90 |
| 5 |
1e |
2e |
3 | 67c |
| 6 |
1f |
2f |
3 | n.d.d |
| 7 |
1g |
2g |
3 | n.d.d |
| 8 |
1h |
2h |
3 | 86 |
| 9 |
1i |
2i |
2 | 79 |
| 10 |
1j |
2j |
6 | 71c |
aUnless otherwise indicated, all reactions were carried out on a 0.5 mmol scale with 5 mol % of AuCl in 5 mL H2O at 55 °C for the indicated time; bunless otherwise indicated, crude yields were reported with >95% purities as determined by 1H NMR; cisolated yield after flash chromatography; dn.d. = not detected.
One of the most remarkable features of this gold(I)-catalyzed Overman rearrangement is that it is performed in water under very mild reaction conditions. Moreover, this method is extremely clean. After completion of the reaction, simple extraction gave the desired product in high purity, and no further purification step was required. To illustrate the potential utility of this method for industrial applications, a gram-scale synthesis of 2a was performed with 2 mol % of AuCl in H2O (Scheme 1). After reacting at 55 °C for 4 h, the desired product was obtained in 92% yield.
Conclusion
In summary, we have developed an efficient gold(I)-catalyzed Overman rearrangement for the synthesis of a series of C3-alkyl substituted allylic trichloroacetamides. This transformation was performed in water under very mild reaction conditions and could be carried out on the gram-scale with low catalyst loading and simple work-up procedure, making it potentially applicable to the industrial community for large-scale synthesis. Further exploration of the substrate scope and the development of an asymmetric version of this transformation are currently underway in our group.
Experimental
Typical procedure: Synthesis of 2,2,2-trichloro-N-(pent-1-en-3-yl)acetamide (2a)
AuCl (5.8 mg, 0.025 mmol) was added to a solution of (E)-pent-2-enyl 2,2,2-trichloroacetimidate (1a) (115 mg, 0.5 mmol) in H2O (5 mL) with vigorously stirring in a 25 mL reaction tube. The reaction mixture was heated at 55 °C for 2 h, then cooled to room temperature, diluted with H2O and extracted with CH2Cl2 three times. The combined organic layers were dried over MgSO4, filtered through a short pad of celite and concentrated in vacuo to provide 107 mg of 2,2,2-trichloro-N-(pent-1-en-3-yl)acetamide (2a) (>95% purity as determined by 1H NMR). Products 2a–2d, 2h and 2j are known compounds and their data were identical to those reported in the literature.
Supporting Information
| Supporting Information File 1: 1H NMR data and NMR spectra of products 2a–2d, 2g–2i. | ||
| Format: PDF | Size: 1.0 MB | Download |
References
-
Overman, L. E. J. Am. Chem. Soc. 1974, 96, 597–599. doi:10.1021/ja00809a054
Return to citation in text: [1] -
Overman, L. E. J. Am. Chem. Soc. 1976, 98, 2901–2910. doi:10.1021/ja00426a038
Return to citation in text: [1] -
Overman, L. E. Acc. Chem. Res. 1980, 13, 218–224. doi:10.1021/ar50151a005
Return to citation in text: [1] -
Overman, L. E. Angew. Chem., Int. Ed. Engl. 1984, 23, 579–586. doi:10.1002/anie.198405791
Return to citation in text: [1] -
Metz, P.; Mues, C.; Schoop, A. Tetrahedron 1992, 48, 1071–1080. doi:10.1016/S0040-4020(01)88203-X
Return to citation in text: [1] -
Johannsen, M.; Jørgensen, K. A. Chem. Rev. 1998, 98, 1689–1708. doi:10.1021/cr970343o
Return to citation in text: [1] -
Overman, L. E.; Carpenter, N. E. In Organic Reactions; Overman, L. E., Ed.; Wiley: Hoboken, NJ, 2005; Vol. 66, pp 653–760.
Return to citation in text: [1] -
Nomura, H.; Richards, C. J. Chem.–Asian J. 2010, 5, 1726–1740. doi:10.1002/asia.201000131
Return to citation in text: [1] -
Hollis, T. K.; Overman, L. E. J. Organomet. Chem. 1999, 576, 290–299. doi:10.1016/S0022-328X(98)01065-1
Return to citation in text: [1] -
Anderson, C. E.; Overman, L. E. J. Am. Chem. Soc. 2003, 125, 12412–12413. doi:10.1021/ja037086r
Return to citation in text: [1] [2] -
Kirsch, S. F.; Overman, L. E.; Watson, M. P. J. Org. Chem. 2004, 69, 8101–8104. doi:10.1021/jo0487092
Return to citation in text: [1] -
Watson, M. P.; Overman, L. E.; Bergman, R. G. J. Am. Chem. Soc. 2007, 129, 5031–5044. doi:10.1021/ja0676962
Return to citation in text: [1] -
Peters, R.; Xin, Z.-q.; Maier, F. Chem.–Asian J. 2010, 5, 1770–1774. doi:10.1002/asia.201000386
Return to citation in text: [1] -
Lutz, R. P. Chem. Rev. 1984, 84, 205–247. doi:10.1021/cr00061a001
Return to citation in text: [1] -
Majumdar, K. C.; Alam, S.; Chattopadhyay, B. Tetrahedron 2008, 64, 597–643. doi:10.1016/j.tet.2007.10.079
Return to citation in text: [1] -
Marion, N.; Nolan, S. P. Angew. Chem., Int. Ed. 2007, 46, 2750–2752. doi:10.1002/anie.200604773
Return to citation in text: [1] -
Zhang, L. J. Am. Chem. Soc. 2005, 127, 16804–16805. doi:10.1021/ja056419c
Return to citation in text: [1] -
Marion, N.; Díez-González, S.; de Frémont, P.; Noble, A. R.; Nolan, S. P. Angew. Chem., Int. Ed. 2006, 45, 3647–3650. doi:10.1002/anie.200600571
Return to citation in text: [1] -
Buzas, A.; Istrate, F.; Gagosz, F. Org. Lett. 2006, 8, 1957–1959. doi:10.1021/ol0606839
Return to citation in text: [1] -
Zhang, L.; Wang, S. J. Am. Chem. Soc. 2006, 128, 1442–1443. doi:10.1021/ja057327q
Return to citation in text: [1] -
Wang, S.; Zhang, L. J. Am. Chem. Soc. 2006, 128, 8414–8415. doi:10.1021/ja062777j
Return to citation in text: [1] -
Buzas, A. K.; Istrate, F. M.; Gagosz, F. Org. Lett. 2007, 9, 985–988. doi:10.1021/ol063031t
Return to citation in text: [1] -
Marion, N.; Gealageas, R.; Nolan, S. P. Org. Lett. 2007, 9, 2653–2656. doi:10.1021/ol070843w
Return to citation in text: [1] -
Gourlaouen, C.; Marion, N.; Nolan, S. P.; Maseras, F. Org. Lett. 2009, 11, 81–84. doi:10.1021/ol802430m
Return to citation in text: [1] -
Jaunzeme, I.; Jirgensons, A. Synlett 2005, 2984–2986. doi:10.1055/s-2005-918952
Return to citation in text: [1] -
Jamieson, A. G.; Sutherland, A. Org. Biomol. Chem. 2006, 4, 2932–2937. doi:10.1039/b607014k
Return to citation in text: [1] -
Swift, M. D.; Sutherland, A. Tetrahedron Lett. 2007, 48, 3771–3773. doi:10.1016/j.tetlet.2007.03.161
Return to citation in text: [1] -
Jaunzeme, I.; Jirgensons, A. Tetrahedron 2008, 64, 5794–5799. doi:10.1016/j.tet.2008.03.099
Return to citation in text: [1] -
Xing, D.; Yang, D. Org. Lett. 2010, 12, 1068–1071. doi:10.1021/ol100056f
Return to citation in text: [1] [2] -
Hutchings, G. J. Catal. Today 2007, 122, 196–200. doi:10.1016/j.cattod.2007.01.018
Return to citation in text: [1] -
Wei, C.; Li, C.-J. J. Am. Chem. Soc. 2003, 125, 9584–9585. doi:10.1021/ja0359299
Return to citation in text: [1] -
Huang, B.; Yao, X.; Li, C.-J. Adv. Synth. Catal. 2006, 348, 1528–1532. doi:10.1002/adsc.200606118
Return to citation in text: [1] -
Lo, V. K.-Y.; Liu, Y.; Wong, M.-K.; Che, C.-M. Org. Lett. 2006, 8, 1529–1532. doi:10.1021/ol0528641
Return to citation in text: [1] -
Xing, D.; Guan, B.; Cai, G.; Fang, Z.; Yang, L.; Shi, Z. Org. Lett. 2006, 8, 693–696. doi:10.1021/ol052830t
Return to citation in text: [1] -
Nishikawa, T.; Asai, M.; Ohyabu, N.; Isobe, M. J. Org. Chem. 1998, 63, 188–192. doi:10.1021/jo9713924
Return to citation in text: [1] -
Rogawski, M. A.; Löscher, W. Nat. Rev. Neurosci. 2004, 5, 553–564. doi:10.1038/nrn1430
Return to citation in text: [1] -
Casara, P. Tetrahedron Lett. 1994, 35, 3049–3050. doi:10.1016/S0040-4039(00)76824-9
Return to citation in text: [1]
| 1. | Overman, L. E. J. Am. Chem. Soc. 1974, 96, 597–599. doi:10.1021/ja00809a054 |
| 2. | Overman, L. E. J. Am. Chem. Soc. 1976, 98, 2901–2910. doi:10.1021/ja00426a038 |
| 16. | Marion, N.; Nolan, S. P. Angew. Chem., Int. Ed. 2007, 46, 2750–2752. doi:10.1002/anie.200604773 |
| 17. | Zhang, L. J. Am. Chem. Soc. 2005, 127, 16804–16805. doi:10.1021/ja056419c |
| 18. | Marion, N.; Díez-González, S.; de Frémont, P.; Noble, A. R.; Nolan, S. P. Angew. Chem., Int. Ed. 2006, 45, 3647–3650. doi:10.1002/anie.200600571 |
| 19. | Buzas, A.; Istrate, F.; Gagosz, F. Org. Lett. 2006, 8, 1957–1959. doi:10.1021/ol0606839 |
| 20. | Zhang, L.; Wang, S. J. Am. Chem. Soc. 2006, 128, 1442–1443. doi:10.1021/ja057327q |
| 21. | Wang, S.; Zhang, L. J. Am. Chem. Soc. 2006, 128, 8414–8415. doi:10.1021/ja062777j |
| 14. | Lutz, R. P. Chem. Rev. 1984, 84, 205–247. doi:10.1021/cr00061a001 |
| 15. | Majumdar, K. C.; Alam, S.; Chattopadhyay, B. Tetrahedron 2008, 64, 597–643. doi:10.1016/j.tet.2007.10.079 |
| 8. | Nomura, H.; Richards, C. J. Chem.–Asian J. 2010, 5, 1726–1740. doi:10.1002/asia.201000131 |
| 9. | Hollis, T. K.; Overman, L. E. J. Organomet. Chem. 1999, 576, 290–299. doi:10.1016/S0022-328X(98)01065-1 |
| 10. | Anderson, C. E.; Overman, L. E. J. Am. Chem. Soc. 2003, 125, 12412–12413. doi:10.1021/ja037086r |
| 11. | Kirsch, S. F.; Overman, L. E.; Watson, M. P. J. Org. Chem. 2004, 69, 8101–8104. doi:10.1021/jo0487092 |
| 12. | Watson, M. P.; Overman, L. E.; Bergman, R. G. J. Am. Chem. Soc. 2007, 129, 5031–5044. doi:10.1021/ja0676962 |
| 13. | Peters, R.; Xin, Z.-q.; Maier, F. Chem.–Asian J. 2010, 5, 1770–1774. doi:10.1002/asia.201000386 |
| 36. | Rogawski, M. A.; Löscher, W. Nat. Rev. Neurosci. 2004, 5, 553–564. doi:10.1038/nrn1430 |
| 3. | Overman, L. E. Acc. Chem. Res. 1980, 13, 218–224. doi:10.1021/ar50151a005 |
| 4. | Overman, L. E. Angew. Chem., Int. Ed. Engl. 1984, 23, 579–586. doi:10.1002/anie.198405791 |
| 5. | Metz, P.; Mues, C.; Schoop, A. Tetrahedron 1992, 48, 1071–1080. doi:10.1016/S0040-4020(01)88203-X |
| 6. | Johannsen, M.; Jørgensen, K. A. Chem. Rev. 1998, 98, 1689–1708. doi:10.1021/cr970343o |
| 7. | Overman, L. E.; Carpenter, N. E. In Organic Reactions; Overman, L. E., Ed.; Wiley: Hoboken, NJ, 2005; Vol. 66, pp 653–760. |
| 37. | Casara, P. Tetrahedron Lett. 1994, 35, 3049–3050. doi:10.1016/S0040-4039(00)76824-9 |
| 10. | Anderson, C. E.; Overman, L. E. J. Am. Chem. Soc. 2003, 125, 12412–12413. doi:10.1021/ja037086r |
| 35. | Nishikawa, T.; Asai, M.; Ohyabu, N.; Isobe, M. J. Org. Chem. 1998, 63, 188–192. doi:10.1021/jo9713924 |
| 25. | Jaunzeme, I.; Jirgensons, A. Synlett 2005, 2984–2986. doi:10.1055/s-2005-918952 |
| 26. | Jamieson, A. G.; Sutherland, A. Org. Biomol. Chem. 2006, 4, 2932–2937. doi:10.1039/b607014k |
| 27. | Swift, M. D.; Sutherland, A. Tetrahedron Lett. 2007, 48, 3771–3773. doi:10.1016/j.tetlet.2007.03.161 |
| 28. | Jaunzeme, I.; Jirgensons, A. Tetrahedron 2008, 64, 5794–5799. doi:10.1016/j.tet.2008.03.099 |
| 23. | Marion, N.; Gealageas, R.; Nolan, S. P. Org. Lett. 2007, 9, 2653–2656. doi:10.1021/ol070843w |
| 24. | Gourlaouen, C.; Marion, N.; Nolan, S. P.; Maseras, F. Org. Lett. 2009, 11, 81–84. doi:10.1021/ol802430m |
| 22. | Buzas, A. K.; Istrate, F. M.; Gagosz, F. Org. Lett. 2007, 9, 985–988. doi:10.1021/ol063031t |
| 30. | Hutchings, G. J. Catal. Today 2007, 122, 196–200. doi:10.1016/j.cattod.2007.01.018 |
| 31. | Wei, C.; Li, C.-J. J. Am. Chem. Soc. 2003, 125, 9584–9585. doi:10.1021/ja0359299 |
| 32. | Huang, B.; Yao, X.; Li, C.-J. Adv. Synth. Catal. 2006, 348, 1528–1532. doi:10.1002/adsc.200606118 |
| 33. | Lo, V. K.-Y.; Liu, Y.; Wong, M.-K.; Che, C.-M. Org. Lett. 2006, 8, 1529–1532. doi:10.1021/ol0528641 |
| 34. | Xing, D.; Guan, B.; Cai, G.; Fang, Z.; Yang, L.; Shi, Z. Org. Lett. 2006, 8, 693–696. doi:10.1021/ol052830t |
© 2011 Xing and Yang; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)