Search results
Search for "photoactivation" in Full Text gives 22 result(s) in Beilstein Journal of Organic Chemistry.
Effect of a photoswitchable rotaxane on membrane permeabilization across lipid compositions
- Udyogi N. K. Conthagamage,
- Lilia Lopez,
- Zuliah A. Abdulsalam and
- Víctor García-López
Beilstein J. Org. Chem. 2025, 21, 2498–2512, doi:10.3762/bjoc.21.192

- . Furthermore, comparison of the absorption spectra of all compounds at the same concentration shows that 1-E and 1-Z exhibit much stronger absorption at 370 and 467 nm than rotaxane 2 and axle 3, which absorb almost negligibly (Supporting Information File 1, Figure S23f). Thus, even if some photoactivation of

Graphical Abstract

Figure 1: a) Structural components of the rotaxanes (PEG, polyethylene glycol chain; BAA (benzylalkylammonium...

Figure 2: Photoisomerization of rotaxane 1.

Figure 3: Reversible photoswitching of rotaxane 1 in LUVs with varying lipid compositions. Left column: UV–vi...

Figure 4: Summary of sulforhodamine B release from LUVs of varying lipid compositions. a) Dye release after 7...

Figure 5: Percentage of sulforhodamine B released from EYPC/Chol 8:2 LUVs upon five irradiation cycles after ...

Figure 6: Percentage of sulforhodamine B released from LUVs containing rotaxane 1 upon five alternating light...

Figure 7: Evaluation of effect of axle 3 upon light exposure. a) Percentage of sulforhodamine B released from...

Figure 8: a) Illustration of rotaxane 4 in its preferred orientation within a lipid bilayer; percentage of su...
Recent advances in oxidative radical difunctionalization of N-arylacrylamides enabled by carbon radical reagents
- Jiangfei Chen,
- Yi-Lin Qu,
- Ming Yuan,
- Xiang-Mei Wu,
- Heng-Pei Jiang,
- Ying Fu and
- Shengrong Guo
Beilstein J. Org. Chem. 2025, 21, 1207–1271, doi:10.3762/bjoc.21.98

Graphical Abstract

Scheme 1: DTBP-mediated oxidative alkylarylation of activated alkenes.

Scheme 2: Iron-catalyzed oxidative 1,2-alkylarylation.

Scheme 3: Possible mechanism for the iron-catalyzed oxidative 1,2-alkylation of activated alkenes.

Scheme 4: A metal-free strategy for synthesizing 3,3-disubstituted oxindoles.

Scheme 5: Iminoxyl radical-promoted cascade oxyalkylation/alkylarylation of alkenes.

Scheme 6: Proposed mechanism for the iminoxyl radical-promoted cascade oxyalkylation/alkylarylation of alkene...

Scheme 7: Bicyclization of 1,n-enynes with alkyl nitriles.

Scheme 8: Possible reaction mechanism for the bicyclization of 1,n-enynes with alkyl nitriles.

Scheme 9: Radical cyclization of N-arylacrylamides with isocyanides.

Scheme 10: Plausible mechanism for the radical cyclization of N-arylacrylamides with isocyanides.

Scheme 11: Electrochemical dehydrogenative cyclization of 1,3-dicarbonyl compounds.

Scheme 12: Plausible mechanism for the dehydrogenative cyclization of 1,3-dicarbonyl compounds.

Scheme 13: Photocatalyzed cyclization of N-arylacrylamide and N,N-dimethylaniline.

Scheme 14: Proposed mechanism for the photocatalyzed cyclization of N-arylacrylamides and N,N-dimethylanilines....

Scheme 15: Electrochemical monofluoroalkylation cyclization of N-arylacrylamides with dimethyl 2-fluoromalonat...

Scheme 16: Proposed mechanism for the electrochemical radical cyclization of N-arylacrylamides with dimethyl 2...

Scheme 17: Photoelectrocatalytic carbocyclization of unactivated alkenes using simple malonates.

Scheme 18: Plausible mechanism for the photoelectrocatalytic carbocyclization of unactivated alkenes with simp...

Scheme 19: Bromide-catalyzed electrochemical trifluoromethylation/cyclization of N-arylacrylamides.

Scheme 20: Proposed mechanism for the electrochemical trifluoromethylation/cyclization of N-arylacrylamides.

Scheme 21: Visible light-mediated trifluoromethylarylation of N-arylacrylamides.

Scheme 22: Plausible reaction mechanism for the visible light-mediated trifluoromethylarylation of N-arylacryl...

Scheme 23: Electrochemical difluoroethylation cyclization of N-arylacrylamides with sodium difluoroethylsulfin...

Scheme 24: Electrochemical difluoroethylation cyclization of N-methyacryloyl-N-alkylbenzamides with sodium dif...

Scheme 25: Photoredox-catalyzed radical aryldifluoromethylation of N-arylacrylamides with S-(difluoromethyl)su...

Scheme 26: Proposed mechanism for the photoredox-catalyzed radical aryldifluoromethylation of N-arylacrylamide...

Scheme 27: Visible-light-induced domino difluoroalkylation/cyclization of N-cyanamide alkenes.

Scheme 28: Proposed mechanism of photoredox-catalyzed radical domino difluoroalkylation/cyclization of N-cyana...

Scheme 29: Palladium-catalyzed oxidative difunctionalization of alkenes.

Scheme 30: Two possible mechanisms of palladium-catalyzed oxidative difunctionalization.

Scheme 31: Silver-catalyzed oxidative 1,2-alkyletherification of unactivated alkenes with α-bromoalkylcarbonyl...

Scheme 32: Photochemical radical cascade cyclization of dienes.

Scheme 33: Proposed mechanism for the photochemical radical cascade 6-endo cyclization of dienes with α-carbon...

Scheme 34: Photocatalyzed radical coupling/cyclization of N-arylacrylamides and.

Scheme 35: Photocatalyzed radical-type couplings/cyclization of N-arylacrylamides with sulfoxonium ylides.

Scheme 36: Possible mechanism of visible-light-induced radical-type couplings/cyclization of N-arylacrylamides...

Scheme 37: Visible-light-promoted difluoroalkylated oxindoles systhesis via EDA complexes.

Scheme 38: Possible mechanism for the visible-light-promoted radical cyclization of N-arylacrylamides with bro...

Scheme 39: A dicumyl peroxide-initiated radical cascade reaction of N-arylacrylamide with DCM.

Scheme 40: Possible mechanism of radical cyclization of N-arylacrylamides with DCM.

Scheme 41: An AIBN-mediated radical cascade reaction of N-arylacrylamides with perfluoroalkyl iodides.

Scheme 42: Possible mechanism for the reaction with perfluoroalkyl iodides.

Scheme 43: Photoinduced palladium-catalyzed radical annulation of N-arylacrylamides with alkyl halides.

Scheme 44: Radical alkylation/cyclization of N-Alkyl-N-methacryloylbenzamides with alkyl halides.

Scheme 45: Possible mechanism for the alkylation/cyclization with unactivated alkyl chlorides.

Scheme 46: Visible-light-driven palladium-catalyzed radical cascade cyclization of N-arylacrylamides with unac...

Scheme 47: NHC-catalyzed radical cascade cyclization of N-arylacrylamides with alkyl bromides.

Scheme 48: Possible mechanism of NHC-catalyzed radical cascade cyclization.

Scheme 49: Electrochemically mediated radical cyclization reaction of N-arylacrylamides with freon-type methan...

Scheme 50: Proposed mechanistic pathway of electrochemically induced radical cyclization reaction.

Scheme 51: Redox-neutral photoinduced radical cascade cylization of N-arylacrylamides with unactivated alkyl c...

Scheme 52: Proposed mechanistic hypothesis of redox-neutral radical cascade cyclization.

Scheme 53: Thiol-mediated photochemical radical cascade cylization of N-arylacrylamides with aryl halides.

Scheme 54: Proposed possible mechanism of thiol-mediated photochemical radical cascade cyclization.

Scheme 55: Visible-light-induced radical cascade bromocyclization of N-arylacrylamides with NBS.

Scheme 56: Possible mechanism of visible-light-induced radical cascade cyclization.

Scheme 57: Decarboxylation/radical C–H functionalization by visible-light photoredox catalysis.

Scheme 58: Plausible mechanism of visible-light photoredox-catalyzed radical cascade cyclization.

Scheme 59: Visible-light-promoted tandem radical cyclization of N-arylacrylamides with N-(acyloxy)phthalimides....

Scheme 60: Plausible mechanism for the tandem radical cyclization reaction.

Scheme 61: Visible-light-induced aerobic radical cascade alkylation/cyclization of N-arylacrylamides with alde...

Scheme 62: Plausible mechanism for the aerobic radical alkylarylation of electron-deficient amides.

Scheme 63: Oxidative decarbonylative [3 + 2]/[5 + 2] annulation of N-arylacrylamide with vinyl acids.

Scheme 64: Plausible mechanism for the decarboxylative (3 + 2)/(5 + 2) annulation between N-arylacrylamides an...

Scheme 65: Rhenium-catalyzed alkylarylation of alkenes with PhI(O2CR)2.

Scheme 66: Plausible mechanism for the rhenium-catalyzed decarboxylative annulation of N-arylacrylamides with ...

Scheme 67: Visible-light-induced one-pot tandem reaction of N-arylacrylamides.

Scheme 68: Plausible mechanism for the visible-light-initiated tandem synthesis of difluoromethylated oxindole...

Scheme 69: Copper-catalyzed redox-neutral cyanoalkylarylation of activated alkenes with cyclobutanone oxime es...

Scheme 70: Plausible mechanism for the copper-catalyzed cyanoalkylarylation of activated alkenes.

Scheme 71: Photoinduced alkyl/aryl radical cascade for the synthesis of quaternary CF3-attached oxindoles.

Scheme 72: Plausible photoinduced electron-transfer (PET) mechanism.

Scheme 73: Photoinduced cerium-mediated decarboxylative alkylation cascade cyclization.

Scheme 74: Plausible reaction mechanism for the decarboxylative radical-cascade alkylation/cyclization.

Scheme 75: Metal-free oxidative tandem coupling of activated alkenes.

Scheme 76: Control experiments and possible mechanism for 1,2-carbonylarylation of alkenes with carbonyl C(sp2...

Scheme 77: Silver-catalyzed acyl-arylation of activated alkenes with α-oxocarboxylic acids.

Scheme 78: Proposed mechanism for the decarboxylative acylarylation of acrylamides.

Scheme 79: Visible-light-mediated tandem acylarylation of olefines with carboxylic acids.

Scheme 80: Proposed mechanism for the radical cascade cyclization with acyl radical via visible-light photored...

Scheme 81: Erythrosine B-catalyzed visible-light photoredox arylation-cyclization of N-arylacrylamides with ar...

Scheme 82: Electrochemical cobalt-catalyzed radical cyclization of N-arylacrylamides with arylhydrazines or po...

Scheme 83: Proposed mechanism of radical cascade cyclization via electrochemical cobalt catalysis.

Scheme 84: Copper-catalyzed oxidative tandem carbamoylation/cyclization of N-arylacrylamides with hydrazinecar...

Scheme 85: Proposed reaction mechanism for the radical cascade cyclization by copper catalysis.

Scheme 86: Visible-light-driven radical cascade cyclization reaction of N-arylacrylamides with α-keto acids.

Scheme 87: Proposed mechanism of visible-light-driven cascade cyclization reaction.

Scheme 88: Peroxide-induced radical carbonylation of N-(2-methylallyl)benzamides with methyl formate.

Scheme 89: Proposed cyclization mechanism of peroxide-induced radical carbonylation with N-(2-methylallyl)benz...

Scheme 90: Persulfate promoted carbamoylation of N-arylacrylamides and N-arylcinnamamides.

Scheme 91: Proposed mechanism for the persulfate promoted radical cascade cyclization reaction of N-arylacryla...

Scheme 92: Photocatalyzed carboacylation with N-arylpropiolamides/N-alkyl acrylamides.

Scheme 93: Plausible mechanism for the photoinduced carboacylation of N-arylpropiolamides/N-alkyl acrylamides.

Scheme 94: Electrochemical Fe-catalyzed radical cyclization with N-arylacrylamides.

Scheme 95: Plausible mechanism for the electrochemical Fe-catalysed radical cyclization of N-phenylacrylamide.

Scheme 96: Substrate scope of the selective functionalization of various α-ketoalkylsilyl peroxides with metha...

Scheme 97: Proposed reaction mechanism for the Fe-catalyzed reaction of alkylsilyl peroxides with methacrylami...

Scheme 98: EDA-complex mediated C(sp2)–C(sp3) cross-coupling of TTs and N-methyl-N-phenylmethacrylamides.

Scheme 99: Proposed mechanism for the synthesis of oxindoles via EDA complex.
Light-enabled intramolecular [2 + 2] cycloaddition via photoactivation of simple alkenylboronic esters
- Lewis McGhie,
- Hannah M. Kortman,
- Jenna Rumpf,
- Peter H. Seeberger and
- John J. Molloy
Beilstein J. Org. Chem. 2025, 21, 854–863, doi:10.3762/bjoc.21.69
- , Germany 10.3762/bjoc.21.69 Abstract The photoactivation of organic molecules via energy transfer (EnT) catalysis is often limited to conjugated systems that have low-energy triplet excited states, with simple alkenes remaining an intractable challenge. The ability to address this limitation, using high
- energy sensitizers, would represent an attractive platform for future reaction design. Here, we disclose the photoactivation of simple alkenylboronic esters established using alkene scrambling as a rapid reaction probe to identify a suitable catalyst and boron motif. Cyclic voltammetry, UV–vis analysis
- prohibitively high in energy for selective reactivity [5]. The inception of energy transfer catalysis (EnT) has expedited discoveries concerning the photoactivation of organic molecules [15][16][17], enabling direct access to the triplet excited state through the use of a photocatalyst (Figure 1A, top

Graphical Abstract

Figure 1: A) Energy transfer catalysis of alkenes in organic synthesis. B) Energy transfer catalysis of conju...

Figure 2: Probing boron effects on reactivity (A) and confirming the generation of a photostationary state eq...

Figure 3: Probing EnT catalysis enabled [2 + 2] cycloaddition of simple alkenylboronic esters.

Scheme 1: Establishing the substrate scope. Conditions: 3 (1 equiv), xanthone (20 mol %), MeCN (0.03 M), unde...

Scheme 2: A) Product derivatization and B) transition-metal EnT catalysis. Reaction conditions A): 4d (1 equi...
Synthesis of HBC fluorophores with an electrophilic handle for covalent attachment to Pepper RNA
- Raphael Bereiter and
- Ronald Micura
Beilstein J. Org. Chem. 2025, 21, 727–735, doi:10.3762/bjoc.21.56
- acids. Rather, halides with enhanced electrophilic potency, such as α-halocarbonyls [14][15][16], nitrogen (half-)mustards [17][18], or epoxides [19][20], Michael acceptors [17], carbamates [17], imidazolides [17], squarates [17], and diaziridines [17] (the latter requiring photoactivation) have been

Graphical Abstract

Figure 1: Structure-guided approach for engineering the (non-covalent) fluorescent light-up aptamer Pepper in...

Scheme 1: Chemical structures of the HBC dye family [7]. Variations to HBC530 highlighted in red color. All dyes...

Scheme 2: Synthesis of bromoalkyl HBC derivatives 7, 8, and 9.

Scheme 3: Synthesis of the HBC ether derivative 11.

Figure 2: Pepper aptamer reacts with different HBC derivatives. Chemical structures of the HBC derivatives us...

Scheme 4: Derivatization of the HBC fluorophore 5 to generate handles with distinct electrophilic groups.

Scheme 5: Synthesis of mesylated HBC fluorophores 16, 17, and 18.

Scheme 6: Synthesis of the bifunctional HBC fluorophore 22. For an application of 22 (pulldown of circular Pe...
Synthesis of π-conjugated polycyclic compounds by late-stage extrusion of chalcogen fragments
- Aissam Okba,
- Pablo Simón Marqués,
- Kyohei Matsuo,
- Naoki Aratani,
- Hiroko Yamada,
- Gwénaël Rapenne and
- Claire Kammerer
Beilstein J. Org. Chem. 2024, 20, 287–305, doi:10.3762/bjoc.20.30
- hydride resulted in the formation of the oxepine ring by a double substitution reaction, to yield the desired dinaphthooxepine 33. The non-planar character of dinaphthooxepine bisimides was confirmed by X-ray crystal structure, and stability towards thermal or photoactivation was also established. Cyclic
- product thiarubrine A [70]. Examples of S-extrusion from annelated 1,2-dithiins under photoactivation (top) or thermal activation (bottom) [71][72]. Synthesis of dibenzo[1,4]dithiapentalene upon photoextrusion of SO2 [78]. Extrusion of SO in naphthotrithiin-2-oxides for the synthesis of 2,5

Graphical Abstract

Scheme 1: “Precursor approach” for the synthesis of π-conjugated polycyclic compounds, with the thermally- or...

Scheme 2: Valence isomerization of chalcogen heteropines and subsequent cheletropic extrusion in the case of ...

Scheme 3: Early example of phenanthrene synthesis via a chemically-induced S-extrusion (and concomitant decar...

Scheme 4: Top: Conversion of dinaphthothiepine bisimides 3a,b and their sulfoxide analogues 4a,b into PBIs 6a,...

Figure 1: Top view (a) and side view (b) of the X-ray crystal structure of thiepine 3b showing its bent confo...

Scheme 5: Modular synthetic route towards dinaphthothiepines 3a–f and the corresponding S-oxides 4a–d, incorp...

Scheme 6: Top: Conversion of dithienobenzothiepine monomeric units into dithienonaphthalenes, upon S-extrusio...

Scheme 7: Synthesis of S-doped extended triphenylene derivative 22 from 3-bromothiophene (17) with the therma...

Scheme 8: Top: Synthesis of thermally-stable O-doped HBC 26a. Bottom: Synthesis of S- and Se-based soluble pr...

Scheme 9: Synthesis of dinaphthooxepine bisimide 33 and conversion into PBI 6f by O-extrusion triggered by el...

Figure 2: Cyclic voltammogram of dinaphthooxepine 33, evidencing the irreversibility of the reduction process...

Scheme 10: Top: Early example of 6-membered ring contraction with concomitant S-extrusion leading to dinaphtho...

Scheme 11: Examples of S-extrusion from annelated 1,2-dithiins under photoactivation (top) or thermal activati...

Scheme 12: Synthesis of dibenzo[1,4]dithiapentalene upon photoextrusion of SO2 [78].

Scheme 13: Extrusion of SO in naphthotrithiin-2-oxides for the synthesis of 2,5-dihydrothiophene 1-oxides [79].

Scheme 14: SO-extrusion as a key step in the synthesis of fullerenes (C60 and C70) encapsulating H2 molecules [80,82]....

Scheme 15: Synthesis of diepoxytetracene precursor 56 and its on-surface conversion into tetracene upon O-extr...

Scheme 16: Soluble precursors of hexacene, decacene and dodecacene incorporating 1,4-epoxides in their hydroca...

Scheme 17: Synthesis of tetraepoxide 59 as soluble precursor of decacene [85].

Figure 3: Constant-height STM measurement of decacene on Au(111) using a CO-functionalized tip (sample voltag...
Recent advancements in iodide/phosphine-mediated photoredox radical reactions
- Tinglan Liu,
- Yu Zhou,
- Junhong Tang and
- Chengming Wang
Beilstein J. Org. Chem. 2023, 19, 1785–1803, doi:10.3762/bjoc.19.131
- amount of ammonium iodide under irradiation in the absence of triphenylphosphine (Scheme 12). The generation of alkyl radicals was attributed to the photoactivation of a transient electron donor–acceptor complex formed between iodide and N-(acyloxy)phthalimide, in line with earlier findings. These

Graphical Abstract

Scheme 1: Photocatalytic decarboxylative transformations mediated by the NaI/PPh3 catalyst system.

Scheme 2: Proposed catalytic cycle of NaI/PPh3 photoredox catalysis.

Scheme 3: Decarboxylative alkenylation of redox-active esters by NaI/PPh3 catalysis.

Scheme 4: Decarboxylative alkenylation mediated by NaI/PPh3 catalysis.

Scheme 5: NaI-mediated photoinduced α-alkenylation of Katritzky salts 7.

Scheme 6: n-Bu4NI-mediated photoinduced decarboxylative olefination.

Scheme 7: Proposed mechanism of the n-Bu4NI-mediated photoinduced decarboxylative olefination.

Scheme 8: Photodecarboxylative alkylation of redox-active esters with diazirines.

Scheme 9: Photoinduced iodine-anion-catalyzed decarboxylative/deaminative C–H alkylation of enamides.

Scheme 10: Photocatalytic C–H alkylation of coumarins mediated by NaI/PPh3 catalysis.

Scheme 11: Photoredox alkylation of aldimines by NaI/PPh3 catalysis.

Scheme 12: Photoredox C–H alkylation employing ammonium iodide.

Scheme 13: NaI/PPh3/CuBr cooperative catalysis for photocatalytic C(sp3)–O/N cross-coupling reactions.

Scheme 14: Proposed mechanism of NaI/PPh3/CuBr cooperative catalysis for photocatalytic C(sp3)–O/N cross-coupl...

Scheme 15: Photocatalytic decarboxylative [3 + 2]/[4 + 2] annulation between enynals and γ,σ-unsaturated N-(ac...

Scheme 16: Proposed mechanism for the decarboxylative [3 + 2]/[4 + 2] annulation.

Scheme 17: Decarboxylative cascade annulation of alkenes/1,6-enynes with N-hydroxyphthalimide esters.

Scheme 18: Decarboxylative radical cascade cyclization of N-arylacrylamides.

Scheme 19: NaI/PPh3-driven photocatalytic decarboxylative radical cascade alkylarylation.

Scheme 20: Proposed mechanism of the NaI/PPh3-driven photocatalytic decarboxylative radical cascade cyclizatio...

Scheme 21: Visible-light-promoted decarboxylative cyclization of vinylcycloalkanes.

Scheme 22: NaI/PPh3-mediated photochemical reduction and amination of nitroarenes.

Scheme 23: PPh3-catalyzed alkylative iododecarboxylation with LiI.

Scheme 24: Visible-light-triggered iodination facilitated by N-heterocyclic carbenes.

Scheme 25: Visible-light-induced photolysis of phosphonium iodide salts for monofluoromethylation.
Strategies to access the [5-8] bicyclic core encountered in the sesquiterpene, diterpene and sesterterpene series
- Cécile Alleman,
- Charlène Gadais,
- Laurent Legentil and
- François-Hugues Porée
Beilstein J. Org. Chem. 2023, 19, 245–281, doi:10.3762/bjoc.19.23

Graphical Abstract

Figure 1: Examples of terpenes containing a bicyclo[3.6.0]undecane motif.

Figure 2: Commercially available first and second generation Grubbs and Hoveyda–Grubbs catalysts.

Figure 3: Examples of strategies to access the fusicoccan and ophiobolin tricyclic core structure by RCM.

Scheme 1: Synthesis of bicyclic core structure 12 of ophiobolin M (13) and cycloaraneosene (14).

Scheme 2: Synthesis of the core structure 21 of ophiobolins and fusicoccanes.

Scheme 3: Ring-closing metathesis attempts starting from thioester 22.

Scheme 4: Total synthesis of ent-fusicoauritone (28).

Figure 4: General structure of ophiobolins and congeners.

Scheme 5: Total synthesis of (+)-ophiobolin A (8).

Scheme 6: Investigation of RCM for the synthesis of ophiobolin A (8). Path A) RCM with TBDPS-protected alcoho...

Scheme 7: Synthesis of the core structure of cotylenin A aglycon, cotylenol (50).

Scheme 8: Synthesis of tricyclic core structure of fusicoccans.

Scheme 9: Total synthesis of (−)-teubrevin G (59).

Scheme 10: Synthesis of the core skeleton 63 of the basmane family.

Scheme 11: Total synthesis of (±)-schindilactone A (68).

Scheme 12: Total synthesis of dactylol (72).

Scheme 13: Ring-closing metathesis for the total synthesis of (±)-asteriscanolide (2).

Scheme 14: Synthesis of the simplified skeleton of pleuromutilin (1).

Scheme 15: Total synthesis of (−)-nitidasin (93) using a ring-closing metathesis to construct the eight-member...

Scheme 16: Total synthesis of (±)-naupliolide (97).

Scheme 17: Synthesis of the A-B ring structure of fusicoccane (101).

Scheme 18: First attempts of TRCM of dienyne substrates.

Scheme 19: TRCM on optimized substrates towards the synthesis of ophiobolin A (8).

Scheme 20: Tandem ring-closing metathesis for the synthesis of variecolin intermediates 114 and 115.

Scheme 21: Synthesis of poitediol (118) using the allylsilane ring-closing metathesis.

Scheme 22: Access to scaffold 122 by a NHK coupling reaction.

Scheme 23: Key step to construct the [5-8] bicyclooctanone core of aquatolide (4).

Scheme 24: Initial strategy to access aquatolide (4).

Scheme 25: Synthetic plan to cotylenin A (130).

Scheme 26: [5-8] Bicyclic structure of brachialactone (7) constructed by a Mizoroki–Heck reaction.

Scheme 27: Influence of the replacement of the allylic alcohol moiety.

Scheme 28: Formation of variecolin intermediate 140 through a SmI2-mediated Barbier-type reaction.

Scheme 29: SmI2-mediated ketyl addition. Pleuromutilin (1) eight-membered ring closure via C5–C14 bond formati...

Scheme 30: SmI2-mediated dialdehyde cyclization cascade of [5-8-6] pleuromutilin scaffold 149.

Scheme 31: A) Modular synthetic route to mutilin and pleuromutilin family members by Herzon’s group. B) Scaffo...

Scheme 32: Photocatalyzed oxidative ring expansion in pleuromutilin (1) total synthesis.

Scheme 33: Reductive radical cascade cyclization route towards (−)-6-epi-ophiobolin N (168).

Scheme 34: Reductive radical cascade cyclization route towards (+)-6-epi-ophiobolin A (173).

Scheme 35: Radical 8-endo-trig-cyclization of a xanthate precursor.

Figure 5: Structural representations of hypoestin A (177), albolic acid (178), and ceroplastol II (179) beari...

Scheme 36: Synthesis of the common [5-8-5] tricyclic intermediate of hypoestin A (177), albolic acid (178), an...

Scheme 37: Asymmetric synthesis of hypoestin A (177), albolic acid (178), and ceroplastol II (179).

Figure 6: Scope of the Pauson–Khand reaction.

Scheme 38: Nazarov cyclization revealing the fusicoauritone core structure 192.

Scheme 39: Synthesis of fusicoauritone (28) through Nazarov cyclization.

Scheme 40: (+)-Epoxydictymene (5) synthesis through a Nicholas cyclization followed by a Pauson–Khand reaction...

Scheme 41: Synthesis of aquatolide (4) by a Mukaiyama-type aldolisation.

Scheme 42: Tandem Wolff/Cope rearrangement furnishing the A-B bicyclic moiety 204 of variecolin.

Scheme 43: Asymmetric synthesis of the A-B bicyclic core 205 and 206 of variecolin.

Scheme 44: Formation of [5-8]-fused rings by cyclization under thermal activation.

Scheme 45: Construction of the [5-8-6] tricyclic core structure of variecolin (3) by Diels–Alder reaction.

Scheme 46: Synthesis of the [6-4-8-5]-tetracyclic skeleton by palladium-mediated cyclization.

Scheme 47: Access to the [5-8] bicyclic core structure of asteriscanolide (227) through rhodium-catalyzed cycl...

Scheme 48: Total syntheses of asterisca-3(15),6-diene (230) and asteriscanolide (2) with a Rh-catalyzed cycliz...

Scheme 49: Photocyclization of 2-pyridones to access the [5-8-5] backbone of fusicoccanes.

Scheme 50: Total synthesis of (+)-asteriscunolide D (245) and (+)-aquatolide (4) through photocyclization.

Scheme 51: Biocatalysis pathway to construct the [5-8-5] tricyclic scaffold of brassicicenes.

Scheme 52: Influence of the CotB2 mutant over the cyclization’s outcome of GGDP.
A photochemical C=C cleavage process: toward access to backbone N-formyl peptides
- Haopei Wang and
- Zachary T. Ball
Beilstein J. Org. Chem. 2021, 17, 2932–2938, doi:10.3762/bjoc.17.202
- give amide 12 with a half-life (t1/2) of 6.4 h. The observation of N-formyl products can be rationalized with a bifurcating mechanism (Figure 5). Following photoactivation, H-atom abstraction and nucleophilic addition of water would produce the key intermediate B. Such hemi-aminal compounds would be

Graphical Abstract

Figure 1: Uncaging of peptide backbone N–H bonds from Chan–Lam-type modification.

Figure 2: Photocleavage of compounds 1 and 6 under basic conditions. Yield of products was calculated from cr...

Figure 3: (a) Photocleavage of compound 6 under acidic conditions. Yields determined by 1H NMR using residual...

Figure 4: Preparation and hydrolysis kinetics (inset) of N-formyl product 11. Dashed line: first-order decay ...

Figure 5: Proposed mechanism for the formation of aldehyde 3 and N-formyl product 8.
When metal-catalyzed C–H functionalization meets visible-light photocatalysis
- Lucas Guillemard and
- Joanna Wencel-Delord
Beilstein J. Org. Chem. 2020, 16, 1754–1804, doi:10.3762/bjoc.16.147

- the variety of possibilities in combining transition metal C–H activation and visible-light photoactivation to reach sustainable synthesis. The particular focus is put on the mechanisms of such complex catalytic systems, highlighting the unique reactivity that arises from the original combination of
- azoles. His research group discovered that the abundant and inexpensive CuI catalyst allowed the direct arylation of benzoxazoles under UV-photoactivation (Figure 46) [106]. Remarkably, this totally site-selective photoinduced C–H arylation took place at room temperature and the use of amino acid ligands

Graphical Abstract

Figure 1: Concept of dual synergistic catalysis.

Figure 2: Classification of catalytic systems involving two catalysts.

Figure 3: General mechanism for the dual nickel/photoredox catalytic system.

Figure 4: General mechanisms for C–H activation catalysis involving different reoxidation strategies.

Figure 5: Indole synthesis via dual C–H activation/photoredox catalysis.

Figure 6: Proposed mechanism for the indole synthesis via dual catalysis.

Figure 7: Oxidative Heck reaction on arenes via the dual catalysis.

Figure 8: Proposed mechanism for the Heck reaction on arenes via dual catalysis.

Figure 9: Oxidative Heck reaction on phenols via the dual catalysis.

Figure 10: Proposed mechanism for the Heck reaction on phenols via dual catalysis.

Figure 11: Carbazole synthesis via dual C–H activation/photoredox catalysis.

Figure 12: Proposed mechanism for the carbazole synthesis via dual catalysis.

Figure 13: Carbonylation of enamides via the dual C–H activation/photoredox catalysis.

Figure 14: Proposed mechanism for carbonylation of enamides via dual catalysis.

Figure 15: Annulation of benzamides via the dual C–H activation/photoredox catalysis.

Figure 16: Proposed mechanism for the annulation of benzamides via dual catalysis.

Figure 17: Synthesis of indoles via the dual C–H activation/photoredox catalysis.

Figure 18: Proposed mechanism for the indole synthesis via dual catalysis.

Figure 19: General concept of dual catalysis merging C–H activation and photoredox catalysis.

Figure 20: The first example of dual catalysis merging C–H activation and photoredox catalysis.

Figure 21: Proposed mechanism for the C–H arylation with diazonium salts via dual catalysis.

Figure 22: Dual catalysis merging C–H activation/photoredox using diaryliodonium salts.

Figure 23: Direct arylation via the dual catalytic system reported by Xu.

Figure 24: Direct arylation via dual catalytic system reported by Balaraman.

Figure 25: Direct arylation via dual catalytic system reported by Guo.

Figure 26: C(sp3)–H bond arylation via the dual Pd/photoredox catalytic system.

Figure 27: Acetanilide derivatives acylation via the dual C–H activation/photoredox catalysis.

Figure 28: Proposed mechanism for the C–H acylation with α-ketoacids via dual catalysis.

Figure 29: Acylation of azobenzenes via the dual catalysis C–H activation/photoredox.

Figure 30: C2-acylation of indoles via the dual C–H activation/photoredox catalysis.

Figure 31: Proposed mechanism for the C2-acylation of indoles with aldehydes via dual catalysis.

Figure 32: C2-acylation of indoles via the dual C–H activation/photoredox catalysis.

Figure 33: Perfluoroalkylation of arenes via the dual C–H activation/photoredox catalysis.

Figure 34: Proposed mechanism for perfluoroalkylation of arenes via dual catalysis.

Figure 35: Sulfonylation of 1-naphthylamides via the dual C–H activation/photoredox catalysis.

Figure 36: Proposed mechanism for sulfonylation of 1-naphthylamides via dual catalysis.

Figure 37: meta-C–H Alkylation of arenes via visible-light metallaphotocatalysis.

Figure 38: Alternative procedure for meta-C–H alkylation of arenes via metallaphotocatalysis.

Figure 39: Proposed mechanism for meta-C–H alkylation of arenes via metallaphotocatalysis.

Figure 40: C–H borylation of arenes via visible-light metallaphotocatalysis.

Figure 41: Proposed mechanism for C–H borylation of arenes via visible-light metallaphotocatalysis.

Figure 42: Undirected C–H aryl–aryl cross coupling via dual gold/photoredox catalysis.

Figure 43: Proposed mechanism for the undirected C–H aryl–aryl cross-coupling via dual catalysis.

Figure 44: Undirected C–H arylation of (hetero)arenes via dual manganese/photoredox catalysis.

Figure 45: Proposed mechanism for the undirected arylation of (hetero)arenes via dual catalysis.

Figure 46: Photoinduced C–H arylation of azoles via copper catalysis.

Figure 47: Photo-induced C–H chalcogenation of azoles via copper catalysis.

Figure 48: Decarboxylative C–H adamantylation of azoles via dual cobalt/photoredox catalysis.

Figure 49: Proposed mechanism for the C–H adamantylation of azoles via dual catalysis.

Figure 50: General mechanisms for the “classical” (left) and Cu-free variant (right) Sonogoshira reaction.

Figure 51: First example of a dual palladium/photoredox catalysis for Sonogashira-type couplings.

Figure 52: Arylation of terminal alkynes with diazonium salts via dual gold/photoredox catalysis.

Figure 53: Proposed mechanism for the arylation of terminal alkynes via dual catalysis.

Figure 54: C–H Alkylation of alcohols promoted by H-atom transfer (HAT).

Figure 55: Proposed mechanism for the C–H alkylation of alcohols promoted by HAT.

Figure 56: C(sp3)–H arylation of latent nucleophiles promoted by H-atom transfer.

Figure 57: Proposed mechanism for the C(sp3)–H arylation of latent nucleophiles promoted by HAT.

Figure 58: Direct α-arylation of alcohols promoted by H-atom transfer.

Figure 59: Proposed mechanism for the direct α-arylation of alcohols promoted by HAT.

Figure 60: C–H arylation of amines via dual Ni/photoredox catalysis.

Figure 61: Proposed mechanism for the C–H arylation of amines via dual Ni/photoredox catalysis.

Figure 62: C–H functionalization of nucleophiles via excited ketone/nickel dual catalysis.

Figure 63: Proposed mechanism for the C–H functionalization enabled by excited ketones.

Figure 64: Selective sp3–sp3 cross-coupling promoted by H-atom transfer.

Figure 65: Proposed mechanism for the selective sp3–sp3 cross-coupling promoted by HAT.

Figure 66: Direct C(sp3)–H acylation of amines via dual Ni/photoredox catalysis.

Figure 67: Proposed mechanism for the C–H acylation of amines via dual Ni/photoredox catalysis.

Figure 68: C–H hydroalkylation of internal alkynes via dual Ni/photoredox catalysis.

Figure 69: Proposed mechanism for the C–H hydroalkylation of internal alkynes.

Figure 70: Alternative procedure for the C–H hydroalkylation of ynones, ynoates, and ynamides.

Figure 71: Allylic C(sp3)–H activation via dual Ni/photoredox catalysis.

Figure 72: Proposed mechanism for the allylic C(sp3)–H activation via dual Ni/photoredox catalysis.

Figure 73: Asymmetric allylation of aldehydes via dual Cr/photoredox catalysis.

Figure 74: Proposed mechanism for the asymmetric allylation of aldehydes via dual catalysis.

Figure 75: Aldehyde C–H functionalization promoted by H-atom transfer.

Figure 76: Proposed mechanism for the C–H functionalization of aldehydes promoted by HAT.

Figure 77: Direct C–H arylation of strong aliphatic bonds promoted by HAT.

Figure 78: Proposed mechanism for the C–H arylation of strong aliphatic bonds promoted by HAT.

Figure 79: Direct C–H trifluoromethylation of strong aliphatic bonds promoted by HAT.

Figure 80: Proposed mechanism for the C–H trifluoromethylation of strong aliphatic bonds.
Photocontrolled DNA minor groove interactions of imidazole/pyrrole polyamides
- Sabrina Müller,
- Jannik Paulus,
- Jochen Mattay,
- Heiko Ihmels,
- Veronica I. Dodero and
- Norbert Sewald
Beilstein J. Org. Chem. 2020, 16, 60–70, doi:10.3762/bjoc.16.8
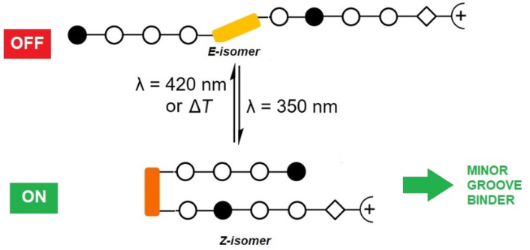
- been used to exert genomic effects on mRNA expression [56]. Therefore, the photoactivation of such interaction may be a future application. The incorporation of the photoswitchable 3-((3-(aminomethyl)phenyl)diazenyl)phenylacetic acid linker upon replacement of the γ-aminobutyric acid linker is a

Graphical Abstract

Scheme 1: Pyrrole–imidazole–azobenzene polyamides and the dsDNA target sequences employed in this study.

Scheme 2: Building blocks required for the synthesis of the photoswitchable Im/Py polyamides. A) Fmoc–Azo–OH 1...

Figure 1: Section of the 1H NMR (600 MHz) spectrum of polyamide P1. A) Initial thermal equilibrium. B) After ...

Figure 2: E/Z isomer ratio of the polyamides P1–P3. Values were obtained from the respective 1H NMR experimen...

Figure 3: Titration experiments of target DNA sequences with P1–P3 in the photostationary Z-state and the the...

Figure 4: Titration of DNA containing single mutations (in bold) with P1–P3 in the photostationary Z-state an...
Light-controllable dithienylethene-modified cyclic peptides: photoswitching the in vivo toxicity in zebrafish embryos
- Sergii Afonin,
- Oleg Babii,
- Aline Reuter,
- Volker Middel,
- Masanari Takamiya,
- Uwe Strähle,
- Igor V. Komarov and
- Anne S. Ulrich
Beilstein J. Org. Chem. 2020, 16, 39–49, doi:10.3762/bjoc.16.6

- ) was recorded, and then in situ illumination (“photoactivation”) was applied to switch into the ring-open photoform and obtain the respective LD50(opened) values (see Figure 2 for the study design and the killing curves). Each time, 12–15 or 18–20 zebrafish embryos at 3 dpf were treated by applying the
- 2.4 ± 0.2 µg/mL GS 1 and 2.1 ± 0.3 µg/mL ring-open 2. With increasing incubation time, lower concentrations were expected to achieve comparable mortality; however, the difference between 1 h and 24 h incubation was low for both peptides (Table 2). The photoactivation assay, in which two photoisomers
- displayed an about 16-fold decrease in the LD50 value after in situ photoactivation. This difference indicates that the ring-open isomer of 2 indeed possesses a higher toxicity than the ring-closed isomer, and it demonstrates in vivo photoswitching of the whole-body toxicity. Since the determined LD50

Graphical Abstract

Figure 1: DAE photoswitch and photoswitchable peptides explored in this study. (A) The reversible photoisomer...

Figure 2: Two versions of the D. rerio embryotoxicity assay for DAE-modified peptides: timelines, peptide pho...

Figure 3: The in vivo toxicity against D. rerio embryos appears to be correlated with the empirical hydrophob...

Figure 4: D. rerio embryotoxicity of GS 1 and the photoswitchable analogues 2–20 correlated with their in vit...

Figure 5: Phototherapeutic cytotoxic action against HeLa cells of GS 1 and its photoswitchable analogues 2–20...
N-Arylphenothiazines as strong donors for photoredox catalysis – pushing the frontiers of nucleophilic addition of alcohols to alkenes
- Fabienne Speck,
- David Rombach and
- Hans-Achim Wagenknecht
Beilstein J. Org. Chem. 2019, 15, 52–59, doi:10.3762/bjoc.15.5
- ion. This was one of the features to use N-phenylphenothiazine for the photoactivation of SF6 for the pentafluorosulfanylation of styrenes [2]. This two photon concept can further be extended to the photoredox catalytically generation of hydrated electrons as very powerful reductants (E = −2.8 V (vs

Graphical Abstract

Figure 1: Reduction potentials (vs SCE) of common photoredox catalysts, pyrene 16 and phenothiazine 2, in com...

Figure 2: Acceptor or donor-modified phenothiazines 1–11 as potential photoredox catalysts.

Figure 3: Normalized UV–vis absorption spectra above 290 nm of N-phenylphenothiazines 1–11 (left) and represe...

Figure 4: Proposed mechanism for the photoredox-catalyzed addition of methanol to α-methylstyrene (13a). (ET ...
Diazirine-functionalized mannosides for photoaffinity labeling: trouble with FimH
- Femke Beiroth,
- Tomas Koudelka,
- Thorsten Overath,
- Stefan D. Knight,
- Andreas Tholey and
- Thisbe K. Lindhorst
Beilstein J. Org. Chem. 2018, 14, 1890–1900, doi:10.3762/bjoc.14.163
- these requirements particularly well. The diazirine photophore is small and its photoactivation is possible at wavelengths around 350 nm and thus does not perturb protein structures. According to the literature, irradiation of a diazirine-functionalized ligand leads to a reactive carbene which can

Graphical Abstract

Figure 1: Principle of photoaffinity labeling of proteins with diazirine derivatives. Photolabile ligands are...

Figure 2: FimH crystal structure (pdb code 1KLF) with docked p-nitrophenyl α-D-mannopyranoside (1, pNPMan). F...

Figure 3: Based on the structure of the known FimH ligands 1 and 2, three photolabile α-D-mannosides, 3–5, we...

Figure 4: Connolly representation of photolabile α-D-mannoside 3 in the closed gate (A, PDB code 1UWF) and op...

Scheme 1: Synthesis of photolabile α-D-mannosides 3 and 4. a) H2, Pd-C, methanol, rt, 6 h, 94%; b) HATU, DIPE...

Figure 5: ESIMS spectra of peptide M2 before (A) and after photoreaction (B) with mannoside 3.

Figure 6: Intact protein ESIMS spectra of FimHtr labeling with diazirine derivative 3 with (A) 50% acetonitri...
Stimuli-responsive oligonucleotides in prodrug-based approaches for gene silencing
- Françoise Debart,
- Christelle Dupouy and
- Jean-Jacques Vasseur
Beilstein J. Org. Chem. 2018, 14, 436–469, doi:10.3762/bjoc.14.32

Graphical Abstract

Scheme 1: Demasking under reducing agents of ON prodrugs modified as phosphotriesters with A) benzyl groups [13] ...

Scheme 2: A) Synthesis via phosphoramidite chemistry and B) demasking under the reducing environment of 2’-O-...

Scheme 3: Synthesis via phosphoramidite chemistry of various 2’-O-alkyldithiomethyl (RSSM)-modified RNAs bear...

Scheme 4: A) siRNA conjugates to cholesterol [19] and B) PNA conjugates to a triphenylphosphonium [20] through a disu...

Scheme 5: Synthesis via phosphoramidite chemistry and deprotection mediated by nitroreductase/NADH of hypoxia...

Scheme 6: Synthesis via phosphoramidite chemistry and conversion mediated by nitroreductase/NADH of hypoxia-a...

Scheme 7: Incorporation of O6-(4-nitrobenzyl)-2’-deoxyguanosine into an ON prone to form a G-quadruplex struc...

Scheme 8: Synthesis and mechanism for the demasking of ON prodrugs from A) S-acylthioethyl phosphotriester [29] a...

Figure 1: Oligothymidylates bearing A) 2,2-bis(ethoxycarbonyl)-3-(pivaloyloxy)propyl- and B) 2-cyano-2(2-phen...

Figure 2: Oligothymidylates containing esterase and thermo-labile (4-acetylthio-2,2-dimethyl-3-oxobutyl) phos...

Scheme 9: Phosphoramidites and the corresponding RNA prodrugs protected as A) t-Bu-SATE, B) OH-SATE and C) A-...

Scheme 10: Mechanism of the hydrolysis of 2’-O-acyloxymethyl ONs mediated by carboxyesterases [46]. The hydrolysis...

Scheme 11: Synthesis of partially 2’-O-PivOM-modified RNAs [49] and 2’-O-PiBuOM-modified RNAs [53] using their corresp...

Figure 3: A) 2’-O-amino and guanidino-containing acetal ester phosphoramidites and B) 2’-O-(amino acid) aceta...

Scheme 12: Prodrugs of tricyclo-ONs functionalized with A) ethyl (tcee-T) and B) hexadecyl (tchd-T) ester func...

Scheme 13: Demasking mechanism of fma thiophosphate triesters in CpG ODN upon heat action [58].

Scheme 14: Thermolytic cleavage of the hydroxy-alkylated thiophosphate and phosphato-/thiophosphato-alkylated ...

Scheme 15: Synthesis via phosphoramidite chemistry and thermolytic cleavage of alkylated (diisopropyl, diethyl...

Scheme 16: Synthesis of thermosensitive prodrugs of ODNs containing fma thiophosphate triesters combined to po...

Scheme 17: Caging of deoxycytidine in methylphosphonate ONs by using the thermolabile phenylsulfonylcarbamoyl ...

Figure 4: Biotinylated 1-(5-(aminomethyl)-2-nitrophenyl)ethyl phosphoramidite used to cage the 5’-end of a si...

Scheme 18: Introduction and cleavage of 1-(4,5-dimethoxy-2-nitrophenyl)ethyl (DMNPE) [74] and cyclododecyl-DMNPE (...

Scheme 19: Post-synthetic introduction of a thioether-enol phosphodiester (TEEP) linkage into a DNAzyme by the...

Scheme 20: A) NPP dT and dG phosphoramidites [91,92] and B) NPOM U and G phosphoramidites [83] used to introduce photocag...

Scheme 21: Introduction of the photocaged 3-NPOM nucleobase into phosphorothioate antisense and morpholino ant...

Scheme 22: Control of the activity of an antisense ODN using a photocaged hairpin [82]. Formation of the hairpin s...

Scheme 23: Control of alternative splicing using phosphorothioate (PS) 2’-OMe-photocaged ONs resulting from th...

Scheme 24: A) Light activation of a photocaged DNAzyme incorporating 3-NPOM thymidine in its catalytic site [87]; ...

Scheme 25: Incorporation of 3-(6-nitropiperonyloxymethyl) (NPOM) thymidine and 4-nitropiperonylethyl (NPE) deo...

Scheme 26: Synthesis of a photocaged DNA decoy from a 3-NPOM thymidine phosphoramidite and release of the NPOM...

Scheme 27: Synthesis of a caged DNA decoy hairpin containing a 7-nitroindole nucleotide and release of the mod...

Figure 5: Caged-2’-adenosines used by MacMillan et al [93,94] (X = O) and Piccirilli et al [95] (X = S) to study RNA mec...

Scheme 28: Synthesis of circular ODNs containing a photolabile linker as described by Tang et al. [101,104].

Scheme 29: Control of RNA digestion with RNase H using light activation of a photocaged hairpin [97].

Scheme 30: Photocontrol of RNA degradation using caged circular antisense ODNs containing a photoresponsive li...

Scheme 31: Control of RNA translation using an “RNA bandage” consisting of two short 2’-OMe ONs linked togethe...

Scheme 32: Control of alternative splicing using photocaged ONs resulting from the incorporation of an o-nitro...

Scheme 33: A) Light deactivation of a photocaged DNAzyme incorporating one photocleavable spacer in its cataly...

Scheme 34: Solid-phase synthesis of a caged vit E-siRNA conjugate and its release upon UV irradiation [106].

Scheme 35: Synthesis of a siRNA conjugated to a nanoparticle (NP) via a cyclooctene heterolinker from a siRNA-...
The reductive decyanation reaction: an overview and recent developments
- Jean-Marc R. Mattalia
Beilstein J. Org. Chem. 2017, 13, 267–284, doi:10.3762/bjoc.13.30
- ) iodide/THF/HMPA at respectively 0 °C and room temperature [123]. Metallic samarium can also promote the decyanation [124]. Doni and Murphy have reported the reductive decyanation of malononitriles and α-cyanoesters by using the neutral organic electron donor 78 (Scheme 25) under photoactivation (method A

Graphical Abstract

Scheme 1: Mechanism for the reduction under metal dissolving conditions.

Scheme 2: Example of decyanation in metal dissolving conditions coupled with deprotection [30]. TBDMS = tert-buty...

Scheme 3: Preparation of α,ω-dienes [18,33].

Scheme 4: Cyclization reaction using a radical probe [18].

Scheme 5: Synthesis of (±)-xanthorrhizol (8) [39].

Scheme 6: Mechanism for the reduction of α-aminonitriles by hydride donors.

Scheme 7: Synthesis of phenanthroindolizidines and phenanthroquinolizidines [71].

Scheme 8: Two-step synthesis of 5-unsubstituted pyrrolidines (25 examples and 1 synthetic application, see be...

Scheme 9: Synthesis of (±)-isoretronecanol 19. DBU = 1,8-diazabicyclo[5.4.0]undec-7-ene [74].

Scheme 10: Proposed mechanism with 14a for the NaBH4 induced decyanation reaction (“BH3” = BH3·THF) [74].

Scheme 11: Reductive decyanation by a sodium hydride–iodide composite (26 examples) [81].

Scheme 12: Proposed mechanism for the reduction by NaH [81].

Scheme 13: Reductive decyanation catalyzed by nickel nanoparticles. Yields are given in weight % from GC–MS da...

Scheme 14: Decyanation of 2-cyanobenzo[b]thiophene [87].

Scheme 15: Simplified pathways involved in transition-metal-promoted reductive decyanations [93,95].

Scheme 16: Fe-catalyzed reductive decyanation. Numbers in square brackets represent turnover numbers. The TONs...

Scheme 17: Rh-catalyzed reductive decyanation of aryl nitriles (18 examples, 2 synthetic applications) [103].

Scheme 18: Rh-catalyzed reductive decyanation of aliphatic nitriles (15 examples, one synthetic application) [103].

Scheme 19: Ni-catalyzed reductive decyanation (method A: 28 examples and 2 synthetic applications; method B: 3...

Scheme 20: Reductive decyanation catalyzed by the nickel complex 58 (method A, 14 examples, yield ≥ 20% and 1 ...

Scheme 21: Proposed catalytic cycle for the nickel complex 58 catalyzed decyanation (method A). Only the cycle...

Scheme 22: Synthesis of bicyclic lactones [119,120].

Scheme 23: Reductive decyanation of malononitriles and cyanoacetates using NHC-boryl radicals (9 examples). Fo...

Scheme 24: Proposed mechanism for the reduction by NHC-boryl radicals. The other possible pathway (addition of ...

Scheme 25: Structures of organic electron-donors. Only the major Z isomer of 80 is shown [125,127].

Scheme 26: Reductive decyanation of malononitriles and cyanoacetates using organic electron-donors (method A, ...

Scheme 27: Photoreaction of dibenzylmalononitrile with 81 [128].

Scheme 28: Examples of decyanation promoted in acid or basic media [129,131,134,135].

Scheme 29: Mechanism proposed for the base-induced reductive decyanation of diphenylacetonitriles [136].

Scheme 30: Reductive decyanation of triarylacetonitriles [140].
A self-assembled cyclodextrin nanocarrier for photoreactive squaraine
- Ulrike Kauscher and
- Bart Jan Ravoo
Beilstein J. Org. Chem. 2016, 12, 2535–2542, doi:10.3762/bjoc.12.248

- the importance of introducing a platform for the immobilization of the squaraines as to provide steric separation and suppress aggregation. In the next step, we investigated the photoactivation under irradiation. To this end, a solution of AdSq was irradiated with light of a wavelength higher than 630

Graphical Abstract

Figure 1: Schematic representation of adamantane-substituted squaraine (AdSq) binding as a divalent guest to ...

Figure 2: Absorption spectra of AdSq in acetonitrile. [AdSq] = 7.5 µM. Top: Absorption at different time poin...

Figure 3: Top: Emission spectra (ex: 630 nm) of AdSq immobilized at CDV. [CDV] = 0–100 µM; [AdSq] = 5 µM. Bot...

Figure 4: Confocal fluorescence microscopy of giant unilamellar vesicles (GUVs) of amphiphilic cyclodextrins ...

Figure 5: Top: Absorption spectra at different time points during irradiation of a CDV solution with AdSq imm...

Figure 6: Synthesis of AdSq (I) NaH, DMF, 1,6-dibromohexane, 24 h, rt. (II) benzothiazole, acetonitrile, 12 h...
Preparative semiconductor photoredox catalysis: An emerging theme in organic synthesis
- David W. Manley and
- John C. Walton
Beilstein J. Org. Chem. 2015, 11, 1570–1582, doi:10.3762/bjoc.11.173
- a reaction partner because on photoactivation it supplies electrons and holes while also donating protons from surface hydroxy groups. An interesting adjunct to these results was our finding that aryloxy-, arylthio- and anilino-acetic acids, carrying electron donor substituents (2-, 3- or 4-MeO, t
- generation of acyl radicals. Radical polymerizations are hugely important industrially and it seems certain SCPC has a role to play in this area too. Production and utilization of h+ and e– by photoactivation of a semiconductor. Photoredox activity of TiO2 with moist air. TiO2 promoted oxidation of

Graphical Abstract

Figure 1: Production and utilization of h+ and e– by photoactivation of a semiconductor.

Figure 2: Photoredox activity of TiO2 with moist air.

Scheme 1: TiO2 promoted oxidation of phenanthrene [29].

Scheme 2: SCPC assisted additions of allylic compounds to diazines and imines [40-42].

Scheme 3: TiO2 promoted addition and addition–cyclization reactions of tert-amines with electron-deficient al...

Scheme 4: Reactions of amines promoted by Pt-TiO2 [48,49].

Scheme 5: P25 Promoted alkylations of N-phenylmaleimide with diverse carboxylic acids [53,54]. aAccompanied by R–R d...

Scheme 6: SCPC cyclizations of aryloxyacetic acids with suitably sited alkene acceptors [54]. aYields in brackets...

Scheme 7: TiO2 promoted reactions of aryloxyacetic acids with maleic anhydride and maleimides [53,54].

Scheme 8: Photoredox addition–cyclization reactions of aryloxyacetic and related acids promoted by maleimide [63]....

Scheme 9: SCPC promoted homo-couplings and macrocyclizations with carboxylic acids [64].

Scheme 10: TiO2 promoted alkylations of alkenes with silanes [66] and thiols [67].

Scheme 11: TiO2 reduction of a nitrochromenone derivative [70].

Scheme 12: TiO2 mediated hydrodehalogenations and cyclizations of organic iodides [71].

Scheme 13: TiO2 promoted hydrogenations of maleimides, maleic anhydride and aromatic aldehydes [79].

Scheme 14: Mechanistic sketch of SCPC hydrogenation of aryl aldehydes.
One-pot functionalisation of N-substituted tetrahydroisoquinolines by photooxidation and tunable organometallic trapping of iminium intermediates
- Joshua P. Barham,
- Matthew P. John and
- John A. Murphy
Beilstein J. Org. Chem. 2014, 10, 2981–2988, doi:10.3762/bjoc.10.316
- -polar solvents, yet low photocatalyst solubility in these solvents precluded photoactivation of 4a. Thus, a solvent switch was used to capitalise on the beneficial properties of both solvents. At this stage, we employed an MeCN/H2O (4:1) solvent system and Ru(bpy)3Cl2 in photoactivations which
- in a 1:4:4 ratio by LC–MS, respectively (Figure 2). First, we sought to rule out the possibility of Ru(bpy)3Cl2 promoting these undesired pathways. Although Ru(bpy)3Cl2 could not be separated from 5a after photoactivation due to their similar polarities, we successfully separated the less polar
- used, allylindium reagents generated and indium trihalide salt byproducts are non-toxic [43]. Our conditions benefit from the absence of amide side-products typically effected by peroxide intermediates in aerobic photoactivation of THIQs [16][22] and so our methodology serves to complement existing

Graphical Abstract

Figure 1: Examples of biologically active 1,2-disubstituted tetrahydroisoquinolines.

Scheme 1: Oxidative C–H functionalisation and examples of previously reported nucleophilic trappings.

Figure 2: Products from allylzinc reagent addition to 5a and 5b.

Figure 3: Proposed mechanism for formation of side-product 8a. Analogous reactivity in the formation of cycli...

Figure 4: Mechanism for dimerisation of the allylzinc halide and β-hydride addition to 5a [36].

Scheme 2: A concise synthesis of methopholine (3).
[3 + 2]-Cycloadditions of nitrile ylides after photoactivation of vinyl azides under flow conditions
- Stephan Cludius-Brandt,
- Lukas Kupracz and
- Andreas Kirschning
Beilstein J. Org. Chem. 2013, 9, 1745–1750, doi:10.3762/bjoc.9.201

Graphical Abstract

Scheme 1: Formation of azirines 2 from vinyl azides 1, photoinduced ring-opening to the nitrile ylides 3, and...

Scheme 2: Solid-phase assisted synthesis of vinyl azides 1 from alkenes 6 under flow conditions [9].

Scheme 3: Schematic presentation of the flow set-up for the synthesis of 2H-azirines 2 under inductive heatin...

Scheme 4: Photoinduced cycloadditions of vinyl azides 1a–f and electron-deficient alkenes 4a–d. All experimen...

Scheme 5: Photoinduced cycloaddtion of vinyl azide 1c and diisopropyl azodicarboxylate (4e). The experiment w...

Scheme 6: Photoinduced cycloaddtion of vinyl azide 1b and alkyne 4f. The experiment was conducted at room tem...

Scheme 7: Formation of 2,5-dihydrooxazole 9 starting from vinyl azide 1g under flow conditions (c = 0.01 M, f...
A chemist and biologist talk to each other about caged neurotransmitters
- Graham C.R. Ellis-Davies
Beilstein J. Org. Chem. 2013, 9, 64–73, doi:10.3762/bjoc.9.8
- rapid photoactivation of a particular enzyme, the Na,K-ATPase. It was the latter group that dubbed such photochemical probes “caged compounds”. This simple term has been adopted by biologists since that time [3][4][5][6][7][8][9], perhaps because the photolabile ATP compound was the one that was used in

Graphical Abstract

Figure 1: Structures of various caging chromophores. Abbreviations: Noc, N-nitrophenethyloxycarbonyl; CNB, ca...

Figure 2: Comparative two-photon uncaging of MNI-Glu and CDNI-Glu on pyramidal neurons in an acutely isolated...
Fine-tuning alkyne cycloadditions: Insights into photochemistry responsible for the double-strand DNA cleavage via structural perturbations in diaryl alkyne conjugates
- Wang-Yong Yang,
- Samantha A. Marrone,
- Nalisha Minors,
- Diego A. R. Zorio and
- Igor V. Alabugin
Beilstein J. Org. Chem. 2011, 7, 813–823, doi:10.3762/bjoc.7.93
- acetamides. The significant protecting effect of the hydroxyl radical and singlet oxygen scavengers to DNA cleavage was shown only with m-lysine conjugate. All three isomeric lysine conjugates inhibited human melanoma cell growth under photoactivation: The p-conjugate had the lowest CC50 (50% cell
- photochemistry is vital for unraveling the mechanistic scenarios that account for DNA cleavage by these compounds (Figure 3) [25]. As illustrated in Figure 3, multiple reaction pathways are potentially unlocked by the photoactivation of alkyne conjugates. In the past, we observed dramatic differences in
- The ability of compounds 1, 6, and 7 to inhibit cell proliferation in human melanoma cell lines was tested in the dark and under photoactivation (Figure 9). According to the control experiments with all three conjugates in the dark, these compounds do not inhibit cell proliferation at concentrations

Graphical Abstract

Figure 1: Structure of C-lysine conjugates.

Figure 2: Alternative pathways of enediyne photoreactivity: photo-Bergman cyclization (left), C1–C5 cyclizati...

Figure 3: Summary of possible mechanistic alternatives for the observed DNA cleavage by monoacetylene conjuga...

Scheme 1: Proposed mechanism of photocycloaddition of acetylene with 1,4-CHD.

Figure 4: p-, m-, and o-amidyl acetylenes and respective lysine conjugates.

Scheme 2: Synthesis of amido-substituted monoacetylenes and lysine conjugates. Reagents and conditions: a. Pd...

Scheme 3: Photochemical reactions of TFP-substituted aryl alkynes with selected π-systems. In short, the reac...

Scheme 4: Photocycloaddition of amido acetylenes with 1,4-CHD.

Scheme 5: Possible mechanism for photochemical hydration of diaryl acetylene moiety catalyzed by the ortho-am...

Figure 5: Stern–Volmer plots of three regioisomers, 3 (blue diamond), 4 (red square), and 5 (green triangle),...

Figure 6: Absorption spectra of three isomers, 3, 4, 5, and Ph-TFP in acetonitrile (10 μM).

Figure 7: Quantified DNA cleavage data for 1 (a), 6 (b) and 7 (c). Blue: Form I (supercoiled) DNA; red: Form ...

Figure 8: Effect of hydroxyl radical/singlet oxygen scavengers (20 mM) on the efficiency of DNA cleavage at p...

Figure 9: Cell proliferation assay using A375 cells (human melanoma) and compound 1 (green square), 6 (red up...
Light-induced olefin metathesis
- Yuval Vidavsky and
- N. Gabriel Lemcoff
Beilstein J. Org. Chem. 2010, 6, 1106–1119, doi:10.3762/bjoc.6.127

- metathesis stands out as both academically motivating and practically useful. Starting from early tungsten heterogeneous photoinitiated metathesis, up to modern ruthenium methods based on complex photoisomerisation or indirect photoactivation, this survey of the relevant literature summarises past and
- present developments in the use of light to expedite olefin ring-closing, ring-opening polymerisation and cross-metathesis reactions. Keywords: catalysis; light activation; olefin metathesis; photoactivation; photoinitiation; photoisomerisation; RCM; ROMP; ruthenium; tungsten; Introduction The metal
- reactions was not explained, even though it is slightly counterintuitive. In order to improve the understanding of the photoactivation mechanism, complex 16g was irradiated by visible light both in the presence and absence of cyclooctene. NMR and UV spectra confirmed the release of p-cymene from the complex

Graphical Abstract

Scheme 1: Light activated metathesis of trans-2-pentene.

Scheme 2: Light induced generation of metathesis active species 2.

Figure 1: Well-defined tungsten photoactive catalysts.

Figure 2: The first ruthenium based complexes for PROMP.

Figure 3: Cyclic strained alkenes for PROMP.

Scheme 3: Proposed mechanism for photoactivation of sandwich complexes.

Figure 4: Ruthenium and osmium complexes with p-cymene and phosphane ligands for PROMP.

Figure 5: Commercially available photoactive ruthenium precatalyst.

Figure 6: Some of the rings produced by photo-RCM.

Scheme 4: Photopromoted ene-yne RCM by cationic allenylidene ruthenium complex 14.

Figure 7: Dihydrofurans synthesised by photopromoted ene-yne RCM.

Figure 8: Ruthenium complexes with p-cymene and NHC ligands.

Scheme 5: Ruthenium NHC complexes for PROMP containing p-cymene and trifluroacetate (17, 19) or phenylisonitr...

Figure 9: Photoactivated cationic ROMP precatalysts.

Figure 10: Different monomers for PROMP.

Scheme 6: Proposed mechanism for photoinitiated polymerisation by 22 and 23.

Figure 11: Light-induced cationic catalysts for ROMP.

Figure 12: Sulfur chelated ruthenium benzylidene pre-catalysts for olefin metathesis.

Scheme 7: Proposed mechanism for the photoactivation of sulfur-chelated ruthenium benzylidene.

Figure 13: Photoacid generators for photoinduced metathesis.

Scheme 8: Synthesis of precatalysts 36 and 37.

Scheme 9: Trapping of proposed intermediate 41.

Figure 14: Encapsulated 39, isolated from the monomer.






