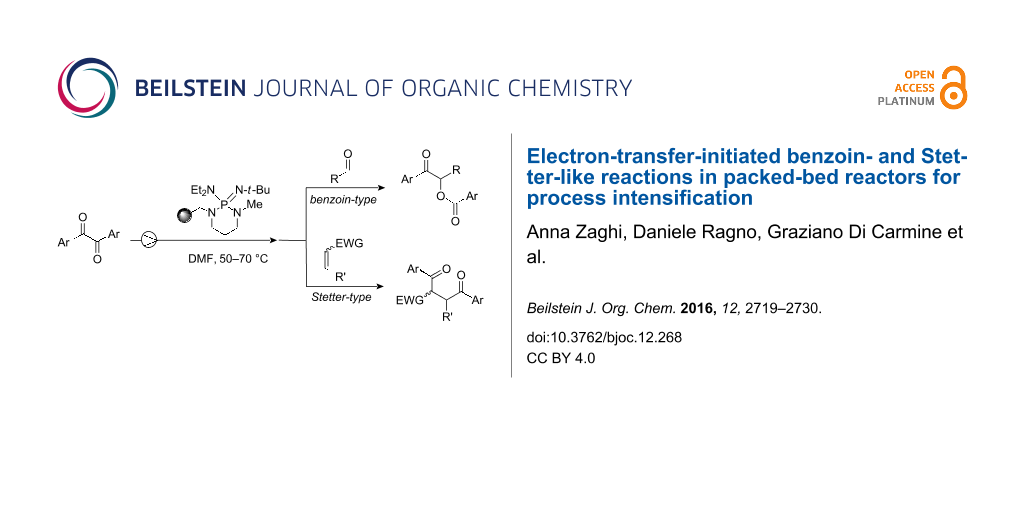
Abstract
A convenient heterogeneous continuous-flow procedure for the polarity reversal of aromatic α-diketones is presented. Propaedeutic batch experiments have been initially performed to select the optimal supported base capable to initiate the two electron-transfer process from the carbamoyl anion of the N,N-dimethylformamide (DMF) solvent to the α-diketone and generate the corresponding enediolate active species. After having identified the 2-tert-butylimino-2-diethylamino-1,3-dimethylperhydro-1,3,2-diazaphosphorine on polystyrene (PS-BEMP) as the suitable base, packed-bed microreactors (pressure-resistant stainless-steel columns) have been fabricated and operated to accomplish the chemoselective synthesis of aroylated α-hydroxy ketones and 2-benzoyl-1,4-diones (benzoin- and Stetter-like products, respectively) with a good level of efficiency and with a long-term stability of the packing material (up to five days).
Graphical Abstract
Introduction
The polarity reversal (umpolung) of carbonyl compounds by N-heterocyclic carbene (NHC) or cyanide catalysis represents a straightforward strategy for the synthesis of valuable molecules such as, among the many examples, α-hydroxy ketones (benzoin reaction) and 1,4-diketones (Stetter reaction) [1-4]. The synthetic utility of the umpolung methodology has therefore spurred intensive research on process intensification through the heterogeneization of NHC catalysts [5-9] for facilitating the post-reaction phase and improving NHCs’ stability towards air and moisture [10,11]. Quite surprisingly, however, implementation of continuos-flow techniques with micro- and meso-reactors is rare in this field [12-17]. Indeed, microreactor technology is today a powerful tool for the fine chemical and pharmaceutical industries facilitating the automation of the production processes with reduced costs and improved safety and sustainability [18-21]. Very recently, Monbaliu and co-workers described a convenient continuous-flow setup for the generation of common free NHCs under homogeneous conditions and their subsequent utilization in transesterification and amidation processes by the reaction telescoping approach [12]. Similarly, the group of Brown reported on the oxidative esterification and amidation of aldehydes in undivided microfluidic electrolysis cells mediated by homogeneous NHCs [13,14]. On the other hand, heterogeneous catalysis in microstructured flow reactors represents a robust synthetic platform, with benefits over the corresponding batch processes such as catalyst stability, lower degradation of supports, and ease of scale-up with minimal changes to the reaction setup [22-24]. An integrated flow system for the synthesis of biodiesel employing an uninterrupted sequence of two fixed-bed reactors packed with a supported acid for esterification of free fatty acids and with an immobilized imidazolidene catalyst for transesterification has been recently described by Lupton and co-workers [15]. Our group also contributed to this area of research fabricating polystyrene monolithic columns functionalized with thiazolium salt pre-catalysts to perform umpolung racemic processes (benzoin, acyloin, and Stetter reactions) with a good level of efficiency [16]. The asymmetric version of acyloin-type reactions was also investigated in our laboratory operating packed-bed bioreactors functionalized with a suitable thiamine diphosphate (ThDP)-dependent enzyme supported on mesoporous silica [17]. Overall, the so far reported umpolung flow processes [12-17] required quite sophisticated procedures, eventually complicated by the separation of homogeneous azolium salt pre-catalysts [25]. In this contribution, we describe a convenient and straightforward continuos-flow protocol for the effective production of benzoin and Stetter-like products that relies on the use of a readily and commercially available supported base as packing material of fixed-bed microreactors. The present study originated from our recent findings on a novel strategy for the umpolung of aromatic α-diketone donors [26] and their peculiar reactivity with aromatic aldehydes or α,β-unsaturated acceptors [27-29]. Indeed, activation of aromatic α-diketones may occur through a double electron-transfer (ET) process triggered by the carbamoyl anion derived from N,N-dimethylformamide (DMF) solvent with catalytic base, which generates an enediolate anion as key reactive species of umpolung catalysis (Figure 1). Significantly, the current investigation on the heterogeneous continuous-flow version of the α-diketone activation process resulted in the fabrication of fixed-bed reactors with elevated stability, allowing their operation for about five days with maintenance of productivity. Moreover, the disclosed flow procedure constituted an equally effective (complete chemoselectivity) and environmentally benign alternative to the analogous batch process towards benzoin- and Stetter-type products mediated by toxic cyanide anions [29,30].
Figure 1: Electron-transfer initiated activation of α-diketones (background) and present study.
Figure 1: Electron-transfer initiated activation of α-diketones (background) and present study.
Results and Discussion
The possibility of transposing the ET-mediated activation process of aromatic α-diketones (benzils) from a homogeneous batch protocol to a heterogeneous flow procedure was initially investigated by testing the efficacy of the commercially available supported bases 4–8 under batch conditions; the benzoin-type reaction of benzil 1a with 2-chlorobenzaldehyde 2a furnishing the benzoylated benzoin 3aa (double aroylation product) was selected as the benchmark (Table 1). Quite surprisingly, the polystyrene-supported 1,8-diazabicyclo [5.4.0]undec-7-ene 4 (PS-DBU) was completely inefficient (DMF, 35 °C, Ar atmosphere) in both catalytic and equimolar amounts despite the detected activity of its homogeneous counterpart [26] (Table 1, entries 1 and 2). Gratifyingly, the highly basic, non-nucleophilic polymer-supported BEMP 5 (PS-BEMP: 2-tert-butylimino-2-diethylamino-1,3-dimethyl-perhydro-1,3,2-diazaphosphorine on polystyrene) afforded the target adduct 3aa in almost quantitative yield (95%) when used in equimolar amounts under an argon atmosphere (Table 1, entry 3). Actually, we previously established the importance of operating under deaerated conditions with homogeneous bases to avoid a marked decrease of the reaction rate (vide infra). By contrast, as demonstrated by the experiment of Table 1, entry 4, the 1a/2a coupling promoted by PS-BEMP 5 was found to be insensitive to the presence of air, thus further improving the practicality of the heterogeneous procedure for the umpolung of benzils. While the utilization of catalytic PS-BEMP 5 (25 mol %) at 35 °C slightly diminished the reaction yield (78%, Table 1, entry 5), the increase of temperature to 50 °C restored the reaction efficiency (91% yield, entry 6). A lower amount of 5 (10 mol %) produced an unsatisfactory yield of 3aa (28%, Table 1, entry 7), whereas the weaker bases diethylamine resin 6, Ambersep 900 OH 7, and the polymer-bound tetraalkylammonium carbonate 8 were completely inefficient (Table 1, entries 8–10). Finally, the conversion efficiency was maintained almost unaltered for recycled PS-BEMP 5 after five runs (Table 1, entry 11). The success of the recycle experiment paved the way for the application of 5 in continuous-flow processes with long-term stability.
Table 1: Optimization of the cross-benzoin-type reaction of benzil 1a with 2-chlorobenzaldehyde 2a promoted by the supported bases 4–8 under batch conditions.a
|
|
|||
| Entry | Base [mol %] | Temp. [°C] | Yield [%]b |
|---|---|---|---|
| 1c | 4 (25) | 35 | <5 |
| 2c | 4 (100) | 35 | <5 |
| 3c | 5 (100) | 35 | 95 |
| 4 | 5 (100) | 35 | 92 |
| 5 | 5 (25) | 35 | 78 |
| 6 | 5 (25) | 50 | 91 |
| 7 | 5 (10) | 50 | 28 |
| 8 | 6 (100) | 50 | <5 |
| 9 | 7 (100) | 50 | <5 |
| 10 | 8 (100) | 50 | <5 |
| 11d | 5 (25) | 50 | 89 |
aReaction Conditions: benzil 1a (0.50 mmol), 2-chlorobenzaldehyde 2a (0.60 mmol), DMF (1.0 mL; water content 0.23% w/w), and the stated amount of base.
bIsolated yield. cReaction conducted under Ar. d5th recycle.
Next, the heterogeneous procedure for the activation of aromatic α-diketones was applied to the model Stetter-like reaction of benzil 1a with chalcone 9a serving as activated α,β-unsaturated acceptor (Table 2). The optimal conditions disclosed for the benzoin-like reaction (25 mol % 5, 50 °C) were not applicable to the 1a/9a coupling (Table 2, entry 1). Also, the use of equimolar 5 gave the target 1,4-dione 10aa in poor yield (26%, Table 2, entry 2) after filtration of 5 and its resuspension in a 10:1 CH2Cl2–AcOH mixture (30 min, rt). This work-up procedure was made necessary because of the sequestering by the basic resin 5 of compounds of type 10 displaying acidic protons at the α-position of carbonyl groups. A higher product yield (45%) was obtained at 70 °C (Table 2, entry 3), while a further increase of temperature (100 °C) and the use of microwave irradiation at 120 °C (1 h) were not beneficial for the reaction outcome (Table 2, entries 4 and 5). The model Stetter-like reaction was finally optimized by varying the 1a/9a ratio (Table 2, entries 6 and 7) and the best yield of 10aa (68%) was achieved at 70 °C with an excess of benzil (1a, 2 equiv; Table 2, entry 6).
Table 2: Optimization of the Stetter-type reaction of benzil (1a) with chalcone 9a promoted by PS-BEMP 5 under batch conditions.a
|
|
||||
| Entry | 5 [mol %] | Temp. [°C] | Time [h] | Yield [%]b |
|---|---|---|---|---|
| 1 | 25 | 50 | 16 | <5 |
| 2 | 100 | 50 | 16 | 26 |
| 3 | 100 | 70 | 8 | 45 |
| 4 | 100 | 100 | 8 | 24 |
| 5c | 100 | 120 | 1 | 31 |
| 6d | 100 | 70 | 8 | 68 |
| 7e | 100 | 70 | 8 | 41 |
aReaction conditions: benzil (1a, 0.50 mmol), chalcone (9a, 0.50 mmol), DMF (1.0 mL; water 0.23% w/w), and the stated amount of 5. bIsolated yield. cReaction warmed by microwave irradiation (Biotage Initiator; temperature was measured externally by an IR sensor). dReaction performed with 1.00 mmol of 1a. eReaction performed with 1.00 mmol of 9a.
On the basis of our previous mechanistic investigation in solution phase [26], the above results may be interpreted as follows. The carbamoyl anion A, which is generated by deprotonation of DMF solvent with PS-BEMP 5, is responsible for two sequential ET to the α-diketone 1 leading to the carbamoyl radical B (non-productive pathway) [26] and the key enediolate intermediate I bound to the polymer as ion pair (Scheme 1). In the case of benzoin-like reactions, the supported species I intercepts the aldehyde acceptor 2 to form the cyclic intermediate III through the first adduct II. Then, the final two ET from III to the α-diketone 1 affords the product 3 regenerating the dianion I ready for a chain process. It is important to underline the beneficial effect on the reaction outcome and practicability of the polymer support, which stabilizes the enediolate functionality through ionic interactions, thus preventing the fast oxidation by oxygen of I to the α-diketone 1 and the consequent slowing down of the reaction as observed under homogeneous conditions. [26]
Scheme 1: Proposed dianionic pathway for the cross-benzoin-like reaction of benzils 1 with aldehydes 2 under heterogeneous conditions.
Scheme 1: Proposed dianionic pathway for the cross-benzoin-like reaction of benzils 1 with aldehydes 2 under ...
In analogy with the study under homogeneous conditions, a trapping experiment was also performed to confirm the crucial role in the catalytic cycle of the enediolate intermediate I. Accordingly, the suspension of benzil (1a) and equimolar PS-BEMP 5 in DMF was treated at 50 °C with an excess (10 equiv) of acetic anhydride recovering the expected O,O’-diacetyl-1,2-diphenylethen-1,2 diol (11) in 6% isolated yield (Scheme 2).
At this stage of our investigation, PS-BEMP 5 was tested as the packing material of fixed-bed reactors with potential long-term stability. A micro-HPLC with minimized extra-column volumes was used as the pumping system. The fixed-bed microreactor R5 was then fabricated by packing a stainless steel column (10 cm length, 4.6 mm internal diameter) with PS-BEMP 5. Pycnometer measurements provided the hold-up volume Vo and the total porosity εtot of R5 [31], whereas the loaded amount of 5 was determined by weighing the filled and empty column. The main features of R5 including the residence time and the observed backpressure are summarized in Table 3.
Table 3: Main features of microreactor R5.a
| Packed 5 [g] | 5 Loading [mmol/g]b | V0 [mL]c | Total porosityd | Time [min]e | Pressure [bar]f |
|---|---|---|---|---|---|
| 0.99 | 2.20 | 1.38 | 0.83 | 138 | 4 |
aGeometric volume (VG) of the stainless-steel column: 1.66 mL. bValue given by the supplier. cDetermined by pycnometry (see the Experimental section). dTotal porosity εtot = V0/VG. eResidence time calculated at 10 μL min−1. fBackpressure measured at 10 μL min−1 (DMF, 50 °C).
Continuous-flow experiments were performed by first considering the benzoin-like reaction of benzil (1a) with 2-chlorobenzaldehyde (2a) (Table 4). Different flow rates and substrate concentrations were initially evaluated to optimize the conversion efficiency and productivity (P) of the process. Hence, portions of the outlet stream were taken at regular intervals (60 min) and analyzed by NMR spectroscopy. While the highest productivity was obtained at 50 °C with a 0.1 M solution of the substrates and a flow rate of 10 μL min−1 (81% conversion; Table 4, entry 1), operating the microreactor R5 at a lower flow rate (5 μL min−1; residence time: 276 min) guaranteed the complete consumption of the reactants (Table 4, entry 2). Under these conditions, the benzoylated benzoin product 3aa could be isolated in pure form by simple evaporation of the solvent. The long-term stability of R5 was next examined to establish the effect of the flow regime on the deactivation rate of the PS-BEMP 5. The analysis of the conversion versus process time plot showed that the steady-state conversion was reached after ca. 3 h at 50 °C and maintained unaltered for about 120 h on stream (Figure 2).
![[1860-5397-12-268-2]](/bjoc/content/figures/1860-5397-12-268-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: Conversion of the 1a/2a coupling in microreactor R5 operated for 150 h at 50 °C.
Figure 2: Conversion of the 1a/2a coupling in microreactor R5 operated for 150 h at 50 °C.
The scope and applicability of the flow cross-benzoin-type reaction were investigated by coupling various α-diketones 1 with aromatic aldehydes 2. Higher efficiencies were detected with α-diketones 1a–c displaying electron-neutral and withdrawing groups with expected lower values of reduction potentials (Table 4, entries 3–13), in agreement with the proposed reaction mechanism. The unreactivity of 4,4’-dimethylbenzil (1d) seemed to confirm our mechanistic hypothesis (Table 4, entry 14).
Table 4: Scope of the continuous-flow benzoin-like reaction.a
|
|
||||||
| Entry | Donor (c [M]) | Acceptor (c [M]) | Flow rate [μL/min] | Time [min]b | Product (Conv. [%])c | Pd |
|---|---|---|---|---|---|---|
| 1 |
1a (0.10) |
2a (0.10) |
10 | 138 |
3aa (81) |
22 |
| 2 |
1a (0.10) |
2a (0.10) |
5 | 276 |
3aa (>95) |
13 |
| 3 |
1a (0.10) |
2b (0.10) |
10 | 138 |
3ab (88) |
24 |
| 4 |
1a (0.10) |
2c (0.10) |
10 | 138 |
3ac (75) |
20 |
| 5 |
1a (0.10) |
2d (0.10) |
5 | 276 |
3ad (62) |
8 |
| 6 |
1a (0.10) |
2e (0.10) |
10 | 138 |
3ae (85) |
23 |
| 7 |
1a (0.10) |
2f (0.10) |
10 | 138 |
3af (90) |
25 |
| 8 |
1a (0.10) |
2g (0.10) |
5 | 276 |
3ag (61) |
8 |
| 9 |
1a (0.10) |
2h (0.10) |
5 | 276 |
3ah (66) |
9 |
| 10 |
1b (0.10) |
2a (0.10) |
10 | 138 |
3ba (77) |
19 |
| 11 |
1c (0.10) |
2b (0.10) |
15 | 207 |
3cb (82) |
34 |
| 12 |
1c (0.10) |
2i (0.10) |
10 | 138 |
3ci (85) |
23 |
| 13 |
1c (0.10) |
2c (0.10) |
10 | 138 |
3cc (69) |
19 |
| 14 |
1d (0.10) |
2a (0.10) |
5 | 276 |
3da (<5) |
– |
aSee the Experimental section for a description of the experimental setup. Experiments performed for 5 h in steady-state regime. Temperature was measured by a thermometer placed inside the thermostated unit containing the reactor. bCalculated residence time. cInstant conversion in steady-state regime as established by 1H NMR analysis. dProductivities are measured in mmol(product) h−1 mmol(catalyst)−1 × 103.
Following the thread of the previous study on the benzoin condensation, the Stetter-like reaction of benzil (1a, 0.1 M) with chalcone 9a (0.05 M) was optimized at 70 °C with a flow rate of 5 μL min−1 (Table 5, entry 1). Because of the partial adsorption of the target 1,4-diketone 10aa onto the basic packing material 5, the reactor R5 was flushed with pure DMF at the end of the coupling experiment, thus permitting the recovery of the whole amount of generated product (see the Experimental section). In general, a lower level of coupling efficiency was detected for the Stetter-like reaction compared to the benzoin condensation as confirmed by the higher residence time (276 min) required to reach satisfactory conversions. Again, benzil 1d proved to be completely ineffective in the addition to α,β-unsaturated acceptors as well (Table 5, entry 7).
Table 5: Scope of the continuous-flow Stetter-like reaction.a
|
|
||||
| Entry | Donor (c [M]) | Acceptor (c [M]) | Product (conv. [%])b | Pc |
|---|---|---|---|---|
| 1 |
1a (0.10) |
9a (0.05) |
10aa (72) |
5 |
| 2 |
1a (0.10) |
9b (0.05) |
10ab (68) |
5 |
| 3 |
1a (0.10) |
9c (0.05) |
10ac (61) |
4 |
| 4 |
1a (0.10) |
9d (0.05) |
10ad (55)d |
4 |
| 5 |
1a (0.10) |
9e (0.05) |
10ae (47)d |
4 |
| 6 |
1a (0.10) |
9f (0.05) |
10af (42)d |
3 |
| 7 |
1d (0.10) |
9a (0.05) |
10da (<5) |
– |
aSee the Experimental section for a description of the experimental setup. Experiments performed for 5 h in steady-state regime. bInstant conversion in steady-state regime as established by 1H NMR analysis. cProductivities are measured in mmol(product) h−1 mmol(catalyst)−1× 103. dDiastereomeric mixture.
Conclusion
In summary, we have disclosed a practical continuous-flow procedure for the umpolung of aromatic α-diketones and demonstrated its efficacy in the chemoselective synthesis of benzoin- and Stetter-like products (aroylated α-hydroxy ketones and 2-benzoyl-1,4-diones, respectively) through the operation of fixed-bed reactors packed with a readily and commercially available polymer-supported base. Together with the ease of product/promoter separation, an important benefit of the flow regime has been the significant long-term stability of the packing bed (ca. 5 five days on streams). Small-scale reactors have been described in this work; nevertheless, an easy scale-up of the disclosed processes may be envisaged by the numbering up approach.
Experimental
Liquid aldehydes were freshly distilled before their utilization. Reactions were monitored by TLC on silica gel 60 F254 with detection by charring with phosphomolybdic acid. Flash column chromatography was performed on silica gel 60 (230–400 mesh). 1H (300 MHz), 13C (101 MHz) and 19F (376 MHz) NMR spectra were recorded for CDCl3 solutions at room temperature unless otherwise specified. Peaks assignments were aided by 1H,1H COSY and gradient-HMQC experiments. For accurate mass measurements, the compounds were analyzed in positive ion mode by Agilent 6520 HPLC-Chip Q/TOF-MS (nanospray) using a quadrupole, a hexapole, and a time-of-flight unit to produce spectra. The capillary source voltage was set at 1700 V; the gas temperature and drying gas were kept at 350 °C and 5 L/min, respectively. The MS analyzer was externally calibrated with ESI-L low concentration tuning mix from m/z 118 to 2700 to yield an accuracy below 5 ppm. Accurate mass data were collected by directly infusing samples in 40/60 H2O/ACN 0.1% TFA into the system at a flow-rate of 0.4 mL/min. Microwave-assisted reactions were carried out using a single-mode cavity dedicated reactor (Biotage InitiatorTM). Reactions were performed with temperature-controlled programs in glass vials (0.5–2 mL) sealed with a Teflon septum. Temperatures were measured externally by an IR sensor. As described in [32], the system used for continuous-flow reactions was composed of an HPLC pump (Agilent 1100 micro series), an in-line pressure transducer, a thermostated microreactor holder (Peltier unit), a system to collect fractions and a data acquisition system (Agilent ChemStation). The units were connected by peek tubing (internal diameter 0.01 inch from Upchurch Scientific). The system hold-up volume was smaller than 80 µL. The temperature was controlled by inserting a thermometer inside the Peltier unit (temperature measurement error: ±0.5 °C). The supported bases 4–8 were purchased from Sigma-Aldrich. All adducts 3 and 10 are known compounds [27-29] apart from compounds 3ab, 3ag, 3cb, 3ci, and 3cc.
Procedure for the model cross-benzoin-like reaction under batch conditions (Table 1)
A mixture of benzil (1a, 105 mg, 0.50 mmol), 2-chlorobenzaldehyde (2a, 56 μL, 0.50 mmol), the stated base (see Table 1 for molar ratio) and DMF (1.0 mL) was stirred at the stated temperature for the stated time, then filtered and concentrated. The resulting residue was analyzed by 1H NMR to determine the conversion. Subsequently, the residue was eluted from a column of silica gel with 20:1 cyclohexane–AcOEt to give isolated 3aa.
Procedure for the model Stetter-like reaction under batch conditions (Table 2)
A mixture of benzil (1a, 105 mg, 0.50 mmol), (E)-3-(4-chlorophenyl)-1-phenylprop-2-en-1-one (9a, 121 mg, 0.50 mmol), PS-BEMP 5 (see Table 2 for molar ratio) and DMF (1.0 mL) was stirred at the stated temperature for the stated time, then filtered and concentrated. The resulting residue was analyzed by 1H NMR to determine the conversion. Subsequently, the residue was eluted from a column of silica gel with 13:1 cyclohexane–AcOEt to give isolated 10aa.
Trapping experiment (Scheme 2)
A mixture of benzil (1a, 210 mg, 1.00 mmol), PS-BEMP 5 (454 mg, 1.00 mmol) and DMF (2 mL) was stirred at 50 °C for 30 min then acetic anhydride (0.94 mL, 10.0 mmol) was added in one portion. The reaction mixture was stirred at 50 °C for 2 h, then cooled to room temperature, filtered, concentrated, and eluted from a column of silica gel with 7:1 cyclohexane–AcOEt to give (Z)-1,2-diphenylethene-1,2-diyl diacetate 11 as a white amorphous solid (17 mg, 6%). 1H NMR (300 MHz, CDCl3) δ 7.30–7.16 (m, 10H, Ar), 2.21 (s, 6H, CH3); 13C{1H} NMR (101 MHz, CDCl3) δ 168.1, 138.5, 133.0, 128.8, 128.8, 128.2, 20.7; HRMS–ESI/Q-TOF (m/z): [M]+ calcd for C18H16O4: 296.1049; found: 296.1105.
Determination of microreactor void-volume
Microreactor void volume (V0) was determined by pycnometry [31]. This method consists in filling the microreactor successively with two distinct solvents (solvent 1: water; solvent 2: n-hexane) and weighing the filled microreactors accurately. Simple math shows that [33]: V0 = (ω1 − ω2) / (δ1 − δ2), where ω1 and ω2 are the weights of the microreactor filled with solvents 1 and 2 and δ1 and δ2 the densities of the solvents.
Continuous-flow cross-benzoin-like reactions (Table 4)
Microreactor R5 was fed with a DMF solution of α-diketone 1 and aldehyde 2 (see Table 4 for molarity concentrations), and operated at the stated temperature and the stated flow rate for 5 h under steady-state conditions. Instant conversion was determined (1H NMR analysis) every hour by taking a sample of the eluate. The collected solution was finally concentrated and eluted from a column of silica gel with the suitable elution system to give the corresponding aroylated α-hydroxy ketone 3.
The long-term stability experiment was performed using benzil (1a, 0.10 M) and 2-chlorobenzaldehyde (2a, 0. 10 M) as the substrates; microreactor R5 was operated at 50 °C with a flow rate of 5 μL min−1 for 150 h. After the achievement of the steady-state regime (ca. 3 h), an almost full conversion of 1a (>95%) was maintained for ca. 120 h, while a progressive loss of catalytic activity was observed after that time.
Continuous-flow Stetter-like reactions (Table 5)
Microreactor R5 was fed with a DMF solution of α-diketone 1 (0.10 M) and chalcone 9 (0.05 M), and operated at 70 °C with a flow rate of 5 μL min−1 for 5 h under steady-state conditions. After that time, the reactor was flushed at room temperature with pure DMF for an additional 5 h. The collected solution was finally concentrated and eluted from a column of silica gel with the suitable elution system to give the corresponding 2-benzoyl-1,4-dione 10.
1-(2-Fluorophenyl)-2-oxo-2-phenylethyl benzoate (3ab). 1H NMR (300 MHz, CDCl3) δ 8.15–8.06 (m, 2H, Ar), 8.06–7.97 (m, 2H, Ar), 7.61–7.50 (m, 3 H, Ar), 7.50–7.40 (m, 5H, Ar, H-1), 7.40–7.31 (m, 1H, Ar), 7.21–7.05 (m, 2H, Ar); 13C{1H} NMR (101 MHz, CDCl3) δ 192.9, 165.9, 160.2 (d, J = 250 Hz), 134.4, 133.9, 133.5, 131.5 (d, J = 8.4 Hz), 130.1 (d, J = 2.5 Hz), 129.3, 129.0, 128.9, 128.7, 128.5, 125.0 (d, J = 3.3 Hz), 121.4 (d, J = 14 Hz), 116.3 (d, J = 22 Hz), 70.7; 19F NMR (376 MHz, CDCl3) δ −116.7 to −116.8 (m); HRMS–ESI/Q-TOF (m/z): [M + Na]+ calcd for C21H15FNaO3: 357.0903; found: 357.0988.
1-(2,6-Dichlorophenyl)-2-oxo-2-phenylethyl benzoate (3ag). 1H NMR (300 MHz, CDCl3) δ 8.19–8.11 (m, 2H, Ar), 7.85–7.78 (m, 3H, Ar, H-1), 7.62–7.51 (m, 1H, Ar), 7.51–7.41 (m, 3H, Ar), 7.41–7.31 (m, 4H, Ar), 7.27–7.18 (m, 1H, Ar); 13C{1H} NMR (101 MHz, CDCl3) δ 192.8, 165.2, 136.7, 134.9, 133.4, 133.2, 132.0, 131.0, 130.2, 129.3, 129.3, 128.5, 128.4, 128.08, 75.1; HRMS–ESI/Q-TOF (m/z): [M + Na]+ calcd for C21H14Cl2NaO3: 407.0218; found: 407.0301.
1-(2-Fluorophenyl)-2-oxo-2-(pyridin-2-yl)ethyl picolinate (3cb). 1H NMR (300 MHz, CDCl3) δ 8.81–8.72 (m, 1H, Ar), 8.59 (m, 1H, Ar), 8.21–8.13 (m, 1H, Ar), 8.08–8.00 (m, 1H, Ar), 7.97 (s, 1H, H-1), 7.86–7.73 (m, 2H, Ar), 7.57–7.36 (m, 3H, Ar), 7.36–7.26 (m, 1H, Ar), 7.16–7.01 (m, 2H, Ar); 13C{1H} NMR (101 MHz, CDCl3) δ 193.3, 164.4, 161.0 (d, J = 250 Hz), 159.7, 151.2, 150.1, 149.1, 147.6, 137.0, 136.9, 131.2 (d, J = 8.4 Hz), 130.7 (d, J = 2.3 Hz), 127.7, 127.1, 125.70, 124.4 (d, J = 3.7 Hz), 122.9, 121.4 (d, J = 14 Hz), 116.2 (d, J = 22 Hz), 72.4; 19F NMR (376 MHz, CDCl3) δ −115.2 to −115.3 (m); HRMS–ESI/Q-TOF (m/z): [M + H]+ calcd for C19H14FN2O3: 337.0988; found: 337.0908.
1-(2-Bromophenyl)-2-oxo-2-(pyridin-2-yl)ethyl picolinate (3ci). 1H NMR (300 MHz, CDCl3) δ 8.81–8.73 (m, 1H, Ar), 8.63–8.55 (m, 1H, Ar), 8.20–8.13 (m, 1H, Ar), 8.09–8.02 (m, 2H, Ar, H-1), 7.86–7.74 (m, 2H, Ar), 7.64 (m, 1H, Ar), 7.50–7.36 (m, 3H, Ar), 7.28–7.13 (m, 2H, Ar); 13C{1H} NMR (101 MHz, CDCl3) δ 194.0, 164.3, 151.3, 150.2, 149.2, 147.6, 137.0, 136.9, 133.8, 133.7, 130.6, 130.5, 127.8, 127.7, 127.1, 125.8, 122.8, 78.0; HRMS–ESI/Q-TOF (m/z): [M + H]+ calcd for C19H14BrN2O3: 397.0188; found: 397.0225.
1-(2-Methoxyphenyl)-2-oxo-2-(pyridin-2-yl)ethyl picolinate (3cc). 1H NMR (300 MHz, CDCl3) δ 8.79–8.68 (m, 1H, Ar), 8.59–8.46 (m, 1H, Ar), 8.17–8.07 (m, 1H, Ar), 8.06–7.99 (m, 2H, Ar, H-1), 7.82–7.70 (m, 2H, Ar), 7.46–7.39 (m, 1H, Ar), 7.39–7.29 (m, 2H, Ar), 7.29–7.23 (m, 1H, Ar), 6.93–6.83 (m, 2H, Ar), 3.83 (s, 3H, CH3); 13C{1H} NMR (101 MHz, CDCl3) δ 194.7, 164.6, 157.8, 151.9, 150.1, 149.0, 147.9, 137.0, 136.8, 130.7, 130.2, 127.4, 127.0, 125.6, 122.7, 122.6, 120.8, 111.7, 73.4, 55.9; HRMS–ESI/Q-TOF (m/z): [M + H]+ calcd for C20H17N2O4: 349.1188; found: 349.1105.
Supporting Information
| Supporting Information File 1: NMR spectra of new compounds. | ||
| Format: PDF | Size: 505.1 KB | Download |
References
-
Menon, R. S.; Biju, A. T.; Nair, V. Beilstein J. Org. Chem. 2016, 12, 444–461. doi:10.3762/bjoc.12.47
Return to citation in text: [1] -
Flanigan, D. M.; Romanov-Michailidis, F.; White, N. A.; Rovis, T. Chem. Rev. 2015, 115, 9307–9387. doi:10.1021/acs.chemrev.5b00060
Return to citation in text: [1] -
Bugaut, X.; Glorius, F. Chem. Soc. Rev. 2012, 41, 3511–3522. doi:10.1039/c2cs15333e
Return to citation in text: [1] -
Phillips, E. P.; Chan, A.; Scheidt, K. A. Aldrichimica Acta 2009, 42, 55–65.
Return to citation in text: [1] -
Wang, L.; Chen, E. Y.-X. ACS Catal. 2015, 5, 6907–6917. doi:10.1021/acscatal.5b01410
Return to citation in text: [1] -
Molina de la Torre, J. A.; Albéniz, A. C. ChemCatChem 2014, 6, 3547–3552. doi:10.1002/cctc.201402767
Return to citation in text: [1] -
Powell, A. B.; Suzuki, Y.; Ueda, M.; Bielawski, C. W.; Cowley, A. H. J. Am. Chem. Soc. 2011, 133, 5218–5220. doi:10.1021/ja200602e
Return to citation in text: [1] -
Zeitler, K.; Mager, I. Adv. Synth. Catal. 2007, 349, 1851–1857. doi:10.1002/adsc.200700174
Return to citation in text: [1] -
Zhao, H.; Foss, F. W., Jr.; Breslow, R. J. Am. Chem. Soc. 2008, 130, 12590–12591. doi:10.1021/ja804577q
Return to citation in text: [1] -
Ueno, A.; Kayaki, Y.; Ikariya, T. Green Chem. 2013, 15, 425–430. doi:10.1039/C2GC36414J
Return to citation in text: [1] -
Zeng, T.; Song, G.; Li, C.-J. Chem. Commun. 2009, 6249–6251. doi:10.1039/b910162d
Return to citation in text: [1] -
Di Marco, L.; Hans, M.; Delaude, L.; Monbaliu, J.-C. M. Chem. – Eur. J. 2016, 22, 4508–4514. doi:10.1002/chem.201505135
Return to citation in text: [1] [2] [3] -
Green, R. A.; Pletcher, D.; Leach, S. G.; Brown, R. C. D. Org. Lett. 2015, 17, 3290–3293. doi:10.1021/acs.orglett.5b01459
Return to citation in text: [1] [2] [3] -
Green, R. A.; Pletcher, D.; Leach, S. G.; Brown, R. C. D. Org. Lett. 2016, 18, 1198–1201. doi:10.1021/acs.orglett.6b00339
Return to citation in text: [1] [2] [3] -
Asadi, M.; Hooper, J. F.; Lupton, D. W. Tetrahedron 2016, 72, 3729–3733. doi:10.1016/j.tet.2016.03.075
Return to citation in text: [1] [2] [3] -
Bortolini, O.; Cavazzini, A.; Dambruoso, P.; Giovannini, P. P.; Caciolli, L.; Massi, A.; Pacifico, S.; Ragno, D. Green Chem. 2013, 15, 2981–2992. doi:10.1039/c3gc41284a
Return to citation in text: [1] [2] [3] -
Giovannini, P. P.; Bortolini, O.; Cavazzini, A.; Greco, R.; Fantin, G.; Massi, A. Green Chem. 2014, 16, 3904–3915. doi:10.1039/C4GC00838C
Return to citation in text: [1] [2] [3] -
Pastre, J. C.; Browne, D. L.; Ley, S. V. Chem. Soc. Rev. 2013, 42, 8849–8869. doi:10.1039/c3cs60246j
Return to citation in text: [1] -
Hessel, V.; Kralisch, D.; Kockmann, N.; Noël, T.; Wang, Q. ChemSusChem 2013, 6, 746–789. doi:10.1002/cssc.201200766
Return to citation in text: [1] -
Roberge, D. M.; Zimmermann, B.; Rainone, F.; Gottsponer, M.; Eyholzer, M.; Kockmann, N. Org. Process Res. Dev. 2008, 12, 905–910. doi:10.1021/op8001273
Return to citation in text: [1] -
Roberge, D. M.; Ducry, L.; Bieler, N.; Cretton, P.; Zimmermann, B. Chem. Eng. Technol. 2005, 28, 318–323. doi:10.1002/ceat.200407128
Return to citation in text: [1] -
Finelli, F. G.; Miranda, L. S. M.; de Souza, R. O. M. A. Chem. Commun. 2015, 51, 3708–3722. doi:10.1039/C4CC08748H
Return to citation in text: [1] -
Atodiresei, I.; Vila, C.; Rueping, M. ACS Catal. 2015, 5, 1972–1985. doi:10.1021/acscatal.5b00002
Return to citation in text: [1] -
Puglisi, A.; Benaglia, M.; Chiroli, V. Green Chem. 2013, 15, 1790–1813. doi:10.1039/c3gc40195b
Return to citation in text: [1] -
Sano, T.; Mizota, I.; Shimizu, M. Chem. Lett. 2013, 42, 995–997. doi:10.1246/cl.130396
Return to citation in text: [1] -
Ragno, D.; Zaghi, A.; Di Carmine, G.; Giovannini, P. P.; Bortolini, O.; Fontagnolo, M.; Molinari, A.; Venturini, A.; Massi, A. Org. Biomol. Chem. 2016, 14, 9823–9835. doi:10.1039/C6OB01868H
Return to citation in text: [1] [2] [3] [4] [5] -
Bortolini, O.; Fantin, G.; Ferretti, V.; Fogagnolo, M.; Giovannini, P. P.; Massi, A.; Pacifico, S.; Ragno, D. Adv. Synth. Catal. 2013, 355, 3244–3252. doi:10.1002/adsc.201300652
Return to citation in text: [1] [2] -
Ragno, D.; Bortolini, O.; Giovannini, P. P.; Massi, A.; Pacifico, S.; Zaghi, A. Org. Biomol. Chem. 2014, 12, 5733–5744. doi:10.1039/C4OB00759J
Return to citation in text: [1] [2] -
Ragno, D.; Bortolini, O.; Fantin, G.; Fogagnolo, M.; Giovannini, P. P.; Massi, A. J. Org. Chem. 2015, 80, 1937–1945. doi:10.1021/jo502582e
Return to citation in text: [1] [2] [3] -
Demir, A. S.; Reis, Ö. Tetrahedron 2004, 60, 3803–3811. doi:10.1016/j.tet.2004.03.016
Return to citation in text: [1] -
McCormick, R. M.; Karger, B. L. Anal. Chem. 1980, 52, 2249–2257. doi:10.1021/ac50064a005
Return to citation in text: [1] [2] -
Bortolini, O.; Caciolli, L.; Cavazzini, A.; Costa, V.; Greco, R.; Massi, A.; Pasti, L. Green Chem. 2012, 14, 992–1000. doi:10.1039/c2gc16673a
Return to citation in text: [1] -
Gritti, F.; Kazakevich, Y.; Guiochon, G. J. Chromatogr. A 2007, 1161, 157–169. doi:10.1016/j.chroma.2007.05.102
Return to citation in text: [1]
| 1. | Menon, R. S.; Biju, A. T.; Nair, V. Beilstein J. Org. Chem. 2016, 12, 444–461. doi:10.3762/bjoc.12.47 |
| 2. | Flanigan, D. M.; Romanov-Michailidis, F.; White, N. A.; Rovis, T. Chem. Rev. 2015, 115, 9307–9387. doi:10.1021/acs.chemrev.5b00060 |
| 3. | Bugaut, X.; Glorius, F. Chem. Soc. Rev. 2012, 41, 3511–3522. doi:10.1039/c2cs15333e |
| 4. | Phillips, E. P.; Chan, A.; Scheidt, K. A. Aldrichimica Acta 2009, 42, 55–65. |
| 18. | Pastre, J. C.; Browne, D. L.; Ley, S. V. Chem. Soc. Rev. 2013, 42, 8849–8869. doi:10.1039/c3cs60246j |
| 19. | Hessel, V.; Kralisch, D.; Kockmann, N.; Noël, T.; Wang, Q. ChemSusChem 2013, 6, 746–789. doi:10.1002/cssc.201200766 |
| 20. | Roberge, D. M.; Zimmermann, B.; Rainone, F.; Gottsponer, M.; Eyholzer, M.; Kockmann, N. Org. Process Res. Dev. 2008, 12, 905–910. doi:10.1021/op8001273 |
| 21. | Roberge, D. M.; Ducry, L.; Bieler, N.; Cretton, P.; Zimmermann, B. Chem. Eng. Technol. 2005, 28, 318–323. doi:10.1002/ceat.200407128 |
| 27. | Bortolini, O.; Fantin, G.; Ferretti, V.; Fogagnolo, M.; Giovannini, P. P.; Massi, A.; Pacifico, S.; Ragno, D. Adv. Synth. Catal. 2013, 355, 3244–3252. doi:10.1002/adsc.201300652 |
| 28. | Ragno, D.; Bortolini, O.; Giovannini, P. P.; Massi, A.; Pacifico, S.; Zaghi, A. Org. Biomol. Chem. 2014, 12, 5733–5744. doi:10.1039/C4OB00759J |
| 29. | Ragno, D.; Bortolini, O.; Fantin, G.; Fogagnolo, M.; Giovannini, P. P.; Massi, A. J. Org. Chem. 2015, 80, 1937–1945. doi:10.1021/jo502582e |
| 12. | Di Marco, L.; Hans, M.; Delaude, L.; Monbaliu, J.-C. M. Chem. – Eur. J. 2016, 22, 4508–4514. doi:10.1002/chem.201505135 |
| 13. | Green, R. A.; Pletcher, D.; Leach, S. G.; Brown, R. C. D. Org. Lett. 2015, 17, 3290–3293. doi:10.1021/acs.orglett.5b01459 |
| 14. | Green, R. A.; Pletcher, D.; Leach, S. G.; Brown, R. C. D. Org. Lett. 2016, 18, 1198–1201. doi:10.1021/acs.orglett.6b00339 |
| 15. | Asadi, M.; Hooper, J. F.; Lupton, D. W. Tetrahedron 2016, 72, 3729–3733. doi:10.1016/j.tet.2016.03.075 |
| 16. | Bortolini, O.; Cavazzini, A.; Dambruoso, P.; Giovannini, P. P.; Caciolli, L.; Massi, A.; Pacifico, S.; Ragno, D. Green Chem. 2013, 15, 2981–2992. doi:10.1039/c3gc41284a |
| 17. | Giovannini, P. P.; Bortolini, O.; Cavazzini, A.; Greco, R.; Fantin, G.; Massi, A. Green Chem. 2014, 16, 3904–3915. doi:10.1039/C4GC00838C |
| 29. | Ragno, D.; Bortolini, O.; Fantin, G.; Fogagnolo, M.; Giovannini, P. P.; Massi, A. J. Org. Chem. 2015, 80, 1937–1945. doi:10.1021/jo502582e |
| 30. | Demir, A. S.; Reis, Ö. Tetrahedron 2004, 60, 3803–3811. doi:10.1016/j.tet.2004.03.016 |
| 10. | Ueno, A.; Kayaki, Y.; Ikariya, T. Green Chem. 2013, 15, 425–430. doi:10.1039/C2GC36414J |
| 11. | Zeng, T.; Song, G.; Li, C.-J. Chem. Commun. 2009, 6249–6251. doi:10.1039/b910162d |
| 25. | Sano, T.; Mizota, I.; Shimizu, M. Chem. Lett. 2013, 42, 995–997. doi:10.1246/cl.130396 |
| 5. | Wang, L.; Chen, E. Y.-X. ACS Catal. 2015, 5, 6907–6917. doi:10.1021/acscatal.5b01410 |
| 6. | Molina de la Torre, J. A.; Albéniz, A. C. ChemCatChem 2014, 6, 3547–3552. doi:10.1002/cctc.201402767 |
| 7. | Powell, A. B.; Suzuki, Y.; Ueda, M.; Bielawski, C. W.; Cowley, A. H. J. Am. Chem. Soc. 2011, 133, 5218–5220. doi:10.1021/ja200602e |
| 8. | Zeitler, K.; Mager, I. Adv. Synth. Catal. 2007, 349, 1851–1857. doi:10.1002/adsc.200700174 |
| 9. | Zhao, H.; Foss, F. W., Jr.; Breslow, R. J. Am. Chem. Soc. 2008, 130, 12590–12591. doi:10.1021/ja804577q |
| 26. | Ragno, D.; Zaghi, A.; Di Carmine, G.; Giovannini, P. P.; Bortolini, O.; Fontagnolo, M.; Molinari, A.; Venturini, A.; Massi, A. Org. Biomol. Chem. 2016, 14, 9823–9835. doi:10.1039/C6OB01868H |
| 15. | Asadi, M.; Hooper, J. F.; Lupton, D. W. Tetrahedron 2016, 72, 3729–3733. doi:10.1016/j.tet.2016.03.075 |
| 17. | Giovannini, P. P.; Bortolini, O.; Cavazzini, A.; Greco, R.; Fantin, G.; Massi, A. Green Chem. 2014, 16, 3904–3915. doi:10.1039/C4GC00838C |
| 22. | Finelli, F. G.; Miranda, L. S. M.; de Souza, R. O. M. A. Chem. Commun. 2015, 51, 3708–3722. doi:10.1039/C4CC08748H |
| 23. | Atodiresei, I.; Vila, C.; Rueping, M. ACS Catal. 2015, 5, 1972–1985. doi:10.1021/acscatal.5b00002 |
| 24. | Puglisi, A.; Benaglia, M.; Chiroli, V. Green Chem. 2013, 15, 1790–1813. doi:10.1039/c3gc40195b |
| 12. | Di Marco, L.; Hans, M.; Delaude, L.; Monbaliu, J.-C. M. Chem. – Eur. J. 2016, 22, 4508–4514. doi:10.1002/chem.201505135 |
| 13. | Green, R. A.; Pletcher, D.; Leach, S. G.; Brown, R. C. D. Org. Lett. 2015, 17, 3290–3293. doi:10.1021/acs.orglett.5b01459 |
| 14. | Green, R. A.; Pletcher, D.; Leach, S. G.; Brown, R. C. D. Org. Lett. 2016, 18, 1198–1201. doi:10.1021/acs.orglett.6b00339 |
| 15. | Asadi, M.; Hooper, J. F.; Lupton, D. W. Tetrahedron 2016, 72, 3729–3733. doi:10.1016/j.tet.2016.03.075 |
| 16. | Bortolini, O.; Cavazzini, A.; Dambruoso, P.; Giovannini, P. P.; Caciolli, L.; Massi, A.; Pacifico, S.; Ragno, D. Green Chem. 2013, 15, 2981–2992. doi:10.1039/c3gc41284a |
| 17. | Giovannini, P. P.; Bortolini, O.; Cavazzini, A.; Greco, R.; Fantin, G.; Massi, A. Green Chem. 2014, 16, 3904–3915. doi:10.1039/C4GC00838C |
| 13. | Green, R. A.; Pletcher, D.; Leach, S. G.; Brown, R. C. D. Org. Lett. 2015, 17, 3290–3293. doi:10.1021/acs.orglett.5b01459 |
| 14. | Green, R. A.; Pletcher, D.; Leach, S. G.; Brown, R. C. D. Org. Lett. 2016, 18, 1198–1201. doi:10.1021/acs.orglett.6b00339 |
| 12. | Di Marco, L.; Hans, M.; Delaude, L.; Monbaliu, J.-C. M. Chem. – Eur. J. 2016, 22, 4508–4514. doi:10.1002/chem.201505135 |
| 16. | Bortolini, O.; Cavazzini, A.; Dambruoso, P.; Giovannini, P. P.; Caciolli, L.; Massi, A.; Pacifico, S.; Ragno, D. Green Chem. 2013, 15, 2981–2992. doi:10.1039/c3gc41284a |
| 26. | Ragno, D.; Zaghi, A.; Di Carmine, G.; Giovannini, P. P.; Bortolini, O.; Fontagnolo, M.; Molinari, A.; Venturini, A.; Massi, A. Org. Biomol. Chem. 2016, 14, 9823–9835. doi:10.1039/C6OB01868H |
| 26. | Ragno, D.; Zaghi, A.; Di Carmine, G.; Giovannini, P. P.; Bortolini, O.; Fontagnolo, M.; Molinari, A.; Venturini, A.; Massi, A. Org. Biomol. Chem. 2016, 14, 9823–9835. doi:10.1039/C6OB01868H |
| 26. | Ragno, D.; Zaghi, A.; Di Carmine, G.; Giovannini, P. P.; Bortolini, O.; Fontagnolo, M.; Molinari, A.; Venturini, A.; Massi, A. Org. Biomol. Chem. 2016, 14, 9823–9835. doi:10.1039/C6OB01868H |
| 31. | McCormick, R. M.; Karger, B. L. Anal. Chem. 1980, 52, 2249–2257. doi:10.1021/ac50064a005 |
| 33. | Gritti, F.; Kazakevich, Y.; Guiochon, G. J. Chromatogr. A 2007, 1161, 157–169. doi:10.1016/j.chroma.2007.05.102 |
| 32. | Bortolini, O.; Caciolli, L.; Cavazzini, A.; Costa, V.; Greco, R.; Massi, A.; Pasti, L. Green Chem. 2012, 14, 992–1000. doi:10.1039/c2gc16673a |
| 27. | Bortolini, O.; Fantin, G.; Ferretti, V.; Fogagnolo, M.; Giovannini, P. P.; Massi, A.; Pacifico, S.; Ragno, D. Adv. Synth. Catal. 2013, 355, 3244–3252. doi:10.1002/adsc.201300652 |
| 28. | Ragno, D.; Bortolini, O.; Giovannini, P. P.; Massi, A.; Pacifico, S.; Zaghi, A. Org. Biomol. Chem. 2014, 12, 5733–5744. doi:10.1039/C4OB00759J |
| 29. | Ragno, D.; Bortolini, O.; Fantin, G.; Fogagnolo, M.; Giovannini, P. P.; Massi, A. J. Org. Chem. 2015, 80, 1937–1945. doi:10.1021/jo502582e |
| 26. | Ragno, D.; Zaghi, A.; Di Carmine, G.; Giovannini, P. P.; Bortolini, O.; Fontagnolo, M.; Molinari, A.; Venturini, A.; Massi, A. Org. Biomol. Chem. 2016, 14, 9823–9835. doi:10.1039/C6OB01868H |
| 31. | McCormick, R. M.; Karger, B. L. Anal. Chem. 1980, 52, 2249–2257. doi:10.1021/ac50064a005 |
© 2016 Zaghi et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)