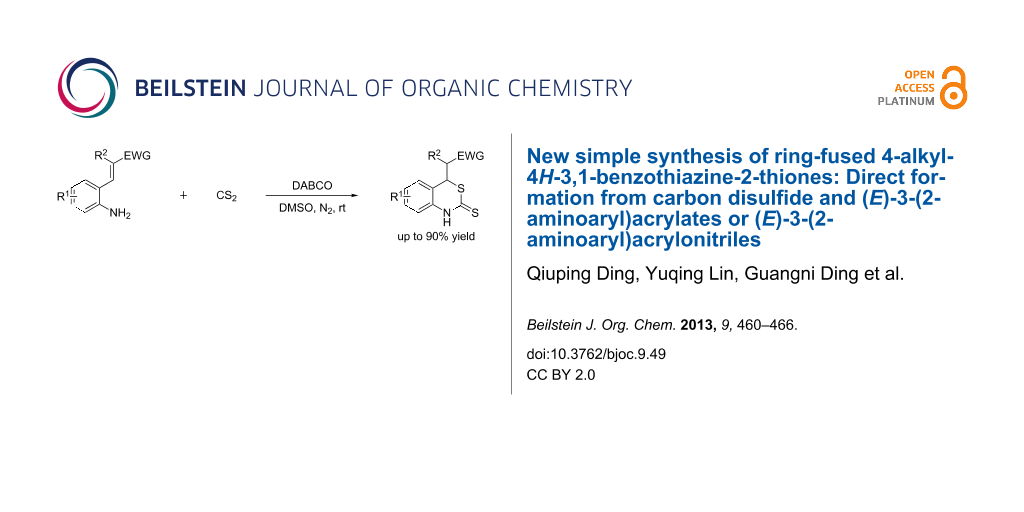
Abstract
A new simple and efficient method to construct ring-fused 4-alkyl-4H-3,1-benzothiazine-2-thione derivatives has been developed from carbon disulfide and (E)-3-(2-aminoaryl)acrylates or (E)-3-(2-aminoaryl)acrylonitriles under mild conditions, without the need for a metal catalyst. The newly developed method tolerates a wide range of substrates in moderate to excellent yields. Moreover, this method is advantageous over previous ones for the easy synthesis of reactants.
Graphical Abstract
Introduction
Molecules containing the 4H-3,1-benzothiazine moiety have received considerable interest from the chemical and medicinal community due to their promising biological activity [1-4] and the applications in recording and photographic materials [5-8]. A number of efficient approaches for their preparation have been reported in the literature [9-15]. 4-Alkyl-4H-3,1-benzothiazine-2-thiones are an important class of 4H-3,1-benzothiazine derivatives. Therefore, 4-alkyl-4H-3,1-benzothiazine-2-thione derivatives are also of potential biological importance. However, only a few practical routes for the synthesis of this class of 4-alkyl-4H-3,1-benzothiazine-2-thione derivatives have been reported [16,17]. Although Kobayashi and co-workers have reported the synthesis of 2-(2-thioxo-4H-3,1-benzothiazin-4-yl)acetic acid derivatives by the reaction of 3-(2-isothiocyanatophenyl)prop-2-enoates with sodium sulfide, this method suffers from the tedious synthesis of the substrates prepared in four steps from 2-iodoaniline [16]. Molina et al. also described the preparation of 4H-3,1-benzothiazine-2-thione derivatives by intramolecular heteroconjugate addition of carbodiimides or isothiocyanates bearing one o-substituted α,β-unsaturated carbonyl fragment promoted by the CS2/TBAF system [17]. However, both the low yields (30–60%) of the products and the substrate limitations outweigh their advantages. As part of a continuing effort in our laboratory toward the development of novel natural-product-like compounds [18-22], we recently reported the practical synthesis of 2-mercapto-4-benzylidene-4H-benzo[d][1,3]thiazines starting from 2-alkynylbenzenamines with CS2, and further transformations to highly functionalized 4-benzylidene-4H-benzo[d][1,3]thiazines (Scheme 1) [9].
Scheme 1: AgNO3-catalyzed tandem reaction of 2-alkynylbenzenamines with CS2 and their further transformation.
Scheme 1: AgNO3-catalyzed tandem reaction of 2-alkynylbenzenamines with CS2 and their further transformation.
Promoted by these results, we envisioned that (E)-3-(2-aminoaryl)acrylates or (E)-3-(2-aminoaryl)acrylonitriles could also be utilized as starting substrates for the synthesis of N-heterocycles. Therefore, we focused on the o-amino-α,β-unsaturated compound 1 (Scheme 2), which would be expected to construct 4-alkyl-4H-3,1-benzothiazine-2-thione derivatives through a one-pot base-promoted intermolecular addition/intramolecular Michael addition reaction.
Scheme 2: Concept for the construction of 4-alkyl-4H-3,1-benzothiazine-2-thione derivatives.
Scheme 2: Concept for the construction of 4-alkyl-4H-3,1-benzothiazine-2-thione derivatives.
Results and Discussion
In our initial study, we examined the tandem reaction with various bases and solvents to optimize the reaction conditions. (E)-Butyl 3-(2-aminophenyl)acrylate (1a) was chosen as a model substrate, and the results are summarized in Table 1. Among the bases screened, DABCO was found to be superior to the other organic or inorganic bases, although DBU, Et3N, and KOH also provided good results (Table 1, entries 1–6). However, no product could be detected in the absence of base (Table 1, entry 7). When a catalytic amount of DABCO (20 mol %) was used, only a 69% yield of product 2a was obtained. Subsequently, the study results showed that the amount of CS2 had a great effect on the reaction (Table 1, entry 1 versus entries 8–10). To reduce the amount of CS2, we finally chose 4.0 equiv of CS2. The results also suggested that the solvent was crucial for this transformation. Low-polar solvents such as toluene and CH2Cl2 inhibited the reaction (Table 1, entry 11 and entry 12). Among the polar solvents screened (Table 1, entries 13–16), DMSO was the best, affording the desired product in 88% yield (Table 1, entry 13). When the reaction was performed at 60 °C in a sealed tube, the yield of product 2a decreased to 65% after a similar reaction time (Table 1, entry 17).
Table 1: Exploring variation of the base and other conditions for the construction of 4-alkyl-4H-3,1-benzothiazine-2-thiones.a
|
|
|||
| entry | base | solvent | yieldb (%) |
|---|---|---|---|
| 1 | DBU | DMF | 80 |
| 2 | Et3N | DMF | 76 |
| 3 | Na2CO3 | DMF | 65 |
| 4 | NaHCO3 | DMF | 60 |
| 5 | KOH | DMF | 82 |
| 6 | DABCO | DMF | 85 |
| 7 | — | DMF | — |
| 8c | DABCO | DMF | 83 |
| 9d | DABCO | DMF | 84 |
| 10e | DABCO | DMF | 44 |
| 11d | DABCO | toluene | — |
| 12d | DABCO | CH2Cl2 | trace |
| 13d | DABCO | DMSO | 88 |
| 14d | DABCO | 1,4-dioxane | 45 |
| 15d | DABCO | CH3CN | 50 |
| 16d | DABCO | THF | 30 |
| 17d,f | DABCO | DMSO | 65 |
aReaction conditions: (E)-butyl 3-(2-aminophenyl)acrylate (1a, 0.3 mmol), CS2 (3 mmol, 10.0 equiv), base (0.3 mmol), rt, 2 d. bIsolated yield based on 1a. cCS2 (1.8 mmol, 6.0 equiv). dCS2 (1.2 mmol, 4.0 equiv). eCS2 (0.9 mmol, 3.0 equiv). fReaction performed in DMSO at 60 °C in sealed tube.
With the preliminary optimized reaction conditions in hand, we next tested the generality of the (E)-3-(2-aminoaryl)acrylates (Table 2). As expected, a series of functional groups on the phenyl ring of the (E)-butyl 3-(2-aminoaryl)acrylates, such as methyl, chloro, fluoro, and nitro were compatible in this procedure, and the corresponding desired products 2b–2e were isolated in 36–86% yields. In general, substrates with electron-donating (methyl) and weakly or moderately electron-withdrawing groups (F, Cl) showed good results in the transformation. For instance, (E)-butyl 3-(2-amino-5-methylphenyl)acrylate (1b) reacted with CS2 leading to the corresponding product 2b in 75% yield (Table 2, entry 2). A slightly higher yield was obtained when (E)-butyl 3-(2-amino-5-fluorophenyl)acrylate (1d) was used as a replacement in the above reaction (86% yield, Table 2, entry 4). It is worth noting that a substrate with strongly electron-withdrawing group (nitro) gave a low yield 36% of the product 2e. Further exploration indicated that various alkyl (methyl, ethyl, tert-butyl) 3-(2-aminophenyl)acrylates 1 were suitable reactants in the transformation, and the desired products 2f–2j were obtained in moderate to good yields (Table 2, entries 6–10). When (E)-ethyl 3-(2-aminophenyl)acrylate (1g) was employed in the reaction, the corresponding product 2g was isolated in 80% yield (Table 2, entry 7). We next examined the reaction of (E)-methyl 3-(2-aminophenyl)-2-methylacrylates 1k–1n with different substituents on the phenyl ring, and the desired products 2k–2n were isolated in 54–74% yield (Table 2, entries 11–14). Furthermore, the reaction conditions proved to be useful for (E)-3-(2-aminoaryl)acrylonitriles (1o–1r, Table 2, entries 15–18). For instance, (E)-3-(2-aminophenyl)acrylonitrile (1o) reacted with CS2 affording the expected product 2o in excellent 90% yield (Table 2, entry 15). However, it was found that reactants 2-(2-aminobenzylidene)malononitrile (1s) and ethyl 3-(2-aminophenyl)-2-cyanoacrylate (1t) were not workable under the standard conditions (Table 2, entries 19 and 20).
Table 2: Preparation of 4-alkyl-4H-3,1-benzothiazine-2-thione derivatives 2.a
|
|
|||
| entry | substrate 1 | product 2 | yieldb (%) |
|---|---|---|---|
| 1 |
1a |
2a |
88 |
| 2 |
1b |
2b |
75 |
| 3 |
1c |
2c |
73 |
| 4 |
1d |
2d |
86 |
| 5 |
1e |
2e |
36 |
| 6 |
1f |
2f |
87 |
| 7 |
1g |
2g |
80 |
| 8 |
1h |
2h |
60 |
| 9 |
1i |
2i |
72 |
| 10 |
1j |
2j |
71 |
| 11 |
1k |
2k |
74 |
| 12 |
1l |
2l |
55 |
| 13 |
1m |
2m |
54 |
| 14 |
1n |
2n |
74 |
| 15 |
1o |
2o |
90 |
| 16 |
1p |
2p |
75 |
| 17 |
1q |
2q |
44 |
| 18 |
1r |
2r |
53 |
| 19 |
1s |
2s |
NR |
| 20 |
1t |
2t |
NR |
aReaction conditions: substrate 1 (0.3 mmol), CS2 (1.2 mmol, 4.0 equiv), base (0.3 mmol, 1.0 equiv), rt, 2 d. bIsolated yield based on 1.
The 2-(2-thioxo-2,4-dihydro-1H-benzo[d][1,3]thiazin-4-yl)acetate 2 could be further elaborated by alkylation with alkyl halide. For example, compound 2g reacted with iodomethane to afford the expected ethyl 2-(2-(methylthio)-4H-benzo[d][1,3]thiazin-4-yl)acetate (3) in 70% yield (Scheme 3).
Scheme 3: Alkylation of 2g with iodomethane.
Scheme 3: Alkylation of 2g with iodomethane.
Conclusion
In summary, we have successfully developed a new simple and efficient method to construct ring-fused 4-alkyl-4H-3,1-benzothiazine-2-thione derivatives. In the context of this method, carbon disulfide reacted with (E)-3-(2-aminoaryl)acrylates or (E)-3-(2-aminoaryl)acrylonitriles under metal-free conditions at room temperature. The newly developed method tolerates a wide range of substrates in moderate to excellent yields and provides promise for further alkylation or arylation. Moreover, this method is advantageous over previous ones [16,17] for the easy synthesis of reactants.
Experimental
General
All reactions were performed in test tubes in air. Flash column chromatography was performed with silica gel (200–300 mesh). Analytical thin-layer chromatography was performed on glass plates precoated with 0.25 mm 230–400 mesh silica gel impregnated with a fluorescent indicator (254 nm). Thin-layer chromatography plates were visualized by exposure to ultraviolet light. Organic solutions were concentrated on rotary evaporators at 25–35 °C. Commercial reagents and solvents were used as received. 1H and 13C NMR spectra were recorded on a Bruker AV 400 at 400 MHz (1H) and 100 MHz (13C) at ambient temperature. Chemical shifts are reported in parts per million (ppm) on the delta scale (δ) and referenced to tetramethylsilane (0 ppm). HRMS analyses were performed in ESI mode on a Bruker mass spectrometer.
General procedure for the synthesis of 2-(2-thioxo-2,4-dihydro-1H-benzo[d][1,3]thiazin-4-yl)acetate, 2: A mixture of 3-(2-aminoaryl)acrylate 1 (0.3 mmol), CS2 (1.2 mmol, 4.0 equiv, 91.2 mg) and DABCO (0.3 mmol, 1.0 equiv, 33.6 mg) was stirred in DMSO (2 mL) at room temperature. After completion of the reaction as indicated by TLC (about 2 d), the reaction was quenched by water and extracted with ethyl acetate. The organic layers were dried with anhydrous MgSO4, the solvent was evaporated under vacuum, and the residue was isolated by column chromatography with EtOAc/petroleum ether (1/5, v/v) as eluent to yield the desired products 2. For details, see Supporting Information File 1.
Supporting Information
| Supporting Information File 1: General procedure, characterization data and copies of spectra. | ||
| Format: PDF | Size: 1.5 MB | Download |
Acknowledgements
Financial support from Jiangxi Educational Committee (GJJ12169), National Natural Science Foundation of China (21002042), and Open Project Program of Key Laboratory of Functional Small Organic Molecule, Ministry of Education, Jiangxi Normal University (No. KLFS-KF-201217) is gratefully acknowledged.
References
-
Nishio, T. J. Org. Chem. 1997, 62, 1106–1111. doi:10.1021/jo961704n
Return to citation in text: [1] -
El-Desoky, S. I.; Kandeel, E. M.; Abd-el-Rahman, A. H.; Schmidt, R. R. J. Heterocycl. Chem. 1999, 36, 153–160. doi:10.1002/jhet.5570360124
Return to citation in text: [1] -
Matysiak, J. Bioorg. Med. Chem. 2006, 14, 2613–2619. doi:10.1016/j.bmc.2005.11.053
Return to citation in text: [1] -
Csomós, P.; Fodor, L.; Bernáth, G.; Sinkkonen, J.; Salminen, J.; Wiinamäki, K.; Pihlaja, K. Tetrahedron 2008, 64, 1002–1011. doi:10.1016/j.tet.2007.09.079
Return to citation in text: [1] -
Obayashi, T.; Okawa, A. Laser heat-mode type recording medium with excellent high sensitivity and storage stability. JP2001253172, Sept 18, 2001.
Return to citation in text: [1] -
Canon, K. K. Electrophotographic developers. JP59197051, Nov 8, 1984.
Return to citation in text: [1] -
Kanagawa, A.; Sadao, I.; Keiso, S.; Hideo, U. Aufzeichnungsmaterial. DE2704724A1, Feb 4, 1977.
Return to citation in text: [1] -
Kanagawa, A.; Sadao, I.; Keiso, S.; Hideo, U. Thiazinderivate und Verfahren zu ihrer Herstellung. DE2658246A1, July 7, 1977.
Return to citation in text: [1] -
Ding, Q.; Liu, X.; Yu, J.; Zhang, Q.; Wang, D.; Cao, B.; Peng, Y. Tetrahedron 2012, 68, 3937–3941. doi:10.1016/j.tet.2012.03.098
Return to citation in text: [1] [2] -
Gimbert, C.; Vallribera, A. Org. Lett. 2009, 11, 269–271. doi:10.1021/ol802346r
Return to citation in text: [1] -
Butin, A. V.; Tsiunchik, F. A.; Abaev, V. T.; Gutnov, A. V.; Cheshkov, D. A. Synthesis 2009, 2616–2626. doi:10.1055/s-0029-1217399
Return to citation in text: [1] -
Otani, T.; Katsurayama, S.; Ote, T.; Saito, T. J. Sulfur Chem. 2009, 30, 250–263. doi:10.1080/17415990902839443
Return to citation in text: [1] -
Abaev, V. T.; Tsiunchik, F. A.; Gutnov, A. V.; Butin, A. V. Tetrahedron Lett. 2006, 47, 4029–4032. doi:10.1016/j.tetlet.2006.04.010
Return to citation in text: [1] -
Fernandes, M. A.; Reid, D. H. Synlett 2003, 2231–2233. doi:10.1055/s-2003-42078
Return to citation in text: [1] -
Fathalla, W. M.; Pazdera, P. Molecules 2002, 7, 96–103. doi:10.3390/70100096
Return to citation in text: [1] -
Fukamachi, S.; Konishi, H.; Kobayashi, K. Helv. Chim. Acta 2011, 94, 111–118. doi:10.1002/hlca.201000340
Return to citation in text: [1] [2] [3] -
Tárraga, A.; Molina, P.; López, J. L. Tetrahedron Lett. 2000, 41, 4895–4899. doi:10.1016/S0040-4039(00)00756-5
Return to citation in text: [1] [2] [3] -
Ding, Q.; Ji, H.; Wang, D.; Lin, Y.; Yu, W.; Peng, Y. J. Organomet. Chem. 2012, 711, 62–67. doi:10.1016/j.jorganchem.2012.03.030
Return to citation in text: [1] -
Ding, Q.; Cao, B.; Zong, Z.; Peng, Y. J. Comb. Chem. 2010, 12, 370–373. doi:10.1021/cc100012a
Return to citation in text: [1] -
Ding, Q.; Cao, B.; Liu, X.; Zong, Z.; Peng, Y. Green Chem. 2010, 12, 1607–1610. doi:10.1039/c0gc00123f
Return to citation in text: [1] -
Ding, Q.; Wang, D.; Sang, X.; Lin, Y.; Peng, Y. Tetrahedron 2012, 68, 8869–8874. doi:10.1016/j.tet.2012.08.039
Return to citation in text: [1] -
Ding, Q.; Liu, X.; Wang, H.; Chen, M.; Peng, Y. Synthesis 2012, 920–926. doi:10.1055/s-0031-1289722
Return to citation in text: [1]
| 1. | Nishio, T. J. Org. Chem. 1997, 62, 1106–1111. doi:10.1021/jo961704n |
| 2. | El-Desoky, S. I.; Kandeel, E. M.; Abd-el-Rahman, A. H.; Schmidt, R. R. J. Heterocycl. Chem. 1999, 36, 153–160. doi:10.1002/jhet.5570360124 |
| 3. | Matysiak, J. Bioorg. Med. Chem. 2006, 14, 2613–2619. doi:10.1016/j.bmc.2005.11.053 |
| 4. | Csomós, P.; Fodor, L.; Bernáth, G.; Sinkkonen, J.; Salminen, J.; Wiinamäki, K.; Pihlaja, K. Tetrahedron 2008, 64, 1002–1011. doi:10.1016/j.tet.2007.09.079 |
| 16. | Fukamachi, S.; Konishi, H.; Kobayashi, K. Helv. Chim. Acta 2011, 94, 111–118. doi:10.1002/hlca.201000340 |
| 16. | Fukamachi, S.; Konishi, H.; Kobayashi, K. Helv. Chim. Acta 2011, 94, 111–118. doi:10.1002/hlca.201000340 |
| 17. | Tárraga, A.; Molina, P.; López, J. L. Tetrahedron Lett. 2000, 41, 4895–4899. doi:10.1016/S0040-4039(00)00756-5 |
| 9. | Ding, Q.; Liu, X.; Yu, J.; Zhang, Q.; Wang, D.; Cao, B.; Peng, Y. Tetrahedron 2012, 68, 3937–3941. doi:10.1016/j.tet.2012.03.098 |
| 10. | Gimbert, C.; Vallribera, A. Org. Lett. 2009, 11, 269–271. doi:10.1021/ol802346r |
| 11. | Butin, A. V.; Tsiunchik, F. A.; Abaev, V. T.; Gutnov, A. V.; Cheshkov, D. A. Synthesis 2009, 2616–2626. doi:10.1055/s-0029-1217399 |
| 12. | Otani, T.; Katsurayama, S.; Ote, T.; Saito, T. J. Sulfur Chem. 2009, 30, 250–263. doi:10.1080/17415990902839443 |
| 13. | Abaev, V. T.; Tsiunchik, F. A.; Gutnov, A. V.; Butin, A. V. Tetrahedron Lett. 2006, 47, 4029–4032. doi:10.1016/j.tetlet.2006.04.010 |
| 14. | Fernandes, M. A.; Reid, D. H. Synlett 2003, 2231–2233. doi:10.1055/s-2003-42078 |
| 15. | Fathalla, W. M.; Pazdera, P. Molecules 2002, 7, 96–103. doi:10.3390/70100096 |
| 5. | Obayashi, T.; Okawa, A. Laser heat-mode type recording medium with excellent high sensitivity and storage stability. JP2001253172, Sept 18, 2001. |
| 6. | Canon, K. K. Electrophotographic developers. JP59197051, Nov 8, 1984. |
| 7. | Kanagawa, A.; Sadao, I.; Keiso, S.; Hideo, U. Aufzeichnungsmaterial. DE2704724A1, Feb 4, 1977. |
| 8. | Kanagawa, A.; Sadao, I.; Keiso, S.; Hideo, U. Thiazinderivate und Verfahren zu ihrer Herstellung. DE2658246A1, July 7, 1977. |
| 16. | Fukamachi, S.; Konishi, H.; Kobayashi, K. Helv. Chim. Acta 2011, 94, 111–118. doi:10.1002/hlca.201000340 |
| 17. | Tárraga, A.; Molina, P.; López, J. L. Tetrahedron Lett. 2000, 41, 4895–4899. doi:10.1016/S0040-4039(00)00756-5 |
| 9. | Ding, Q.; Liu, X.; Yu, J.; Zhang, Q.; Wang, D.; Cao, B.; Peng, Y. Tetrahedron 2012, 68, 3937–3941. doi:10.1016/j.tet.2012.03.098 |
| 18. | Ding, Q.; Ji, H.; Wang, D.; Lin, Y.; Yu, W.; Peng, Y. J. Organomet. Chem. 2012, 711, 62–67. doi:10.1016/j.jorganchem.2012.03.030 |
| 19. | Ding, Q.; Cao, B.; Zong, Z.; Peng, Y. J. Comb. Chem. 2010, 12, 370–373. doi:10.1021/cc100012a |
| 20. | Ding, Q.; Cao, B.; Liu, X.; Zong, Z.; Peng, Y. Green Chem. 2010, 12, 1607–1610. doi:10.1039/c0gc00123f |
| 21. | Ding, Q.; Wang, D.; Sang, X.; Lin, Y.; Peng, Y. Tetrahedron 2012, 68, 8869–8874. doi:10.1016/j.tet.2012.08.039 |
| 22. | Ding, Q.; Liu, X.; Wang, H.; Chen, M.; Peng, Y. Synthesis 2012, 920–926. doi:10.1055/s-0031-1289722 |
| 17. | Tárraga, A.; Molina, P.; López, J. L. Tetrahedron Lett. 2000, 41, 4895–4899. doi:10.1016/S0040-4039(00)00756-5 |
© 2013 Ding et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)