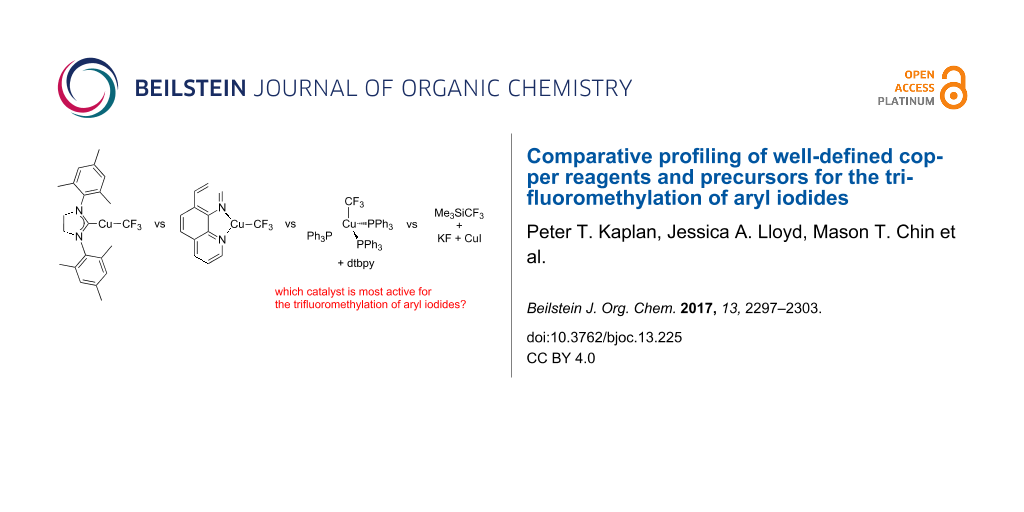
Abstract
A number of copper reagents were compared for their effectiveness in trifluoromethylating 4-iodobiphenyl, 4-iodotoluene, and 2-iodotoluene. Yields over time were plotted in order to refine our understanding of each reagent performance, identify any bottlenecks, and provide more insight into the rates of the reactions. Interestingly, differences in reactivity were observed when a well-defined [LCuCF3] complex was employed directly or generated in situ from precursors by published reports. Relative reactivities were also found to highly dependent on the nature of the iodoarenes.
Graphical Abstract
Introduction
Selectively fluorinated molecules that bear the trifluoromethyl group have great importance in the life sciences and materials fields as well as discovery chemistry in general [1-3]. Consequently, transition-metal-catalyzed methods for preparing aromatic trifluoromethyl compounds from readily available aryl halides are an area that has seen rapid growth in the past ten years. Copper is one of the most successfully used metals for mediating the trifluoromethylation of aryl halides, and the active form of the reagents is typically a copper(I) complex bearing a trifluoromethyl ligand, i.e., [LnCu–CF3]. Sporadic examples of trifluoromethylation ‘catalysis’ using copper have been observed [4-9], but these reactions typically only work for aryl iodides and have a low substrate scope, low turn-over values, and/or involve decarboxylation reactions at high temperatures. Stoichiometric trifluoromethylating agents are therefore more commonly used in benchtop trifluoromethylation chemistry. Ancillary ligands (L) are known to play a large role in the reactivity of such [LnCu–CF3] reagents, and recent work has focused on developing new ligands that not only allow for better control of reactivity but also provide stability to facilitate meaningful comparative studies, such as structural and electrochemical ones [2]. N-Heterocyclic carbene (NHC) complexes of copper such as A1 (Scheme 1) were the first well-defined and structurally characterized copper–CF3 complexes that display activity for the trifluoromethylation of aryl halides [10,11]. [(SIMes)CuCF3] (1, SIMes = 1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidene), which is in equilibrium with [(SIMes)2Cu][Cu(CF3)2] (2), can either be used directly or prepared in situ through the reaction of [(SIMes)Cu(O-t-Bu)] (3) with Me3SiCF3 (Scheme 2) [10]. Phenanthroline complexes of copper B1 were reported shortly after the NHC counterparts [5,12] and have reached much success in chemical synthesis due to the ease of preparation and the low cost of the phenanthroline ancillary ligand. [(phen)CuCF3] can now be purchased commercially, or prepared in situ by a variety of methods including the reaction of [Cu(O-t-Bu)]4 with Me3SiCF3 and phen (B2, Scheme 1) [12] or by reaction of [(MeO)3BCF3] with CuI and phen (B3, Scheme 1) [8]. The compound [(PPh3)3CuCF3] has been for a long time [13], however, its trifluoromethylating ability and structure determination was not reported until 2011. Trifluoromethylations with [(PPh3)3CuCF3] are only efficient when the reactions are performed in neat aryl iodode [14]. Less side-products and higher yields are observed for trifluoromethylations with [(PPh3)3CuCF3] when dtbpy (dtbpy = 4,4′-di-tert-butylbipyridine) is added to reaction mixtures (C1) to presumably generate a dtbpy complex of CuCF3 [14]. Finally, conditions that generate “ligandless” [CuCF3] (D1, for example) are also amenable for the trifluoromethylation of aryl iodides [15], but it is unclear how the reactivity profile of the ligandless complex compares to systems A–C described in Scheme 1. An important issue is that only single time point yields have been reported for systems B–D, and the significantly different reaction conditions employed for each system have made it impossible to truly compare reagent performance based on the available literature data. For this reason, we sought to run trifluoromethylation reactions with systems A–D under both identical and the reported optimal reaction conditions in order to track how yields change over time for each reagent for comparative studies. We also sought to explore whether there were differences in reactivity when a well-defined [LCuCF3] complex was employed directly or generated in situ by published reports. If so, it will be informative to know the extent of differences in reagent performance over time.
Scheme 1: Reagents and precursors used for trifluoromethylation reactions.
Scheme 1: Reagents and precursors used for trifluoromethylation reactions.
Scheme 2: Preparation of [(SIMes)2Cu][Cu(CF3)2].
Scheme 2: Preparation of [(SIMes)2Cu][Cu(CF3)2].
Results and Discussion
Because the phenanthroline-based system described as B1 (Scheme 1) is the most widely used reagent for trifluoromethylations, we modeled our “standard” comparative conditions similar to those reported by Hartwig in 2011 [12]. These conditions involve reacting 4-iodo-1,1’-biphenyl with a [Cu–CF3] source at 50 °C in DMF (Scheme 3). Somewhat more diluted reaction conditions relative to the published procedure were used to ensure homogeneity for all the different complexes described in Scheme 1. Conversions to 4-(trifluoromethyl)-1,1’-biphenyl were then monitored by gas chromatography relative to a calibrated internal standard. Experiments were performed in triplicate, and the average yields over time are plotted graphically in Figure 1.
Scheme 3: General protocol for reactions described in Figure 1.
Scheme 3: General protocol for reactions described in Figure 1.
![[1860-5397-13-225-1]](/bjoc/content/figures/1860-5397-13-225-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: Yields of 4-(trifluoromethyl)-1,1’-biphenyl over time for the systems described in Scheme 1. These runs represent “standard” conditions described in Scheme 3 and the Experimental section. Yields were monitored by gas chromatography relative to a calibrated internal standard.
Figure 1: Yields of 4-(trifluoromethyl)-1,1’-biphenyl over time for the systems described in Scheme 1. These runs rep...
As shown in Figure 1, conditions where the [(phen)CuCF3] was generated in situ (B2) provided the best yields of 4-(trifluoromethyl)-1,1’-biphenyl after 24 hours, with yields and conversion of aryl iodide (data not shown) both near 65%. When commercially purchased [(phen)CuCF3] (B1) was used, yields up to the five hour mark were comparable to those of B2, but were ≈15% lower after the full 24 hours. Yields of product were 50%, with consumption of biphenyl iodide at 62%. Importantly, traditional single time point yields would not be able to highlight the loss of activity at the longer reaction times. The related phen system B3, which uses K[(MeO)3BCF3] as the trifluoromethyl source, performed significantly lower than B1 and B2 and gave a final overall yield of 31%. Consumption of the biphenyl iodide was found to be 51%. The data is intriguing because systems B1, B2, and B3 are all expected to involve [(phen)CuCF3] as the active trifluoromethylating agent, yet there are clear differences in reactivities for only slight changes in chemical components in the reaction mixtures. For the same conditions in DMF at 50 °C, A1, C1, and D1 all performed poorly relative to [(phen)CuCF3] and gave product yields less than 10% (Figure 1).
We then compared B2, the highest performing [(phen)CuCF3] system in DMF, to the performance of isolated A1 and in situ generated (A2) [(SIMes)CuCF3] as well as to the [(PPh3)3CuCF3 + dtbpy] combination (C1) under their reported optimized solvent conditions to explore the effect of the solvent on the lower performing systems in Figure 1 [10,14]. The results are shown in Figure 2 and highlight the fact that the solvent plays a key role in reagent performance, even for reasonably comparable LCuCF3 complexes. The system C1 shows high yields at early reaction times, but then suffers a severe leveling out effect after approximately ten hours. Based on the data, it is tempting to suggest that system C1 might be worthy of a thorough mechanistic analysis, as if one can fully understand why the reagent suffers a rapid deactivation then a performance improvement may be possible. System A1, on the other hand, displays sluggish reactivity at early reaction times, but steadily produces 4-(trifluoromethyl)-1,1’-biphenyl in yields that are slightly higher than C1 after 30 hours. [(SIMes)Cu(CF3)], generated in situ from [(SIMes)Cu(O-t-Bu)] and TMSCF3 (A2) afforded the highest yields at extended reaction times. It is interesting to note here, for the trifluoromethylation of 4-iodobiphenyl with SIMes copper complexes under the reported literature conditions, that the better performer was not the isolated and well-defined LCu–CF3 complex, but instead the LCu–CF3 complex generated in situ from the copper tert-butoxide precursor. This trend in reactivity mirrors that which was observed for the phen-based systems B1 and B2 in Figure 1. The reported optimized conditions for A1 and A2, however, involve using copper as the limiting agent with a five-fold excess of aryl halide [4]. Therefore, while yields of the SIMes-based systems can provide good yields of product, the phen-based systems remain far more practical. Ligandless CuCF3 was also tested under the reported optimized reaction conditions [15], but in our hands the protocol afforded CHCF3 as the major fluorine-containing product.
![[1860-5397-13-225-2]](/bjoc/content/figures/1860-5397-13-225-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: Yields of 4-(trifluoromethyl)-1,1’-biphenyl over time for the systems described in Scheme 1. Conditions for C1: [(PPh3)3Cu(CF3)] + dtbpy: toluene, 80 °C. Conditions for A1: [(SIMes)Cu(CF3)] and A2 ([(SIMes)Cu(O-t-Bu)] + TMSCF3: DMI/benzene (1.5:7.5), 50 °C. DMI = 1,3-dimethyl-2-imidazolidinone. Conditions for B2: [(Cu(O-t-Bu)]4 + phen +TMSCF3: DMF, 50 °C. Yields were monitored by gas chromatography relative to a calibrated internal standard.
Figure 2: Yields of 4-(trifluoromethyl)-1,1’-biphenyl over time for the systems described in Scheme 1. Conditions for ...
Because our group has developed the NHC-based copper reagents for trifluoromethylation reactions [10,11], we were interested in comparing the effects of electronics and sterics of the aryl halides using the NHC-based systems A1 and A2 with the phen system B2. First, in order to explore reactivities with more electron rich aryl iodides, we investigated the use of 4-iodotoluene as a substrate for trifluoromethylation reactions. Because the product 1-methyl-4-(trifluoromethyl)benzene had similar retention times as the solvents in the gas chromatography analyses, we monitored the reactions of the iodotoluenes by quantitative 19F NMR spectroscopy. Using the same solvent systems employed for the reactions in Figure 2, conversions were measured over a 22 hour period (Figure 3). In this case, [(SIMes)Cu(CF3)] performed just as well as the [(phen)Cu(CF3)] generated in situ at the 22 hour mark. However, the yield versus time plot revealed that the phen-based reagent was clearly better at early reaction times. The plots revealed other interesting information. For the electron-rich aryl iodides, the reactivity difference for the SIMes copper complexes was opposite from what was observed previously in Figure 2. Here, the isolated and well-defined [(SIMes)Cu(CF3)] outperformed the in situ-generated counterpart, although at the five hour mark both performed equally well. Single time point yields would not have been able to identify the leveling out of reactivity of A2 for the more electron-rich aryl halide. Moreover, an induction period was observed for both A1 and A2. We noted a detection limit of approximately 2% with our NMR spectrometer, so we believe this induction period with the electron-rich substrates is real and not stemming from the different analytical method used for determining yields for Figure 2 and Figure 3. Why an induction period is observed for the iodotoluenes but not for the iodobiphenyl substrate is still not well-understood and is currently under investigation.
![[1860-5397-13-225-3]](/bjoc/content/figures/1860-5397-13-225-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: Reaction of 1-iodo-4-methylbenzene with systems A1, A2, and B2 to produce 1-methyl-4-(trifluoromethyl)benzene. Conditions for B2: DMF, 50 °C. Conditions for A1 and A2: DMI/benzene (1.5:7.5), 50 °C. Yields were calculated by 19F NMR spectroscopy versus an internal standard.
Figure 3: Reaction of 1-iodo-4-methylbenzene with systems A1, A2, and B2 to produce 1-methyl-4-(trifluorometh...
Reactions were then run with 2-iodotoluene in order to gauge steric effects in the trifluoromethylation reactions. It should be noted here that the promoting effect of ortho substituents in trifluoromethylation reactions is well-known. For example, the rate of trifluoromethylation of o-MeC6H4Br was found to be 3.5 times faster than that for bromobenzene by CuCF3 in DMF [16]. Figure 4 describes the results of the trifluoromethylations of 2-iodotoluene with systems A1, A2, and B2. For this iodoarene substrate, the phen- and the NHC-based copper reagents performed nearly equally well at almost all time periods. The well-defined and isolated A1 again showed a noticeable induction period, whereas the induction period for A2 was short.
![[1860-5397-13-225-4]](/bjoc/content/figures/1860-5397-13-225-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: Reaction of 2-iodotoluene with systems A1, A2, and B2 to produce 1-methyl-2-(trifluoromethyl)benzene. Conditions for B2: DMF, 50 °C. Conditions for A1 and A2: DMI/benzene (1.5:7.5), 50 °C. Yields were calculated by 19F NMR spectroscopy versus an internal standard.
Figure 4: Reaction of 2-iodotoluene with systems A1, A2, and B2 to produce 1-methyl-2-(trifluoromethyl)benzen...
Conclusion
In summary, we have determined that in DMF the best trifluoromethylating agent was generated in situ using the [Cu(O-t-Bu)]4/Me3SiCF3/phen combination. However, when optimized solvents were employed, other metal complexes and precursors approached and even exceeded the [(phen)Cu(CF3)] system, albeit with much higher metal loadings. In order to rigorously assess future trifluoromethylating agents and minimize issues of reproducibility, we encourage others to provide comparative data (yields versus time) for any newly developed trifluoromethylation reaction with a well-established reagent using identical reaction vessels and performed by the same experimentalist. The work shown here reveals the importance of comparing trifluoromethylation reactions using a number of different variables (solvent, sterics, and electronics) in order to adequately describe a catalyst’s performance for the community. Explicit benchmarking in catalysis science is rarely reported in the literature (less than 500 mentions in approximately 1 × 106 articles describing catalytic phenomena) [17], and as methodologies for trifluoromethylation reactions continue to develop it will be important to have protocols for assessing new reagents.
Experimental
General
[(Phen)Cu(CF3)] was purchased from Aspira Scientific (Lot #40C906, 90% purity) and used without further purification. All other copper reagents were prepared according to reported procedures and were verified by 1H NMR and 19F NMR for purity. Copper salt precursors were purchased from Sigma-Aldrich. Trimethyl(trifluoromethyl)silane (99% purity) was purchased from SynQuest Labs, Inc. and used without further purification. All other chemicals were verified by 1H NMR for purity and used without further purification. Purity of reagents used, CuCl (97%), KF (≥99%), 1,10-phenanthroline (≥99%), NaO-t-Bu (98%), KO-t-Bu (98%), K2CO3 (≥99%), undecane (≥99%), fluorobenzene (≥99%), 4-iodobiphenyl (97%), 4-iodotoluene (97%), 2-iodotoluene (97%), and 4,4′-di-tert-butyl-2,2′-dipyridyl (98%). All solvents were purified by passing through activated alumina and/or copper in a solvent purification system supplied by Pure Process Technology or purchased anhydrous from Fisher Scientific (toluene, acetonitrile, DMF, and DMI). The quantitative analyses were accomplished using a Shimadzu GC-2010 Plus Gas Chromatograph and flame ionization detector (FID). A Rxi-5ms (fused silica), low-polarity phase, crossbond diphenyl dimethyl polysiloxane, 15.0 m length column was used. Parameters were: injection volume of 4.0 µL, 25:1 split ratio, linear velocity of 57.0 cm/s, total flow of 65.3 mL/min, and temperature program starting at 40 °C held for one minute, followed by a temperature ramp of 20.0 °C per minute to the final temperature of 250 °C which was held for 4 minutes. All peaks were well separated. All manipulations were performed using standard Schlenk and high vacuum techniques or were performed in a nitrogen filled glovebox. The quantitative NMR analyses were accomplished using a Bruker Ascend 400 MHz spectrometer by 19F NMR spectra referenced to internal standard of fluorobenzene. Solution 1H NMR spectra were recorded at ambient temperature on a Bruker Ascend 400 MHz spectrometer and referenced to residual proton solvent signals. 19F NMR spectra were recorded on the Bruker Ascend NMR spectrometer operating at 376 MHz and referenced to trifluorotoluene set at δ −63.7. All graphical data were treated with a best fit curve generated by the Origin 9.0.0 program. The exponential fit with function ExpGro1 was selected for all data.
Updated procedure for the preparation of [(SIMes)2Cu][Cu(CF3)2] (2): A solution of [(SIMes)Cu(O-t-Bu)] (220 mg, 0.50 mmol) and CF3Si(CH3)3 (0.110 mL, 0.74 mmol) in 6.0 mL THF was stirred at room temperature. The conversion to product was monitored by 19F NMR spectroscopy, and after 1.5 h the volatiles were evaporated on a high vacuum line. The white residue was filtered and washed twice with 5 mL toluene and then twice with 5 mL of pentane. The yield of [(SIMes)2Cu][Cu(CF3)2] was 81%. The spectroscopic data matched literature values [10]. 1H NMR (25 °C, CD2Cl2) δ 1.84 (s, 12H), 2.39 (s, 6H), 3.80 (s, 4H), 6.89 (s, 4H); 19F NMR (25 °C, CD2Cl2) δ −31.33 (s, 3F).
Updated procedure for the preparation of [(SIMes)Cu(O-t-Bu)] (3): A suspension of [(SIMes)CuCl] (330 mg, 0.81 mmol) and t-BuONa (78 mg, 0.81 mmol) in 6.0 mL THF was stirred for 2 h at room temperature and then filtered through a pad of Celite. The Celite was washed two times with 4 mL of THF. The solvents were then removed on a high vacuum line, and the resulting light yellow residue was dissolved in benzene and then filtered again through a pad of Celite. The Celite was washed two times with 4 mL of benzene, and the filtrate was evaporated on a high vacuum line. The resulting white solid was washed with pentane, filtered, and dried. Yield 92%. The spectroscopic data matched literature values [10]. 1H NMR (C6D6) δ 1.31 (s, 9H), 2.12 (s, 6H), 2.14 (s, 12H), 3.01 (s, 4H), 6.73 (s, 4H).
General procedure for the standard conditions of trifluoromethylation of 4-iodobiphenyl (systems A1, B1, and C1 in Figure 1). To a 20 mL vial was added copper trifluoromethyl reagent (0.28 mmol) in 5.4 mL of DMF. In the case of [(PPh3)3CuCF3] [14], 77.2 mg (0.28 mmol) of dtbpy was also added. Then 67.1 mg (0.23 mmol) of 4-iodobiphenyl and 60.5 µL (0.28 mmol) of undecane as internal standard, were added to the vial. The solution was then allowed to stir for five minutes. Then 0.6 mL aliquots were taken and transferred into 5 mL air-tight ampules fitted with a stirring bar. The ampules were sealed and placed in an oil bath at 50 °C. The reactions were removed from the oil bath at various time intervals and quenched with 0.6 mL of methanol in air. Aliquots of each solution were injected into a GC-FID and the reactions were monitored for the formation of the 4-trifluoromethylbiphenyl product.
General procedure for the standard conditions of trifluoromethylation of 4-iodobiphenyl using in situ generated reagents (systems B2, B3, and D1 in Figure 1). The preparation of each reagent (B2, B3, and D1) is described below. After preparation of the reagent, 60.50 µL (0.2851 mmol) of undecane was added as internal standard. Each solution was allowed to stir for five minutes, and then 0.60 mL aliquots were taken and transferred into 5 mL air-tight resealable ampules. The ampules were sealed and placed in an oil bath at 50 °C and the solutions were stirred. The reactions were removed from the oil bath at various time intervals and quenched with 0.6 mL of methanol in air. Aliquots of each solution were injected into a GC-FID and the reactions were monitored for the formation of the 4-trifluoromethylbiphenyl product.
Generation of B2 in Figure 1: For the generation of (phen)CuCF3 in situ, a vial was charged with 28.4 mg (0.28 mmol) of CuCl, 32.3 mg (0.28 mmol) of KO-t-Bu, and 51.5 mg (0.28 mmol) of 1,10-phenanthroline in 5.4 mL of DMF. The solution was stirred for 0.5 h before the addition of 0.042 mL (0.28 mmol) of (Me)3SiCF3. The solution was stirred for an additional hour before the introduction of 0.23 mmol of 4-iodobiphenyl.
Generation of B3 in Figure 1: A vial was charged with 0.232 mmol of 4-iodobiphenyl, 53.6 mg (0.28 mmol) of CuI, 60.5 mg (0.28 mmol) of [K][B(OMe3)(CF3)] [8], and 51.5 mg (0.28 mmol) of 1,10-phenanthroline in 5.4 mL of DMF.
Generation of D1 in Figure 1: A vial was charged with (0.23 mmol) of 4-iodobiphenyl, 53.6 mg (0.28 mmol) of CuI, 16.7 mg (0.28 mmol) of KF, and 0.042 mL (0.28 mmol) of (Me)3SiCF3 in 5.4 mL of DMF as solvent.
General procedure for the ‘best’ conditions of trifluoromethylation of 4-iodobiphenyl (systems A1, A2, and C1 in Figure 2)
Reaction conditions employing [(SIMes)2Cu][Cu(CF3)2] (system A1): A vial was charged with 1.15 mmol of 4-iodobiphenyl and 105 mg (0.12 mmol) of [(SIMes)2Cu][Cu(CF3)2] in 5.4 mL of DMI/benzene (1.5:7.5) with 60.5 µL (0.29 mmol) of undecane, as internal standard. After the solution was allowed to stir for five minutes, 0.6 mL aliquots were taken and transferred into 5 mL air-tight ampules. The ampules were sealed and placed in an oil bath at 50 °C. The reactions were removed from the oil bath at various time intervals and quenched with 0.6 mL of methanol in air. Aliquots of each solution were injected into a GC-FID and the reactions were monitored for the formation of the 4-trifluoromethylbiphenyl product.
Reaction conditions employing [(SIMes)Cu(O-t-Bu)] + TMSCF3 (system A2): A vial was charged with 1.15 mmol of 4-iodobiphenyl, 106 mg (0.24 mmol) of [(SIMes)Cu(O-t-Bu)] and 0.053 mL (0.359 mmol) of (Me)3SiCF3 in 5.4 mL of DMI/benzene (1.5:7.5) with 60.5 µL (0.29 mmol) of undecane, as internal standard. After the solution was allowed to stir for five minutes, 0.6 mL aliquots were taken and transferred into 5 mL air-tight ampules. The ampules were sealed and placed in an oil bath at 50 °C. The reactions were removed from the oil bath at various time intervals and quenched with 0.6 mL of methanol in air. Aliquots of each solution were injected into a GC-FID and the reactions were monitored for the formation of the 4-trifluoromethylbiphenyl product.
Reaction conditions employing [(PPh3)3CuCF3] (system C1): A vial was charged with 0.31 mmol of 4-iodobiphenyl, 264 mg (0.29 mmol) of (PPh3)3CuCF3, and 85.0 mg (0.32 mmol) of dtbpy in 5.4 mL of toluene with 60.5 µL (0.29 mmol) of undecane, as internal standard. After the solution was allowed to stir for five minutes, 0.6 mL aliquots were taken and transferred into 5 mL air-tight ampules. The ampules were sealed and placed in an oil bath at 80 °C. The reactions were removed from the oil bath at various time intervals and quenched with 0.6 mL of methanol in air. Aliquots of each solution were injected into a GC-FID and the reactions were monitored for the formation of the 4-trifluoromethylbiphenyl product.
General procedure for the trifluoromethylation of 2-iodotoluene and 4-iodotoluene (system A1, A2 and B2 in Figure 3 and Figure 4)
Reaction conditions employing [(SIMes)2Cu][Cu(CF3)2] (system A1): A vial was charged with 270 mg (1.20 mmol) of 2-iodotoluene or 4-iodotoluene and 105 mg (0.12 mmol) of [(SIMes)2Cu][Cu(CF3)2] in 5.4 mL of DMI/benzene (1.5:7.5) with 90.0 µL (0.95 mmol) of fluorobenzene, as internal standard. After the solution was allowed to stir for five minutes, 0.6 mL aliquots were taken and transferred into 5 mL air-tight ampules. The ampules were sealed and placed in an oil bath at 50 °C. The reactions were removed from the oil bath at various time intervals and quenched with 0.6 mL of methanol in air. Each aliquot was monitored by 19F NMR spectroscopy for formation of the respective 2-trifluoromethyltoluene or 4-trifluoromethyltoluene product.
Reaction conditions employing [(SIMes)Cu(O-t-Bu)] + TMSCF3 (system A2): A vial was charged with 270 mg (1.20 mmol) of 2-iodotoluene or 4-iodotoluene, 106 mg (0.24 mmol) of [(SIMes)Cu(O-t-Bu)] and 0.053 mL (0.36 mmol) of (Me)3SiCF3 in 5.4 mL of DMI/benzene (1.5:7.5) with 90.0 µL (0.95 mmol) of fluorobenzene, as internal standard. After the solution was allowed to stir for five minutes, 0.60 mL aliquots were taken and transferred into 5 mL air-tight ampules. The ampules were sealed and placed in an oil bath at 50 °C. The reactions were removed from the oil bath at various time intervals and quenched with 0.6 mL of methanol in air. Each aliquot was monitored by 19F NMR spectroscopy for formation of the respective 2-trifluoromethyltoluene or 4-trifluoromethyltoluene product.
Reaction conditions employing [(phen)Cu(O-t-Bu)]4 + TMSCF3 (system B2): For the generation of (phen)CuCF3 in situ, a vial was charged with 28.4 mg (0.28 mmol) of CuCl, 32.3 mg (0.28 mmol) of KO-t-Bu, and 51.5 mg (0.28 mmol) of 1,10-phenanthroline. To the vial, 5.4 mL of DMF was added. The solution was stirred for 0.5 h before the addition of 0.042 mL (0.28 mmol) of Me3SiCF3. The solution was stirred for an additional hour before the introduction of 52.1 mg (0.23 mmol) of 2-iodotoluene or 4-iodotoluene and 90.0 µL (0.95 mmol) of fluorobenzene, as internal standard. After the solution was allowed to stir for five minutes, 0.60 mL aliquots were taken and transferred into 5 mL air-tight ampules. The ampules were sealed and placed in an oil bath at 50 °C. The reactions were removed from the oil bath at various time intervals and quenched with 0.6 mL of methanol in air. Each aliquot was monitored by 19F NMR spectroscopy for formation of the respective 2-trifluoromethyltoluene or 4-trifluoromethyltoluene product.
References
-
Tomashenko, O. A.; Grushin, V. V. Chem. Rev. 2011, 111, 4475–4521. doi:10.1021/cr1004293
Return to citation in text: [1] -
Wang, H.; Vicic, D. A. Synlett 2013, 24, 1887–1898. doi:10.1055/s-0033-1339435
Return to citation in text: [1] [2] -
Roy, S.; Gregg, B. T.; Gribble, G. W.; Le, V.-D.; Roy, S. Tetrahedron 2011, 67, 2161–2195. doi:10.1016/j.tet.2011.01.002
Return to citation in text: [1] -
Schareina, T.; Wu, X.-F.; Zapf, A.; Cotté, A.; Gotta, M.; Beller, M. Top. Catal. 2012, 55, 426–431. doi:10.1007/s11244-012-9824-0
Return to citation in text: [1] [2] -
Oishi, M.; Kondo, H.; Amii, H. Chem. Commun. 2009, 1909–1911. doi:10.1039/b823249k
Return to citation in text: [1] [2] -
Weng, Z.; Lee, R.; Jia, W.; Yuan, Y.; Wang, W.; Feng, X.; Huang, K.-W. Organometallics 2011, 30, 3229–3232. doi:10.1021/om200204y
Return to citation in text: [1] -
Nakamura, Y.; Fujiu, M.; Murase, T.; Itoh, Y.; Serizawa, H.; Aikawa, K.; Mikami, K. Beilstein J. Org. Chem. 2013, 9, 2404–2409. doi:10.3762/bjoc.9.277
Return to citation in text: [1] -
Knauber, T.; Arikan, F.; Roeschenthaler, G.-V.; Goossen, L. J. Chem. – Eur. J. 2011, 17, 2689–2697. doi:10.1002/chem.201002749
Return to citation in text: [1] [2] [3] -
Chen, Q.-Y.; Wu, S.-W. J. Chem. Soc., Chem. Commun. 1989, 705–706. doi:10.1039/c39890000705
Return to citation in text: [1] -
Dubinina, G. G.; Ogikubo, J.; Vicic, D. A. Organometallics 2008, 27, 6233–6235. doi:10.1021/om800794m
Return to citation in text: [1] [2] [3] [4] [5] [6] -
Dubinina, G. G.; Furutachi, H.; Vicic, D. A. J. Am. Chem. Soc. 2008, 130, 8600–8601. doi:10.1021/ja802946s
Return to citation in text: [1] [2] -
Morimoto, H.; Tsubogo, T.; Litvinas, N. D.; Hartwig, J. F. Angew. Chem., Int. Ed. 2011, 50, 3793–3798. doi:10.1002/anie.201100633
Return to citation in text: [1] [2] [3] -
Usui, Y.; Noma, J.; Hirano, M.; Komiya, S. Inorg. Chim. Acta 2000, 309, 151–154. doi:10.1016/S0020-1693(00)00248-6
Return to citation in text: [1] -
Tomashenko, O. A.; Escudero-Adán, E. C.; Martínez Belmonte, M.; Grushin, V. V. Angew. Chem., Int. Ed. 2011, 50, 7655–7659. doi:10.1002/anie.201101577
Return to citation in text: [1] [2] [3] [4] -
Urata, H.; Fuchikami, T. Tetrahedron Lett. 1991, 32, 91–94. doi:10.1016/S0040-4039(00)71226-3
Return to citation in text: [1] [2] -
Konovalov, A. I.; Lishchynskyi, A.; Grushin, V. V. J. Am. Chem. Soc. 2014, 136, 13410–13425. doi:10.1021/ja507564p
Return to citation in text: [1] -
Bligaard, T.; Bullock, R. M.; Campbell, C. T.; Chen, J. G.; Gates, B. C.; Gorte, R. J.; Jones, C. W.; Jones, W. D.; Kitchin, J. R.; Scott, S. L. ACS Catal. 2016, 6, 2590–2602. doi:10.1021/acscatal.6b00183
Return to citation in text: [1]
| 17. | Bligaard, T.; Bullock, R. M.; Campbell, C. T.; Chen, J. G.; Gates, B. C.; Gorte, R. J.; Jones, C. W.; Jones, W. D.; Kitchin, J. R.; Scott, S. L. ACS Catal. 2016, 6, 2590–2602. doi:10.1021/acscatal.6b00183 |
| 10. | Dubinina, G. G.; Ogikubo, J.; Vicic, D. A. Organometallics 2008, 27, 6233–6235. doi:10.1021/om800794m |
| 11. | Dubinina, G. G.; Furutachi, H.; Vicic, D. A. J. Am. Chem. Soc. 2008, 130, 8600–8601. doi:10.1021/ja802946s |
| 16. | Konovalov, A. I.; Lishchynskyi, A.; Grushin, V. V. J. Am. Chem. Soc. 2014, 136, 13410–13425. doi:10.1021/ja507564p |
| 1. | Tomashenko, O. A.; Grushin, V. V. Chem. Rev. 2011, 111, 4475–4521. doi:10.1021/cr1004293 |
| 2. | Wang, H.; Vicic, D. A. Synlett 2013, 24, 1887–1898. doi:10.1055/s-0033-1339435 |
| 3. | Roy, S.; Gregg, B. T.; Gribble, G. W.; Le, V.-D.; Roy, S. Tetrahedron 2011, 67, 2161–2195. doi:10.1016/j.tet.2011.01.002 |
| 10. | Dubinina, G. G.; Ogikubo, J.; Vicic, D. A. Organometallics 2008, 27, 6233–6235. doi:10.1021/om800794m |
| 4. | Schareina, T.; Wu, X.-F.; Zapf, A.; Cotté, A.; Gotta, M.; Beller, M. Top. Catal. 2012, 55, 426–431. doi:10.1007/s11244-012-9824-0 |
| 10. | Dubinina, G. G.; Ogikubo, J.; Vicic, D. A. Organometallics 2008, 27, 6233–6235. doi:10.1021/om800794m |
| 11. | Dubinina, G. G.; Furutachi, H.; Vicic, D. A. J. Am. Chem. Soc. 2008, 130, 8600–8601. doi:10.1021/ja802946s |
| 15. | Urata, H.; Fuchikami, T. Tetrahedron Lett. 1991, 32, 91–94. doi:10.1016/S0040-4039(00)71226-3 |
| 12. | Morimoto, H.; Tsubogo, T.; Litvinas, N. D.; Hartwig, J. F. Angew. Chem., Int. Ed. 2011, 50, 3793–3798. doi:10.1002/anie.201100633 |
| 4. | Schareina, T.; Wu, X.-F.; Zapf, A.; Cotté, A.; Gotta, M.; Beller, M. Top. Catal. 2012, 55, 426–431. doi:10.1007/s11244-012-9824-0 |
| 5. | Oishi, M.; Kondo, H.; Amii, H. Chem. Commun. 2009, 1909–1911. doi:10.1039/b823249k |
| 6. | Weng, Z.; Lee, R.; Jia, W.; Yuan, Y.; Wang, W.; Feng, X.; Huang, K.-W. Organometallics 2011, 30, 3229–3232. doi:10.1021/om200204y |
| 7. | Nakamura, Y.; Fujiu, M.; Murase, T.; Itoh, Y.; Serizawa, H.; Aikawa, K.; Mikami, K. Beilstein J. Org. Chem. 2013, 9, 2404–2409. doi:10.3762/bjoc.9.277 |
| 8. | Knauber, T.; Arikan, F.; Roeschenthaler, G.-V.; Goossen, L. J. Chem. – Eur. J. 2011, 17, 2689–2697. doi:10.1002/chem.201002749 |
| 9. | Chen, Q.-Y.; Wu, S.-W. J. Chem. Soc., Chem. Commun. 1989, 705–706. doi:10.1039/c39890000705 |
| 10. | Dubinina, G. G.; Ogikubo, J.; Vicic, D. A. Organometallics 2008, 27, 6233–6235. doi:10.1021/om800794m |
| 14. | Tomashenko, O. A.; Escudero-Adán, E. C.; Martínez Belmonte, M.; Grushin, V. V. Angew. Chem., Int. Ed. 2011, 50, 7655–7659. doi:10.1002/anie.201101577 |
| 13. | Usui, Y.; Noma, J.; Hirano, M.; Komiya, S. Inorg. Chim. Acta 2000, 309, 151–154. doi:10.1016/S0020-1693(00)00248-6 |
| 14. | Tomashenko, O. A.; Escudero-Adán, E. C.; Martínez Belmonte, M.; Grushin, V. V. Angew. Chem., Int. Ed. 2011, 50, 7655–7659. doi:10.1002/anie.201101577 |
| 14. | Tomashenko, O. A.; Escudero-Adán, E. C.; Martínez Belmonte, M.; Grushin, V. V. Angew. Chem., Int. Ed. 2011, 50, 7655–7659. doi:10.1002/anie.201101577 |
| 8. | Knauber, T.; Arikan, F.; Roeschenthaler, G.-V.; Goossen, L. J. Chem. – Eur. J. 2011, 17, 2689–2697. doi:10.1002/chem.201002749 |
| 15. | Urata, H.; Fuchikami, T. Tetrahedron Lett. 1991, 32, 91–94. doi:10.1016/S0040-4039(00)71226-3 |
| 8. | Knauber, T.; Arikan, F.; Roeschenthaler, G.-V.; Goossen, L. J. Chem. – Eur. J. 2011, 17, 2689–2697. doi:10.1002/chem.201002749 |
| 12. | Morimoto, H.; Tsubogo, T.; Litvinas, N. D.; Hartwig, J. F. Angew. Chem., Int. Ed. 2011, 50, 3793–3798. doi:10.1002/anie.201100633 |
| 10. | Dubinina, G. G.; Ogikubo, J.; Vicic, D. A. Organometallics 2008, 27, 6233–6235. doi:10.1021/om800794m |
| 5. | Oishi, M.; Kondo, H.; Amii, H. Chem. Commun. 2009, 1909–1911. doi:10.1039/b823249k |
| 12. | Morimoto, H.; Tsubogo, T.; Litvinas, N. D.; Hartwig, J. F. Angew. Chem., Int. Ed. 2011, 50, 3793–3798. doi:10.1002/anie.201100633 |
| 14. | Tomashenko, O. A.; Escudero-Adán, E. C.; Martínez Belmonte, M.; Grushin, V. V. Angew. Chem., Int. Ed. 2011, 50, 7655–7659. doi:10.1002/anie.201101577 |
| 10. | Dubinina, G. G.; Ogikubo, J.; Vicic, D. A. Organometallics 2008, 27, 6233–6235. doi:10.1021/om800794m |
© 2017 Kaplan et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)