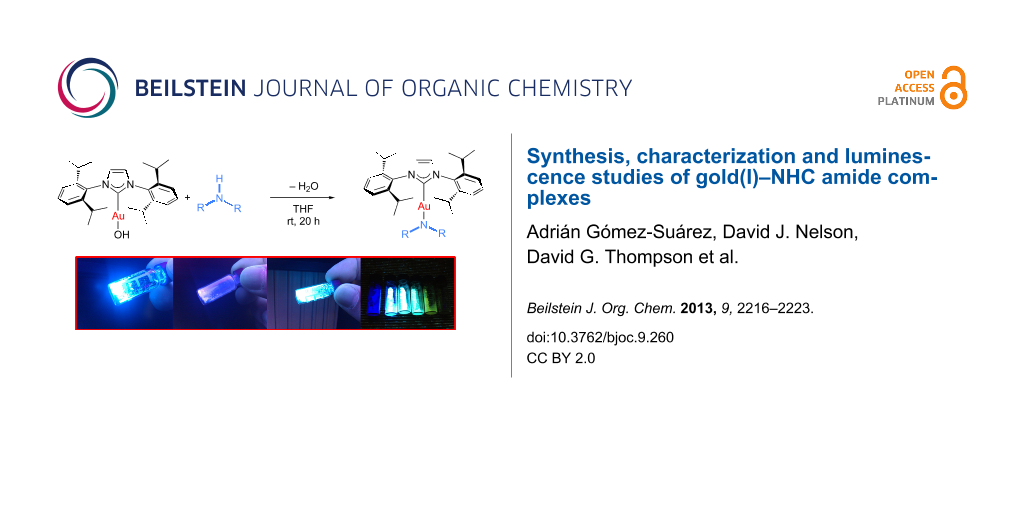
Abstract
A flexible, efficient and straightforward methodology for the synthesis of N-heterocyclic carbene gold(I)–amide complexes is reported. Reaction of the versatile building block [Au(OH)(IPr)] (1) (IPr = 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene) with a series of commercially available (hetero)aromatic amines leads to the synthesis of several [Au(NRR’)(IPr)] complexes in good yields and with water as the sole byproduct. Interestingly, these complexes present luminescence properties. UV–vis and fluorescence measurements have allowed the identification of their excitation and emission wavelengths (λmax). These studies revealed that by selecting the appropriate amine ligand the emission can be easily tuned to achieve a variety of colors, from violet to green.
Graphical Abstract
Introduction
The synthesis of organogold complexes has recently attracted wide attention due to their considerable range of applications, in areas such as materials and medicinal chemistry, as well as their potential role as reaction intermediates or new catalysts in gold-catalyzed processes [1-7]. This has led several research groups to investigate general, green and straightforward methodologies for the synthesis of organogold complexes. We have focused on the synthesis and study of transition metal complexes bearing N-heterocyclic carbene (NHC) ligands [8-10]. Recently, we have been very active in the synthesis and characterization of new gold(I)–NHC complexes and the study of their reactivity, with a special focus on the development of straightforward methodologies [11,12]. As a result of our investigations, we have recently reported the synthesis of [AuX(NHC)] (X = Cl, Br, I) complexes, directly from imidazolium and imidazolidinium salts and a suitable gold source, such as [AuCl(SMe2)], using K2CO3 as a base [13]. Moreover, we have also reported the synthesis of the first mononuclear gold(I) hydroxide species, [Au(OH)(IPr)] (1) (IPr = 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene), using [AuCl(IPr)] and an excess of KOH in THF [14,15]. This complex has proven to be an excellent synthon for the preparation of a wide variety of organogold(I) species [16-23]. Two approaches have been developed for the synthesis of such species using hydroxide 1: a) via transmetallation from either boronic acids or siloxides, or b) via deprotonation reactions of suitable substrates. We have applied the latter methodology to the synthesis of organogold–NHC complexes bearing several trans-ligands, such as acetylene, phenyl and phenol derivatives. These reactions can be carried out using reagent-grade materials, without the requirement to exclude light, air or moisture; providing an easy, straightforward 3-step route for the synthesis of organogold–NHC complexes from readily available gold sources, such as [Au(SMe2)Cl] (Scheme 1).
Scheme 1: Straightforward synthesis of organogold complexes via deprotonation reactions, using 1.
Scheme 1: Straightforward synthesis of organogold complexes via deprotonation reactions, using 1.
Toste and Bergman have recently reported the synthesis, characterization and reactivity studies of a series of [Au(NRR’)(NHC)] complexes [24]. This study represents the first reported synthesis of gold(I)–NHC amide complexes. The procedure employed for the synthesis of these species required the use of lithium amide reagents that are not stable towards air or moisture [24]. Therefore, the development of a more robust approach would be desirable. In addition to the interesting reactivity shown by Toste and Bergman, Hoffman and Viseux have probed the anticancer properties of gold amide complexes, reacting modified triflamide compounds with phosphine-bearing gold chloride species [25]. Taking into account the interest in these type of complexes, we sought to use hydroxide 1 to develop an easy, green (in such deprotonation reactions, water is the sole byproduct) and straightforward methodology for the synthesis of gold(I)–amide complexes.
Results and Discussion
Synthesis and characterization of gold(I)–amide complexes
We began our studies by exposing hydroxide 1 to a series of alkyl- and arylamines. While no reaction was observed with either morpholine or isopropylamine, the use of 1 equiv of aniline or 2-aminopyridine led to the isolation of complexes 2 and 3 respectively, in good yields after overnight reaction at room temperature. These results are consistent with the known pKa of [Au(OH)(IPr)] (30.3) [26]; the scope of this preparative route is, as with other deprotonation reactions with this building block, limited to substrates with a pKa lower than 30.
Encouraged by these exploratory reactions, a series of (hetero)aromatic amines were employed to prepare the corresponding gold(I)–amide complexes. The desired complexes were obtained by reaction of [Au(OH)(IPr)] (1) with each (hetero)aromatic amine in THF at room temperature for 20 h. A range of aromatic amines were employed, including aniline, diphenylamine, pyridines, a pyrimidine and one isoquinoline. The corresponding complexes were obtained in analytically pure form and in good yields as yellow or white microcrystalline powders after a simple work-up (Scheme 2). All complexes were characterized by 1H and 13C{1H} NMR spectroscopy and elemental analysis (see Supporting Information File 1). The new species are bench stable in the solid state, and do not require special handling. However, some do decompose slowly in solution over the course of a number of hours.
Scheme 2: Scope of the reaction between 1 and several (hetero)aromatic amines. Reaction conditions: 1 (1 equiv), amine (1 equiv), THF (0.5–2 mL), rt, 20 h.
Scheme 2: Scope of the reaction between 1 and several (hetero)aromatic amines. Reaction conditions: 1 (1 equi...
A selected number of these complexes were characterized by X-ray crystallography, as further support to confirm atom connectivity and molecular geometry (Figure 1) [27].
![[1860-5397-9-260-1]](/bjoc/content/figures/1860-5397-9-260-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: X-ray crystal structures of complexes 2, 3, 7, 8, 10, 11 and 12. Hydrogen atoms are omitted for clarity.
Figure 1: X-ray crystal structures of complexes 2, 3, 7, 8, 10, 11 and 12. Hydrogen atoms are omitted for cla...
All complexes were found to display the expected two-coordinate linear geometry around the metal center, with all C–Au–N angles in the range 173–179°. There was no evidence of any interactions between the gold center and the heteroatoms in complexes 3, 7, 8, 10 or 11. However, for 3, 7, 8, 10 and 11 there is an intermolecular contact between the aromatic nitrogen atom and the NHC ligand backbone proton (dN-H = 2.27 Å–2.34 Å). All gold–carbon bond lengths were in the range 1.95 Å (8) to 2.02 Å (2), while gold–nitrogen bond lengths varied from 1.98 Å (8) to 2.06 Å (2).
During the characterization of the gold–NHC amide complexes we observed that some of the complexes possessed luminescent properties when exposed to ultraviolet light (λ = 366 nm) in the solid state (Figure 2).
![[1860-5397-9-260-2]](/bjoc/content/figures/1860-5397-9-260-2.jpg?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: Selected examples of gold–NHC amide complexes under UV light (λ = 366 nm).
Figure 2: Selected examples of gold–NHC amide complexes under UV light (λ = 366 nm).
A number of luminescent organogold complexes have been reported in the literature, often demonstrating interesting properties that may lead to their application in materials science and the preparation of optical materials [28-34]. Amongst these, NHC-bearing gold complexes have been shown by several research groups to be very useful luminescent materials [19,35-39], and have attracted industrial interest [40-42]. Thus, we decided to explore the potential of these gold amide species as luminescent materials.
We began our luminescence studies by recording the UV–vis spectra of the aforementioned gold–amide complexes using a dilute (ca. 0.2 mmol/L) CH2Cl2 solution. The wavelengths of the absorption maxima on the UV–vis spectra were in the range of 250–350 nm for most complexes, with the exception of 2-amino-5-nitropyridine derived 9 and 3-aminoisoquinoline-derived 10, which exhibited absorption maxima at ca. 430 nm (see Supporting Information File 1). These data are consistent with the physical appearance of complexes 2–12 in the solution state, i.e. complexes 9 and 10 are yellow.
The excitation and emission maxima were then determined. These measurements were conducted on more concentrated (ca. 4 mmol/L) CH2Cl2 solutions, compared to those used for UV–vis spectroscopy. In each case, the relevant maxima could be identified. An example dataset is presented in Figure 3. Lifetimes for all luminescence measurements were ≤1 μs (at the limit of the apparatus), suggesting that the luminescence was due to fluorescence, rather than phosphorescence.
![[1860-5397-9-260-3]](/bjoc/content/figures/1860-5397-9-260-3.png?scale=2.5&max-width=1024&background=FFFFFF)
Figure 3: Excitation (blue) and emission (pink) data for complex 3, bearing a 2-pyridine ligand (see inset).
Figure 3: Excitation (blue) and emission (pink) data for complex 3, bearing a 2-pyridine ligand (see inset).
The wavelengths of emission maxima spans the range 390 to 516 nm, demonstrating that by selecting the appropriate amine ligand the emission can be easily tuned to achieve a variety of colors, i.e. from violet (3, λmax = 409 nm) to green (4, λmax = 516 nm). In each case, there is a considerable Stokes shift (27 to 166 nm). In terms of the intensity of emission, a wide range of values were recorded, although complexes could be considered as part of one of three distinct groups. Phenyl-bearing complexes (such as 2 and 12), 4-aminopyridine-derived 5 and electron-poor 9 showed relatively weak emission (<10 AU). Electron-poor 2-aminopyridine derivatives, 3-aminopyridine-derived 4 and 2-amino-5-chloropyrimidine-derived 11 exhibited moderate emission intensity (ca. 10–50 AU). The most intense fluorescence was observed with complexes 3, 6 and 10: 2-pyridine derivatives without electron-withdrawing substituents (ca. 120–290 AU) (Table 1).
Table 1: Fluorescence measurement data.a
| entry | substituent | λmax/excitation (nm) | λmax/emission (nm) | intensitymax (AU) |
|---|---|---|---|---|
| 1 |
2 |
396 | 423 | 3.8 |
| 2 |
12 |
410 | 470 | 4.61 |
| 3 |
5 |
352 | 390 | 7.25 |
| 4 |
9 |
350 | 516 | 1.09 |
| 5 |
4 |
398 | 454 | 21.2 |
| 6 |
8 |
368 | 406 | 12.8 |
| 7 |
7 |
395 | 428 | 45.8 |
| 8 |
11 |
385 | 435 | 36.0 |
| 9 |
3 |
379 | 409 | 126 |
| 10 |
6 |
389 | 420 | 119 |
| 11 |
10 |
471 | 505 | 293 |
aAt ca. 4 mmol/L in CH2Cl2 solution.
DFT calculations were used to probe the nature of the frontier orbitals of complex 3 (at the M06-L/SDD level of theory) [43] using Gaussian 09 [44]. The LUMO, HOMO and HOMO-1 are pictured in Figure 4. While the HOMO and HOMO-1 are centered predominantly on the amide ligand, the LUMO is localized on the aryl ring of the NHC ligand; we propose that the fluorescence behavior is due to a HOMO-to-LUMO transition. Therefore, the use of different NHC ligands should also allow access to different fluorescence behavior.
![[1860-5397-9-260-4]](/bjoc/content/figures/1860-5397-9-260-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: (a) LUMO, (b) HOMO and (c) HOMO-1 of complex 3.
Figure 4: (a) LUMO, (b) HOMO and (c) HOMO-1 of complex 3.
Conclusion
A novel series of NHC-bearing gold(I)–amide complexes have been prepared using a simple, straightforward synthetic route that can be conducted using reagent-grade materials on the laboratory bench in air. The resulting species have been characterized using a number of methods, including NMR spectroscopy and X-ray crystal diffraction. These new species have been shown to be fluorescent, and their absorbance and emission maxima have been determined. Notably, there are key trends in the fluorescence behavior of these materials, with more electron-rich 2-pyridine derivatives showing strong emission, and isoquinoline-derived complexes showing the strongest fluorescence. While the present study has been conducted using commercial reagent-grade amines, there is significant scope to prepare a much wider range of gold(I)–amide complexes, including those prepared using designed aromatic amines. Further work is underway in our group to explore both the potential of gold(I)–NHC amide complexes, and further applications of gold(I) hydroxides as building blocks and catalysts.
Supporting Information
| Supporting Information File 1: Experimental part. | ||
| Format: PDF | Size: 1.7 MB | Download |
Acknowledgements
SPN thanks the ERC (Advanced Investigator Award-FUNCAT), EPSRC and Syngenta for support, and Umicore AG for their generous gift of materials. D.G. and S.P.N. are Royal Society Wolfson Merit Award holders. We thank EaStCHEM for computational support through the EaStCHEM Research Computing Facility.
References
-
Marcon, G.; Messori, L.; Orioli, P. Expert Rev. Anticancer Ther. 2002, 2, 337–346. doi:10.1586/14737140.2.3.337
Return to citation in text: [1] -
Hashmi, A. S. K.; Hutchings, G. J. Angew. Chem., Int. Ed. 2006, 45, 7896–7936. doi:10.1002/anie.200602454
Return to citation in text: [1] -
Hashmi, A. S. K. Gold Bull. 2009, 42, 275–279. doi:10.1007/BF03214949
Return to citation in text: [1] -
He, X.; Yam, V. W.-W. Coord. Chem. Rev. 2011, 255, 2111–2123. doi:10.1016/j.ccr.2011.02.003
Return to citation in text: [1] -
Liu, L.-P.; Hammond, G. B. Chem. Soc. Rev. 2012, 41, 3129–3139. doi:10.1039/c2cs15318a
Return to citation in text: [1] -
Schmidbaur, H.; Schier, A. Chem. Soc. Rev. 2012, 41, 370–412. doi:10.1039/c1cs15182g
Return to citation in text: [1] -
Gómez-Suárez, A.; Nolan, S. P. Angew. Chem., Int. Ed. 2012, 51, 8156–8159. doi:10.1002/anie.201203587
Return to citation in text: [1] -
Díez-González, S.; Marion, N.; Nolan, S. P. Chem. Rev. 2009, 109, 3612–3676. doi:10.1021/cr900074m
Return to citation in text: [1] -
Nelson, D. J.; Nolan, S. P. Chem. Soc. Rev. 2013, 42, 6723–6753. doi:10.1039/c3cs60146c
Return to citation in text: [1] -
de Frémont, P.; Marion, N.; Nolan, S. P. Coord. Chem. Rev. 2009, 253, 862–892. doi:10.1016/j.ccr.2008.05.018
Return to citation in text: [1] -
Marion, N.; Nolan, S. P. Chem. Soc. Rev. 2008, 37, 1776–1782. doi:10.1039/b711132k
Return to citation in text: [1] -
Nolan, S. P. Acc. Chem. Res. 2011, 44, 91–100. doi:10.1021/ar1000764
Return to citation in text: [1] -
Collado, A.; Gómez-Suárez, A.; Martin, A. R.; Slawin, A. M. Z.; Nolan, S. P. Chem. Commun. 2013, 49, 5541–5543. doi:10.1039/c3cc43076f
Return to citation in text: [1] -
Gaillard, S.; Slawin, A. M. Z.; Nolan, S. P. Chem. Commun. 2010, 46, 2742–2744. doi:10.1039/c0cc00018c
Return to citation in text: [1] -
Gómez-Suárez, A.; Ramón, R. S.; Slawin, A. M. Z.; Nolan, S. P. Dalton Trans. 2012, 41, 5461–5463. doi:10.1039/c2dt30294b
Return to citation in text: [1] -
Dupuy, S.; Slawin, A. M. Z.; Nolan, S. P. Chem.–Eur. J. 2012, 18, 14923–14928. doi:10.1002/chem.201202299
Return to citation in text: [1] -
Dupuy, S.; Crawford, L.; Bühl, M.; Slawin, A. M. Z.; Nolan, S. P. Adv. Synth. Catal. 2012, 354, 2380–2386. doi:10.1002/adsc.201200233
Return to citation in text: [1] -
Dupuy, S.; Lazreg, F.; Slawin, A. M. Z.; Cazin, C. S. J.; Nolan, S. P. Chem. Commun. 2011, 47, 5455–5457. doi:10.1039/c1cc10917k
Return to citation in text: [1] -
Fortman, G. C.; Poater, A.; Levell, J. W.; Gaillard, S.; Slawin, A. M. Z.; Samuel, I. D. W.; Cavallo, L.; Nolan, S. P. Dalton Trans. 2010, 39, 10382–10390. doi:10.1039/c0dt00276c
Return to citation in text: [1] [2] -
Gaillard, S.; Rix, D.; Slawin, A. M. Z.; Lacour, J.; Nolan, S. P. Dalton Trans. 2012, 41, 8235–8237. doi:10.1039/c2dt30440f
Return to citation in text: [1] -
Konkolewicz, D.; Gaillard, S.; West, A. G.; Cheng, Y. Y.; Gray-Weale, A.; Schmidt, T. W.; Nolan, S. P.; Perrier, S. Organometallics 2011, 30, 1315–1318. doi:10.1021/om200103f
Return to citation in text: [1] -
Gaillard, S.; Nun, P.; Slawin, A. M. Z.; Nolan, S. P. Organometallics 2010, 29, 5402–5408. doi:10.1021/om100456b
Return to citation in text: [1] -
Egbert, J. D.; Slawin, A. M. Z.; Nolan, S. P. Organometallics 2013, 32, 2271–2274. doi:10.1021/om301187a
Return to citation in text: [1] -
Johnson, M. W.; Shevick, S. L.; Toste, F. D.; Bergman, R. G. Chem. Sci. 2013, 4, 1023–1027. doi:10.1039/c2sc21519e
Return to citation in text: [1] [2] -
Newcombe, S.; Bobin, M.; Shrikhande, A.; Gallop, C.; Pace, Y.; Yong, H.; Gates, R.; Chaudhuri, S.; Roe, M.; Hoffmann, E.; Viseux, E. M. E. Org. Biomol. Chem. 2013, 11, 3255–3260. doi:10.1039/c3ob27460h
Return to citation in text: [1] -
Boogaerts, I. I. F.; Nolan, S. P. J. Am. Chem. Soc. 2010, 132, 8858–8859. doi:10.1021/ja103429q
Return to citation in text: [1] -
CCDC 949310 (2), 949311 (3), 949312 (7), 949313 (8), 949314 (10), 949315 (11) and 949316 (12) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre (CCDC) via http://www.ccdc.cam.ac.uk/data_request/cif.
Return to citation in text: [1] -
Vogler, A.; Kunkely, H. Coord. Chem. Rev. 2001, 219–221, 489–507. doi:10.1016/S0010-8545(01)00348-4
Return to citation in text: [1] -
Ito, H.; Saito, T.; Oshima, N.; Kitamura, N.; Ishizaka, S.; Hinatsu, Y.; Wakeshima, M.; Kato, M.; Tsuge, K.; Sawamura, M. J. Am. Chem. Soc. 2008, 130, 10044–10045. doi:10.1021/ja8019356
Return to citation in text: [1] -
Carlos Lima, J.; Rodriguez, L. Chem. Soc. Rev. 2011, 40, 5442–5456. doi:10.1039/c1cs15123a
Return to citation in text: [1] -
Lu, W.; Kwok, W.-M.; Ma, C.; Chan, C. T.-L.; Zhu, M.-X.; Che, C.-M. J. Am. Chem. Soc. 2011, 133, 14120–14135. doi:10.1021/ja205831v
Return to citation in text: [1] -
Cámara, J.; Crespo, O.; Gimeno, M. C.; Koshevoy, I. O.; Laguna, A.; Ospino, I.; Smirnova, E. S.; Tunik, S. P. Dalton Trans. 2012, 41, 13891–13898. doi:10.1039/C2DT31019H
Return to citation in text: [1] -
Partyka, D. V.; Teets, T. S.; Zeller, M.; Updegraff, J. B.; Hunter, A. D.; Gray, T. G. Chem.–Eur. J. 2012, 18, 2100–2112. doi:10.1002/chem.201101891
Return to citation in text: [1] -
Heckler, J. E.; Zeller, M.; Hunter, A. D.; Gray, T. G. Angew. Chem., Int. Ed. 2012, 51, 5924–5928. doi:10.1002/anie.201201744
Return to citation in text: [1] -
Gao, L.; Partyka, D. V.; Updegraff, J. B., III; Deligonul, N.; Gray, T. G. Eur. J. Inorg. Chem. 2009, 2711–2719. doi:10.1002/ejic.200900307
Return to citation in text: [1] -
Hirtenlehner, C.; Krims, C.; Hölbling, J.; List, M.; Zabel, M.; Fleck, M.; Berger, R. J. F.; Schoefberger, W.; Monkowius, U. Dalton Trans. 2011, 40, 9899–9910. doi:10.1039/c1dt11175b
Return to citation in text: [1] -
To, W.-P.; Tong, G. S.-M.; Lu, W.; Ma, C.; Liu, J.; Chow, A. L.-F.; Che, C.-M. Angew. Chem., Int. Ed. 2012, 51, 2654–2657. doi:10.1002/anie.201108080
Return to citation in text: [1] -
Gimeno, M. C.; Laguna, A.; Visbal, R. Organometallics 2012, 31, 7146–7157. doi:10.1021/om300571m
Return to citation in text: [1] -
Visbal, R.; Ospino, I.; López-de-Luzuriaga, J. M.; Laguna, A.; Gimeno, M. C. J. Am. Chem. Soc. 2013, 135, 4712–4715. doi:10.1021/ja401523x
Return to citation in text: [1] -
Fujimura, O.; Fukunaga, K.; Honma, T.; Machida, T. Substituted phenylethynyl gold-nitrogenated heterocyclic carbene complex. WO Patent 2007139001 A1, Dec 6, 2004; pp 42 ff.
Return to citation in text: [1] -
Fujimura, O.; Fukunaga, K.; Honma, T.; Machida, T. Gold complex, method for production of the gold complex, and organic ultraviolet electroluminescent element using the gold complex. WO Patent 2008050733 A1, May 2, 2008; pp 16 ff.
Return to citation in text: [1] -
Fujimura, O.; Fukunaga, K.; Honma, T.; Machida, T. Substituted phenylethynyl gold-nitrogen-containing heterocyclic carbene complex. Jpn Patent JP 2008179550 A, 2008; pp 41 ff.
Return to citation in text: [1] -
Zhao, Y.; Truhlar, D. G. Acc. Chem. Res. 2008, 41, 157–167. doi:10.1021/ar700111a
Return to citation in text: [1] -
Gaussian 03, Revision D.02; Gaussian Inc.: Wallingford, CT, 2004.
Return to citation in text: [1]
| 1. | Marcon, G.; Messori, L.; Orioli, P. Expert Rev. Anticancer Ther. 2002, 2, 337–346. doi:10.1586/14737140.2.3.337 |
| 2. | Hashmi, A. S. K.; Hutchings, G. J. Angew. Chem., Int. Ed. 2006, 45, 7896–7936. doi:10.1002/anie.200602454 |
| 3. | Hashmi, A. S. K. Gold Bull. 2009, 42, 275–279. doi:10.1007/BF03214949 |
| 4. | He, X.; Yam, V. W.-W. Coord. Chem. Rev. 2011, 255, 2111–2123. doi:10.1016/j.ccr.2011.02.003 |
| 5. | Liu, L.-P.; Hammond, G. B. Chem. Soc. Rev. 2012, 41, 3129–3139. doi:10.1039/c2cs15318a |
| 6. | Schmidbaur, H.; Schier, A. Chem. Soc. Rev. 2012, 41, 370–412. doi:10.1039/c1cs15182g |
| 7. | Gómez-Suárez, A.; Nolan, S. P. Angew. Chem., Int. Ed. 2012, 51, 8156–8159. doi:10.1002/anie.201203587 |
| 14. | Gaillard, S.; Slawin, A. M. Z.; Nolan, S. P. Chem. Commun. 2010, 46, 2742–2744. doi:10.1039/c0cc00018c |
| 15. | Gómez-Suárez, A.; Ramón, R. S.; Slawin, A. M. Z.; Nolan, S. P. Dalton Trans. 2012, 41, 5461–5463. doi:10.1039/c2dt30294b |
| 43. | Zhao, Y.; Truhlar, D. G. Acc. Chem. Res. 2008, 41, 157–167. doi:10.1021/ar700111a |
| 13. | Collado, A.; Gómez-Suárez, A.; Martin, A. R.; Slawin, A. M. Z.; Nolan, S. P. Chem. Commun. 2013, 49, 5541–5543. doi:10.1039/c3cc43076f |
| 11. | Marion, N.; Nolan, S. P. Chem. Soc. Rev. 2008, 37, 1776–1782. doi:10.1039/b711132k |
| 12. | Nolan, S. P. Acc. Chem. Res. 2011, 44, 91–100. doi:10.1021/ar1000764 |
| 19. | Fortman, G. C.; Poater, A.; Levell, J. W.; Gaillard, S.; Slawin, A. M. Z.; Samuel, I. D. W.; Cavallo, L.; Nolan, S. P. Dalton Trans. 2010, 39, 10382–10390. doi:10.1039/c0dt00276c |
| 35. | Gao, L.; Partyka, D. V.; Updegraff, J. B., III; Deligonul, N.; Gray, T. G. Eur. J. Inorg. Chem. 2009, 2711–2719. doi:10.1002/ejic.200900307 |
| 36. | Hirtenlehner, C.; Krims, C.; Hölbling, J.; List, M.; Zabel, M.; Fleck, M.; Berger, R. J. F.; Schoefberger, W.; Monkowius, U. Dalton Trans. 2011, 40, 9899–9910. doi:10.1039/c1dt11175b |
| 37. | To, W.-P.; Tong, G. S.-M.; Lu, W.; Ma, C.; Liu, J.; Chow, A. L.-F.; Che, C.-M. Angew. Chem., Int. Ed. 2012, 51, 2654–2657. doi:10.1002/anie.201108080 |
| 38. | Gimeno, M. C.; Laguna, A.; Visbal, R. Organometallics 2012, 31, 7146–7157. doi:10.1021/om300571m |
| 39. | Visbal, R.; Ospino, I.; López-de-Luzuriaga, J. M.; Laguna, A.; Gimeno, M. C. J. Am. Chem. Soc. 2013, 135, 4712–4715. doi:10.1021/ja401523x |
| 8. | Díez-González, S.; Marion, N.; Nolan, S. P. Chem. Rev. 2009, 109, 3612–3676. doi:10.1021/cr900074m |
| 9. | Nelson, D. J.; Nolan, S. P. Chem. Soc. Rev. 2013, 42, 6723–6753. doi:10.1039/c3cs60146c |
| 10. | de Frémont, P.; Marion, N.; Nolan, S. P. Coord. Chem. Rev. 2009, 253, 862–892. doi:10.1016/j.ccr.2008.05.018 |
| 40. | Fujimura, O.; Fukunaga, K.; Honma, T.; Machida, T. Substituted phenylethynyl gold-nitrogenated heterocyclic carbene complex. WO Patent 2007139001 A1, Dec 6, 2004; pp 42 ff. |
| 41. | Fujimura, O.; Fukunaga, K.; Honma, T.; Machida, T. Gold complex, method for production of the gold complex, and organic ultraviolet electroluminescent element using the gold complex. WO Patent 2008050733 A1, May 2, 2008; pp 16 ff. |
| 42. | Fujimura, O.; Fukunaga, K.; Honma, T.; Machida, T. Substituted phenylethynyl gold-nitrogen-containing heterocyclic carbene complex. Jpn Patent JP 2008179550 A, 2008; pp 41 ff. |
| 25. | Newcombe, S.; Bobin, M.; Shrikhande, A.; Gallop, C.; Pace, Y.; Yong, H.; Gates, R.; Chaudhuri, S.; Roe, M.; Hoffmann, E.; Viseux, E. M. E. Org. Biomol. Chem. 2013, 11, 3255–3260. doi:10.1039/c3ob27460h |
| 27. | CCDC 949310 (2), 949311 (3), 949312 (7), 949313 (8), 949314 (10), 949315 (11) and 949316 (12) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre (CCDC) via http://www.ccdc.cam.ac.uk/data_request/cif. |
| 24. | Johnson, M. W.; Shevick, S. L.; Toste, F. D.; Bergman, R. G. Chem. Sci. 2013, 4, 1023–1027. doi:10.1039/c2sc21519e |
| 28. | Vogler, A.; Kunkely, H. Coord. Chem. Rev. 2001, 219–221, 489–507. doi:10.1016/S0010-8545(01)00348-4 |
| 29. | Ito, H.; Saito, T.; Oshima, N.; Kitamura, N.; Ishizaka, S.; Hinatsu, Y.; Wakeshima, M.; Kato, M.; Tsuge, K.; Sawamura, M. J. Am. Chem. Soc. 2008, 130, 10044–10045. doi:10.1021/ja8019356 |
| 30. | Carlos Lima, J.; Rodriguez, L. Chem. Soc. Rev. 2011, 40, 5442–5456. doi:10.1039/c1cs15123a |
| 31. | Lu, W.; Kwok, W.-M.; Ma, C.; Chan, C. T.-L.; Zhu, M.-X.; Che, C.-M. J. Am. Chem. Soc. 2011, 133, 14120–14135. doi:10.1021/ja205831v |
| 32. | Cámara, J.; Crespo, O.; Gimeno, M. C.; Koshevoy, I. O.; Laguna, A.; Ospino, I.; Smirnova, E. S.; Tunik, S. P. Dalton Trans. 2012, 41, 13891–13898. doi:10.1039/C2DT31019H |
| 33. | Partyka, D. V.; Teets, T. S.; Zeller, M.; Updegraff, J. B.; Hunter, A. D.; Gray, T. G. Chem.–Eur. J. 2012, 18, 2100–2112. doi:10.1002/chem.201101891 |
| 34. | Heckler, J. E.; Zeller, M.; Hunter, A. D.; Gray, T. G. Angew. Chem., Int. Ed. 2012, 51, 5924–5928. doi:10.1002/anie.201201744 |
| 24. | Johnson, M. W.; Shevick, S. L.; Toste, F. D.; Bergman, R. G. Chem. Sci. 2013, 4, 1023–1027. doi:10.1039/c2sc21519e |
| 16. | Dupuy, S.; Slawin, A. M. Z.; Nolan, S. P. Chem.–Eur. J. 2012, 18, 14923–14928. doi:10.1002/chem.201202299 |
| 17. | Dupuy, S.; Crawford, L.; Bühl, M.; Slawin, A. M. Z.; Nolan, S. P. Adv. Synth. Catal. 2012, 354, 2380–2386. doi:10.1002/adsc.201200233 |
| 18. | Dupuy, S.; Lazreg, F.; Slawin, A. M. Z.; Cazin, C. S. J.; Nolan, S. P. Chem. Commun. 2011, 47, 5455–5457. doi:10.1039/c1cc10917k |
| 19. | Fortman, G. C.; Poater, A.; Levell, J. W.; Gaillard, S.; Slawin, A. M. Z.; Samuel, I. D. W.; Cavallo, L.; Nolan, S. P. Dalton Trans. 2010, 39, 10382–10390. doi:10.1039/c0dt00276c |
| 20. | Gaillard, S.; Rix, D.; Slawin, A. M. Z.; Lacour, J.; Nolan, S. P. Dalton Trans. 2012, 41, 8235–8237. doi:10.1039/c2dt30440f |
| 21. | Konkolewicz, D.; Gaillard, S.; West, A. G.; Cheng, Y. Y.; Gray-Weale, A.; Schmidt, T. W.; Nolan, S. P.; Perrier, S. Organometallics 2011, 30, 1315–1318. doi:10.1021/om200103f |
| 22. | Gaillard, S.; Nun, P.; Slawin, A. M. Z.; Nolan, S. P. Organometallics 2010, 29, 5402–5408. doi:10.1021/om100456b |
| 23. | Egbert, J. D.; Slawin, A. M. Z.; Nolan, S. P. Organometallics 2013, 32, 2271–2274. doi:10.1021/om301187a |
| 26. | Boogaerts, I. I. F.; Nolan, S. P. J. Am. Chem. Soc. 2010, 132, 8858–8859. doi:10.1021/ja103429q |
© 2013 Gómez-Suárez et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)