Abstract
The synthesis of oligosaccharides is still a challenging task as there is no universal glycosyl donor for the synthesis of all oligosaccharides. The gold catalysis for glycosidation reactions, in which alkynylated glycosides are used, has emerged as one of the versatile options in this regard. A cleavage of the interglycosidic bond that was thought to be due to the higher reaction temperature and the acidic medium was observed during the synthesis of trisaccharides. In addition, a very little percentage of deprotection of benzyl protecting groups at the C-6 position was observed and no deprotection of benzyl ethers in aliphatic molecules was noticed. In order to overcome this fact, a collection of leaving groups that contain an alkynyl moiety were screened. It was found that 1-ethynylcyclohexanyl (Ech) glycosides are suitable for carrying out the glycosidation at 25 °C in the presence of 5 mol % each of AuCl3 and AgSbF6. Subsequently, Ech-glycosides were observed to be suitable for the synthesis of trisaccharides under gold catalysis conditions.
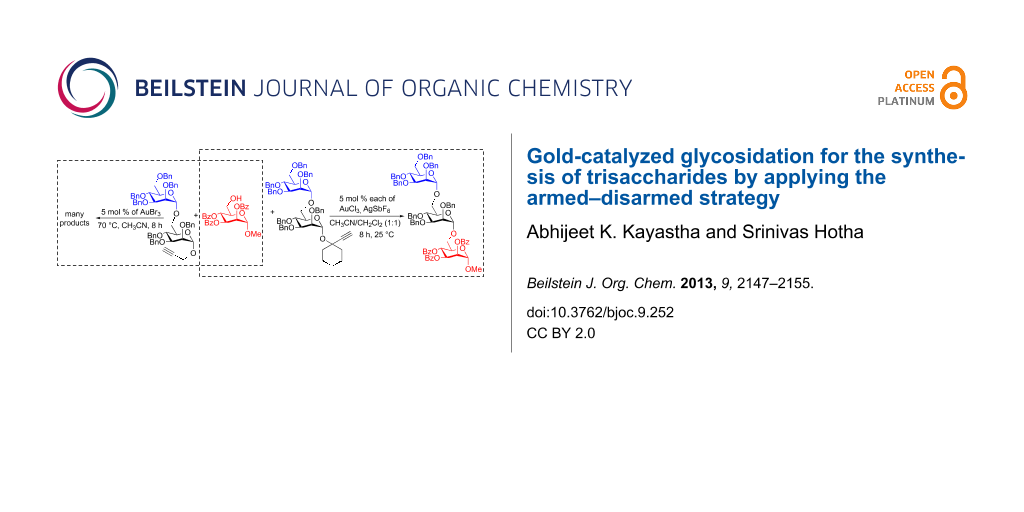
Graphical Abstract
Introduction
Observations that gold(III) has a great affinity for alkynes placed the chemistry of gold in an enviable situation that culminated into the total synthesis of several natural products, in which gold-mediated reactions are a key step [1-7]. Over the last two decades, chemistry with gold complexes has gained immense significance and thus been investigated for a variety of organic transformations in homogeneous and heterogeneous reaction media [8-14]. The use of gold catalysts in carbohydrate chemistry was first reported for the oxidation of alcohols [11-16]. However, until recently these catalysts were scarcely applied. Glycosidation is one of the key reactions in chemistry of carbohydrates, in which a nucleophile attaches to a saccharide to form a glycoside. In this process, the saccharide unit that is donating its glycon is called a glycosyl donor, whereas the saccharide that is accepting the glycon is referred to as glycosyl acceptor or aglycon. The synthesis of oligosaccharides is still a formidable task in spite of the development of various methods. There is still no universal glycosyl donor [17,18], although the first glycoside was reported by Emil Fischer more than a century ago.
A series of observations in our laboratory led to the identification of a gold(III)-catalyzed glycosidation reaction that uses alkynyl glycosides as glycosyl donors [19-21]. The salient features of this glycosidation reaction are the requirement of a catalytic amount of gold salts, good reaction yields and mild reaction conditions [22]. The alkynophilicity of gold(III) salts has been found to be beneficial for the synthesis of 1,2-trans-glycosides [23], amino acid glycoconjugates [24], carbohydrate epitopes present on the cell surface of infectious bacteria [25], glycopolypeptides [26], glycopolyacrylates [27], and glycomimetics [28]. The remarkable reactivity and chemoselectivity have also attracted other groups to investigate gold catalysts for glycosidation [29-34].
Esters at the C-2 position of the saccharide are known to impede the glycoside formation whereas ethers (–OBn) facilitate the reaction. Fraser-Reid applied the terms disarmed to deactivated glycosyl donors [e.g., esters], and armed to the activated donors [e.g., ethers] [35,36]. During the synthesis of oligosaccharides by sequentially adding saccharides, armed–disarmed effects can effectively be utilized to tune the reactivity of the glycosyl donors by placing appropriate protecting groups at the C-2 position. Similar armed and disarmed effects were noticed during several gold-catalyzed glycosidations [22-28]. Propargyl mannopyranosides as glycosyl donors are ideal for investigating armed–disarmed strategies for the synthesis of oligosaccharides, because the gold-catalyzed glycosidation proceeds in a highly 1,2-trans diastereoselective fashion [22]. Accordingly, the armed mannosyl donor 1 was allowed to react with the disarmed aglycon 2, under the standard conditions for a gold-catalyzed glycosidation (AuBr3, CH3CN, 70 °C), to observe the formation of disaccharide 3, in which the propargyl substitution is disarmed due to the presence of benzoates. Subsequently, the disarmed disaccharide 3 was transformed into an armed glycosyl donor 4 by simple saponification followed by etherification. The reaction between armed donor 4 and disarmed aglycon 2, which was carried out under the aforementioned conditions did not result in the formation of desired trisaccharide. Instead, disaccharide 3 (53%) and 1,6-anhydro sugar 5 (20%) were isolated as major products [37]. Interestingly, propargyl mannoside 1 (12%) along with benzyl glycoside 6 and lactol 7 were noticed in 5% and 4% yield, respectively (Scheme 1).
Scheme 1: Gold-catalyzed synthesis of a disaccharide.
Scheme 1: Gold-catalyzed synthesis of a disaccharide.
The Brønsted acid (HBr) released from AuBr3 in the presence of the aglycon can protonate the exocyclic oxygen present in the disaccharide 4. The protonation of the exocyclic oxygen and subsequent cleavage could give rise to oxocarbenium ion intermediates A and B as shown in Scheme 2. The formation of 1,6-anhydro sugar 5 can be easily envisioned by the intramolecular attack of C-6-OH on the intermediate A. The surprising cleavage of the interglycosidic linkage leads to intermediate B, which can be trapped by various nucleophiles that are present in the reaction mixture. The trapping of the intermediate B by propargyl alcohol gives propargyl mannoside 1 (12%), the addition of OH− due to moisture results in lactol 7 (4%), the addition of aglycon 2 gives rise to disaccharide 3. The formation of benzyl mannoside 6 (5%) can be explained by the attack of BnO− on the intermediate B. The presence of BnO− could be explained due to the hydrolysis of the primary benzyl ether.
Scheme 2: Mechanistic rationale for the cleavage of the interglycosidic linkage.
Scheme 2: Mechanistic rationale for the cleavage of the interglycosidic linkage.
Results and Discussion
In order to further understand the cleavage of the C-6 benzyl ether, the model propargyl mannoside 8 was treated with 5 mol % of AuBr3 under aforementioned conditions. LC–MS analysis of the reaction mixture showed the formation of anhydro sugar 4 (13%), p-methylbenzyl mannoside 9 (9%) and lactol 10 (6%), which indicated the hydrolysis of the primary benzyl ether. The gold-catalyzed hydrolysis of benzyl ethers was not observed in the case of non-carbohydrate benzyl ether 11 (Scheme 3). For example, per-O-benzylated glycerol 11 did not show any benzyl deprotection, whereas the more acid-sensitive p-methoxybenzyl derivative 12 underwent deprotection of the p-methoxybenzyl moiety to give alcohol 13 with 88% yield. The deprotection of the p-methoxybenzyl moiety can be utilized for the one-pot synthesis of glycerol mannosides from mannosyl donor 1 and compound 12 in 67% yield under gold-catalysis conditions (Scheme 3). Importantly, the hydrolysis of benzyl ethers was not observed when the gold catalysis reactions were performed at room temperature [23-28,38].
Scheme 3: C-6 Benzyl ether hydrolysis and synthesis of a glycerol mannoside.
Scheme 3: C-6 Benzyl ether hydrolysis and synthesis of a glycerol mannoside.
From the above observations, the high temperature (70 °C) of the glycosidation and the oxophilicity of gold salts were observed to be major impediments for the synthesis of oligosaccharides. In order to overcome this problem, a systematic investigation of various leaving groups that bear an alkynyl moiety was carried out. The aim was to find a better leaving group, which would facilitate the glycosidation at ambient temperature. Accordingly, a panel of alkynylated glycosyl donors (15a–j) was synthesized and subjected to the glycosidation with three widely available gold salts, namely AuBr3, AuCl3 and HAuCl4, at 25 °C for 12 h in acetonitirile (Table 1). Substitutions at the terminal alkyne carbon (15b,e) were not tolerated. The gem-dimethyl alkyne 15g showed a substantial improvement of the performance at 25 °C compared to the other alkynyl donors 15a–f (Table 1). However, the gem-dimethyl donor 15g was not preferred due to the shorter shelf life. The alicyclic derivatives 15h–j gave comparable yields to 15g and were observed to be much more stable. Furthermore, 15h needs to be prepared from cyclohexanone, while 15i is costly compared to 15j. Thus, further studies were performed with 15j only. The alkyne moiety is really essential for the transglycosylation reaction as only very little formation of the desired product was noticed in the case of the donors 15k and 15l. Subsequently, it was found that the addition of 5 mol % of AgSbF6 along with AuCl3 would increase the yield of disaccharide 17 to 96%. However, the disaccharide formation was not observed with AgSbF6 alone [38].
Table 1: Room temperature activation.
|
|
|||||||
| LG | % yield of disaccharide 17 with catalyst | LG | % yield of disaccharide 17 with catalyst | ||||
|---|---|---|---|---|---|---|---|
| AuCl3 | AuBr3 | HAuCl4 | AuCl3 | AuBr3 | HAuCl4 | ||
|
15a |
8 | 15 | 0 |
15g |
30 | 8 | 10 |
|
15b |
10 | 15 | 9 |
15h |
30 | 13 | 16 |
|
15c |
17 | 0 | 8 |
15i |
23 | 8 | 14 |
|
15d |
20 | 5 | 0 |
15j |
32 | 16 | 15 |
|
15e |
12 | 0 | 5 |
15k |
7 | 3 | 5 |
|
15f |
0 | 0 | 15 |
15l |
2 | 0 | 2 |
In addition, armed mannosyl donor 15j reacted with aglycon 19 in the presence of 5 mol % each of AuCl3/AgSbF6 in CH3CN/CH2Cl2 (1:1) at 25 °C for 4 h to give 1,2-trans menthyl mannoside 20. The leaving group 21 could be removed easily by applying high vacuum. Disarmed donors 18a and 18b failed to react with menthol (19) under aforementioned modified gold-catalysis conditions (Scheme 4).
Scheme 4: Armed–disarmed effect in Ech-glycosides during gold-catalyzed reactions.
Scheme 4: Armed–disarmed effect in Ech-glycosides during gold-catalyzed reactions.
The strong armed–disarmed effects that were observed for the Ech-donors at 25 °C encouraged us to continue the use of the armed–disarmed strategy for the trisaccharide synthesis. Accordingly, the armed mannosyldonor 15j was allowed to react with disarmed aglycon 22 in the presence of AuCl3 (5 mol %)/AgSbF6 (5 mol %) in CH3CN/CH2Cl2 (1:1) at 25 °C for 4 h to obtain the disarmed disaccharide 23 in 85% yield. Further, the armed disaccharide 24 was synthesized from 23 by saponification followed by the etherification in 84% over two steps. The glycosylation between disaccharide 24 and disarmed aglycon 16 was performed under aforementioned conditions for a gold-catalyzed transglycosidation. Purification by conventional silica gel column chromatography enabled us to characterize the anticipated trisaccharide 25 (21%) along with disaccharide 17 and anhydro sugar 5 (Scheme 5). In trisaccharide 25, three anomeric protons were noticed at δ 4.88 (d, J = 1.6 Hz, 1H), 4.91 (d, J = 1.6 Hz, 1H), 5.61 (dd, J = 1.6, 3.2 Hz, 1H) ppm. The 13C NMR spectrum revealed that there are three mannose residues with 1,2-trans configuration as their anomeric carbon atoms were noticed at δ 98.1, 98.2 and 98.5 ppm and the molecular weight was found to be 1483.586 ([M + 23]+ for the Na adduct). The rest of the resonances in the spectrum were completely in agreement with the assigned structure of trisaccharide 25. Formation of disaccharide 17 (34%) and anhydro sugar 5 (16%) can be rationalized on the basis of an interglycosidic bond cleavage.
Scheme 5: Gold-catalyzed glycosidation at ambient temperature for the synthesis of trimannoside 25.
Scheme 5: Gold-catalyzed glycosidation at ambient temperature for the synthesis of trimannoside 25.
The hydrolysis of the interglycosidic bond during the gold-catalyzed transglycosidation reaction depends on the nature of interglycosidic linkage. Generally the glycosyl donors with axial hydroxy groups are considered to be more reactive than the glycosyl donors without axial hydroxy group. For example, β-D-glucose is less reactive than α-D-glucose or α-D-mannose. In order to verify the effect of the differences in reactivity on the cleavage of the interglycosidic bond, armed disaccharide 26 was prepared and allowed to react with menthol (19) under aforementioned conditions for 6 h to obtain the anticipated menthyl glycoside (27) in 32% yield. Similarly, the reactions with 4-penten-1-ol (28) and methyl 2,3,4-tri-O-benzyl α-D-glucopyranoside (30) gave the corresponding transglycosides 29 and 31 in 37% and 23% yield, respectively (Scheme 6).
Scheme 6: Gold-catalyzed glycosidation at ambient temperature for the synthesis of higher saccharides.
Scheme 6: Gold-catalyzed glycosidation at ambient temperature for the synthesis of higher saccharides.
Finally, the gold-catalyzed transglycosidation reaction between disaccharide 32 and aglycon 16 gave the corresponding trisaccharide 33 in 76% yield. A cleavage of the interglycosidic bond was not observed, which shows the importance of the protecting groups in gold-catalyzed glycosidation reactions (Scheme 7).
Scheme 7: Gold-catalyzed glycosidation for synthesis of higher saccharides.
Scheme 7: Gold-catalyzed glycosidation for synthesis of higher saccharides.
Conclusion
In conclusion, the armed–disarmed effect in propargyl glycosides in the presence of a catalytic amount of gold salts is studied. The high temperature of the glycosidation was found to be partially responsible for the cleavage of the interglycosidic bond along with side reactions like benzyl deprotection. These observations were then successfully applied for PMB deprotection and one-pot glycosidation. Subsequent experiments proved the significance of the alkyne moiety. It was also observed that the addition of the silver salt AgSbF6 during the gold-mediated transglycosidation reaction helps in reducing the reaction temperature to 25 °C. This was successfully utilized for activating 1-ethynylcyclohexanyl donors at 25 °C. Trisaccharides were synthesized under identified conditions in moderate yields.
Experimental
All the reactions were performed under argon atmosphere. Products obtained as solids or syrups were dried under high vacuum. Gold and silver salts were purchased from Sigma-Aldrich. Analytical thin-layer chromatography was performed on pre-coated Merck silica plates (F254, 0.25 mm thickness); compounds were visualized by UV light or by staining with anisaldehyde spray. Optical rotations were measured on a JASCO P-1020 or Rudolph polarimeter. NMR spectra were recorded either on a Bruker AC 200, AV 400, AV 500 or JEOL ECX 400 or Bruker Avance 500 with CDCl3 as the solvent and tetramethylsilane as internal standard. High resolution mass spectroscopy (HRMS) was performed on ABI–MALDI–TOF using TiO2 as the solid matrix.
Compound characterization data
Characterization data for compound 15j [38]: [α]D25 +28.2 (CHCl3, c 1.00); 1H NMR (200.13 MHz, CDCl3) δ 1.10–2.15 (m, 10H), 2.40 (s, 1H), 3.65–4.12 (m, 6H), 4.59 (s, 2H), 4.60 (ABq, J = 12.6 Hz, 2H), 4.71 (ABq, J = 10.6 Hz, 2H), 4.76 (s, 2H), 5.56 (d, J = 1.8 Hz, 1H), 7.13–7.42 (m, 20H); 13C NMR (50.32 MHz, CDCl3) δ 22.7, 22.7, 25.0, 37.6, 38.2, 69.3, 71.9, 72.1, 72.3, 73.3, 74.1, 75.0, 75.2, 75.2, 75.5, 80.0, 84.6, 94.0, 127.3–128.3, 138.4, 138.5, 138.5, 138.5; HRMS (MALDI–TOF, m/z): [M + Na]+ calcd for C42H46NaO6, 669.3192; found, 669.3173.
Characterization data for compound 25: [α]D25 −10.6 (CHCl3, c 1.00); 1H NMR (500.13 MHz, CDCl3) δ 3.42 (s, 3H), 3.51–3.69 (m, 8H), 3.80 (dd, J = 3.9, 11.6 Hz, 1H), 3.84–3.88 (m, 4H), 3.95 (dt, J = 9.4, 25.7 Hz, 2H), 4.14 (dt, J = 4.2, 9.6 Hz, 1H), 4.35–4.62 (m, 8H), 4.41 (ABq, J = 11.0 Hz, 2H), 4.61 (s, 2H), 4.84 (ABq, J = 11.0 Hz, 2H), 4.88 (d, J = 1.5 Hz, 1H), 4.90 (d, J = 1.5 Hz, 1H), 5.03 (t, J = 10.0 Hz, 1H), 5.06 (d, J = 1.3 Hz, 1H), 5.84 (dd, J = 3.3, 10.2 Hz, 1H), 7.11–7.51 (m, 44H), 7.81–8.08 (m, 6H); 13C NMR (125.76 MHz, CDCl3) δ 55.4, 65.6, 66.6, 69.0, 69.1, 69.8, 70.6, 71.3, 71.7, 71.7, 71.7, 71.7, 72.2, 72.7, 73.2, 74.2, 74.6, 74.8, 74.9, 74.9, 75.0, 79.2, 80.2, 98.1, 98.2, 98.4, 127.2–129.8, 133.1, 133.3, 133.5, 138.3, 138.3, 138.4, 138.4, 138.6, 138.6, 138.7, 165.3, 165.4, 165.5; HRMS (MALDI–TOF, m/z): [M + Na]+ calcd for C89H88NaO19, 1483.5818; found, 1483.5837.
Characterization data for compound 29: [α]D25 18.4 (CHCl3, c 1.00); 1H NMR (399.78 MHz, CDCl3) δ 1.54 (t, J = 7.2 Hz, 2H), 1.59 (s, 2H), 1.99 (m, 2H), 3.26–3.98 (m, 12H), 4.25–5.06 (m, 18H), 5.73 (m, 1H), 7.15–7.37 (m, 35H); 13C NMR (100.53 MHz, CDCl3) δ 28.4, 30.2, 66.8, 68.9, 69.0, 71.3, 72.0, 72.6, 73.4, 74.7, 74.7, 74.9, 74.9, 74.9, 75.0, 75.7, 77.8, 80.2, 82.0, 84.6, 97.7, 104.0, 114.8, 126.9–128.5, 137.9, 138.1, 138.2, 138.3, 138.5, 138.5, 138.6; HRMS (MALDI–TOF, m/z): [M + Na]+ calcd for C66H72NaO11, 1063.4972; found, 1063.4994.
Characterization data for compound 31: [α]D25 +23.1 (CHCl3, c 1.00); 1H NMR (399.78 MHz, CDCl3) δ 3.17 (s, 3H), 3.25–3.94 (m, 17H), 4.11 (dd, J = 1.8, 9.1 Hz, 1H), 4.24–4.96 (m, 23H), 7.04–7.32 (m, 50H); 13C NMR (100.53 MHz, CDCl3) δ 55.0, 65.6, 68.7, 69.0, 69.8, 71.3, 71.8, 72.5, 73.2, 73.4, 73.4, 74.5, 74.6, 74.7, 74.8, 74.9, 74.9, 74.9, 74.9, 77.5, 77.8,79.5, 79.9, 82.0, 82.0, 84.6, 97.6, 98.1, 104.0, 127.3–128.4, 138.1, 138.1, 138.1, 138.2, 138.3, 138.3, 138.5, 138.6, 138.6, 138.7; HRMS (MALDI–TOF, m/z): [M + Na]+ calcd for C89H94NaO16, 1441.6440; found, 1441.6457.
Characterization data for compound 33: [α]D25 −63.8 (CHCl3, c 1.00); 1H NMR (399.78 MHz, CDCl3) δ 1.95 (s, 3H), 1.99 (s, 3H), 2.01 (s, 3H), 2.02 (s, 3H), 3.50–4.21 (m, 13H), 4.33–4.37 (m, 3H), 4.52 (d, J = 11.4 Hz, 1H), 4.58 (s, 2H), 4.90–4.96 (m, 3H), 5.01 (t, J = 9.8 Hz, 1H), 5.03 (t, J = 10.1 Hz, 1H), 5.13 (t, J = 9.4 Hz, 1H), 5.63 (dd, J = 1.8, 3.2 Hz, 1H), 5.84 (dd, J = 3.2, 9.8 Hz, 1H), 7.23–7.54 (m, 24H), 7.81–8.11 (m, 6H); 13C NMR (100.53 MHz, CDCl3) δ 20.5, 20.5, 20.6, 20.6, 55.4, 61.8, 66.6, 67.8, 68.3, 68.7, 69.1, 69.9, 70.5, 71.0, 71.1, 71.5, 71.5, 72.5, 72.8, 74.4, 74.6, 74.8, 80.1, 98.0, 98.4, 100.9, 127.4–129.8, 133.0, 133.3, 133.4, 138.2, 138.3, 138.5, 165.3, 165.4, 165.5, 169.0, 169.4, 170.3, 170.6; HRMS (MALDI–TOF, m/z): [M + Na]+ calcd for C69H72NaO23, 1291.4362; found, 1291.4377.
Supporting Information
| Supporting Information File 1: Detailed experimental data. | ||
| Format: PDF | Size: 1.1 MB | Download |
References
-
Ghosh, N.; Nayak, S.; Sahoo, A. K. J. Org. Chem. 2011, 76, 500–511. doi:10.1021/jo101995g
Return to citation in text: [1] -
Trost, B. M.; Dong, G. Nature 2008, 456, 485–488. doi:10.1038/nature07543
Return to citation in text: [1] -
Trost, B. M.; O’Boyle, B. M.; Hund, D. J. Am. Chem. Soc. 2009, 131, 15061–15074. doi:10.1021/ja906056v
Return to citation in text: [1] -
Fang, C.; Pang, Y.; Forsyth, C. J. Org. Lett. 2010, 12, 4528–4531. doi:10.1021/ol101833h
Return to citation in text: [1] -
Tlais, S. F.; Dudley, G. B. Beilstein J. Org. Chem. 2011, 7, 570–577. doi:10.3762/bjoc.7.66
Return to citation in text: [1] -
Benson, S.; Collin, M.-P.; Arlt, A.; Gabor, B.; Goddard, R.; Fürstner, A. Angew. Chem., Int. Ed. 2011, 50, 8739–8744. doi:10.1002/anie.201103270
Return to citation in text: [1] -
Nakajima, R.; Ogino, T.; Yokoshima, S.; Fukuyama, T. J. Am. Chem. Soc. 2010, 132, 1236–1237. doi:10.1021/ja9103233
Return to citation in text: [1] -
Herrmann, W. A.; Cornils, B. Angew. Chem., Int. Ed. 1997, 36, 1048–1067. doi:10.1002/anie.199710481
Return to citation in text: [1] -
Bond, G. C.; Sermon, P. A.; Webb, G.; Buchanan, D. A.; Wells, P. B. J. Chem. Soc., Chem. Commun. 1973, 444b–445. doi:10.1039/C3973000444B
Return to citation in text: [1] -
Hashmi, A. S. K.; Schwarz, L.; Choi, J.-H.; Frost, T. M. Angew. Chem., Int. Ed. 2000, 39, 2285–2288. doi:10.1002/1521-3773(20000703)39:13<2285::AID-ANIE2285>3.0.CO;2-F
Return to citation in text: [1] -
Haruta, M. Nature 2005, 437, 1098–1099. doi:10.1038/4371098a
Return to citation in text: [1] [2] -
Hutchings, G. J. Catal. Today 2005, 100, 55–61. doi:10.1016/j.cattod.2004.12.016
Return to citation in text: [1] [2] -
Haruta, M.; Kobayashi, T.; Sano, H.; Yamada, N. Chem. Lett. 1987, 16, 405–408. doi:10.1246/cl.1987.405
Return to citation in text: [1] [2] -
Hutchings, G. J. J. Catal. 1985, 96, 292–295. doi:10.1016/0021-9517(85)90383-5
Return to citation in text: [1] [2] -
Prati, L.; Rossi, M. J. Catal. 1998, 176, 552–560. doi:10.1006/jcat.1998.2078
Return to citation in text: [1] -
Biella, S.; Prati, L.; Rossi, M. J. Catal. 2002, 206, 242–247. doi:10.1006/jcat.2001.3497
Return to citation in text: [1] -
Paulsen, H. Angew. Chem. 1982, 94, 184–201. doi:10.1002/ange.19820940304
Return to citation in text: [1] -
Paulsen, H. Angew. Chem., Int. Ed. Engl. 1982, 21, 155–173. doi:10.1002/anie.198201553
Return to citation in text: [1] -
Maurya, S. K.; Hotha, S. Tetrahedron Lett. 2006, 47, 3307–3310. doi:10.1016/j.tetlet.2006.03.016
Return to citation in text: [1] -
Kashyap, S.; Hotha, S. Tetrahedron Lett. 2006, 47, 2021–2023. doi:10.1016/j.tetlet.2006.01.048
Return to citation in text: [1] -
Kashyap, S.; Vidadala, S. R.; Hotha, S. Tetrahedron Lett. 2007, 48, 8960–8962. doi:10.1016/j.tetlet.2007.10.144
Return to citation in text: [1] -
Hotha, S.; Kashyap, S. J. Am. Chem. Soc. 2006, 128, 9620–9621. doi:10.1021/ja062425c
Return to citation in text: [1] [2] [3] -
Sureshkumar, G.; Hotha, S. Tetrahedron Lett. 2007, 48, 6564–6568. doi:10.1016/j.tetlet.2007.07.015
Return to citation in text: [1] [2] [3] -
Shaikh, A. Y.; Sureshkumar, G.; Pati, D.; Gupta, S. S.; Hotha, S. Org. Biomol. Chem. 2011, 9, 5951–5959. doi:10.1039/c1ob05056g
Return to citation in text: [1] [2] [3] -
Sureshkumar, G.; Hotha, S. Glycoconjugate J. 2012, 29, 221–230. doi:10.1007/s10719-012-9400-7
Return to citation in text: [1] [2] [3] -
Pati, D.; Shaikh, A. Y.; Hotha, S.; Gupta, S. S. Polym. Chem. 2011, 2, 805–811. doi:10.1039/c0py00412j
Return to citation in text: [1] [2] [3] -
Thadke, S. A.; Kar, M.; Gupta, S. S.; Hotha, S. Carbohydr. Res. 2011, 346, 1511–1518. doi:10.1016/j.carres.2011.04.018
Return to citation in text: [1] [2] [3] -
Vidadala, S. R.; Pimpalpalle, T. M.; Linker, T.; Hotha, S. Eur. J. Org. Chem. 2011, 2426–2430. doi:10.1002/ejoc.201100134
Return to citation in text: [1] [2] [3] -
Mamidyala, S. K.; Finn, M. G. J. Org. Chem. 2009, 74, 8417–8420. doi:10.1021/jo901857x
Return to citation in text: [1] -
Götze, S.; Fitzner, R.; Kunz, H. Synlett 2009, 3346–3348. doi:10.1055/s-0029-1218356
Return to citation in text: [1] -
Li, Y.; Yang, Y.; Yu, B. Tetrahedron Lett. 2008, 49, 3604–3608. doi:10.1016/j.tetlet.2008.04.017
Return to citation in text: [1] -
Li, Y.; Tang, P.; Chen, Y.; Yu, B. J. Org. Chem. 2008, 73, 4323–4325. doi:10.1021/jo8003875
Return to citation in text: [1] -
Yang, F.; Wang, Q.; Yu, B. Tetrahedron Lett. 2012, 53, 5231–5234. doi:10.1016/j.tetlet.2012.07.059
Return to citation in text: [1] -
Adhikari, S.; Baryal, K. N.; Zhu, D.; Li, X.; Zhu, J. ACS Catal. 2013, 3, 57–60. doi:10.1021/cs300670k
Return to citation in text: [1] -
Fraser-Reid, B.; Wu, Z.; Udodong, U. E.; Ottosso, H. J. Org. Chem. 1990, 55, 6068–6070. doi:10.1021/jo00312a004
Return to citation in text: [1] -
Mootoo, D. R.; Konradsson, P.; Udodong, U.; Fraser-Reid, B. J. Am. Chem. Soc. 1988, 110, 5583–5584. doi:10.1021/ja00224a060
Return to citation in text: [1] -
Kayastha, A. K.; Hotha, S. Tetrahedron Lett. 2010, 51, 5269–5272. doi:10.1016/j.tetlet.2010.07.157
Return to citation in text: [1] -
Kayastha, A. K.; Hotha, S. Chem. Commun. 2012, 48, 7161–7163. doi:10.1039/c2cc32649c
Return to citation in text: [1] [2] [3]
| 38. | Kayastha, A. K.; Hotha, S. Chem. Commun. 2012, 48, 7161–7163. doi:10.1039/c2cc32649c |
| 37. | Kayastha, A. K.; Hotha, S. Tetrahedron Lett. 2010, 51, 5269–5272. doi:10.1016/j.tetlet.2010.07.157 |
| 23. | Sureshkumar, G.; Hotha, S. Tetrahedron Lett. 2007, 48, 6564–6568. doi:10.1016/j.tetlet.2007.07.015 |
| 24. | Shaikh, A. Y.; Sureshkumar, G.; Pati, D.; Gupta, S. S.; Hotha, S. Org. Biomol. Chem. 2011, 9, 5951–5959. doi:10.1039/c1ob05056g |
| 25. | Sureshkumar, G.; Hotha, S. Glycoconjugate J. 2012, 29, 221–230. doi:10.1007/s10719-012-9400-7 |
| 26. | Pati, D.; Shaikh, A. Y.; Hotha, S.; Gupta, S. S. Polym. Chem. 2011, 2, 805–811. doi:10.1039/c0py00412j |
| 27. | Thadke, S. A.; Kar, M.; Gupta, S. S.; Hotha, S. Carbohydr. Res. 2011, 346, 1511–1518. doi:10.1016/j.carres.2011.04.018 |
| 28. | Vidadala, S. R.; Pimpalpalle, T. M.; Linker, T.; Hotha, S. Eur. J. Org. Chem. 2011, 2426–2430. doi:10.1002/ejoc.201100134 |
| 38. | Kayastha, A. K.; Hotha, S. Chem. Commun. 2012, 48, 7161–7163. doi:10.1039/c2cc32649c |
| 1. | Ghosh, N.; Nayak, S.; Sahoo, A. K. J. Org. Chem. 2011, 76, 500–511. doi:10.1021/jo101995g |
| 2. | Trost, B. M.; Dong, G. Nature 2008, 456, 485–488. doi:10.1038/nature07543 |
| 3. | Trost, B. M.; O’Boyle, B. M.; Hund, D. J. Am. Chem. Soc. 2009, 131, 15061–15074. doi:10.1021/ja906056v |
| 4. | Fang, C.; Pang, Y.; Forsyth, C. J. Org. Lett. 2010, 12, 4528–4531. doi:10.1021/ol101833h |
| 5. | Tlais, S. F.; Dudley, G. B. Beilstein J. Org. Chem. 2011, 7, 570–577. doi:10.3762/bjoc.7.66 |
| 6. | Benson, S.; Collin, M.-P.; Arlt, A.; Gabor, B.; Goddard, R.; Fürstner, A. Angew. Chem., Int. Ed. 2011, 50, 8739–8744. doi:10.1002/anie.201103270 |
| 7. | Nakajima, R.; Ogino, T.; Yokoshima, S.; Fukuyama, T. J. Am. Chem. Soc. 2010, 132, 1236–1237. doi:10.1021/ja9103233 |
| 19. | Maurya, S. K.; Hotha, S. Tetrahedron Lett. 2006, 47, 3307–3310. doi:10.1016/j.tetlet.2006.03.016 |
| 20. | Kashyap, S.; Hotha, S. Tetrahedron Lett. 2006, 47, 2021–2023. doi:10.1016/j.tetlet.2006.01.048 |
| 21. | Kashyap, S.; Vidadala, S. R.; Hotha, S. Tetrahedron Lett. 2007, 48, 8960–8962. doi:10.1016/j.tetlet.2007.10.144 |
| 22. | Hotha, S.; Kashyap, S. J. Am. Chem. Soc. 2006, 128, 9620–9621. doi:10.1021/ja062425c |
| 23. | Sureshkumar, G.; Hotha, S. Tetrahedron Lett. 2007, 48, 6564–6568. doi:10.1016/j.tetlet.2007.07.015 |
| 24. | Shaikh, A. Y.; Sureshkumar, G.; Pati, D.; Gupta, S. S.; Hotha, S. Org. Biomol. Chem. 2011, 9, 5951–5959. doi:10.1039/c1ob05056g |
| 25. | Sureshkumar, G.; Hotha, S. Glycoconjugate J. 2012, 29, 221–230. doi:10.1007/s10719-012-9400-7 |
| 26. | Pati, D.; Shaikh, A. Y.; Hotha, S.; Gupta, S. S. Polym. Chem. 2011, 2, 805–811. doi:10.1039/c0py00412j |
| 27. | Thadke, S. A.; Kar, M.; Gupta, S. S.; Hotha, S. Carbohydr. Res. 2011, 346, 1511–1518. doi:10.1016/j.carres.2011.04.018 |
| 28. | Vidadala, S. R.; Pimpalpalle, T. M.; Linker, T.; Hotha, S. Eur. J. Org. Chem. 2011, 2426–2430. doi:10.1002/ejoc.201100134 |
| 17. | Paulsen, H. Angew. Chem. 1982, 94, 184–201. doi:10.1002/ange.19820940304 |
| 18. | Paulsen, H. Angew. Chem., Int. Ed. Engl. 1982, 21, 155–173. doi:10.1002/anie.198201553 |
| 22. | Hotha, S.; Kashyap, S. J. Am. Chem. Soc. 2006, 128, 9620–9621. doi:10.1021/ja062425c |
| 11. | Haruta, M. Nature 2005, 437, 1098–1099. doi:10.1038/4371098a |
| 12. | Hutchings, G. J. Catal. Today 2005, 100, 55–61. doi:10.1016/j.cattod.2004.12.016 |
| 13. | Haruta, M.; Kobayashi, T.; Sano, H.; Yamada, N. Chem. Lett. 1987, 16, 405–408. doi:10.1246/cl.1987.405 |
| 14. | Hutchings, G. J. J. Catal. 1985, 96, 292–295. doi:10.1016/0021-9517(85)90383-5 |
| 15. | Prati, L.; Rossi, M. J. Catal. 1998, 176, 552–560. doi:10.1006/jcat.1998.2078 |
| 16. | Biella, S.; Prati, L.; Rossi, M. J. Catal. 2002, 206, 242–247. doi:10.1006/jcat.2001.3497 |
| 29. | Mamidyala, S. K.; Finn, M. G. J. Org. Chem. 2009, 74, 8417–8420. doi:10.1021/jo901857x |
| 30. | Götze, S.; Fitzner, R.; Kunz, H. Synlett 2009, 3346–3348. doi:10.1055/s-0029-1218356 |
| 31. | Li, Y.; Yang, Y.; Yu, B. Tetrahedron Lett. 2008, 49, 3604–3608. doi:10.1016/j.tetlet.2008.04.017 |
| 32. | Li, Y.; Tang, P.; Chen, Y.; Yu, B. J. Org. Chem. 2008, 73, 4323–4325. doi:10.1021/jo8003875 |
| 33. | Yang, F.; Wang, Q.; Yu, B. Tetrahedron Lett. 2012, 53, 5231–5234. doi:10.1016/j.tetlet.2012.07.059 |
| 34. | Adhikari, S.; Baryal, K. N.; Zhu, D.; Li, X.; Zhu, J. ACS Catal. 2013, 3, 57–60. doi:10.1021/cs300670k |
| 8. | Herrmann, W. A.; Cornils, B. Angew. Chem., Int. Ed. 1997, 36, 1048–1067. doi:10.1002/anie.199710481 |
| 9. | Bond, G. C.; Sermon, P. A.; Webb, G.; Buchanan, D. A.; Wells, P. B. J. Chem. Soc., Chem. Commun. 1973, 444b–445. doi:10.1039/C3973000444B |
| 10. | Hashmi, A. S. K.; Schwarz, L.; Choi, J.-H.; Frost, T. M. Angew. Chem., Int. Ed. 2000, 39, 2285–2288. doi:10.1002/1521-3773(20000703)39:13<2285::AID-ANIE2285>3.0.CO;2-F |
| 11. | Haruta, M. Nature 2005, 437, 1098–1099. doi:10.1038/4371098a |
| 12. | Hutchings, G. J. Catal. Today 2005, 100, 55–61. doi:10.1016/j.cattod.2004.12.016 |
| 13. | Haruta, M.; Kobayashi, T.; Sano, H.; Yamada, N. Chem. Lett. 1987, 16, 405–408. doi:10.1246/cl.1987.405 |
| 14. | Hutchings, G. J. J. Catal. 1985, 96, 292–295. doi:10.1016/0021-9517(85)90383-5 |
| 35. | Fraser-Reid, B.; Wu, Z.; Udodong, U. E.; Ottosso, H. J. Org. Chem. 1990, 55, 6068–6070. doi:10.1021/jo00312a004 |
| 36. | Mootoo, D. R.; Konradsson, P.; Udodong, U.; Fraser-Reid, B. J. Am. Chem. Soc. 1988, 110, 5583–5584. doi:10.1021/ja00224a060 |
| 25. | Sureshkumar, G.; Hotha, S. Glycoconjugate J. 2012, 29, 221–230. doi:10.1007/s10719-012-9400-7 |
| 27. | Thadke, S. A.; Kar, M.; Gupta, S. S.; Hotha, S. Carbohydr. Res. 2011, 346, 1511–1518. doi:10.1016/j.carres.2011.04.018 |
| 24. | Shaikh, A. Y.; Sureshkumar, G.; Pati, D.; Gupta, S. S.; Hotha, S. Org. Biomol. Chem. 2011, 9, 5951–5959. doi:10.1039/c1ob05056g |
| 28. | Vidadala, S. R.; Pimpalpalle, T. M.; Linker, T.; Hotha, S. Eur. J. Org. Chem. 2011, 2426–2430. doi:10.1002/ejoc.201100134 |
| 23. | Sureshkumar, G.; Hotha, S. Tetrahedron Lett. 2007, 48, 6564–6568. doi:10.1016/j.tetlet.2007.07.015 |
| 38. | Kayastha, A. K.; Hotha, S. Chem. Commun. 2012, 48, 7161–7163. doi:10.1039/c2cc32649c |
| 22. | Hotha, S.; Kashyap, S. J. Am. Chem. Soc. 2006, 128, 9620–9621. doi:10.1021/ja062425c |
| 26. | Pati, D.; Shaikh, A. Y.; Hotha, S.; Gupta, S. S. Polym. Chem. 2011, 2, 805–811. doi:10.1039/c0py00412j |
© 2013 Kayastha and Hotha; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)