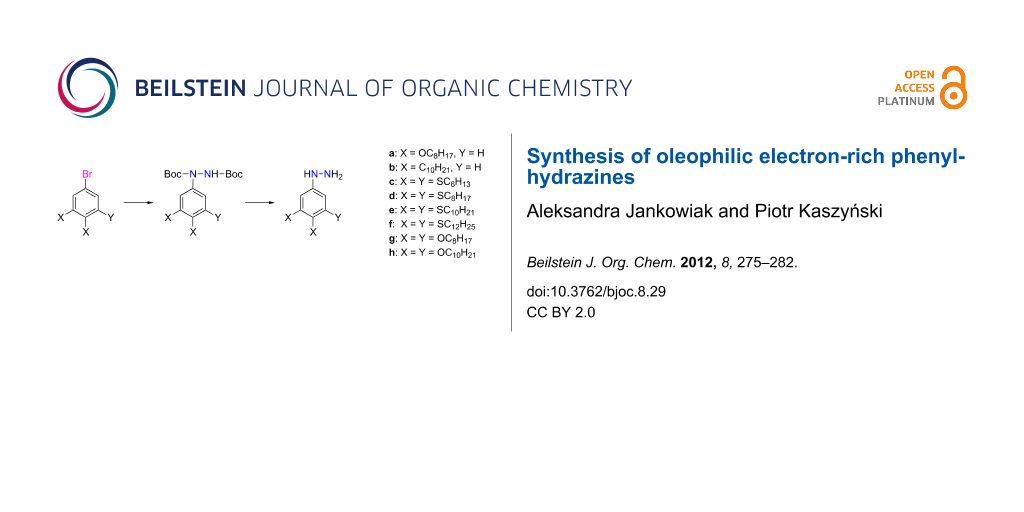
Abstract
Phenylhydrazines 1 substituted with two or three long-chain alkyl, alkoxy or alkylsulfanyl groups were successfully prepared by acid-induced removal of the Boc group in hydrazides 2. The reaction is carried out with 5 equivalents of TfOH in CF3CH2OH/CH2Cl2 at −40 °C for 1.5 min. Under these conditions, the deprotected hydrazine 1 is fully protonated, which increases its stability in the reaction medium. The hydrazines were isolated in 60–86% yields and purities >90%. The hydrazides 2 were obtained in 43–71% yields from aryl bromides 5, which were lithiated with t-BuLi and subsequently reacted with di-tert-butyl azodicarboxylate (DTBAD).
Graphical Abstract
Introduction
Mono-arylhydrazines I are important intermediates in the synthesis of a number of heterocycles, including indoles [1] and some azoles (for example [2,3]), many of which exhibit biological activity and are used in drug development [4-6]. Arylhydrazines are also key intermediates in the preparation of stable radicals such as verdazyl [7-9] and benzo[1,2,4]triazinyls [10-12].
The parent phenylhydrazine and many of its electron-deficient derivatives, such as p-nitrophenylhydrazine, are stable under ambient conditions and are conveniently obtained by using classical methods, such as the reduction of diazonium salts [13-15]. In contrast, electron-rich arylhydrazines are far less numerous and their preparation is complicated by oxidative instability.
To access functionalized and sensitive arylhydrazines several methods involving the deprotection of hydrazides II have been developed (Figure 1). Hydrazides II are efficiently obtained by the addition of organometallic reagents III, prepared from aryl halide IV, to azodicarboxylate diesters (AD) [16,17]. Alternatively, II can be obtained in the Pd(0)- or Cu2+-catalyzed reaction of arylboronic acid V to AD [18-20]. The latter method is especially suited for arylhydrazides substituted with sensitive functional groups. Protected electron-rich arylhydrazines, hydrazides II, containing the 2,2,2-trichloroethyl group (R = CH2CCl3) are conveniently prepared by direct electrophilic amination of arenes VI with bis(2,2,2-trichloroethyl) azodicarboxylate (BTCEAD) under Lewis [21,22] or Brønsted [23] acid conditions.
Figure 1: Selected methods for the preparation of arylhydrazines I through hydrazides II.
Figure 1: Selected methods for the preparation of arylhydrazines I through hydrazides II.
By judicious choice of the substituent R, the removal of the protecting group in II and formation of arylhydrazines I can be accomplished under acidic (R = t-Bu) [16], reductive (R = CH2CCl3) [24], or nearly neutral (R = CH2CH2TMS) conditions [22,25]. Among the three methods, the most straightforward is the removal of the Boc group under acidic conditions. Unfortunately, the literature method for deprotection (HCl in isopropanol, 70 °C) has limited scope, and electron-rich 3,4-dimethoxyphenylhydrazine could not be obtained under these conditions, although 4-pentyloxyphenylhydrazine hydrochloride was isolated in 60% yield [16]. The controlled reduction of 2,2,2-trichloroethyl esters (II, R = CH2CCl3) with Zn in aqueous MeOH containing NH4OAc gave access to a number of small, electron-rich phenylhydrazines, including 3,4-dimethoxyphenylhydrazine isolated in 76% yield as hydrochloride [24].
In the context of our research program in liquid-crystalline verdazyl derivatives [26], we needed phenylhydrazines 1 (Figure 2) substituted with multiple long-chain alkyl, alkoxy and alkylsulfanyl groups. Here we demonstrate an efficient method for the preparation of several hydrophobic di- and tri-substituted phenylhydrazines in purities sufficient for further chemical transformations. Finally, we demonstrate the application of one of the phenylhydrazines for the preparation of a discotic liquid crystal.
Figure 2: The structures for hydrazines 1a–1h.
Figure 2: The structures for hydrazines 1a–1h.
Results and Discussion
Our initial attempts at the preparation of 3,4-dioctyloxyphenylhydrazine (1a) focused on deprotection of the trichloroethyl ester 3a under buffered reductive conditions, according to the general literature procedure [24]. In aqueous MeOH hydrazide 3a was practically insoluble, and the reaction mixture was triphasic. Under these conditions no formation of hydrazine 1a was observed. Changing MeOH to EtOH and increasing its volume by two-fold gave homogenous solutions within which the desired hydrazine 1a was formed along with significant quantities of 4 as the major products (Scheme 1). The deamination product 4 was isolated and identified by comparison with the authentic sample. The yield and proportions of the two products, 1a and 4, varied from run to run, according to the 1H NMR spectra. Therefore, we focused on the acid-catalyzed deprotection of Boc-substituted hydrazines (Scheme 2), hydrazides 2, expecting that the reaction could be performed under fully homogenous conditions.
Scheme 1: Formation and deprotection of 3a under reductive conditions.
Scheme 1: Formation and deprotection of 3a under reductive conditions.
Scheme 2: General mechanism for the deprotection of arylhydrazides. G represents a substituent.
Scheme 2: General mechanism for the deprotection of arylhydrazides. G represents a substituent.
Analysis of the reaction mechanism for the deprotection of 2 under acidic conditions shows that removal of the Boc group generates t-Bu+, which reacts with the solvent, or alternatively it can alkylate the benzene ring of arylhydrazine (Scheme 2). For less reactive arylhydrazines the former process is faster, k1 << k2, and deprotection with HCl in iPrOH is effective [16]. For dialkoxyphenylhydrazines apparently k1 >> k2 and the desired hydrazine is not obtained [16].
The nucleophilicity of the hydrazine can be suppressed by its fast and complete protonation with a strong acid (Scheme 2). In this situation, the transient t-Bu+ is trapped with the solvent, forming volatile products, which simplifies isolation of the hydrazine as a crude product. We have focused on this approach to arylhydrazines employing trifluoromethanesulfonic acid (TfOH), which was used as an effective catalyst in the deprotection of tert-butyl aryl ethers [27].
Addition of catalytic amounts of the TfOH acid (10 mol %) to solutions of hydrazide 2a (Figure 3) in a mixture of CF3CH2OH/CH2Cl2 at −40 °C gave little conversion to hydrazine 1a. With 1.5 equiv of TfOH, hydrazide 2a was only partially converted to hydrazine 1a. With 5 equiv of TfOH the reaction was complete in less than 2 min and the crude hydrazine 1a was isolated as the sole product. Reaction times under 2 min appear to be optimum; the purity of the hydrazine decreased with increasing reaction times.
By using this protocol, hydrazines 1 were isolated as viscous oils in purities >90% and yields of 60–86%, according to 1H NMR analysis with 1,4-dimethoxybenzene as the internal standard (Scheme 3). Attempts at the preparation of crystalline hydrochlorides of 1 were unsuccessful and the viscous salts rapidly darkened and decomposed.
Scheme 3: Synthesis of arylhydrazines 1. Substituents X and Y are defined in Figure 2.
Scheme 3: Synthesis of arylhydrazines 1. Substituents X and Y are defined in Figure 2.
The Boc-protected arylhydrazines, hydrazides 2, were conveniently obtained by direct addition of aryllithium to di-tert-butyl azodicarboxylate (DTBAD, Scheme 3). The latter was prepared by lithiation of aryl bromides 5 with t-BuLi to avoid the formation of n-BuBr with n-BuLi and N-butylation of hydrazide 2. Hydrazide 2a was also obtained by the Cu2+-catalyzed addition [18] of arylboronic acid 6a [28] to DTBAD. The yields of both syntheses of 2a were comparable.
The trichloroethyl hydrazide 3a was prepared by acid-catalyzed amination of 1,2-dioctyloxybenzene (4) with BTCEAD in the presence of catalytic amounts of TfOH, according to a general literature procedure [23] (Scheme 1).
The requisite bromobenzene 5a was prepared by bromination of 1,2-dioctyloxybenzene (4) [29] with CuBr2 in MeCN according to a literature method [30] (Scheme 4). This method is a convenient alternative to the alkylation of the less readily accessible 4-bromocatechol (7) [28].
1-Bromo-3,4-didecylbenzene (5b) was obtained by bromination of 1,2-didecylbenzene (8) [31], obtained by the Kumada method [32], with Br2 in acetic anhydride (Scheme 5). Typically, the electrophilic bromination of 1,2-dialkylbenzenes results in 4,5-dibromo derivatives as the major products [33,34]. In contrast, the present method permits selective monobromination, although the bromo derivative 5b was isolated only in about 85% purity. The product could not be purified rigorously from several unidentified contaminants either by chromatography or by distillation due to the lack of separation or partial decomposition. Therefore, crude 5b was used for the preparation of hydrazide 2b, which was easily purified by chromatographic methods.
The attempted monoiodination of 8 with BTMA·ICl2 by using a general literature method [35] gave only traces of the product and nearly all of the starting material was recovered. Iodination under the Kern conditions [36,37] (HIO3/I2) gave a mixture of mono- and diiodo derivatives, which were difficult to separate. Manipulation of the reaction time and temperature failed to give the desired monoiodo derivative as the major product.
The preparation of bromobenzenes substituted with alkylsulfanyl groups, 5c–5f, is described elsewhere [38]. Bromides 5g [39,40] and 5h [41] were obtained according to the respective literature procedures by alkylation of 5-bromopyrogallol.
The 3,4,5-trialkylsulfanylphenylhydrazines 1c–1f have been used in the preparation of 6-oxoverdazyl derivatives that exhibit liquid-crystalline properties [26]. For instance, radical 9, prepared from 1d (Figure 4), exhibits a monotropic columnar rectangular phase (Cr 62 (Colr 60) I), a broad absorption band in the visible region, and redox potentials E0/+11/2 = +0.99 V and E0/−11/2 = −0.45 V versus SCE. Photovoltaic studies of 9 demonstrated hole mobility μh = 1.52 × 10−3 cm2 V−1s−1 in the mesophase with an activation energy Ea = 0.06 ± 0.01 eV.
Figure 4: The structure of verdazyl radical 9 and a texture of the Colr phase.
Figure 4: The structure of verdazyl radical 9 and a texture of the Colr phase.
Conclusion
We have developed a synthetic protocol for the efficient preparation of electron-rich phenylhydrazines 1 substituted with alkylsulfanyl, alkyl and alkoxy groups from Boc hydrazides 2. Experiments demonstrate that the addition of hydrazides 2 to a large excess of TfOH (5 equiv) at −40 °C gives hydrazines 1 in yields ranging from 60–86% and with purity >90%, which is sufficient for subsequent chemical transformations. The optimum reaction time is less than 2 min, typically 90 sec, and longer times lead to a lower purity of the product.
The presented method for the preparation of phenylhydrazines is an attractive alternative to Leblanc’s method, which relies on the reductive deprotection of trichloroethyl hydrazide 3 under heterogenous conditions. Our method involves homogenous solutions, low temperatures and short reaction times, and is particularly suited to oleophilic (“greasy”) arylhydrazines such as 1, which are important intermediates for the preparation of verdazyls and other heterocycles that may exhibit, e.g., liquid-crystalline properties (e.g., 9). In comparison with Leblanc’s protocol, our method is also a regiocontrolled hydrazinylation of the aromatics with the more accessible DTBAD through the organolithium. Although we focus on long-chain-substituted phenylhydrazines, we believe that this method can be used for other electron-rich arylhydrazines.
Experimental
Reagents and solvents were obtained commercially. Reactions were carried out under Ar. 1H NMR spectra were obtained at 400 MHz in CDCl3 and referenced to the solvent, unless specified otherwise.
Arylhydrazines 1
General procedure
A solution of hydrazide 2 (1 mmol) in a mixture of CH2Cl2 (3 mL)/CF3CH2OH (1 mL) was rapidly added to a solution of TfOH (0.750 g, 0.44 mL, 5 mmol) in CF3CH2OH (1 mL) at −40 °C under Ar. The mixture was stirred for 1.5 min, and CH2Cl2 (5 mL) followed by sat. NaHCO3 (10 mL) were added under very vigorous stirring. The organic layer was separated and the aqueous layer extracted (3 × CH2Cl2). Then the extracts were dried (Na2SO4) and the solvents were evaporated to give crude arylhydrazine 1 in purities typically >90% as a viscous, yellow to orange oil that darkened upon standing. The quantitative analysis of the deprotection reaction was conducted with 0.2 mmol of 2 as described above. The yield of the hydrazines was established by adding known quantities of 1,4-dimethoxybenzene (2.0 mL of 25 mM solution in CH2Cl2, 0.05 mmol) to the CH2Cl2 extract, evaporation of the resulting solution, and integration of the low-field 1H NMR signals.
3,4-Dioctyloxyphenylhydrazine (1a): 1H NMR (400 MHz, CDCl3) δ 0.88 (t, J = 6.8 Hz, 6H), 1.26–1.36 (m, 16H), 1.37–1.47 (m, 4H), 1.70–1.85 (m, 4H), 3.92 (t, J = 6.7 Hz, 2H), 3.96 (t, J = 6.7 Hz, 2H), 6.34 (dd, J1 = 8.5 Hz, J2 = 2.6 Hz, 1H), 6.46 (d, J = 2.6 Hz, 1H), 6.82 (d, J = 8.5 Hz, 1H); 1H NMR (500 MHz, DMSO-d6) δ 0.86 (t, J = 6.7 Hz, 6H), 1.20–1.36 (m, 16H), 1.37–1.46 (m, 4H), 1.61 (quint, J = 7.0 Hz, 2H), 1.68 (quint, J = 6.9 Hz, 2H), 3.78 (t, J = 6.4 Hz, 2H), 3.87 (t, J = 6.3 Hz, 2H), 6.24 (dd, J1 = 8.5 Hz, J2 = 2.3 Hz, 1H), 6.47 (d, J = 2.3 Hz, 1H), 6.71 (d, J = 8.6 Hz, 1H).
3,4-Didecylphenylhydrazine (1b): 1H NMR (500 MHz, CDCl3) δ 0.88 (t, J = 6.9 Hz, 6H), 1.22–1.40 (m, 28H), 1.47–1.58 (m, 4H), 2.51 (t, J = 7.0 Hz, 2H), 2.53 (t, J = 7.1 Hz, 2H), 2.6 (brs, 3H), 6.59–6.65 (m, 2H), 7.01 (d, J = 8.0 Hz, 1H).
3,4,5-Trihexylsulfanylphenylhydrazine (1c): 1H NMR (400 MHz, CDCl3) δ 0.87 (t, J = 6.9 Hz, 3H), 0.89 (t, J = 6.8 Hz, 6H), 1.20–1.35 (m, 12H), 1.36–1.52 (m, 6H), 1.59 (quint, J = 7.5 Hz, 2H), 1.71 (quint, J = 7.4 Hz, 4H), 2.77 (t, J = 7.4 Hz, 2H), 2.83 (t, J = 7.3 Hz, 4H), 3.2 (brs, 3H), 6.41 (s, 2H).
3,4,5-Trioctylsulfanylphenylhydrazine (1d): 1H NMR (500 MHz, CDCl3) δ 0.87 (t, J = 6.9 Hz, 3H), 0.88 (t, J = 6.6 Hz, 6H), 1.20–1.34 (m, 24H), 1.38–1.43 (m, 2H), 1.44–1.53 (m, 4H), 1.59 (quint, J = 7.5 Hz, 2H), 1.72 (quint, J = 7.5 Hz, 4H), 2.77 (t, J = 7.5 Hz, 2H), 2.84 (t, J = 7.4 Hz, 4H), 6.40 (s, 2H).
3,4,5-Tridecylsulfanylphenylhydrazine (1e): 1H NMR (400 MHz, CDCl3) δ 0.87 (t, J = 6.8 Hz, 3H), 0.88 (t, J = 6.8 Hz, 6H), 1.20–1.35 (m, 36H), 1.36–1.52 (m, 6H), 1.59 (quint, J = 7.6 Hz, 2H), 1.71 (quint, J = 7.3 Hz, 4H), 2.76 (t, J = 7.5 Hz, 2H), 2.83 (t, J = 7.3 Hz, 4H), 6.40 (s, 2H).
3,4,5-Tridodecylsulfanylphenylhydrazine (1f): 1H NMR (500 MHz, CDCl3) δ 0.88 (t, J = 6.8 Hz, 9H), 1.20–1.35 (m, 48H), 1.36–1.51 (m, 6H), 1.59 (quint, J = 7.5 Hz, 2H), 1.71 (quint, J = 7.4 Hz, 4H), 2.77 (t, J = 7.4 Hz, 2H), 2.84 (t, J = 7.1 Hz, 4H), 6.40 (s, 2H).
3,4,5-Trioctyloxyphenylhydrazine (1g): Soft yellow solid; 1H NMR (400 MHz, CDCl3) δ 0.88 (t, J = 6.7 Hz, 9H), 1.22–1.38 (m, 24H), 1.42–1.53 (m, 6H), 1.72 (quint, J = 7.1 Hz, 2H), 1.79 (quint, J = 7.1 Hz, 4H), 3.86 (t, J = 6.6 Hz, 2H), 3.95 (t, J = 6.6 Hz, 4H), 6.06 (s, 2H).
3,4,5-Tridecyloxyphenylhydrazine (1h): 1H NMR (400 MHz, CDCl3) δ 0.88 (t, J = 6.8 Hz, 9H), 1.21–1.38 (m, 36H), 1.39–1.64 (m, 6H), 1.65–1.84 (m, 6H), 3.86 (t, J = 6.6 Hz, 2H), 3.95 (t, J = 6.6 Hz, 4H), 6.06 (s, 2H); 1H NMR (400 MHz, C6D6) δ 0.92 (t, J = 6.8 Hz, 9H), 1.22–1.58 (m, 38H), 1.63–1.73 (m, 4H), 1.78 (quint, J = 7.1 Hz, 4H), 1.97 (quint, J = 8.3 Hz, 2H), 3.89 (t, J = 6.4 Hz, 4H), 4.23 (t, J = 6.5 Hz, 2H), 6.03 (s, 2H).
Preparation of hydrazides 2
General procedure
To a solution of the substituted bromobenzene 5 (1.0 mmol) in dry THF (10 mL), t-BuLi (1.7 M in pentane, 2.2 mmol) was added under Ar at −78 °C. After 1.5 h a THF (1 mL) solution of di-tert-butyl azodicarboxylate (DTBAD, 345 mg, 1.5 mmol) was added dropwise. The mixture was stirred at −78 °C for 0.5 h, then 1 h at rt, and quenched with 5% HCl. The organic products were extracted (Et2O), the extracts dried (Na2SO4), the solvents evaporated, and the residue was passed through a short silica-gel column (hexane/CH2Cl2 then CH2Cl2) to give hydrazides 2 as white solids.
1,2-Bis(tert-butoxycarbonyl)-1-(3,4-dioctyloxyphenyl)hydrazine (2a): Yield 71%; mp 55–57 °C; 1H NMR (500 MHz, CDCl3) δ 0.88 (t, J = 6.9 Hz, 6H), 1.22–1.38 (m, 16H), 1.39–1.51 (m, 4H), 1.49 (s, 18H), 1.73–1.84 (m, 4H), 3.96 (t, J = 6.6 Hz, 2H), 3.97 (t, J = 6.6 Hz, 2H), 6.71 (brs, 1H), 6.80 (d, J = 8.6 Hz, 1H), 6.86–6.92 (m, 1H), 6.93–7.02 (m, 1H); Anal. calcd for C32H56N2O6: C, 68.05; H, 9.99; N, 4.96; found: C, 68.35; H, 9.82; N, 5.02.
Method B: To a solution of 3,4-dioctyloxyphenylboronic acid (6a, 50 mg, 0.13 mmol) in THF (2 mL), di-tert-butyl azodicarboxylate (DTBAD, 30 mg, 0.13 mmol) was added followed by Cu(OAc)2 (cat) under an Ar atmosphere. The mixture was stirred at rt overnight, the solvent was evaporated and the residue was purified on a short silica-gel column (CH2Cl2) to give 50 mg (68% of yield) of hydrazide 2a.
1,2-Bis(tert-butoxycarbonyl)-1-(3,4-didecylphenyl)hydrazine (2b): Yield 63%; mp 37–38 °C; 1H NMR (500 MHz, CDCl3) δ 0.88 (t, J = 6.8 Hz, 6H), 1.23–1.40 (m, 28H), 1.49 (s, 18H), 1.48–1.59 (m, 4H), 2.52–2.59 (m, 4H), 6.70 (brs, 1H), 7.06 (d, J = 8.2 Hz, 1H), 7.08–7.21 (br m, 2H); Anal. calcd for C36H64N2O4: C, 73.42; H, 10.95; N, 4.76; found: C, 73.06; H, 10.88; N, 4.74.
1,2-Bis(tert-butoxycarbonyl)-1-(3,4,5-trihexylsulfanylphenyl)hydrazine (2c): Yield 43%; mp 74–75 °C; 1H NMR (400 MHz, CDCl3) δ 0.87 (t, J = 6.9 Hz, 3H), 0.88 (t, J = 6.9 Hz, 6H), 1.23–1.37 (m, 12H), 1.38–1.52 (m, 6H), 1.51 (s, 18H), 1.60 (quint, J = 7.4 Hz, 2H), 1.72 (quint, J = 7.2 Hz, 4H), 2.81 (t, J = 7.5 Hz, 2H), 2.84 (t, J = 7.4 Hz, 4H), 6.69 (brs, 1H), 6.99 (brs, 2H); Anal. calcd for C34H60N2O4S3: C, 62.15; H, 9.20; N, 4.26; found: C, 62.35; H, 9.34; N, 4.22.
1,2-Bis(tert-butoxycarbonyl)-1-(3,4,5-trioctylsulfanylphenyl)hydrazine (2d): Yield 55% yield; mp 51–52 oC; 1H NMR (400 MHz, CDCl3) δ 0.84–0.91 (m, 9H), 1.21–1.35 (m, 24H), 1.36–1.50 (m, 6H), 1.51 (s, 18H), 1.60 (quint, J = 7.4 Hz, 2H), 1.72 (quint, J = 7.5 Hz, 4H), 2.81 (t, J = 7.5 Hz, 4H), 2.83 (t, J = 7.4 Hz, 2H), 6.69 (brs, 1H), 6.98 (brs, 2H); the analytically pure sample was obtained by recrystallization (MeCN); Anal. calcd for C40H72N2O4S3: C, 64.82; H, 9.79; N, 3.78; found: C, 64.92; H, 9.56; N, 3.91.
1,2-Bis(tert-butoxycarbonyl)-1-(3,4,5-tridecylsulfanylphenyl)hydrazine (2e): Yield 56%; mp 50–51 °C; 1H NMR (500 MHz, CDCl3) δ 0.87 (t, J = 6.8 Hz, 3H), 0.88 (t, J = 7.0 Hz, 6H), 1.21–1.36 (m, 38H), 1.37–1.51 (m, 6H), 1.51 (s, 18H), 1.60 (quint, J = 7.4 Hz, 2H), 1.71 (quint, J = 7.4 Hz, 4H), 2.81 (t, J = 7.6 Hz, 2H), 2.83 (t, J = 7.4 Hz, 4H), 6.70 (brs, 1H), 6.98 (brs, 2H); Anal. calcd for C46H86N2O4S3: C, 66.78; H, 10.48; N, 3.39; found: C, 66.75; H, 10.07; N, 3.43.
1,2-Bis(tert-butoxycarbonyl)-1-(3,4,5-tridodecylsulfanylphenyl)hydrazine (2f): Yield 50%; mp 49–51 °C; 1H NMR (500 MHz, CDCl3) δ 0.88 (t, J = 6.7 Hz, 9H), 1.22–1.36 (m, 50H), 1.35–1.51 (m, 6H), 1.51 (s, 18H), 1.60 (quint, J = 7.4 Hz, 2H), 1.71 (quint, J = 7.2 Hz, 4H), 2.81 (t, J = 7.4 Hz, 2H), 2.83 (t, J = 7.2 Hz, 4H), 6.69 (brs, 1H), 6.98 (brs, 2H); Anal. calcd for C52H98N2O4S3: C, 68.52; H, 10.84; N, 3.07; found: C, 68.82; H, 10.83; N, 3.06.
1,2-Bis(tert-butoxycarbonyl)-1-(3,4,5-trioctyloxyphenyl)hydrazine (2g): Yield 45%; white crystals (MeCN/EtOAc); mp 73–74 °C; 1H NMR (400 MHz, CDCl3) δ 0.88 (t, J = 6.8 Hz, 9H), 1.23–1.38 (m, 24H), 1.38–1.52 (m, 6H), 1.50 (s, 18H), 1.73 (quint, J = 7.4 Hz, 2H), 1.77 (quint, J = 6.9 Hz, 4H), 3.92 (t, J = 6.8 Hz, 2H), 3.93 (t, J = 6.6 Hz, 4H), 6.64 (brs, 2H), 6.68 (brs, 1H); Anal. calcd for C40H72N2O7: C, 69.32; H, 10.47; N, 4.04; found: C, 69.61; H, 10.43; N, 3.91.
1,2-Bis(tert-butoxycarbonyl)-1-(3,4,5-tridecyloxyphenyl)hydrazine (2h): Yield 64%; mp 55–57 °C; 1H NMR (400 MHz, CDCl3) δ 0.88 (t, J = 6.8 Hz, 9H), 1.21–1.38 (m, 38H), 1.41–1.54 (m, 6H), 1.50 (s, 18H), 1.67–1.82 (m, 6H), 3.91 (t, J = 6.8 Hz, 2H), 3.93 (t, J = 6.6 Hz, 4H), 6.64 (brs, 2H), 6.68 (brs, 1H); Anal. calcd for C46H86N2O7: C, 70.91; H, 11.12; N, 3.60; found: C, 71.31; H, 11.08; N, 3.65.
1,2-Bis(2,2,2-trichloroethoxycarbonyl)-1-(3,4-dioctyloxyphenyl)hydrazine (3a): To the solution of 1,2-dioctyloxybenzene (4, 1.10 g, 3.31 mmol) in dry CH2Cl2 (20 mL), one drop of CF3SO3H was added under Ar at −78 °C followed by a solution of bis(2,2,2-trichloroethyl) azodicarboxylate (BTCEAD, 1.50 g, 3.97 mmol) in CH2Cl2 (3 mL). The reaction mixture was stirred for 20 min, warmed up to rt, stirred for 10 min, and quenched with 25% NH4OAc. The organic products were extracted (CH2Cl2), the extracts dried (Na2SO4), and the solvent evaporated. The viscous residue was passed through a silica-gel plug (hexane/CH2Cl2 then CH2Cl2) to give 1.03 g (36% yield) of the hydrazide 3a as a viscous oil: 1H NMR (400 MHz, CDCl3) δ 0.88 (t, J = 6.7 Hz, 3H), 0.89 (t, J = 7.2 Hz, 3H), 1.24–1.39 (m, 16H), 1.41–1.50 (m, 4H), 1.80 (quint, J = 7.0 Hz, 4H), 3.96 (t, J = 6.6 Hz, 2H), 3.99 (t, J = 6.6 Hz, 2H), 4.82 (s, 4H), 6.83 (d, J = 8.6 Hz, 1H), 6.97 (d, J = 8.2 Hz, 1H), 7.02 (brs, 1H), 7.39 (brs, 1H); Anal. calcd for C28H42Cl6N2O6: C, 47.01; H, 5.92; N, 3.92; found: C, 46.27; H, 5.72; N, 3.92.
1-Bromo-3,4-didecylbenzene (5b): To a solution of 1,2-didecylbenzene (8, 1.00 g, 2.8 mmol) in a mixture of Ac2O (3 mL) and CH2Cl2 (3 mL), Br2 (0.30 mL, 5.6 mmol) and catalytic amounts of I2 were added. The reaction mixture was stirred overnight at rt, water was added, the organic products were extracted (hexane), the extracts dried (Na2SO4), and the solvents evaporated. The residue was passed through a silica-gel plug (hexane) to give 1.20 g (~85% yield, based on NMR, contained ~15% of at least two impurities) of 4-bromo-1,2-didecylbenzene (5b) as a colorless oil: 1H NMR (500 MHz, CDCl3) major signals δ 0.88 (t, J = 6.8 Hz, 6H), 1.20–1.40 (m, 28H), 1.49–1.58 (m, 4H), 2.53 (t, J = 7.4 Hz, 2H), 2.55 (t, J = 7.5 Hz, 2H), 6.98 (d, J = 8.2 Hz, 1H), 7.22 (dd, J1 = 8.2 Hz, J2 = 1.8 Hz, 1H), 7.25–7.27 (m, 1H); 1H NMR (400 MHz, CD2Cl2) major signals δ 0.86 (t, J = 6.8 Hz, 6H), 1.20–1.40 (m, 28H), 1.49–1.58 (m, 4H), 2.52 (t, J = 7.4 Hz, 2H), 2.55 (t, J = 7.5 Hz, 2H), 6.99 (d, J = 8.2 Hz, 1H), 7.19 (dd, J1 = 8.1 Hz, J2 = 2.2 Hz, 1H), 7.25 (d, J = 2.1 Hz, 1H); HRMS-EI (m/z): [M]+ calcd for C26H45Br, 436.2705; found, 436.2726; since 5b undergoes partial decomposition during attempted short-path distillation (>260 °C/0.2 mmHg), it was used without further purification for the preparation of 2b.
1,2-Didecylbenzene (8): Following a general procedure [31], a solution of 1,2-dichlorobenzene (10.0 g, 68.0 mmol), Ni(dppp)Cl2 (370 mg, 0.68 mmol), and n-decylmagnesium bromide (272 mmol) in a dry THF (100 mL) was heated under reflux overnight. The crude product was passed through a silica-gel plug (hexane) and short-path distilled (220–230 °C/0.3 mmHg) to collect 11.4 g (48% yield) of 1,2-didecylbenzene (8) as a colorless oil: 1H NMR (400 MHz, CDCl3) δ 0.88 (t, J = 6.8 Hz, 6H), 1.20–1.43 (m, 28H), 1.57 (quint, J = 7.7 Hz, 4H), 2.59 (t, J = 8.0 Hz, 4H), 7.06–7.16 (m, 4H); HRMS–EI (m/z): [M]+ calcd for C26H46, 358.3600; found, 358.3583.
References
-
Robinson, B. The Fisher Indole Synthesis; Wiley & Sons: New York, 1982.
Return to citation in text: [1] -
Elguero, J. Pyrazoles. In Comprehensive Heterocyclic Chemistry II; Katritzky, A. R.; Rees, C. W.; Scriven, E. F. V., Eds.; Pergamon, 1996; Vol. 3, pp 1–75. doi:10.1016/B978-008096518-5.00059-9
Return to citation in text: [1] -
Garratt, P. J. 1,2,4-Triazoles. In Comprehensive Heterocyclic Chemistry II; Katritzky, A. R.; Rees, C. W.; Scriven, E. F. V., Eds.; Pergamon, 1996; Vol. 4, pp 127–163. doi:10.1016/B978-008096518-5.00080-0
Return to citation in text: [1] -
Duffy, K. J.; Darcy, M. G.; Delorme, E.; Dillon, S. B.; Eppley, D. F.; Erickson-Miller, C.; Giampa, L.; Hopson, C. B.; Huang, Y.; Keenan, R. M.; Lamb, P.; Leong, L.; Liu, N.; Miller, S. G.; Price, A. T.; Rosen, J.; Shah, R.; Shaw, T. N.; Smith, H.; Stark, K. C.; Tian, S.-S.; Tyree, C.; Wiggall, K. J.; Zhang, L.; Luengo, J. I. J. Med. Chem. 2001, 44, 3730. doi:10.1021/jm010283l
Return to citation in text: [1] -
He, L.; Chang, H.-X.; Chou, T.-C.; Savaraj, N.; Cheng, C. C. Eur. J. Med. Chem. 2003, 38, 101. doi:10.1016/S0223-5234(02)01420-4
Return to citation in text: [1] -
Sugimoto, A.; Tanaka, H.; Eguchi, Y.; Ito, S.; Takashima, Y.; Ishikawa, M. J. Med. Chem. 1984, 27, 1300. doi:10.1021/jm00376a013
Return to citation in text: [1] -
Wiley, P. F. Verdazyls. Chemistry of 1,2,3-Triazines and 1,2,4-Triazines, Tetrazines and Pentazines; The Chemistry of Heterocyclic Compounds; Willey & Sons: New York , 1978; pp 1225–1246.
Return to citation in text: [1] -
Koivisto, B. D.; Hicks, R. G. Coord. Chem. Rev. 2005, 249, 2612. doi:10.1016/j.ccr.2005.03.012
Return to citation in text: [1] -
Hicks, R. G. Verdazyls and Related Radicals Containing the Hydrazyl [R2N-NR] Group. In Stable Radicals: Fundamentals and Applied Aspects of Odd-Electron Compounds; Hicks, R. G., Ed.; Wiley & Sons, 2010; pp 245–280.
And references therein.
Return to citation in text: [1] -
Neugebauer, F. A.; Umminger, I. Chem. Ber. 1980, 113, 1205. doi:10.1002/cber.19801130402
Return to citation in text: [1] -
Constantinides, C. P.; Koutentis, P. A.; Krassos, H.; Rawson, J. M.; Tasiopoulos, A. J. J. Org. Chem. 2011, 76, 2798. doi:10.1021/jo200210s
Return to citation in text: [1] -
Koutentis, P. A.; Lo Re, D. Synthesis 2010, 2075. doi:10.1055/s-0029-1218782
Return to citation in text: [1] -
Hunsberger, I. M.; Shaw, E. R.; Fugger, J.; Ketcham, R.; Lednicer, D. J. Org. Chem. 1956, 21, 394. doi:10.1021/jo01110a004
Return to citation in text: [1] -
Bandgar, B. P.; Thite, C. S. Synth. Commun. 1997, 27, 635. doi:10.1080/00397919708003336
Return to citation in text: [1] -
Browne, D. L.; Baxendale, I. R.; Ley, S. V. Tetrahedron 2011, 67, 10296. doi:10.1016/j.tet.2011.09.146
Return to citation in text: [1] -
Demers, J. P.; Klaubert, D. H. Tetrahedron Lett. 1987, 28, 4933. doi:10.1016/S0040-4039(00)96662-0
Return to citation in text: [1] [2] [3] [4] [5] -
Velarde-Ortiz, R.; Guijarro, A.; Rieke, R. D. Tetrahedron Lett. 1998, 39, 9157. doi:10.1016/S0040-4039(98)02108-X
Return to citation in text: [1] -
Kabalka, G. W.; Guchhait, S. K. Org. Lett. 2003, 5, 4129. doi:10.1021/ol035544v
Return to citation in text: [1] [2] -
Uemura, T.; Chatani, N. J. Org. Chem. 2005, 70, 8631. doi:10.1021/jo051387x
Return to citation in text: [1] -
Muñiz, K.; Iglesias, A. Angew. Chem., Int. Ed. 2007, 46, 6350. doi:10.1002/anie.200700288
Return to citation in text: [1] -
Zaltsgendler, I.; Leblanc, Y.; Bernstein, M. A. Tetrahedron Lett. 1993, 34, 2441. doi:10.1016/S0040-4039(00)60436-7
Return to citation in text: [1] -
Mitchell, H.; Leblanc, Y. J. Org. Chem. 1994, 59, 682. doi:10.1021/jo00082a035
Return to citation in text: [1] [2] -
Leblanc, Y.; Boudreault, N. J. Org. Chem. 1995, 60, 4268. doi:10.1021/jo00118a052
Return to citation in text: [1] [2] -
Dufresne, C.; Leblanc, Y.; Berthelette, C.; McCooeye, C. Synth. Commun. 1997, 27, 3613. doi:10.1080/00397919708007084
Return to citation in text: [1] [2] [3] -
Hudlicky, T.; Seoane, G.; Pettus, T. J. Org. Chem. 1989, 54, 4239. doi:10.1021/jo00278a052
Return to citation in text: [1] -
Jankowiak, A.; Pociecha, D.; Szczytko, J.; Monobe, H.; Kaszyński, P. J. Am. Chem. Soc. 2012, 134, 2465. doi:10.1021/ja209467h
Return to citation in text: [1] [2] -
Holcombe, J. L.; Livinghouse, T. J. Org. Chem. 1986, 51, 111. doi:10.1021/jo00351a028
Return to citation in text: [1] -
Sienkowska, M. J.; Farrar, J. M.; Zhang, F.; Kusuma, S.; Heiney, P. A.; Kaszynski, P. J. Mater. Chem. 2007, 17, 1399. doi:10.1039/b615545f
Return to citation in text: [1] [2] -
Collins, R. F.; Davis, M. J. Chem. Soc. C 1966, 366. doi:10.1039/J39660000366
Return to citation in text: [1] -
Bhatt, S.; Nayak, S. K. Synth. Commun. 2007, 37, 1381. doi:10.1080/00908320701230026
Return to citation in text: [1] -
Mohr, B.; Enkelmann, V.; Wegner, G. J. Org. Chem. 1994, 59, 635. doi:10.1021/jo00082a022
Return to citation in text: [1] [2] -
Tamao, K.; Sumitani, K.; Kiso, Y.; Zembayashi, M.; Fujioka, A.; Kodama, S.-i.; Nakajima, I.; Minato, A.; Kumada, M. Bull. Chem. Soc. Jpn. 1976, 49, 1958. doi:10.1246/bcsj.49.1958
Return to citation in text: [1] -
Sonoda, M.; Sakai, Y.; Yoshimura, T.; Tobe, Y.; Kamada, K. Chem. Lett. 2004, 33, 972. doi:10.1246/cl.2004.972
Return to citation in text: [1] -
Hanack, M.; Haisch, P.; Lehmann, H.; Subramanian, L. R. Synthesis 1993, 387. doi:10.1055/s-1993-25869
Return to citation in text: [1] -
Kajigaeshi, S.; Kakinami, T.; Moriwaki, M.; Tanaka, T.; Fujisaki, S.; Okamoto, T. Bull. Chem. Soc. Jpn. 1989, 62, 439. doi:10.1246/bcsj.62.439
Return to citation in text: [1] -
Wirth, H. O.; Königstein, O.; Kern, W. Justus Liebigs Ann. Chem. 1960, 634, 84. doi:10.1002/jlac.19606340109
Return to citation in text: [1] -
Jasiński, M.; Jankowiak, A.; Januszko, A.; Bremer, M.; Pauluth, D.; Kaszyński, P. Liq. Cryst. 2008, 35, 343. doi:10.1080/02678290701817318
Return to citation in text: [1] -
Jankowiak, A.; Dębska, Z.; Romański, J.; Kaszyński, P. J. Sulfur Chem. 2012, 33. doi:10.1080/17415993.2011.644554
Return to citation in text: [1] -
Maeda, H.; Haketa, Y.; Nakanishi, T. J. Am. Chem. Soc. 2007, 129, 13661. doi:10.1021/ja074435z
Return to citation in text: [1] -
Yasuda, T.; Shimizu, T.; Liu, F.; Ungar, G.; Kato, T. J. Am. Chem. Soc. 2011, 133, 13437. doi:10.1021/ja2035255
Return to citation in text: [1] -
Ma, C.-Q.; Pisula, W.; Weber, C.; Feng, X.-L.; Müllen, K.; Bäuerle, P. Chem.–Eur. J. 2011, 17, 1507. doi:10.1002/chem.201002198
Return to citation in text: [1]
| 32. | Tamao, K.; Sumitani, K.; Kiso, Y.; Zembayashi, M.; Fujioka, A.; Kodama, S.-i.; Nakajima, I.; Minato, A.; Kumada, M. Bull. Chem. Soc. Jpn. 1976, 49, 1958. doi:10.1246/bcsj.49.1958 |
| 33. | Sonoda, M.; Sakai, Y.; Yoshimura, T.; Tobe, Y.; Kamada, K. Chem. Lett. 2004, 33, 972. doi:10.1246/cl.2004.972 |
| 34. | Hanack, M.; Haisch, P.; Lehmann, H.; Subramanian, L. R. Synthesis 1993, 387. doi:10.1055/s-1993-25869 |
| 35. | Kajigaeshi, S.; Kakinami, T.; Moriwaki, M.; Tanaka, T.; Fujisaki, S.; Okamoto, T. Bull. Chem. Soc. Jpn. 1989, 62, 439. doi:10.1246/bcsj.62.439 |
| 10. | Neugebauer, F. A.; Umminger, I. Chem. Ber. 1980, 113, 1205. doi:10.1002/cber.19801130402 |
| 11. | Constantinides, C. P.; Koutentis, P. A.; Krassos, H.; Rawson, J. M.; Tasiopoulos, A. J. J. Org. Chem. 2011, 76, 2798. doi:10.1021/jo200210s |
| 12. | Koutentis, P. A.; Lo Re, D. Synthesis 2010, 2075. doi:10.1055/s-0029-1218782 |
| 24. | Dufresne, C.; Leblanc, Y.; Berthelette, C.; McCooeye, C. Synth. Commun. 1997, 27, 3613. doi:10.1080/00397919708007084 |
| 7. | Wiley, P. F. Verdazyls. Chemistry of 1,2,3-Triazines and 1,2,4-Triazines, Tetrazines and Pentazines; The Chemistry of Heterocyclic Compounds; Willey & Sons: New York , 1978; pp 1225–1246. |
| 8. | Koivisto, B. D.; Hicks, R. G. Coord. Chem. Rev. 2005, 249, 2612. doi:10.1016/j.ccr.2005.03.012 |
| 9. |
Hicks, R. G. Verdazyls and Related Radicals Containing the Hydrazyl [R2N-NR] Group. In Stable Radicals: Fundamentals and Applied Aspects of Odd-Electron Compounds; Hicks, R. G., Ed.; Wiley & Sons, 2010; pp 245–280.
And references therein. |
| 26. | Jankowiak, A.; Pociecha, D.; Szczytko, J.; Monobe, H.; Kaszyński, P. J. Am. Chem. Soc. 2012, 134, 2465. doi:10.1021/ja209467h |
| 4. | Duffy, K. J.; Darcy, M. G.; Delorme, E.; Dillon, S. B.; Eppley, D. F.; Erickson-Miller, C.; Giampa, L.; Hopson, C. B.; Huang, Y.; Keenan, R. M.; Lamb, P.; Leong, L.; Liu, N.; Miller, S. G.; Price, A. T.; Rosen, J.; Shah, R.; Shaw, T. N.; Smith, H.; Stark, K. C.; Tian, S.-S.; Tyree, C.; Wiggall, K. J.; Zhang, L.; Luengo, J. I. J. Med. Chem. 2001, 44, 3730. doi:10.1021/jm010283l |
| 5. | He, L.; Chang, H.-X.; Chou, T.-C.; Savaraj, N.; Cheng, C. C. Eur. J. Med. Chem. 2003, 38, 101. doi:10.1016/S0223-5234(02)01420-4 |
| 6. | Sugimoto, A.; Tanaka, H.; Eguchi, Y.; Ito, S.; Takashima, Y.; Ishikawa, M. J. Med. Chem. 1984, 27, 1300. doi:10.1021/jm00376a013 |
| 22. | Mitchell, H.; Leblanc, Y. J. Org. Chem. 1994, 59, 682. doi:10.1021/jo00082a035 |
| 25. | Hudlicky, T.; Seoane, G.; Pettus, T. J. Org. Chem. 1989, 54, 4239. doi:10.1021/jo00278a052 |
| 26. | Jankowiak, A.; Pociecha, D.; Szczytko, J.; Monobe, H.; Kaszyński, P. J. Am. Chem. Soc. 2012, 134, 2465. doi:10.1021/ja209467h |
| 2. | Elguero, J. Pyrazoles. In Comprehensive Heterocyclic Chemistry II; Katritzky, A. R.; Rees, C. W.; Scriven, E. F. V., Eds.; Pergamon, 1996; Vol. 3, pp 1–75. doi:10.1016/B978-008096518-5.00059-9 |
| 3. | Garratt, P. J. 1,2,4-Triazoles. In Comprehensive Heterocyclic Chemistry II; Katritzky, A. R.; Rees, C. W.; Scriven, E. F. V., Eds.; Pergamon, 1996; Vol. 4, pp 127–163. doi:10.1016/B978-008096518-5.00080-0 |
| 16. | Demers, J. P.; Klaubert, D. H. Tetrahedron Lett. 1987, 28, 4933. doi:10.1016/S0040-4039(00)96662-0 |
| 31. | Mohr, B.; Enkelmann, V.; Wegner, G. J. Org. Chem. 1994, 59, 635. doi:10.1021/jo00082a022 |
| 21. | Zaltsgendler, I.; Leblanc, Y.; Bernstein, M. A. Tetrahedron Lett. 1993, 34, 2441. doi:10.1016/S0040-4039(00)60436-7 |
| 22. | Mitchell, H.; Leblanc, Y. J. Org. Chem. 1994, 59, 682. doi:10.1021/jo00082a035 |
| 16. | Demers, J. P.; Klaubert, D. H. Tetrahedron Lett. 1987, 28, 4933. doi:10.1016/S0040-4039(00)96662-0 |
| 39. | Maeda, H.; Haketa, Y.; Nakanishi, T. J. Am. Chem. Soc. 2007, 129, 13661. doi:10.1021/ja074435z |
| 40. | Yasuda, T.; Shimizu, T.; Liu, F.; Ungar, G.; Kato, T. J. Am. Chem. Soc. 2011, 133, 13437. doi:10.1021/ja2035255 |
| 18. | Kabalka, G. W.; Guchhait, S. K. Org. Lett. 2003, 5, 4129. doi:10.1021/ol035544v |
| 19. | Uemura, T.; Chatani, N. J. Org. Chem. 2005, 70, 8631. doi:10.1021/jo051387x |
| 20. | Muñiz, K.; Iglesias, A. Angew. Chem., Int. Ed. 2007, 46, 6350. doi:10.1002/anie.200700288 |
| 24. | Dufresne, C.; Leblanc, Y.; Berthelette, C.; McCooeye, C. Synth. Commun. 1997, 27, 3613. doi:10.1080/00397919708007084 |
| 41. | Ma, C.-Q.; Pisula, W.; Weber, C.; Feng, X.-L.; Müllen, K.; Bäuerle, P. Chem.–Eur. J. 2011, 17, 1507. doi:10.1002/chem.201002198 |
| 16. | Demers, J. P.; Klaubert, D. H. Tetrahedron Lett. 1987, 28, 4933. doi:10.1016/S0040-4039(00)96662-0 |
| 17. | Velarde-Ortiz, R.; Guijarro, A.; Rieke, R. D. Tetrahedron Lett. 1998, 39, 9157. doi:10.1016/S0040-4039(98)02108-X |
| 36. | Wirth, H. O.; Königstein, O.; Kern, W. Justus Liebigs Ann. Chem. 1960, 634, 84. doi:10.1002/jlac.19606340109 |
| 37. | Jasiński, M.; Jankowiak, A.; Januszko, A.; Bremer, M.; Pauluth, D.; Kaszyński, P. Liq. Cryst. 2008, 35, 343. doi:10.1080/02678290701817318 |
| 13. | Hunsberger, I. M.; Shaw, E. R.; Fugger, J.; Ketcham, R.; Lednicer, D. J. Org. Chem. 1956, 21, 394. doi:10.1021/jo01110a004 |
| 14. | Bandgar, B. P.; Thite, C. S. Synth. Commun. 1997, 27, 635. doi:10.1080/00397919708003336 |
| 15. | Browne, D. L.; Baxendale, I. R.; Ley, S. V. Tetrahedron 2011, 67, 10296. doi:10.1016/j.tet.2011.09.146 |
| 23. | Leblanc, Y.; Boudreault, N. J. Org. Chem. 1995, 60, 4268. doi:10.1021/jo00118a052 |
| 38. | Jankowiak, A.; Dębska, Z.; Romański, J.; Kaszyński, P. J. Sulfur Chem. 2012, 33. doi:10.1080/17415993.2011.644554 |
| 16. | Demers, J. P.; Klaubert, D. H. Tetrahedron Lett. 1987, 28, 4933. doi:10.1016/S0040-4039(00)96662-0 |
| 24. | Dufresne, C.; Leblanc, Y.; Berthelette, C.; McCooeye, C. Synth. Commun. 1997, 27, 3613. doi:10.1080/00397919708007084 |
| 16. | Demers, J. P.; Klaubert, D. H. Tetrahedron Lett. 1987, 28, 4933. doi:10.1016/S0040-4039(00)96662-0 |
| 28. | Sienkowska, M. J.; Farrar, J. M.; Zhang, F.; Kusuma, S.; Heiney, P. A.; Kaszynski, P. J. Mater. Chem. 2007, 17, 1399. doi:10.1039/b615545f |
| 31. | Mohr, B.; Enkelmann, V.; Wegner, G. J. Org. Chem. 1994, 59, 635. doi:10.1021/jo00082a022 |
| 29. | Collins, R. F.; Davis, M. J. Chem. Soc. C 1966, 366. doi:10.1039/J39660000366 |
| 30. | Bhatt, S.; Nayak, S. K. Synth. Commun. 2007, 37, 1381. doi:10.1080/00908320701230026 |
| 28. | Sienkowska, M. J.; Farrar, J. M.; Zhang, F.; Kusuma, S.; Heiney, P. A.; Kaszynski, P. J. Mater. Chem. 2007, 17, 1399. doi:10.1039/b615545f |
| 23. | Leblanc, Y.; Boudreault, N. J. Org. Chem. 1995, 60, 4268. doi:10.1021/jo00118a052 |
| 27. | Holcombe, J. L.; Livinghouse, T. J. Org. Chem. 1986, 51, 111. doi:10.1021/jo00351a028 |
| 18. | Kabalka, G. W.; Guchhait, S. K. Org. Lett. 2003, 5, 4129. doi:10.1021/ol035544v |
© 2012 Jankowiak and Kaszyński; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)