Search results
Search for "radicals" in Full Text gives 226 result(s) in Beilstein Journal of Nanotechnology. Showing first 200.
Structural, optical, and bioimaging characterization of carbon quantum dots solvothermally synthesized from o-phenylenediamine
Beilstein J. Nanotechnol. 2023, 14, 165–174, doi:10.3762/bjnano.14.17

- −polyoxypropylene−polyoxyethylene Pluronic 68 generate singlet oxygen through energy transfer to molecular oxygen [21]. But CQDs prepared from o-phenylenediamine do not generate singlet oxygen or OH radicals through energy or electron transfer, because the condensation process of these dots includes NH2 groups in
- their structure whereas the presence of pyrrolic and pyridinic nitrogen is really minor. Thus reaction centers for ROS generation (dominantly pyridinic N) do not exist in o-phenylenediamine CQDs [40]. Hydroxyl radical production To examine the production of hydroxyl radicals, two measurements at
- indicate a low level of PL intensity after all measured time periods (0‒300 min) and (0‒440 min). This fact shows that there is no production of hydroxyl radicals in the CQDs/PU composite samples at the two excitation wavelengths (365 and 405 nm). In the previous section we established that the CQDs do not
Non-stoichiometric magnetite as catalyst for the photocatalytic degradation of phenol and 2,6-dibromo-4-methylphenol – a new approach in water treatment
Beilstein J. Nanotechnol. 2022, 13, 1531–1540, doi:10.3762/bjnano.13.126

- , petroleum constituents, and volatile organic compounds. Furthermore, the rate of ozonation is accelerated in alkaline media because hydroxide ions catalyze the decomposition of ozone and produce hyperactive hydroxyl radicals (•OH). Photocatalysis is a promising technique for removing POPs from water using
- hydroxyl radicals (•OH) generated during water ozonation are described by the SBH model [30][31][32] for neutral pH or the TFG model [33][34][35] for pH > 7. Considering the process conditions during the study (pH 8), we are interested in the TFG model. The rate constants used in the model were determined
- by Chelkowska et al. [36]. The TFG model includes the following reactions: The reaction in Equation 6 shows that the ozone decomposition process is initiated by hydroxy anions. Two-electron transfer of the oxygen atom produces the –OOH anion, which is necessary for the generation of hydroxyl radicals
Recent trends in Bi-based nanomaterials: challenges, fabrication, enhancement techniques, and environmental applications
Beilstein J. Nanotechnol. 2022, 13, 1316–1336, doi:10.3762/bjnano.13.109

- conduction band (CB) through visible light [39]. The holes in the valence band of the catalyst split water to hydroxyl radicals (•OH). Electrons in the CB of a semiconductor photocatalyst can generate the superoxide anion (•O2−) when they interact with oxygen molecules. During the photocatalytic oxidative
- obtained. They found that Bi5O7Br effectively converts molecular oxygen to superoxide radicals and hydroxyl radicals in visible light. Under UV–vis irradiation, Bi5O7Br showed a higher photocatalytic activity in the degradation of rhodamine B (RhB) dye than BiOBr. The addition of Bi5O7Br photocatalysis to
- inside the oxygen vacancies have a longer lifetime than those in the CB. Therefore, electrons in defect states have the potential to react with oxygen that has been adsorbed by oxygen vacancies, which results in the production of superoxide •O2− radicals. These may subsequently be employed to drive
Rapid fabrication of MgO@g-C3N4 heterojunctions for photocatalytic nitric oxide removal
Beilstein J. Nanotechnol. 2022, 13, 1141–1154, doi:10.3762/bjnano.13.96

- scavenger also reduces the NO decomposition by about 1.3 times. The weak contribution of •OH radicals in the NO degradation is clearly shown, with a reduction in efficiency by only about 1%. Hence, electrons and holes are the main contributors to the photocatalytic NO degradation. Also, ESR was used to
- determine accurately the reaction mechanism of the material. Figure 11b shows that under visible light and using DMPO–H2O and DMPO–OH, the material generates •OH and •O2 radicals. In contrast, only •OH radicals are generated in the dark, but to a very low extent. Hence, the generation of •O2 radicals
- ). The holes can degrade NO directly by oxidizing NO into NO2 (Equations 9 and 10) [47][69]. Simultaneously, at the CB of g-C3N4, electrons are excited and react with O2 to produce •O2− radicals. In addition, these electrons also migrate across the CB of MgO, creating excess electrons in MgO and avoiding
Green synthesis of zinc oxide nanoparticles toward highly efficient photocatalysis and antibacterial application
Beilstein J. Nanotechnol. 2022, 13, 1108–1119, doi:10.3762/bjnano.13.94

- ]. These photogenerated electrons and holes migrate to the surface of ZnO to react with H2O and O2 to generate O2•− and •OH radicals, which oxidize organic substances. In addition, ZnO nanoparticles (NPs) have high antibacterial activity against bacteria, high biocompatibility, and are nontoxic to human
- holes in VB and electrons in CB. The electrons reduce the oxygen absorbed on the ZnO surface to form •O2−. These •O2− species continue reacting with H2O to form H2O2 and •OH. The hole in VB will react with H2O to produce •OH radicals. These •OH radicals are strong oxidizing radicals and are mainly
- present in solution which can degraded dyes. These radicals can attract MO and MB molecules, oxidize the dye molecules to degradation products, and finally completely degrade the dyes to CO2 and H2O [35][36]. In addition, when ZnO NPs get in contact with E. coli, reactive oxygen species (ROS), such as •OH
Recent advances in green carbon dots (2015–2022): synthesis, metal ion sensing, and biological applications
Beilstein J. Nanotechnol. 2022, 13, 1068–1107, doi:10.3762/bjnano.13.93

- Fe3+/Fe2+ in water, H2O2 can be split into hydroxyl radicals, and the ensuing radical is an incredibly potent oxidizing species. The treatment time lasted from 10 to 60 min, with 40 min yielding the highest CD luminescence. The resulting CDs (2–10 nm) have excellent penetration into HeLa cells
Spindle-like MIL101(Fe) decorated with Bi2O3 nanoparticles for enhanced degradation of chlortetracycline under visible-light irradiation
Beilstein J. Nanotechnol. 2022, 13, 1038–1050, doi:10.3762/bjnano.13.91

- speculated and shown in Figure 7. Two possible degradation pathways were deduced through hydroxylation, dehydration, and ring opening [59]. First, dechlorination of CTC due to the attack of radicals resulted in the formation of P1 intermediates with m/z of 444. Then P2 (m/z 432) was obtained from P1 through
- investigated by radical trapping test. Ethylenediaminetetraacetic acid disodium (EDTA-2Na), isopropanol (IPA), and vitamin C (VC) were used as scavengers for holes (h+), hydroxyl radicals (•OH), and superoxide radicals (•O2−), respectively. Figure 8a shows the degradation rate of CTC in the presence of three
- degradation efficiency of CTC decreased to 73.6% and 65.1%, respectively, indicating that h+ and •OH also played a role in the photocatalytic process to some extent. To further confirm the free radicals produced in the photocatalytic degradation of CTC, ESR spectra of the samples were measured. As given in
Solar-light-driven LaFexNi1−xO3 perovskite oxides for photocatalytic Fenton-like reaction to degrade organic pollutants
Beilstein J. Nanotechnol. 2022, 13, 882–895, doi:10.3762/bjnano.13.79

- substances in wastewater [16]. Among these procedures, the Fenton method causes numerous interests due to its convenience and effectiveness. Notably, the Fenton method can produce many hydroxyl radicals (∙OH) by introducing divalent iron solution and hydrogen peroxide, as shown in Equation 1 below. The
- complexes might compete with the hydroxyl radicals, eliciting a degradation of the reaction performance [18]. In recent years, the Fenton method has gradually developed into a new scenario of oxidation method, called photo-Fenton, which is facilitated or driven by the light source. Compared with a typical
- hydrogen peroxide can be remarkably transformed into redox radicals, followed by destroying the organic pollutants. Meanwhile, the remaining divalent iron complexes in the system can return to the circulation of hydrogen peroxide reaction and continuously form new hydroxide radicals [20]. Therefore, based
Hierarchical Bi2WO6/TiO2-nanotube composites derived from natural cellulose for visible-light photocatalytic treatment of pollutants
Beilstein J. Nanotechnol. 2022, 13, 745–762, doi:10.3762/bjnano.13.66

- photocatalytic reduction of Cr(VI), while hydroxyl radicals and reactive holes contributed to the photocatalytic degradation of rhodamine B. Keywords: biomimetic synthesis; cellulose; nanoarchitectonics; nanocomposite; nanotubes; photocatalysis; pollutants; Introduction The direct emission of untreated
- Cr(VI), IPA (0.1 M), EDTA-2Na (10.0 mM), KIO3 (0.1 M), and AgNO3 (0.1 M) were added into the reaction solution to shield the hydroxyl radicals (•OH), reactive holes (h+), superoxide radicals (•O2−) and electrons (e−) species, respectively, generated during the photocatalysis. Similarly, IPA (0.1 M
Correction: Coordination-assembled myricetin nanoarchitectonics for sustainably scavenging free radicals
Beilstein J. Nanotechnol. 2022, 13, 570–571, doi:10.3762/bjnano.13.48

Ethosomal (−)-epigallocatechin-3-gallate as a novel approach to enhance antioxidant, anti-collagenase and anti-elastase effects
Beilstein J. Nanotechnol. 2022, 13, 491–502, doi:10.3762/bjnano.13.41

- responsible for the elasticity and resistance of the skin in the dermis, (i.e., the middle layer of the skin) are collagen and elastin, and the changes in these two components play an important role in the skin aging process [1][2]. The production of reactive oxygen species (ROS) or free radicals through
- ultraviolet (UV) radiation, smoking, pollution, and normal endogenous metabolic processes triggers the skin aging process. Elastase and collagenase enzymes induced by the formation of ROS accelerate the aging process and cause loss of collagen and elastin fibrils. With the formation of free radicals, lipid
- peroxides and free oxygen radicals increase. Damage to phospholipids, which contain large amounts of unsaturated fatty acids and are sensitive to degradation by hydroxyl radicals, deteriorates the structure of the cell membrane [3]. Antioxidants are compounds that capture and stabilize free radicals and
Investigation of electron-induced cross-linking of self-assembled monolayers by scanning tunneling microscopy
Beilstein J. Nanotechnol. 2022, 13, 462–471, doi:10.3762/bjnano.13.39

- attachment process at ≈6 eV and suggested that the cross-linking of aromatic SAMs proceeds in a radical chain reaction [53]. Above the ionization potential, a direct electron impact ionization is believed to cause C–H cleavage and generate radicals [63]. Neumann et al. found a strong energy dependence for
- initiates a reaction with an adjacent molecule and generates another new radical carbon center, which may react with other molecules. According to Amiaud et al. [53], the formation of first radicals can be caused either by electronic rearrangement or dissociative electron attachment of the negative ion
Coordination-assembled myricetin nanoarchitectonics for sustainably scavenging free radicals
Beilstein J. Nanotechnol. 2022, 13, 284–291, doi:10.3762/bjnano.13.23

- signal pathways and, thus, of sustainably scavenging radicals. However, Myr is poorly soluble in water, which limits its bioavailability for biomedical applications, and even its clinical therapeutic potential. The antioxidant peptide glutathione (GSH) plays a role as antioxidant in cells and possesses
- not only chelating intracellular transition metal ions for removing reactive oxygen species (ROS) [20], but also of activating antioxidant enzymes and the AMPK/NRF2 signal pathway [21], yielding sustainable scavenging of radicals. Myr can inherently increase body resistance to carcinogens, viruses
- (equivalent concentration of Myr: 0.25, 0.5, 1, 1.5, and 2 µg·mL−1, equivalent concentration of GSH: 1, 2, 3, and 4 µg·mL−1) gradually decreased, suggesting that MZG nanoparticles scavenged radicals. When the concentration of MZG nanoparticles was equivalent to 2 µg·mL−1 of Myr and 4 µg·mL−1 of GSH, the
Engineered titania nanomaterials in advanced clinical applications
Beilstein J. Nanotechnol. 2022, 13, 201–218, doi:10.3762/bjnano.13.15

- photocatalytic activity. Upon UV irradiation, the electrons in the valence band get excited to the conduction band, leading to the formation of electron–hole pairs and the generation of ROS. Subsequently, the generated holes (h+) convert water/hydroxide molecules to peroxide/hydroxyl radicals by oxidation. The
- generated free electrons (e−) react with molecular oxygen to generate superoxide radicals by reduction. Several factors contribute to the photocatalytic performance of TiO2, such as the structural phase (anatase, brookite, or rutile), defects in the lattice, the degree of crystallinity, morphology
- convenient recombination electrons and holes, the electrons and holes live long enough for a continuous ROS generation on the surface, which is a highly demanded feature of TiO2 nps for the eradication of surface microorganisms [78]. Some studies showed that anatase could produce •OH radicals in a
A comprehensive review on electrospun nanohybrid membranes for wastewater treatment
Beilstein J. Nanotechnol. 2022, 13, 137–159, doi:10.3762/bjnano.13.10

Tin dioxide nanomaterial-based photocatalysts for nitrogen oxide oxidation: a review
Beilstein J. Nanotechnol. 2022, 13, 96–113, doi:10.3762/bjnano.13.7

- working scheme of semiconductor photocatalysts for NO oxidation. Light generates holes (h+) in the valence band (VB) and electrons (e–) in the conduction band (CB) of the photocatalytic material. Electrons at the material surface will react with oxygen molecules to form superoxide radicals (•O2
- −, similarly holes react with water to form hydroxyl radicals). Free radicals and strong oxidizing agents react with NOx to produce NO3−, deposited on the photocatalyst surface. The NO3− product formed on the surface of the catalyst can be easily separated for further treatment by washing with water due [11
- SnO2 NPs, and this is the first report on using a SnO2 photocatalyst with NP morphology for the NO degradation. The photocatalytic mechanism of SnO2 NPs is based on electrons and holes to generate reactive radicals. Figure 7 shows that the photocatalytic NO removal efficacy of SnO2 NPs achieved 63.37
Sputtering onto liquids: a critical review
Beilstein J. Nanotechnol. 2022, 13, 10–53, doi:10.3762/bjnano.13.2
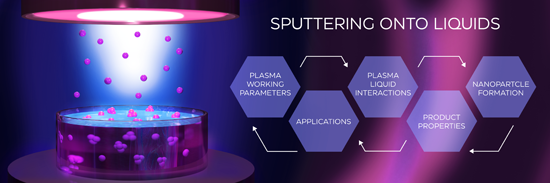
- multiply charged ions (Ar2+, M2+). If the plasma is generated in an argon/reactive gas mixture R+, R2+, R2+, and MRx+ ions (with R being, e.g., O or N) can also be found; (3) chemically reactive radicals such as O or N atoms produced through dissociation reactions during reactive sputtering. O atoms can
Comprehensive review on ultrasound-responsive theranostic nanomaterials: mechanisms, structures and medical applications
Beilstein J. Nanotechnol. 2021, 12, 808–862, doi:10.3762/bjnano.12.64

- ]. In other cases, disruption and destabilization of the complex nanostructure subsequent to US vibration leads to drug release [28][29][30]. In addition, the ultrasonication of certain complexes can generate free radicals that can cause cell damage or activation of cellular signaling pathways [31
- components in water-based media, which plays a role in both therapeutic and diagnostic applications [148][149]. Due to the toxicity of free radicals, some chemical compounds called sonosensitizers have been used as sonodynamic therapy agents which produce synergistic effects with US irradiation by generating
- free radicals [150]. Masuda et al. proposed that there is a relation between the quality and quantity of free radical formation and the frequency of the US applied in the presence of MBs [151]. The combination of free-radical-generating components and other materials could lead to multifunctional
A review of defect engineering, ion implantation, and nanofabrication using the helium ion microscope
Beilstein J. Nanotechnol. 2021, 12, 633–664, doi:10.3762/bjnano.12.52

The impact of molecular tumor profiling on the design strategies for targeting myeloid leukemia and EGFR/CD44-positive solid tumors
Beilstein J. Nanotechnol. 2021, 12, 375–401, doi:10.3762/bjnano.12.31

A review on the biological effects of nanomaterials on silkworm (Bombyx mori)
Beilstein J. Nanotechnol. 2021, 12, 190–202, doi:10.3762/bjnano.12.15

- [124]. This occurs as a result of the imbalance of free radicals in the organism [125]. ROS generation in living organisms is essential as it activates cell defense mechanisms and their antioxidant enzymes [126][127]. Xu et al. [128] reported that silkworm larvae exposed to 10–70 μg/mL of ZnO NPs via
- in silkworms. It was shown that AgNPC were able to improve the survival rate of the silkworm from 22 to 67% when compared to the control untreated group. Adherence of the virus to AgNps led to the production of free radicals which penetrated and disintegrated the virus capsids, proteins, and DNA of
Unravelling the interfacial interaction in mesoporous SiO2@nickel phyllosilicate/TiO2 core–shell nanostructures for photocatalytic activity
Beilstein J. Nanotechnol. 2020, 11, 1834–1846, doi:10.3762/bjnano.11.165

- , the electron–hole recombination can be inhibited by loading metals, such as Ni [12], V, Fe [13], Ag [14], and Cu–Ni [15], on the TiO2 surface, which accelerates the formation of hydroxyl radicals and, consequently, improves the photocatalytic activity of TiO2. In contrast, the doping of TiO2 with
- ][67]. These oxygen vacancies easily act as hole traps that lower the charge-carrier recombination rate, resulting in more free electrons that can give rise to more superoxide radicals upon reaction with adsorbed surface oxygen [23]. Furthermore, the flake-like NiPS morphology could act as a suitable
- while holes are created in the valence band allowing for the separation of electrons and holes (Figure 6a). The electrons in the conduction band can react with oxygen to form reactive superoxide radicals, which oxidize the MV dye molecules [70]. Also, the holes in the valence band react with H2O to
Nanocasting synthesis of BiFeO3 nanoparticles with enhanced visible-light photocatalytic activity
Beilstein J. Nanotechnol. 2020, 11, 1822–1833, doi:10.3762/bjnano.11.164

- the complete mineralization of dyes is achieved by the generation of hydroxyl and superoxide anion radicals [10][11]. Traditional photocatalysts, such as TiO2 or ZnO, provide chemical stability and facile preparation methods [12][13]. However, their environmental benefit in large-scale industrial
- to an interference in the degradation process [51]. In addition, the concentration of the active species, that is, hydroxyl radicals, decreases at pH > 7 due to their reaction with hydroxy anions and the formation of less oxidizing species such as O−. In accordance with the literature, the
- radicals (•O2−) and hydrogen peroxide (H2O2), while the photogenerated electron hole h+ reacts with H2O to form hydroxyl radicals (•OH). The latter species can additionally be formed by disproportionation of •O2− radicals and a subsequent chain reaction. It has been reported previously that hydroxyl
Cu2O nanoparticles for the degradation of methyl parathion
Beilstein J. Nanotechnol. 2020, 11, 1546–1555, doi:10.3762/bjnano.11.137

- degradation time while increasing the degradation efficacy. Our results suggest that the surface basicity of Cu2O NPs leads to degradation of MP without the need of other chemical substances or the use of photocatalysts that generate free radicals. The presence of free radicals is undesired since there is a
- as others have already reported on the literature [15][16][22][23][24][25][26][49]. One major difference in this work is the absence of free radicals since the degradation is not photocatalytic. This absence of free radicals makes Cu2O NPs a reliable source for the degradation of MP in natural waters
Antimicrobial metal-based nanoparticles: a review on their synthesis, types and antimicrobial action
Beilstein J. Nanotechnol. 2020, 11, 1450–1469, doi:10.3762/bjnano.11.129

- microorganisms [151]. Superoxide radicals (O2−), hydroxyl radicals (•OH), hydrogen peroxide (H2O2), and singlet oxygen (1O2) are the most well-known ROS. The mechanism that better explains the synthesis of ROS from NPs is based on their photocatalytic activity (Figure 5). Metal compounds receive enough energy
- , and tocopherol, microorganisms have an enzymatic antioxidant defense system, including catalase and superoxide dismutase (SOD), which controls the oxidative stress, reducing lipid peroxidation and the effects of ROS radicals, such as OH2•− and OH•. At normal aerobic microorganism conditions, the
- , genes related to the general stress response were upregulated. Genes protecting against hydrogen peroxide oxidative damage, catalase/hydroperoxidase, superoxide radicals degradation genes, superoxide dismutase, and superoxide removal transcriptional activator, were upregulated in a range varying from










































































































































































































































