Abstract
The synthesis of (αS,2R)-(2,5-dihydro-1H-pyrrol-2-yl)glycine (22, normethylazafuranomycin) by the gold-catalyzed cycloisomerization of α-aminoallene 17 is described. The target molecule was synthesized in 13 linear steps from Cbz-protected Garner aldehyde (R)-2 in an overall yield of 2.4%. The approach was first examined in model studies, which afforded the alkylated azafuranomycin derivative 13a in 2.9% yield over 12 steps.
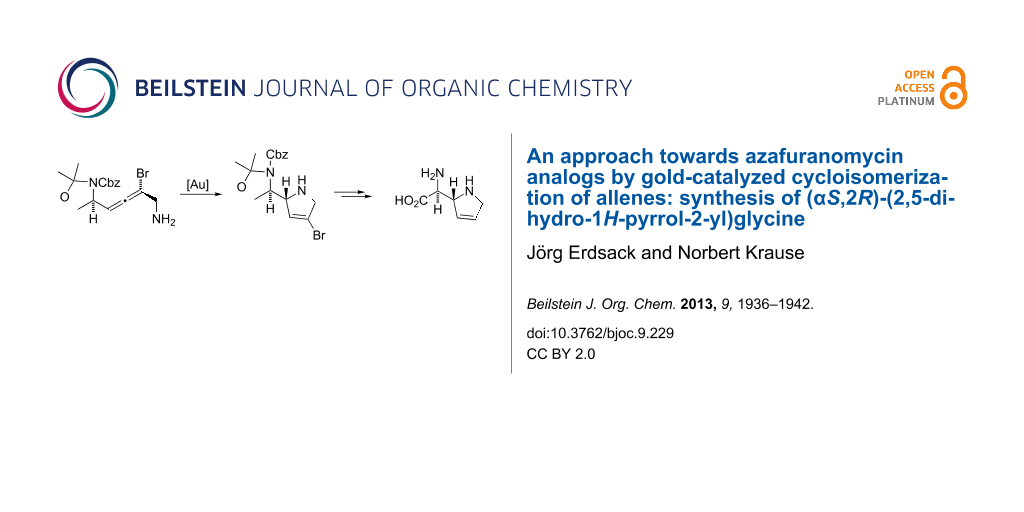
Graphical Abstract
Introduction
In 1967, Katagiri et al. reported the isolation of a novel antibiotic from the culture broth of the fungus Streptomyces threomyceticus [1]. The compound acts as a competitive antagonist for isoleucine in vitro and hampers the growth of several microorganisms, including the E. coli, S. aureus and M. tuberculosis. (+)-Furanomycin (1a, Figure 1, X = O) was identified as a non-proteinogenic amino acid bearing a characteristic 2,5-dihydrofuran ring. The correct (αS,2R,5S)-stereochemistry was established in 1980 by the first total synthesis by Joullié and co-workers [2] and the X-ray analysis of the N-acetyl derivative of the natural product by Shiro et al. [3]. (+)-Furanomycin belongs to the smallest natural antibiotics [4]. Therefore, the compound found considerable interest in synthetic chemistry. Until today, five total syntheses were published [2,5-8] as well as numerous reports dedicated to derivatives and stereoisomers [9-20]. Examination of structure–activity relationship (SAR) revealed a loss of antibiotic activity upon shifting of the methyl group to different positions, removal of the double bond, or change of the relative configuration [14,16]. Likewise, carbafuranomycin (1b) showed insufficient biological activity [17].
Figure 1: Structure of furanomycin and its carba- and aza-anolgue.
Figure 1: Structure of furanomycin and its carba- and aza-anolgue.
In 2007, we reported a synthesis of furanomycin derivatives by gold-catalyzed endo-selective cycloisomerization of α-hydroxyallenes [19]. This method opens an efficient stereoselective access to chiral 2,5-dihydrofurans by axis-to-center chirality transfer (Scheme 1) [21-32] and was applied to the total synthesis of various natural products [29-37]. Likewise, the corresponding gold-catalyzed cycloisomerization of various protected or unprotected α-aminoallenes affords 2,5-dihydropyrroles [29-32,38,39]. Due to the difference in biological activity of furanomycin (1a) and carbafuranomycin (1b), we became interested in the synthesis of derivatives of the (so far unknown) azafuranomycin (1c). Here, we describe the first results of this study.
Scheme 1: Gold-catalyzed cycloisomerization of α-functionalized allenes.
Scheme 1: Gold-catalyzed cycloisomerization of α-functionalized allenes.
Results and Discussion
Since Boc-protected intermediates tend to decompose during late-stage oxidation to the carboxylic acid [19], we selected the Cbz-protected Garner aldehyde 2 as starting material instead of the commonly used Boc-protected analog [40,41]. We prepared aldehyde (S)/(R)-2 on a multigram scale in three steps starting from commercial available (S)/(R)-serine methylester hydrochloride by treatment with Cbz-Cl [42], acetalization with dimethoxypropane [43], and ester reduction with DIBAL-H [44]. In our hands, this pathway was most effective as only for the reduction step Schlenk technique was necessary. Addition of lithiated t-butyldimethylprop-2-ynyloxysilane 3 [45] to (S)-2 in THF at –78 °C in the presence of HMPA afforded alcohol 4 [46-50] in 74% yield and high anti-selectivity (>95:5; Scheme 2). Only traces of the syn-isomer were detected by TLC. Conversion of 4 into tosylate 5a and acetate 5b by standard conditions (p-TsCl/cat. DMAP in pyridine and acetic anhydride/cat. DMAP/triethylamine, respectively) proceeded in 81 and 88% yield, respectively. In contrast, treatment of alcohol 4 with diethyl chlorophosphate and n-BuLi or cat. DMAP in pyridine gave the phosphate 5c in low yield. Here, direct quenching of the acetylide formed from (S)-2 and lithiated 3 with diethyl chlorophosphate was more effective and afforded phosphate 5c in 54% yield. With these propargylic electrophiles in hand, we studied the allene synthesis by copper-mediated SN2’-substitution [51] (Table 1, see below).
Scheme 2: Synthesis of propargylic electrophiles 5.
Scheme 2: Synthesis of propargylic electrophiles 5.
In order to establish suitable reaction conditions, we first examined the synthesis of the butyl-substituted model substrate 6a. Treatment of propargyl tosylate 5a with the organocopper reagent formed in situ from n-BuMgCl, CuBr·SMe2, and LiBr [52] afforded allene 6a with up to 74% yield (Table 1, entries 1–3). In order to achieve complete conversion, a large excess of the nucleophile is required. The yield could be raised further by using the cyanocuprate n-BuCu(CN)MgBr [53-55] or the heterocuprate n-BuCu(SPh)Li [56] (Table 1, entries 4 and 5). In the latter case, no formal reduction product (6, R2 = H) was observed which might have been formed by hydrolysis of a stable copper(III) intermediate [51,56]. As expected, all SN2’-substitutions proceeded with excellent anti-stereoselectivity [51]. With propargyl acetate 5b as starting material, allene 6a was obtained in 85% yield using n-BuCu(CN)MgBr·2LiCl as nucleophile (Table 1, entry 6). In contrast, use of the heterocuprate n-BuCu(SPh)Li led to decomposition (Table 1, entry 7). Propargyl phosphate 5c is also a suitable precursor of allene 6a (Table 1, entries 8 and 9).
Table 1: Copper-promoted SN2’-substitution of propargylic electrophiles 5 to afford allenes 6.
|
|
||||
| Entry | 5 | Conditions | 6 | Yield/% |
|---|---|---|---|---|
| 1 | 5a | 4 equiv n-BuCuMgBr2·LiCl, THF, –60 → 0 °C, 90 min | 6a | —a |
| 2 | 5a | 8 equiv n-BuCuMgBr2·LiCl, THF, –60 °C, 30 min | 6a | 55 |
| 3 | 5a | 10 equiv n-BuCuMgBr2·LiCl, THF, –60 → 0 °C, 90 min | 6a | 74 |
| 4 | 5a | 10 equiv n-BuCu(CN)MgBr·2LiCl, THF, –78 °C, 30 min | 6a | 83 |
| 5 | 5a | 4 equiv n-BuCu(SPh)Li, Et2O, –78 °C, 30 min | 6a | 80 |
| 6 | 5b | 10 equiv n-BuCu(CN)MgBr·2LiCl, THF, –78 °C → rt, 12 h | 6a | 85 |
| 7 | 5b | 4 equiv n-BuCu(SPh)Li, Et2O, –78 °C, 30 min | 6a | —b |
| 8 | 5c | 10 equiv n-BuCuMgBr2·LiCl, THF, –78 °C, 60 min | 6a | 83 |
| 9 | 5c | 10 equiv n-BuCu(CN)MgBr·2LiCl, THF, –78 °C, 3 h | 6a | 71 |
| 10 | 5a | 6 equiv LiCuBr2, THF, reflux, 12 h | 6b | 68 |
| 11 | 5a | 1.2 equiv (PhMe2Si)2CuCNLi2, THF, –78 °C, 30 min | 6c | 77 |
| 12 | 5b | 1.2 equiv (PhMe2Si)2CuCNLi2, THF, –78 °C, 30 min | 6c | —b |
aIncomplete conversion. bDecomposition.
After these successful model studies, we introduced substituents into the allene which can be removed at a later stage. Treatment of propargyl tosylate 5a with lithium dibromocuprate [57-59] or the silylcuprate (PhMe2Si)2CuCNLi2 [60,61] afforded the allenes 6b and 6c with 68 and 77% yield, respectively (Table 1, entries 10 and 11). Also these SN2’-substitution took place with complete anti-stereoselectivity. In contrast, decomposition occurred when propargyl acetate 5b was treated with the silyl cuprate (Table 1, entry 12).
The next steps towards the substrates of the gold-catalyzed cycloisomerization proceeded uneventfully (Scheme 3). Desilylation of allenes 6a and 6b with tetrabutylammonium fluoride trihydrate afforded the α-hydroxyallenes 7a/b in high yield, and these were converted into the aminoallenes 8a/b under standard Mitsunobu conditions (DEAD, PPh3, phthalimide; then hydrazine monohydrate) [38,39,62]. Unfortunately, fluoride-mediated desilylation of allene 6c caused complete epimerization of the allenic chirality axis. Therefore, the silylallene was not used in further studies.
Scheme 3: Synthesis of α-hydroxyallenes 7 and α-aminoallenes 8.
Scheme 3: Synthesis of α-hydroxyallenes 7 and α-aminoallenes 8.
The results of the gold-catalyzed cycloisomerization of the allenes 7 and 8 to the five-membered heterocycles 9/10 are summarized in Table 2. Treatment of the α-hydroxyallene 7a with 1 mol % AuCl3 in THF [21-23] afforded the desired 2,5-dihydrofuran 9a with 84% yield (Table 2, entry 1). The temperature was decreased to 5 °C to avoid acetal cleavage by the Lewis-acidic gold catalyst [19]. For the corresponding cyclization of the bromoallene 7b, the temperature had to be raised to 50°C in order to achieve complete conversion (Table 2, entry 2). Only traces of the acetal cleavage product were detected by TLC. However, the cycloisomerization was accompanied by partial epimerization of the allene, so that dihydrofuran 9b was isolated as a 4:1-mixture of diastereomers.
Table 2: Gold-catalyzed cycloisomerization of allenes 7 and 8.
|
|
||||
| Entry | 7/8 | Conditions | 9/10 | Yield/% |
|---|---|---|---|---|
| 1 | 7a | 1 mol % AuCl3,THF, 5 °C, 12 h | 9a | 84 |
| 2 | 7b | 2 mol % AuCl3, THF, 50 °C, 5 h | 9b | 77a |
| 3 | 8a | 10 mol % AuCl, 10 mol % imidazole, DCE, 80 °C, 12 h | 10a | 66b |
| 4 | 8a | 10 mol % AuCl, 10 mol % imidazole, toluene, 80 °C, 12 h | 10a | 78 |
| 5 | 8b | 7 mol % Ph3PAuCl, 7 mol % AgBF4, 7 mol % imidazole, toluene, 100 °C, 12 h | 10b | 47c |
adr = 4:1. bThe N-chloroethylated dihydropyrrole was formed as side product (29% yield). cYield of the twofold protected dihydropyrrole 11b obtained by treatment of 10b with CbzCl and DMAP; 2’-epi-11b was obtained as minor product (4% yield).
As expected, the cycloisomerization of allenes 8 bearing an unprotected amino group is much slower [38,39] and requires rather forcing conditions. For a complete conversion of α-aminoallene 8a, 10 mol % of AuCl [38,39], 10 mol % of imidazole as stabilizing ligand and an elevated temperature (80 °C) are necessary. With dichloroethane as solvent, dihydropyrrole 10a was obtained in 66% yield (Table 2, entry 3); however, this was accompanied by 29% of the corresponding N-chloroethylated product. This undesired side product could be avoided by using toluene as the solvent (Table 2, entry 4). Application of these conditions to the brominated α-aminoallene 8b gave incomplete conversion. Full conversion was achieved with 7 mol % each of Ph3PAuCl, AgBF4 and imidazole in toluene at 100 °C (Table 2, entry 5). The dihydropyrrole 10b thus formed could not purified completely even after repeated column chromatography. Therefore, the crude product was treated with CbzCl and DMAP [63] to give the twofold protected dihydropyrrole 11b (formula not shown) with 47% yield over two steps. Additionally, we isolated the epimeric compound 2’-epi-11b in 4% yield, indicating a minimal epimerization of bromoallene 8b during the gold-catalyzed cyclization.
The synthesis of azafuranomycin analogs was continued with the twofold Cbz-protected heterocycle 11a which was obtained with 79% yield by treatment of 10a with CbzCl and DMAP [63] (Scheme 4). This protection step was carried out in order to avoid dehydrogenation or chlorination of the secondary amine in the subsequent oxidation steps [64,65]. Acetal cleavage under mild protic conditions furnished the hydroxycarbamate, which underwent two-step oxidation with Dess–Martin periodinane [66,67] and sodium chlorite in buffered solution [68] in the presence of resorcine [19]. The carboxylic acid 12a was isolated in 65% yield. Finally, the protecting groups were removed with trifluoracetic acid in the presence of thioanisole [69] to afford the azafuranomycin analog 13a with 31% yield. Later, we found that a higher excess of thioanisole, which captures benzylic cations in the deprotection step, affords higher yields of the amino acid (Scheme 5).
Scheme 4: Synthesis of azafuranomycin analog 13a.
Scheme 4: Synthesis of azafuranomycin analog 13a.
Scheme 5: Synthesis of (αS,2R)-(2,5-dihydro-1H-pyrrol-2-yl)glycine (22).
Scheme 5: Synthesis of (αS,2R)-(2,5-dihydro-1H-pyrrol-2-yl)glycine (22).
After successful conclusion of the model studies, the synthesis of (αS,2R)-(2,5-dihydro-1H-pyrrol-2-yl)glycine (22, normethylazafuranomycin) was carried out (Scheme 5). The aldehyde (R)-2, which was prepared from D-serine [42-44], underwent chelation-controlled nucleophilic addition of metallated alkyne 3 [45] to give the propargyl alcohol 14 in 84% yield and a syn-diastereoselectivity of >95:5 [7,46,47]. After tosylation of 14 (85% yield), synthesis of the bromoallene 15 with lithium dibromocuprate resulted in an unexpected epimerization of the allene moiety to afford an inseparable 2:1-mixture of diastereomers in 66% yield. Addition of stabilizing ligands ((n-Bu)3P or (n-BuO)3P) did not affect this loss of stereoselectivity. Fortunately, the correct stereoisomer was enriched at the stage of the dihydropyrrole 18 due to several purification steps.
Desilylation of 15 with tetrabutylammonium fluoride trihydrate (94% yield) and conversion of 16 into the α-aminoallene 17 under Mitsunobu conditions (45% yield) [38,39,62] set the stage for the gold-catalyzed cycloisomerization. This was carried out with 10 mol % each of AuCl and imidazole in toluene at 100 °C to give the desired dihydropyrrole 18 in ca. 67% yield. Similar to the diastereomer 10b, compound 18 could not be purified in a sufficient manner even after repeated column chromatography. Treatment of 18 with CbzCl/DMAP [63] gave the fully protected heterocycle 19 in 42% yield over two steps from 17. The spectroscopic data are identical with those of 2’-epi-11b, except for the sign of the optical rotation {19: [α]19D +37.9 (c 1.27, CHCl3); 2’-epi-11b: [α]20D –47.0 (c 0.10, CHCl3)}.
For the debromination of dihydropyrrole 19, we first tested radical conditions ((n-Bu)3SnH/AIBN), but these led to complete decomposition. This is surprising since carbamates are known to be stable under radical conditions [70]. Indeed, treatment of diastereomer 11b with (n-Bu)3SnH/AIBN afforded the desired dehalogenated dihydropyrrole with 66% yield (formula not shown). For the conversion of 19 to 20, we applied a bromine–lithium exchange with 2 equivalents of t-BuLi in diethyl ether at –90 °C [71], followed by hydrolysis. Even though oxazolidines are known to be sensitive towards organolithium compounds [19,72-74], dehalogenated dihydropyrrole 20 was obtained in 60% yield, together with 22% of reisolated 19. The remaining steps towards azafuranomycin analog 22 followed those established for 13a: acetal cleavage (71% yield), two-step oxidation which afforded protected amino acid 21 with 72% yield [19,66-68], and final deprotection according to the procedure of Kiso et al. (50 equiv thioanisole and 270 equiv TFA per Cbz-group) [69] gave the target molecule 22 with 66% yield after purification by ion exchange chromatography (DOWEX 50W X8).
Conclusion
We have developed the first synthesis of the azafuranomycin analog (αS, 2R)-(2,5-dihydro-1H-pyrrol-2-yl)glycine (22) in 13 linear steps with an overall yield of 2.4% starting from the Cbz-protected Garner aldehyde (R)-2. The key step is the gold-catalyzed cycloisomerization of α-aminoallene 17 to dihydropyrrole 18. The sequence was first tested in model studies which afforded butyl-substituted azafuranomycin derivative 13a in 12 linear step with an overall yield of 2.9% starting from (S)-2.
Supporting Information
| Supporting Information File 1: Experimental part. | ||
| Format: PDF | Size: 283.7 KB | Download |
References
-
Katagiri, K.; Tori, K.; Kimura, Y.; Yoshida, T.; Nagasaki, T.; Minato, H. J. Med. Chem. 1967, 10, 1149. doi:10.1021/jm00318a035
Return to citation in text: [1] -
Semple, J. E.; Wang, P. C.; Lysenko, Z.; Joullié, M. M. J. Am. Chem. Soc. 1980, 102, 7505. doi:10.1021/ja00545a018
Return to citation in text: [1] [2] -
Shiro, M.; Nakai, H.; Tori, K.; Nishikawa, J.; Yashimura, Y.; Katagiri, K. J. Chem. Soc., Chem. Commun. 1980, 375a. doi:10.1039/C3980000375A
Return to citation in text: [1] -
von Nussbaum, F.; Brands, M.; Hinzen, B.; Weigand, S.; Häbich, D. Angew. Chem., Int. Ed. 2006, 45, 5072. doi:10.1002/anie.200600350
Return to citation in text: [1] -
Kang, S. H.; Lee, S. B. Chem. Commun. 1998, 761. doi:10.1039/a800727f
Return to citation in text: [1] -
Zhang, J. H.; Clive, D. L. J. J. Org. Chem. 1999, 64, 1754. doi:10.1021/jo982205k
Return to citation in text: [1] -
VanBrunt, M. P.; Standaert, R. F. Org. Lett. 2000, 2, 705. doi:10.1021/ol005569j
Return to citation in text: [1] [2] -
Zimmermann, P. J.; Blanarikova, J.; Jäger, V. Angew. Chem., Int. Ed. 2000, 39, 910. doi:10.1002/(SICI)1521-3773(20000303)39:5<910::AID-ANIE910>3.0.CO;2-9
Return to citation in text: [1] -
Masamune, T.; Ono, M. Chem. Lett. 1975, 625. doi:10.1246/cl.1975.625
Return to citation in text: [1] -
Divanfard, H. R.; Lysenko, Z.; Semple, J. E.; Wang, P.-C.; Joullié, M. M. Heterocycles 1981, 16, 1975. doi:10.3987/R-1981-11-1975
Return to citation in text: [1] -
Robins, M. J.; Parker, J. M. R. Can. J. Chem. 1983, 61, 317. doi:10.1139/v83-057
Return to citation in text: [1] -
Wiliams, R. M.; Sinclair, P. J.; Zhai, D.; Chen, D. J. Am. Chem. Soc. 1988, 110, 1547. doi:10.1021/ja00213a031
Return to citation in text: [1] -
Braithwaite, D. H.; Holzapfel, C. W.; Wiliams, D. B. G. J. Chem. Res., Synop. 1999, 108. doi:10.1039/a807128d
Return to citation in text: [1] -
Kazmaier, U.; Pähler, S.; Endermann, R.; Häbich, D.; Kroll, H.-P.; Riedl, B. Bioorg. Med. Chem. 2002, 10, 3905. doi:10.1016/S0968-0896(02)00317-6
Return to citation in text: [1] [2] -
Chattopadhyay, S. K.; Sarkar, K.; Karmakar, S. Synlett 2005, 2083. doi:10.1055/s-2005-871944
Return to citation in text: [1] -
Zimmermann, P. J.; Lee, J. Y.; Hlobilova (neé Blanarikova), I.; Endermann, R.; Häbich, D.; Jäger, V. Eur. J. Org. Chem. 2005, 3450. doi:10.1002/ejoc.200500148
Return to citation in text: [1] [2] -
Lee, J. Y.; Schiffer, G.; Jäger, V. Org. Lett. 2005, 7, 2317. doi:10.1021/ol0504493
Return to citation in text: [1] [2] -
Jirgensons, A.; Marinozzi, M.; Pellicciari, R. Tetrahedron 2005, 61, 373. doi:10.1016/j.tet.2004.10.091
Return to citation in text: [1] -
Erdsack, J.; Krause, N. Synthesis 2007, 3741. doi:10.1055/s-2007-990860
Return to citation in text: [1] [2] [3] [4] [5] [6] [7] -
Avenoza, A.; Busto, J. H.; Canal, N.; Corzana, F.; Peregrina, J. M.; Pérez-Fernández, M.; Rodríguez, F. J. Org. Chem. 2010, 75, 545. doi:10.1021/jo9025258
Return to citation in text: [1] -
Hoffmann-Röder, A.; Krause, N. Org. Lett. 2001, 3, 2537. doi:10.1021/ol016205+
Return to citation in text: [1] [2] -
Krause, N.; Hoffmann-Röder, A.; Canisius, J. Synthesis 2002, 1759. doi:10.1055/s-2002-33707
Return to citation in text: [1] [2] -
Deutsch, C.; Gockel, B.; Hoffmann-Röder, A.; Krause, N. Synlett 2007, 1790. doi:10.1055/s-2007-982561
Return to citation in text: [1] [2] -
Aksιn, Ö.; Krause, N. Adv. Synth. Catal. 2008, 350, 1106. doi:10.1002/adsc.200800050
Return to citation in text: [1] -
Winter, C.; Krause, N. Green Chem. 2009, 11, 1309. doi:10.1039/b905823k
Return to citation in text: [1] -
Asikainen, M.; Krause, N. Adv. Synth. Catal. 2009, 351, 2305. doi:10.1002/adsc.200900434
Return to citation in text: [1] -
Aksın-Artok, Ö.; Krause, N. Adv. Synth. Catal. 2011, 353, 385. doi:10.1002/adsc.201000903
Return to citation in text: [1] -
Minkler, S. R. K.; Lipshutz, B. H.; Krause, N. Angew. Chem., Int. Ed. 2011, 50, 7820. doi:10.1002/anie.201101396
Return to citation in text: [1] -
Krause, N.; Belting, V.; Deutsch, C.; Erdsack, J.; Fan, H.-T.; Gockel, B.; Hoffmann-Röder, A.; Morita, N.; Volz, F. Pure Appl. Chem. 2008, 80, 1063. doi:10.1351/pac200880051063
Return to citation in text: [1] [2] [3] -
Krause, N.; Aksin-Artok, Ö.; Breker, V.; Deutsch, C.; Gockel, B.; Poonoth, M.; Sawama, Y.; Sawama, Y.; Sun, T.; Winter, C. Pure Appl. Chem. 2010, 82, 1529. doi:10.1351/PAC-CON-09-09-23
Return to citation in text: [1] [2] [3] -
Krause, N.; Winter, C. Chem. Rev. 2011, 111, 1994. doi:10.1021/cr1004088
Return to citation in text: [1] [2] [3] -
Krause, N.; Aksin-Artok, Ö.; Asikainen, M.; Breker, V.; Deutsch, C.; Erdsack, J.; Fan, H.-T.; Gockel, B.; Minkler, S.; Poonoth, M.; Sawama, Y.; Sawama, Y.; Sun, T.; Volz, F.; Winter, C. J. Organomet. Chem. 2012, 704, 1. doi:10.1016/j.jorganchem.2012.01.008
Return to citation in text: [1] [2] [3] -
Volz, F.; Krause, N. Org. Biomol. Chem. 2007, 5, 1519. doi:10.1039/b703995f
Return to citation in text: [1] -
Volz, F.; Wadman, S. H.; Hoffmann-Röder, A.; Krause, N. Tetrahedron 2009, 65, 1902. doi:10.1016/j.tet.2008.11.104
Return to citation in text: [1] -
Miura, T.; Shimada, M.; de Mendoza, P.; Deutsch, C.; Krause, N.; Murakami, M. J. Org. Chem. 2009, 74, 6050. doi:10.1021/jo900987w
Return to citation in text: [1] -
Sun, T.; Deutsch, C.; Krause, N. Org. Biomol. Chem. 2012, 10, 5965. doi:10.1039/c2ob25069a
Return to citation in text: [1] -
Gao, Z.; Li, Y.; Cooksey, J. P.; Snaddon, T. N.; Schunk, S.; Viseux, E. M. E.; McAteer, S. M.; Kocienski, P. J. Angew. Chem., Int. Ed. 2009, 48, 5022. doi:10.1002/anie.200901608
Return to citation in text: [1] -
Morita, N.; Krause, N. Org. Lett. 2004, 6, 4121. doi:10.1021/ol0481838
Return to citation in text: [1] [2] [3] [4] [5] -
Morita, N.; Krause, N. Eur. J. Org. Chem. 2006, 4634. doi:10.1002/ejoc.200600438
Return to citation in text: [1] [2] [3] [4] [5] -
Garner, P. Tetrahedron Lett. 1984, 25, 5855. doi:10.1016/S0040-4039(01)81703-2
Return to citation in text: [1] -
Garner, P.; Park, J. M. J. Org. Chem. 1987, 52, 2361. doi:10.1021/jo00388a004
Return to citation in text: [1] -
Hassall, C. H.; Thomas, J. O. J. Chem. Soc. C 1968, 1495.
Return to citation in text: [1] [2] -
Chhabra, S. R.; Mahajan, A.; Chan, W. C. J. Org. Chem. 2002, 67, 4017. doi:10.1021/jo010456e
Return to citation in text: [1] [2] -
McKillop, A.; Taylor, R. J. K.; Watson, R. J.; Lewis, N. Synthesis 1994, 31. doi:10.1055/s-1994-25398
Return to citation in text: [1] [2] -
Tsou, H.-R.; Mamuya, N.; Johnson, B. D.; Reich, M. F.; Gruber, B. C.; Ye, F.; Nilakantan, R.; Shen, R.; Discafani, C.; DeBlanc, R.; Davis, R.; Koehn, F. E.; Greenberger, L. M.; Wang, Y.-F.; Wissner, A. J. Med. Chem. 2001, 44, 2719. doi:10.1021/jm0005555
Return to citation in text: [1] [2] -
Herold, P. Helv. Chim. Acta 1988, 71, 354. doi:10.1002/hlca.19880710208
Return to citation in text: [1] [2] -
Gruza, H.; Kiciak, K.; Krasiński, A.; Jurczak, J. Tetrahedron: Asymmetry 1997, 8, 2627. doi:10.1016/S0957-4166(97)00306-6
Return to citation in text: [1] [2] -
Chun, J.; Byun, H.-S.; Bitman, R. J. Org. Chem. 2003, 68, 348. doi:10.1021/jo026240+
Return to citation in text: [1] -
Masuda, Y.; Mori, K. Eur. J. Org. Chem. 2005, 4789. doi:10.1002/ejoc.200500357
Return to citation in text: [1] -
Erdsack, J.; Schürmann, M.; Preut, H.; Krause, N. Acta Crystallogr., Sect. E: Struct. Rep. Online 2008, E64, o1171. doi:10.1107/S1600536808014906
Return to citation in text: [1] -
Krause, N.; Hoffmann-Röder, A. Tetrahedron 2004, 60, 11671. doi:10.1016/j.tet.2004.09.094
Review.
Return to citation in text: [1] [2] [3] -
Elsevier, C. J.; Vermeer, P. J. Org. Chem. 1989, 54, 3726. doi:10.1021/jo00276a040
Return to citation in text: [1] -
Yanagisawa, A.; Noritake, Y.; Nomura, N.; Yamamoto, H. Synlett 1991, 251. doi:10.1055/s-1991-20696
Return to citation in text: [1] -
Yanagisawa, A.; Nomura, N.; Yamamoto, H. Synlett 1993, 689. doi:10.1055/s-1993-22573
Return to citation in text: [1] -
Torneiro, M.; Fall, Y.; Castedo, L.; Mouriño, A. J. Org. Chem. 1997, 62, 6344. doi:10.1021/jo970604u
Return to citation in text: [1] -
Nantz, M. H.; Bender, D. M.; Janaki, S. Synthesis 1993, 577. doi:10.1055/s-1993-25909
Return to citation in text: [1] [2] -
Montury, M.; Goré, J. Synth. Commun. 1980, 10, 873. doi:10.1080/00397918008062772
Return to citation in text: [1] -
Elsevier, C. J.; Vermeer, P.; Gedanken, A.; Runge, W. J. Org. Chem. 1985, 50, 364. doi:10.1021/jo00203a015
Return to citation in text: [1] -
Boukouvalas, J.; Pouliot, M.; Robichaud, J.; MacNeil, S.; Snieckus, V. Org. Lett. 2006, 8, 3597. doi:10.1021/ol061385e
Return to citation in text: [1] -
Jin, J.; Weinreb, S. M. J. Am. Chem. Soc. 1997, 119, 2050. doi:10.1021/ja963900h
Return to citation in text: [1] -
Aoyagi, S.; Hirashima, S.; Saito, K.; Kibayashi, C. J. Org. Chem. 2002, 67, 5517. doi:10.1021/jo0200466
Return to citation in text: [1] -
Mitsunobu, O. Synthesis 1981, 1. doi:10.1055/s-1981-29317
Return to citation in text: [1] [2] -
Ashley, E. R.; Cruz, E.; Stoltz, B. M. J. Am. Chem. Soc. 2003, 125, 15000. doi:10.1021/ja039223q
Return to citation in text: [1] [2] [3] -
Nicolaou, K. C.; Mathison, C. J. N.; Montagnon, T. J. Am. Chem. Soc. 2004, 126, 5192. doi:10.1021/ja0400382
Return to citation in text: [1] -
Hayes, C. J.; Sherlock, A. E.; Selby, M. D. Org. Biomol. Chem. 2006, 4, 193. doi:10.1039/b516311k
Return to citation in text: [1] -
Dess, D. B.; Martin, J. C. J. Org. Chem. 1983, 48, 4155. doi:10.1021/jo00170a070
Return to citation in text: [1] [2] -
Boeckmann, R. K., Jr.; Shao, P.; Mullins, J. Org. Synth. 2004, Coll. Vol. 10, 696.
Return to citation in text: [1] [2] -
Bal, B. S.; Childers, W. E., Jr.; Pinnick, H. W. Tetrahedron 1981, 37, 2091. doi:10.1016/S0040-4020(01)97963-3
Return to citation in text: [1] [2] -
Kiso, Y.; Ukawa, K.; Akita, T. J. Chem. Soc., Chem. Commun. 1980, 101. doi:10.1039/C39800000101
Return to citation in text: [1] [2] -
Muratake, H.; Natsume, M.; Nakai, H. Tetrahedron 2006, 62, 7093. doi:10.1016/j.tet.2006.04.086
Return to citation in text: [1] -
Neumann, H.; Seebach, D. Chem. Ber. 1978, 111, 2785. doi:10.1002/cber.19781110807
Return to citation in text: [1] -
Reginato, G.; Mordini, A.; Degl’Innocenti, A.; Caracciolo, M. Tetrahedron Lett. 1995, 36, 8275. doi:10.1016/0040-4039(95)01725-W
Return to citation in text: [1] -
Reginato, G.; Mordini, A.; Caracciolo, M. J. Org. Chem. 1997, 62, 6187. doi:10.1021/jo970619s
Return to citation in text: [1] -
Govek, S. P.; Overman, L. E. Tetrahedron 2007, 63, 8499. doi:10.1016/j.tet.2007.05.127
Return to citation in text: [1]
| 38. | Morita, N.; Krause, N. Org. Lett. 2004, 6, 4121. doi:10.1021/ol0481838 |
| 39. | Morita, N.; Krause, N. Eur. J. Org. Chem. 2006, 4634. doi:10.1002/ejoc.200600438 |
| 62. | Mitsunobu, O. Synthesis 1981, 1. doi:10.1055/s-1981-29317 |
| 21. | Hoffmann-Röder, A.; Krause, N. Org. Lett. 2001, 3, 2537. doi:10.1021/ol016205+ |
| 22. | Krause, N.; Hoffmann-Röder, A.; Canisius, J. Synthesis 2002, 1759. doi:10.1055/s-2002-33707 |
| 23. | Deutsch, C.; Gockel, B.; Hoffmann-Röder, A.; Krause, N. Synlett 2007, 1790. doi:10.1055/s-2007-982561 |
| 68. | Bal, B. S.; Childers, W. E., Jr.; Pinnick, H. W. Tetrahedron 1981, 37, 2091. doi:10.1016/S0040-4020(01)97963-3 |
| 64. | Nicolaou, K. C.; Mathison, C. J. N.; Montagnon, T. J. Am. Chem. Soc. 2004, 126, 5192. doi:10.1021/ja0400382 |
| 65. | Hayes, C. J.; Sherlock, A. E.; Selby, M. D. Org. Biomol. Chem. 2006, 4, 193. doi:10.1039/b516311k |
| 66. | Dess, D. B.; Martin, J. C. J. Org. Chem. 1983, 48, 4155. doi:10.1021/jo00170a070 |
| 67. | Boeckmann, R. K., Jr.; Shao, P.; Mullins, J. Org. Synth. 2004, Coll. Vol. 10, 696. |
| 63. | Ashley, E. R.; Cruz, E.; Stoltz, B. M. J. Am. Chem. Soc. 2003, 125, 15000. doi:10.1021/ja039223q |
| 63. | Ashley, E. R.; Cruz, E.; Stoltz, B. M. J. Am. Chem. Soc. 2003, 125, 15000. doi:10.1021/ja039223q |
| 38. | Morita, N.; Krause, N. Org. Lett. 2004, 6, 4121. doi:10.1021/ol0481838 |
| 39. | Morita, N.; Krause, N. Eur. J. Org. Chem. 2006, 4634. doi:10.1002/ejoc.200600438 |
| 38. | Morita, N.; Krause, N. Org. Lett. 2004, 6, 4121. doi:10.1021/ol0481838 |
| 39. | Morita, N.; Krause, N. Eur. J. Org. Chem. 2006, 4634. doi:10.1002/ejoc.200600438 |
| 69. | Kiso, Y.; Ukawa, K.; Akita, T. J. Chem. Soc., Chem. Commun. 1980, 101. doi:10.1039/C39800000101 |
| 42. | Hassall, C. H.; Thomas, J. O. J. Chem. Soc. C 1968, 1495. |
| 43. | Chhabra, S. R.; Mahajan, A.; Chan, W. C. J. Org. Chem. 2002, 67, 4017. doi:10.1021/jo010456e |
| 44. | McKillop, A.; Taylor, R. J. K.; Watson, R. J.; Lewis, N. Synthesis 1994, 31. doi:10.1055/s-1994-25398 |
| 45. | Tsou, H.-R.; Mamuya, N.; Johnson, B. D.; Reich, M. F.; Gruber, B. C.; Ye, F.; Nilakantan, R.; Shen, R.; Discafani, C.; DeBlanc, R.; Davis, R.; Koehn, F. E.; Greenberger, L. M.; Wang, Y.-F.; Wissner, A. J. Med. Chem. 2001, 44, 2719. doi:10.1021/jm0005555 |
| 19. | Erdsack, J.; Krause, N. Synthesis 2007, 3741. doi:10.1055/s-2007-990860 |
| 66. | Dess, D. B.; Martin, J. C. J. Org. Chem. 1983, 48, 4155. doi:10.1021/jo00170a070 |
| 67. | Boeckmann, R. K., Jr.; Shao, P.; Mullins, J. Org. Synth. 2004, Coll. Vol. 10, 696. |
| 68. | Bal, B. S.; Childers, W. E., Jr.; Pinnick, H. W. Tetrahedron 1981, 37, 2091. doi:10.1016/S0040-4020(01)97963-3 |
| 69. | Kiso, Y.; Ukawa, K.; Akita, T. J. Chem. Soc., Chem. Commun. 1980, 101. doi:10.1039/C39800000101 |
| 71. | Neumann, H.; Seebach, D. Chem. Ber. 1978, 111, 2785. doi:10.1002/cber.19781110807 |
| 19. | Erdsack, J.; Krause, N. Synthesis 2007, 3741. doi:10.1055/s-2007-990860 |
| 72. | Reginato, G.; Mordini, A.; Degl’Innocenti, A.; Caracciolo, M. Tetrahedron Lett. 1995, 36, 8275. doi:10.1016/0040-4039(95)01725-W |
| 73. | Reginato, G.; Mordini, A.; Caracciolo, M. J. Org. Chem. 1997, 62, 6187. doi:10.1021/jo970619s |
| 74. | Govek, S. P.; Overman, L. E. Tetrahedron 2007, 63, 8499. doi:10.1016/j.tet.2007.05.127 |
| 63. | Ashley, E. R.; Cruz, E.; Stoltz, B. M. J. Am. Chem. Soc. 2003, 125, 15000. doi:10.1021/ja039223q |
| 70. | Muratake, H.; Natsume, M.; Nakai, H. Tetrahedron 2006, 62, 7093. doi:10.1016/j.tet.2006.04.086 |
| 7. | VanBrunt, M. P.; Standaert, R. F. Org. Lett. 2000, 2, 705. doi:10.1021/ol005569j |
| 46. | Herold, P. Helv. Chim. Acta 1988, 71, 354. doi:10.1002/hlca.19880710208 |
| 47. | Gruza, H.; Kiciak, K.; Krasiński, A.; Jurczak, J. Tetrahedron: Asymmetry 1997, 8, 2627. doi:10.1016/S0957-4166(97)00306-6 |
| 38. | Morita, N.; Krause, N. Org. Lett. 2004, 6, 4121. doi:10.1021/ol0481838 |
| 39. | Morita, N.; Krause, N. Eur. J. Org. Chem. 2006, 4634. doi:10.1002/ejoc.200600438 |
| 62. | Mitsunobu, O. Synthesis 1981, 1. doi:10.1055/s-1981-29317 |
| 1. | Katagiri, K.; Tori, K.; Kimura, Y.; Yoshida, T.; Nagasaki, T.; Minato, H. J. Med. Chem. 1967, 10, 1149. doi:10.1021/jm00318a035 |
| 2. | Semple, J. E.; Wang, P. C.; Lysenko, Z.; Joullié, M. M. J. Am. Chem. Soc. 1980, 102, 7505. doi:10.1021/ja00545a018 |
| 5. | Kang, S. H.; Lee, S. B. Chem. Commun. 1998, 761. doi:10.1039/a800727f |
| 6. | Zhang, J. H.; Clive, D. L. J. J. Org. Chem. 1999, 64, 1754. doi:10.1021/jo982205k |
| 7. | VanBrunt, M. P.; Standaert, R. F. Org. Lett. 2000, 2, 705. doi:10.1021/ol005569j |
| 8. | Zimmermann, P. J.; Blanarikova, J.; Jäger, V. Angew. Chem., Int. Ed. 2000, 39, 910. doi:10.1002/(SICI)1521-3773(20000303)39:5<910::AID-ANIE910>3.0.CO;2-9 |
| 4. | von Nussbaum, F.; Brands, M.; Hinzen, B.; Weigand, S.; Häbich, D. Angew. Chem., Int. Ed. 2006, 45, 5072. doi:10.1002/anie.200600350 |
| 43. | Chhabra, S. R.; Mahajan, A.; Chan, W. C. J. Org. Chem. 2002, 67, 4017. doi:10.1021/jo010456e |
| 3. | Shiro, M.; Nakai, H.; Tori, K.; Nishikawa, J.; Yashimura, Y.; Katagiri, K. J. Chem. Soc., Chem. Commun. 1980, 375a. doi:10.1039/C3980000375A |
| 2. | Semple, J. E.; Wang, P. C.; Lysenko, Z.; Joullié, M. M. J. Am. Chem. Soc. 1980, 102, 7505. doi:10.1021/ja00545a018 |
| 40. | Garner, P. Tetrahedron Lett. 1984, 25, 5855. doi:10.1016/S0040-4039(01)81703-2 |
| 41. | Garner, P.; Park, J. M. J. Org. Chem. 1987, 52, 2361. doi:10.1021/jo00388a004 |
| 29. | Krause, N.; Belting, V.; Deutsch, C.; Erdsack, J.; Fan, H.-T.; Gockel, B.; Hoffmann-Röder, A.; Morita, N.; Volz, F. Pure Appl. Chem. 2008, 80, 1063. doi:10.1351/pac200880051063 |
| 30. | Krause, N.; Aksin-Artok, Ö.; Breker, V.; Deutsch, C.; Gockel, B.; Poonoth, M.; Sawama, Y.; Sawama, Y.; Sun, T.; Winter, C. Pure Appl. Chem. 2010, 82, 1529. doi:10.1351/PAC-CON-09-09-23 |
| 31. | Krause, N.; Winter, C. Chem. Rev. 2011, 111, 1994. doi:10.1021/cr1004088 |
| 32. | Krause, N.; Aksin-Artok, Ö.; Asikainen, M.; Breker, V.; Deutsch, C.; Erdsack, J.; Fan, H.-T.; Gockel, B.; Minkler, S.; Poonoth, M.; Sawama, Y.; Sawama, Y.; Sun, T.; Volz, F.; Winter, C. J. Organomet. Chem. 2012, 704, 1. doi:10.1016/j.jorganchem.2012.01.008 |
| 33. | Volz, F.; Krause, N. Org. Biomol. Chem. 2007, 5, 1519. doi:10.1039/b703995f |
| 34. | Volz, F.; Wadman, S. H.; Hoffmann-Röder, A.; Krause, N. Tetrahedron 2009, 65, 1902. doi:10.1016/j.tet.2008.11.104 |
| 35. | Miura, T.; Shimada, M.; de Mendoza, P.; Deutsch, C.; Krause, N.; Murakami, M. J. Org. Chem. 2009, 74, 6050. doi:10.1021/jo900987w |
| 36. | Sun, T.; Deutsch, C.; Krause, N. Org. Biomol. Chem. 2012, 10, 5965. doi:10.1039/c2ob25069a |
| 37. | Gao, Z.; Li, Y.; Cooksey, J. P.; Snaddon, T. N.; Schunk, S.; Viseux, E. M. E.; McAteer, S. M.; Kocienski, P. J. Angew. Chem., Int. Ed. 2009, 48, 5022. doi:10.1002/anie.200901608 |
| 17. | Lee, J. Y.; Schiffer, G.; Jäger, V. Org. Lett. 2005, 7, 2317. doi:10.1021/ol0504493 |
| 29. | Krause, N.; Belting, V.; Deutsch, C.; Erdsack, J.; Fan, H.-T.; Gockel, B.; Hoffmann-Röder, A.; Morita, N.; Volz, F. Pure Appl. Chem. 2008, 80, 1063. doi:10.1351/pac200880051063 |
| 30. | Krause, N.; Aksin-Artok, Ö.; Breker, V.; Deutsch, C.; Gockel, B.; Poonoth, M.; Sawama, Y.; Sawama, Y.; Sun, T.; Winter, C. Pure Appl. Chem. 2010, 82, 1529. doi:10.1351/PAC-CON-09-09-23 |
| 31. | Krause, N.; Winter, C. Chem. Rev. 2011, 111, 1994. doi:10.1021/cr1004088 |
| 32. | Krause, N.; Aksin-Artok, Ö.; Asikainen, M.; Breker, V.; Deutsch, C.; Erdsack, J.; Fan, H.-T.; Gockel, B.; Minkler, S.; Poonoth, M.; Sawama, Y.; Sawama, Y.; Sun, T.; Volz, F.; Winter, C. J. Organomet. Chem. 2012, 704, 1. doi:10.1016/j.jorganchem.2012.01.008 |
| 38. | Morita, N.; Krause, N. Org. Lett. 2004, 6, 4121. doi:10.1021/ol0481838 |
| 39. | Morita, N.; Krause, N. Eur. J. Org. Chem. 2006, 4634. doi:10.1002/ejoc.200600438 |
| 14. | Kazmaier, U.; Pähler, S.; Endermann, R.; Häbich, D.; Kroll, H.-P.; Riedl, B. Bioorg. Med. Chem. 2002, 10, 3905. doi:10.1016/S0968-0896(02)00317-6 |
| 16. | Zimmermann, P. J.; Lee, J. Y.; Hlobilova (neé Blanarikova), I.; Endermann, R.; Häbich, D.; Jäger, V. Eur. J. Org. Chem. 2005, 3450. doi:10.1002/ejoc.200500148 |
| 9. | Masamune, T.; Ono, M. Chem. Lett. 1975, 625. doi:10.1246/cl.1975.625 |
| 10. | Divanfard, H. R.; Lysenko, Z.; Semple, J. E.; Wang, P.-C.; Joullié, M. M. Heterocycles 1981, 16, 1975. doi:10.3987/R-1981-11-1975 |
| 11. | Robins, M. J.; Parker, J. M. R. Can. J. Chem. 1983, 61, 317. doi:10.1139/v83-057 |
| 12. | Wiliams, R. M.; Sinclair, P. J.; Zhai, D.; Chen, D. J. Am. Chem. Soc. 1988, 110, 1547. doi:10.1021/ja00213a031 |
| 13. | Braithwaite, D. H.; Holzapfel, C. W.; Wiliams, D. B. G. J. Chem. Res., Synop. 1999, 108. doi:10.1039/a807128d |
| 14. | Kazmaier, U.; Pähler, S.; Endermann, R.; Häbich, D.; Kroll, H.-P.; Riedl, B. Bioorg. Med. Chem. 2002, 10, 3905. doi:10.1016/S0968-0896(02)00317-6 |
| 15. | Chattopadhyay, S. K.; Sarkar, K.; Karmakar, S. Synlett 2005, 2083. doi:10.1055/s-2005-871944 |
| 16. | Zimmermann, P. J.; Lee, J. Y.; Hlobilova (neé Blanarikova), I.; Endermann, R.; Häbich, D.; Jäger, V. Eur. J. Org. Chem. 2005, 3450. doi:10.1002/ejoc.200500148 |
| 17. | Lee, J. Y.; Schiffer, G.; Jäger, V. Org. Lett. 2005, 7, 2317. doi:10.1021/ol0504493 |
| 18. | Jirgensons, A.; Marinozzi, M.; Pellicciari, R. Tetrahedron 2005, 61, 373. doi:10.1016/j.tet.2004.10.091 |
| 19. | Erdsack, J.; Krause, N. Synthesis 2007, 3741. doi:10.1055/s-2007-990860 |
| 20. | Avenoza, A.; Busto, J. H.; Canal, N.; Corzana, F.; Peregrina, J. M.; Pérez-Fernández, M.; Rodríguez, F. J. Org. Chem. 2010, 75, 545. doi:10.1021/jo9025258 |
| 21. | Hoffmann-Röder, A.; Krause, N. Org. Lett. 2001, 3, 2537. doi:10.1021/ol016205+ |
| 22. | Krause, N.; Hoffmann-Röder, A.; Canisius, J. Synthesis 2002, 1759. doi:10.1055/s-2002-33707 |
| 23. | Deutsch, C.; Gockel, B.; Hoffmann-Röder, A.; Krause, N. Synlett 2007, 1790. doi:10.1055/s-2007-982561 |
| 24. | Aksιn, Ö.; Krause, N. Adv. Synth. Catal. 2008, 350, 1106. doi:10.1002/adsc.200800050 |
| 25. | Winter, C.; Krause, N. Green Chem. 2009, 11, 1309. doi:10.1039/b905823k |
| 26. | Asikainen, M.; Krause, N. Adv. Synth. Catal. 2009, 351, 2305. doi:10.1002/adsc.200900434 |
| 27. | Aksın-Artok, Ö.; Krause, N. Adv. Synth. Catal. 2011, 353, 385. doi:10.1002/adsc.201000903 |
| 28. | Minkler, S. R. K.; Lipshutz, B. H.; Krause, N. Angew. Chem., Int. Ed. 2011, 50, 7820. doi:10.1002/anie.201101396 |
| 29. | Krause, N.; Belting, V.; Deutsch, C.; Erdsack, J.; Fan, H.-T.; Gockel, B.; Hoffmann-Röder, A.; Morita, N.; Volz, F. Pure Appl. Chem. 2008, 80, 1063. doi:10.1351/pac200880051063 |
| 30. | Krause, N.; Aksin-Artok, Ö.; Breker, V.; Deutsch, C.; Gockel, B.; Poonoth, M.; Sawama, Y.; Sawama, Y.; Sun, T.; Winter, C. Pure Appl. Chem. 2010, 82, 1529. doi:10.1351/PAC-CON-09-09-23 |
| 31. | Krause, N.; Winter, C. Chem. Rev. 2011, 111, 1994. doi:10.1021/cr1004088 |
| 32. | Krause, N.; Aksin-Artok, Ö.; Asikainen, M.; Breker, V.; Deutsch, C.; Erdsack, J.; Fan, H.-T.; Gockel, B.; Minkler, S.; Poonoth, M.; Sawama, Y.; Sawama, Y.; Sun, T.; Volz, F.; Winter, C. J. Organomet. Chem. 2012, 704, 1. doi:10.1016/j.jorganchem.2012.01.008 |
| 46. | Herold, P. Helv. Chim. Acta 1988, 71, 354. doi:10.1002/hlca.19880710208 |
| 47. | Gruza, H.; Kiciak, K.; Krasiński, A.; Jurczak, J. Tetrahedron: Asymmetry 1997, 8, 2627. doi:10.1016/S0957-4166(97)00306-6 |
| 48. | Chun, J.; Byun, H.-S.; Bitman, R. J. Org. Chem. 2003, 68, 348. doi:10.1021/jo026240+ |
| 49. | Masuda, Y.; Mori, K. Eur. J. Org. Chem. 2005, 4789. doi:10.1002/ejoc.200500357 |
| 50. | Erdsack, J.; Schürmann, M.; Preut, H.; Krause, N. Acta Crystallogr., Sect. E: Struct. Rep. Online 2008, E64, o1171. doi:10.1107/S1600536808014906 |
| 44. | McKillop, A.; Taylor, R. J. K.; Watson, R. J.; Lewis, N. Synthesis 1994, 31. doi:10.1055/s-1994-25398 |
| 45. | Tsou, H.-R.; Mamuya, N.; Johnson, B. D.; Reich, M. F.; Gruber, B. C.; Ye, F.; Nilakantan, R.; Shen, R.; Discafani, C.; DeBlanc, R.; Davis, R.; Koehn, F. E.; Greenberger, L. M.; Wang, Y.-F.; Wissner, A. J. Med. Chem. 2001, 44, 2719. doi:10.1021/jm0005555 |
| 57. | Montury, M.; Goré, J. Synth. Commun. 1980, 10, 873. doi:10.1080/00397918008062772 |
| 58. | Elsevier, C. J.; Vermeer, P.; Gedanken, A.; Runge, W. J. Org. Chem. 1985, 50, 364. doi:10.1021/jo00203a015 |
| 59. | Boukouvalas, J.; Pouliot, M.; Robichaud, J.; MacNeil, S.; Snieckus, V. Org. Lett. 2006, 8, 3597. doi:10.1021/ol061385e |
| 60. | Jin, J.; Weinreb, S. M. J. Am. Chem. Soc. 1997, 119, 2050. doi:10.1021/ja963900h |
| 61. | Aoyagi, S.; Hirashima, S.; Saito, K.; Kibayashi, C. J. Org. Chem. 2002, 67, 5517. doi:10.1021/jo0200466 |
| 51. |
Krause, N.; Hoffmann-Röder, A. Tetrahedron 2004, 60, 11671. doi:10.1016/j.tet.2004.09.094
Review. |
| 56. | Nantz, M. H.; Bender, D. M.; Janaki, S. Synthesis 1993, 577. doi:10.1055/s-1993-25909 |
| 51. |
Krause, N.; Hoffmann-Röder, A. Tetrahedron 2004, 60, 11671. doi:10.1016/j.tet.2004.09.094
Review. |
| 53. | Yanagisawa, A.; Noritake, Y.; Nomura, N.; Yamamoto, H. Synlett 1991, 251. doi:10.1055/s-1991-20696 |
| 54. | Yanagisawa, A.; Nomura, N.; Yamamoto, H. Synlett 1993, 689. doi:10.1055/s-1993-22573 |
| 55. | Torneiro, M.; Fall, Y.; Castedo, L.; Mouriño, A. J. Org. Chem. 1997, 62, 6344. doi:10.1021/jo970604u |
| 56. | Nantz, M. H.; Bender, D. M.; Janaki, S. Synthesis 1993, 577. doi:10.1055/s-1993-25909 |
| 51. |
Krause, N.; Hoffmann-Röder, A. Tetrahedron 2004, 60, 11671. doi:10.1016/j.tet.2004.09.094
Review. |
| 52. | Elsevier, C. J.; Vermeer, P. J. Org. Chem. 1989, 54, 3726. doi:10.1021/jo00276a040 |
© 2013 Erdsack and Krause; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)