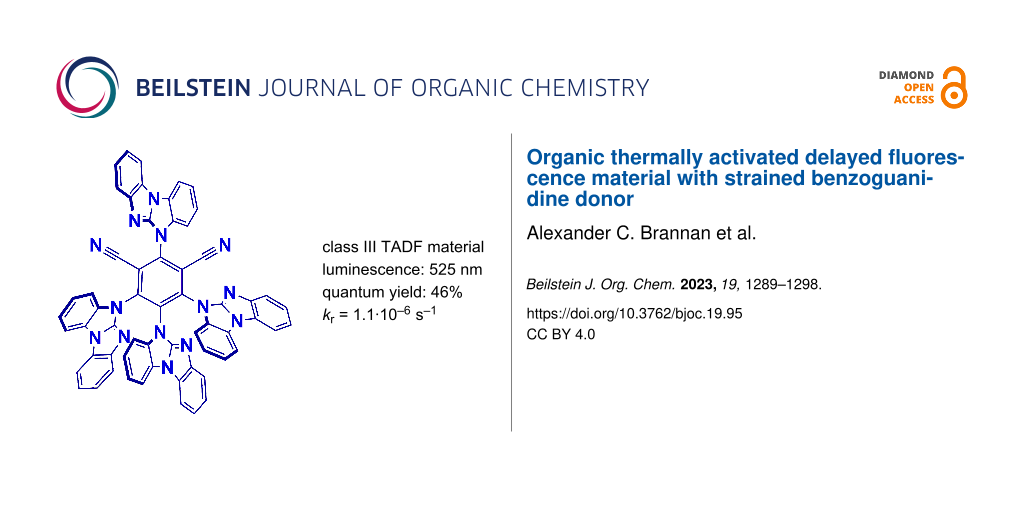
Abstract
Organic thermally activated delayed fluorescence (TADF) materials have been widely investigated due to their impressive electronic properties and applied potential for the third generation of organic light-emitting diodes (OLED). We present organic TADF material (4BGIPN) based on the strained benzoguanidine donor and compare it with the benchmark carbazole-based material (4CzIPN). Extended π-conjugation in 4BGIPN material results in yellow-green luminescence at 512 nm with a fast radiative rate of 5.5 × 10−5 s−1 and a photoluminescence quantum yield of 46% in methylcyclohexane solution. Such a nitrogen-rich 4BGIPN material has a significantly stabilized highest occupied molecular orbital (HOMO) at −6.4 eV while the lowest unoccupied molecular orbital (LUMO) at −4.0 eV, indicating potential suitability for application as the electron transport layer or TADF class III emitter in OLEDs.
Graphical Abstract
Introduction
Thermally activated delayed fluorescence (TADF) is a photoluminescence mechanism where excitons undergo thermally-assisted reverse-intersystem crossing from an excited triplet state to a higher-lying in energy singlet state to emit delayed fluorescence [1-3]. Organic TADF emitters have gained substantial attention in recent years for their prospective application in organic light-emitting diodes (OLEDs), photocatalysis, bioimaging, and sensors [4-6]. The ability to harvest both singlet and triplet excitons enable organic TADF emitters to compete with classic phosphorescent emitters that employ scarce metals such as iridium and platinum [7-9]. Since its first report in 2012 by Uoyama et al., 1,2,3,5-tetrakis(carbazol-9-yl)-4,6-dicyanobenzene (4CzIPN) has been a benchmark TADF emitter due to its high quantum yields and excellent performance in OLED devices [1]. 4CzIPN is a donor–acceptor-type system where carbazole donor ligands are bound to the benzonitrile acceptor core moiety. In this work we have substituted the carbazole donors with 5H-benzo[d]benzo[4,5]imidazo[1,2-a]imidazole (benzoguanidine) ligands to give 4BGIPN, see Figure 1. Benzoguanidine has an extended π-conjugation compared with carbazole and is more nitrogen-rich (three N-atoms vs one in carbazole). Thompson et al. recently reported a series of carbene–metal–amide (CMA) (metal = Cu, Ag, Au) emitters employing a benzoguanidine ligand [10]. The extended π-conjugation of benzoguanidine induced a larger hole–electron separation resulting in a smaller energy gap between the excited singlet and triplet states (S1 and T1) and ΔEST resulting in faster radiative rates. This study aimed to synthesize and explore the luminescent properties of the 4BGIPN material containing a rigid benzoguanidine ligand in its molecular structure.
Figure 1: The molecular structures of the title compound 4BGIPN and the benchmark TADF emitter 4CzIPN.
Figure 1: The molecular structures of the title compound 4BGIPN and the benchmark TADF emitter 4CzIPN.
Results and Discussion
Synthesis and structure
4BGIPN was prepared in 70% yield by aromatic nucleophilic substitution reaction from 2,4,5,6-tetrafluoroisophthalonitrile and 5H-benzo[d]benzo[4,5]imidazo[1,2-a]imidazole (benzoguanidine) after deprotonation the latter with sodium hydride base. The compound shows poor solubility in most common organic solvents with moderate solubility in dichloromethane, 1,2-dichlorobenzene and dimethyl sulfoxide (DMSO). Compound 4BGIPN was characterized by high-resolution mass spectrometry (HRMS), elemental analysis, and 1H/13C NMR spectroscopy. Proton NMR shows a complicated set of overlapping multiplets indicating that the reaction results in the formation of various isomers (rotamers) which are different by relative orientation of the benzoguanidine donor moieties with respect to each other (Figure 2, see Supporting Information File 1 for NMR). In DMSO-d6 solution, 4BGIPN isomers do not show interconversion even upon warming to 120 °C, resulting in a similar set of signals. Excellent fit between HRMS and elemental analysis further supports the formation of the isomeric mixture of 4BGIPN as evidenced by the identical molecular peak ion and C, H, N values within acceptable deviation of 0.4%. The decomposition temperature (Td, corresponds to 5% weight loss) was measured by thermogravimetric analysis (TGA) indicating excellent thermal stability for 4BGIPN with Td = 425 °C, which is similar to the benchmark material 4CzIPN, having a Td in the range of 402–449 °C (Td range is dependent on the type of the 4CzIPN polymorph) [11,12]. Differential scanning calorimetry (DSC) shows an endothermic process in the range of 237 to 265 °C which can be associated with the glass transition temperature (Tg) for the isomeric mixture of 4BGIPN. This is expectedly higher than the analogous Tg of 176 °C for the 4CzIPN material [13] due to a lower molecular mass of the latter (Figure S1, Supporting Information File 1).
![[1860-5397-19-95-2]](/bjoc/content/figures/1860-5397-19-95-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: Crystal structure for compound 4BGIPN in monoclinic form ((a) top view and (b) side view) where black arrows show opposite orientation of the benzoguanidine moieties around the central benzene ring. Representative example for the torsion angle α is shown in red; (c) Crystal structure for compound 4BGIPN in triclinic form; (d) packing diagram with key geometrical parameters and intermolecular contacts shown as a cyan dashed line; (e) possible isomers for 4BGIPN material. Ellipsoids are shown at the 50% level where hydrogen atoms are omitted for clarity.
Figure 2: Crystal structure for compound 4BGIPN in monoclinic form ((a) top view and (b) side view) where bla...
Single crystals for X-ray diffraction study were obtained by slow layer diffusion of hexanes into dichloromethane solution for 4BGIPN at room temperature (Figure 2). The title compound crystallizes with two independent molecules in the unit cell of the triclinic (P−1, Figure 2c, yellow plates) and monoclinic chiral space group P21 (Figure 2a,b,d, yellow blocks). Due to very weak reflection data, the structure of 4BGIPN in triclinic form was refined in isotropic model, therefore, we do not discuss in detail the structural parameters. We only note that both forms are not due to the polymorphism but rather due to rotational isomerism of the 4BGIPN material, i.e., different orientation of the benzoguanidine donor ligands above or below the central 4,6-dicyanobenzene ring. In the monoclinic form the two independent molecules of 4BGIPN are related by a pseudo glide plane that do not completely align when superimposed through a glide operation. There is no evidence for systematic absences relating to the presence of a glide plane in the data supporting the refinement in the chiral P21 space group. The structure was refined as a two-component inversion twin; the crystal structure as a whole is a racemic mixture of both orientations. The Cbenzene–Nbenzoguanidine bond length varies within the error of the experiment from 1.402(5) to 1.420(5), giving an average of 1.407(13) Å for 4BGIPN, which is closely similar to 1.405(8) Å reported for the benchmark 4CZIPN compound.
Unlike carbazole, the benzoguanidine ligand lacks C2 rotational symmetry, thus enabling the benzoguanidine ligands to project above and below the plane of the central benzene ring. In both molecules in the asymmetric unit, the benzoguanidine moiety bound to the benzene carbon neighboring two nitrile groups, is orientated in the opposing projection about the plane of the benzene ring to the remaining benzoguanidine moieties (Figure 2b). Unlike monoclinic, the triclinic form of 4BGIPN possesses two donor moieties pointing down at C1 and C3 carbon atoms while donor moieties at C2 and C5 are pointed up (Figure 2c). Several possible 4BGIPN rotational isomers are demonstrated in Figure 2e, however, not isolated in this work. Compound 4BGIPN possesses a twisted orientation between the donor (benzoguanidine) and acceptor (benzonitrile) ligands (Figure 2) due to steric hindrance imposed by benzoguanidine ligands and reflected by the torsion angle (α) laying in the range of 42.5(2)–64.3(2)º. We compared it with the more narrow torsion angle range of 55.1(2)–60.2(2)º for 4CzIPN thus indicating that various carbazole donor ligands possess a very similar twist orientation [12]. The donor–acceptor twist angle has been demonstrated to be one of the key structural parameters enabling fast radiative rates for purely organic TADF materials since it’s directly related with the overlap integral between HOMO and LUMO orbitals and influences the energy differences between first singlet and triplet excited states [14]. Therefore, we expect a marked difference in the photophysical properties for 4BGIPN, vide infra.
Analysis of the intermolecular interactions revealed that 4BGIPN molecules experience face to face intermolecular π–π stacking interactions between the benzoguanidine moieties similar to 4CzIPN (reported by Etherington et al., [12]). The average interplanar distance for close neighbor benzoguanidine moieties in 4BGIPN is 3.322(3) Å, which is significantly shorter (0.4 Å) than the 3.74(3) Å average distance between nearest neighbor carbazole ligands in 4CzIPN, indicative for much stronger intermolecular interactions.
Cyclic voltammetry was used to analyze the redox behavior of 4BGIPN in THF solution containing [n-Bu4N]PF6 as supporting electrolyte (Figure 3, Table 1). The reduction wave has a quasi-reversible character with the E1/2 at −1.50 V, which is 260 mV shifted to higher potential when compared with 4CzIPN (−1.76 V) under similar conditions in THF [15]. Compounds 4BGIPN and 4CzIPN experience a reduction process at the benzonitrile core (see, the LUMO isosurface in Figure 6, vide infra). Therefore, the higher reduction potential for 4BGIPN suggests that the benzonitrile core has a lower electron density, which is likely associated with extended π-conjugation and two additional electron withdrawing aza-type nitrogen atoms in the benzoguanidine moieties. This explains the ca. 0.2 eV more stabilized LUMO energy level for compound 4BGIPN compared with 4CzIPN. Both 4CzIPN and 4BGIPN exhibit an irreversible oxidation wave observed at +1.25 V for 4BGIPN in THF and +0.94 V for 4CzIPN in MeCN [15]. A higher oxidation potential (Ep) for 4BGIPN compared with the 4CzIPN corroborates with the electron deficient nature of the benzoguanidine moiety thus making it harder to oxidize compared with the more electron-rich carbazole moiety. This results in stabilization of the HOMO energy level at −6.4 eV for 4BGIPN. Significant stabilization for both HOMO and LUMO energy levels indicates the potential suitability of 4BGIPN material for application not only as emitter in the emitting layer but also as an electron transport layer in the fabrication of OLEDs.
![[1860-5397-19-95-3]](/bjoc/content/figures/1860-5397-19-95-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: Full range cyclic voltammogram for 4BGIPN. Recorded using a glassy carbon electrode in THF solution (1.4 mM) with [n-Bu4N]PF6 as supporting electrolyte (0.13 M), scan rate 0.1 Vs−1.
Figure 3: Full range cyclic voltammogram for 4BGIPN. Recorded using a glassy carbon electrode in THF solution...
Table 1: Formal electrode potentials (peak position Ep for irreversible and E1/2 for quasi-reversible processes (*), V, vs FeCp2), onset potentials (E, V, vs FeCp2), peak-to-peak separation in parentheses for quasi-reversible processes (ΔEp in mV), EHOMO/ELUMO (eV) and band gap values (ΔE, eV) for the redox changes exhibited by 4BGIPN.a
| Complex | Reduction |
ELUMO
eVb |
Oxidation |
EHOMO
eVb |
ΔE
eV |
||
| E1st | Eonset red | E1st | Eonset ox | ||||
| 4BGIPN |
−1.50
(167) |
−1.41 | −3.98 | +1.25 | +1.01 | −6.40 | 2.42 |
aIn THF solution, recorded using a glassy carbon electrode, concentration 1.4 mM, supporting electrolyte [n-Bu4N][PF6] (0.13 M), measured at 0.1 V s−1. bCalculated according to EHOMO = –(Eonset ox Fc/Fc+ + 5.39) and ELUMO = –(Eonset red Fc/Fc+ + 5.39) eV.
Photophysical properties and theoretical considerations
UV–vis and photoluminescence (PL) spectra for 4BGIPN are shown in Figure 4 and Figure 5 while data in various media is collected in Table 2 and Table 3, respectively. UV–vis absorption spectra of 4BGIPN show a strong π–π* intraligand transition (IL, benzoguanidine) at 290 nm with ε = 42000 M−1cm−1. Unlike 4CzIPN, we do not observe any vibronically resolved carbazole absorption peaks which are commonly present at 325 nm [15]. Similar to 4CzIPN [14,15], the UV–vis profile has two broad regions: localized charge transfer (loCT) over 320–380 nm region with ε up to 14000 M−1 cm−1 and a delocalized charge transfer (deCT) broad shoulder over the region of ca. 380–460 nm with ε up to 1900 M−1 cm−1 (Table 2). Both loCT and deCT bands are observed for the benchmark material 4CzIPN [16] while originating from HOMO to LUMO transition in line with the theoretical calculations (Tables S1, S3, and S4, Supporting Information File 1). All CT bands experience a very weak solvatochromic effect with increasing solvent polarity from cyclohexane to dichloromethane. This indicates only minor change of the dipole moment upon vertical excitation from S0 (6.4 D) to S1 (7.2 D) excited states according to the TD-DFT theoretical calculations (Table S2, Supporting Information File 1).
![[1860-5397-19-95-4]](/bjoc/content/figures/1860-5397-19-95-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: UV–vis absorption spectra for compound 4BGIPN in various solvents.
Figure 4: UV–vis absorption spectra for compound 4BGIPN in various solvents.
Table 2: UV–vis data for compounds 4BGIPN and 4CzIPN [15,17] in various solvents.
The photoluminescence (PL) characteristics of 4BGIPN have been studied in methylcyclohexane solution (MCH, concentration 3.2 × 10−5 M) and Zeonex polymer films (0.1% concentration by weight) at 298 K and 77 K, which is shown in Figure 5 with data collected in Table 3. Compound 4BGIPN exhibits a featureless yellow CT-type luminescence with λmax = 525 nm that is 44 and 25 nm red-shifted compared to 4CzIPN (λmax = 481 and 500 nm in Zeonex and MCH, respectively) [12]. The solution photoluminescent quantum yield (PLQY) of 4BGIPN is 46% under inert atmosphere and decreases down to 18% in aerated MCH solution. The reduction in quantum yield on exposure to oxygen is due to quenching of the triplet excited states indicating a TADF luminescence mechanism. PLQY in Zeonex films is 39% in air, which is lower than the PLQY of 87% reported for 4CzIPN [17].
![[1860-5397-19-95-5]](/bjoc/content/figures/1860-5397-19-95-5.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: Photoluminescence spectra for 4BGIPN at 295 and 77 K in (top left) MCH solution; (bottom left) Zeonex 0.1 wt % films; (top right) steady state and delayed PL in frozen glass MCH at 77 K with delays of 1.8 and 720 ms; (bottom right) excited state lifetime at various temperatures in the range from 16 to 296 K.
Figure 5: Photoluminescence spectra for 4BGIPN at 295 and 77 K in (top left) MCH solution; (bottom left) Zeon...
Table 3: Photophysical properties of 4BGIPN in various media at 296 and 77 K.
| λem (nm) |
τ
(ns) |
Φ
(%)a |
kr
(105 s−1)b |
knr
(105 s−1)c |
1LE/3LE/1CT
(eV)d |
λem
(nm) |
τ | |
| methylcyclohexane (MCH) solution 296 K | 77 K | |||||||
| 4BGIPN | 512 |
13 (50%)
1666 (50%) |
46 (N2)
(18 air) |
5.48 | 6.43 | 3.12/2.73/2.70 | 405 (1LE); | 13 ns |
| 470 (3LE); | 1.8 s | |||||||
| 530 (3CT) |
126 (36%) µs
1038 (64%) µs |
|||||||
| 0.1 wt % Zeonex matrix 296 K 16 K | ||||||||
| 4BGIPN | 517 |
12 (35%)
2023 (75%) |
39 | 2.56 | 4.0 | –/–/2.66 | 498 |
4.35 (31%) ms
36.8 (37%) ms 212 (32%) ms |
aAbsolute quantum yields determined using an integrating sphere; bradiative rate constant kr = Φ/τ; cnonradiative constant knr = (1 – Φ)/τ; dCT/LE energies based on the onset values of the emission spectra blue edge at 77 K and 295 K.
The two-component excited state lifetime with prompt and delayed fluorescence is characteristic for the TADF-type luminescence [1]. The excited state lifetime of 4BGIPN has a biexponential decay with a prompt fluorescence τp = 13 ns and a delayed fluorescence τd = 1655 ns components in MCH solution. Zeonex films of 4BGIPN exhibit a similar prompt τp = 12 ns, but an almost two-fold longer delayed fluorescence τd = 2.4 μs when compared to MCH solution. The archetype material 4CzIPN possesses a similar prompt fluorescence component of 8 ns, whereas a delayed component is nearly ten-times longer, 8.9 and 8.8 μs, in MCH and Zeonex films, respectively [12]. These measurements correlate well with lower PLQY values for 4BGIPN compared with 4CzIPN, thus indicating that the use of a larger benzoguanidine donor ligand may open more nonradiative processes. This is reflected in the larger distribution in the torsion angles for 4BGIPN compared with 4CzIPN, vide supra.
We collected steady state luminescence and PL after long time delays for 4BGIPN at 77 K to further support the assignment of the TADF mechanism and attempt to characterize LE and CT triplet states. The emission profiles experienced minor narrowing upon cooling to 77 K, while the PL profile remained broad and featureless (Figure 5) in Zeonex matices. Notably, a frozen MCH glass exhibits a new vibronically resolved component at 405 and 470 nm, together with a broad CT profile. The first resolved high-energy PL component at 405 nm (Figure 5 top right) has a lifetime of 13 ns, which we assigned to singlet locally excited fluorescence (1LE) from the benzoguanidine donor ligand. The second high-energy PL component at 470 nm becomes visible only after a long-time delay (720 ms, see Figure 5 black profile), therefore, we ascribed it to a phosphorescence from a higher lying 3LE state localized on a donor benzoguanidine moiety. Unlike 1LE-fluorescence, 3LE-phosphorescence has a very long lifetime of 1.8 s. Excitation spectra of the broad and resolved bands for 4BGIPN in MCH glass at 77 K (Figure S4, Supporting Information File 1) follow a mirror image rule when compared with emission spectra showing both broad and resolved components, thus supporting the assignments of the 3CT and 3LE(donor) excited states. The excited state lifetime of the broad CT component has a multiexponential decay with averaged lifetimes of 0.7 ms in MCH glass and up to 212 ms in Zeonex films, which we assigned as phosphorescence from a 3CT state. A more than 100-fold increase in radiative lifetime on cooling to 77 K is characteristic for the organic TADF emitters [1]. Upon cooling Zeonex matrices of 4BGIPN from 296 K to 16 K (Figure 5), the excited state lifetime shows an order of magnitude increase down to 60 K. However, only marginal increase of lifetime measured in the range of 60 to 16 K indicating a phosphorescence PL nature below 60 K.
The charge transfer singlet (1CT) and local excited singlet (1LE), triplet (3LE) state energies were estimated from the onset values of the blue emission edge of the PL spectra at 295 K for 1CT and 720 ms delayed PL at 77 K for 3LE, respectively (Figure 5 and Table 3). It was expected to have a large energy difference between the states of similar character (LE) but different multiplicity, i.e., 1LE singlet state is 3.12 eV whereas 3LE state is 2.73 eV. The energy of the singlet 1CT state (2.70 eV) is only 0.03 eV lower compared to the energy of the 3LE state. Therefore, we ascribe compound 4BGIPN to the class III TADF material where the 3LE is higher in energy than the manifold of the CT states as shown on Figure 6 [18]. The energy difference between singlet and triplet excited states is −0.03 eV for ΔE1CT-3LE. Such small energy ΔEST values further support the assignment of the TADF mechanism for the compound 4BGIPN. Theoretical results (Tables S3 and S4, Supporting Information File 1) support our experimental observations, suggesting that low energy triplet states (T1, T2 and T3) possess a mixed CT/LE character with an energy difference up to 0.2 eV to the first singlet state S1. At the same time, class III TADF (E3LE > ECT) [19] and class II TADF materials (E3LE ≈ ECT) [20] are reported to have shorter excited state lifetimes compared with class I TADF materials, for instance 4CzIPN (ΔE1CT-3LE = +0.09 eV [12]). The short 1.6 microsecond excited state lifetime for 4BGIPN (regardless of somewhat lower PLQY) is in line with those reported for other TADF class III systems [19]. We have been unable to resolve the 3LE state for 4BGIPN in Zeonex matrices regardless of numerous efforts and cooling the films down to 16 K and applying long time delays.
![[1860-5397-19-95-6]](/bjoc/content/figures/1860-5397-19-95-6.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 6: Energy state diagram and natural transition orbitals HONTO and LUNTO for compound 4BGIPN in excited S1, T1, T2 and T3 states calculated from the crystal S0 geometry.
Figure 6: Energy state diagram and natural transition orbitals HONTO and LUNTO for compound 4BGIPN in excited...
Conclusion
We have synthesized and characterized a donor–acceptor-type thermally activated delayed fluorescent emitter 4BGIPN with four terminal benzoguanidine donor moieties surrounding the benzonitrile acceptor core. We found that the material is formed as a mixture of the rotational isomers that do not experience interconversion upon heating the 4BGIPN solution in DMSO to 120 °C. Two rotational isomers were successfully crystallized to show different up and down orientations of the benzoguanidine donor ligands around the central 4,6-dicyanobenzene core. Unlike the 4CzIPN compound, the 4BGIPN emitter can crystallize in a chiral P21 space group due to the parallel and antiparallel orientation of the benzoguanidine donors with respect to each other, lack of C2 rotational symmetry and extended π-conjugation. The twisted structure of the 4BGIPN ensures that the donor groups accommodate the highest occupied molecular orbital (HOMO) while the acceptor 4,6-dicyanobenzene moiety contains the lowest unoccupied molecular orbital (LUMO) and is supported by the TD-DFT calculations. A comparison of the electronic parameters between benchmark 4CzIPN and new 4BGIPN materials revealed that benzoguanidine acts as a weaker donor ligand compared with carbazole, resulting in greater stabilization of the HOMO energy level down to −6.4 eV rather than LUMO. The significant stabilization of both HOMO and LUMO energy levels, along with multiple electron-withdrawing aza-nitrogen atoms in the structure of 4BGIPN, suggests its potential suitability as an electron transport layer in OLED (organic light-emitting diode) devices. Variable temperature photoluminescence studies revealed that 4BGIPN corresponds to the class III TADF system (E3LE > E1CT), while having a small energy difference between singlet and triplet excited states of −0.03 eV for ΔE1CT-3LE. Theoretical calculations support that the first three triplet excited states possess a mixed CT/LE character while benzoguanidine singlet 1LE state is up to 0.4 eV higher in energy than the singlet 1CT state. The high quantum yields of up to 46% indicate that the yellow-green 4BGIPN emitter shows a high promise as a platform for developing bright 4BGIPN-TADF class III type compounds with unity PLQY. Future works may benefit in isolating a particular isomer that could show superior photophysical TADF characteristics important for fabricating TADF OLED devices with improved operating stability.
Experimental
General considerations
All reactions were performed under a N2 atmosphere. Solvents were dried as required. Sodium hydride was washed from mineral oil with diethyl ether and dried prior to use. 5H-Benzo[d]benzo[4,5]imidazo[1,2-a]imidazole (benzoguanidine) was obtained according to the literature protocol [10] while 2,4,5,6-tetrafluoroisophthalonitrile was purchased from Fluorochem Ltd. and used as received. 1H and 13C{1H} NMR spectra were recorded using a Bruker AVIII HD 500 MHz NMR spectrometer. 1H NMR spectra (500.19 MHz) and 13C{1H} (125.79 MHz) were referenced to dichloromethane-d2 at δ 5.32 (13C, δ 53.84). All electrochemical experiments were performed using an Autolab PGSTAT 302N computer-controlled potentiostat. Cyclic voltammetry (CV) was performed using a three-electrode configuration consisting of a glassy carbon macrodisk working electrode (GCE) (diameter of 3 mm; BASi, Indiana, U.S.A.) combined with a Pt wire counter electrode (99.99%; GoodFellow, Cambridge, U.K.) and an Ag wire pseudoreference electrode (99.99%; GoodFellow, Cambridge, U.K.). The GCE was polished between experiments using alumina slurry (0.3 μm), rinsed in distilled water and subjected to brief sonication to remove any adhering alumina microparticles. The metal electrodes were then dried in an oven at 100 °C to remove residual traces of water, the GCE was left to air dry and residual traces of water were removed under vacuum. The Ag wire pseudoreference electrodes were calibrated to the ferrocene/ferrocenium couple in THF at the end of each run to allow for any drift in potential, following IUPAC recommendations [16]. All electrochemical measurements were performed at ambient temperature under an inert N2 atmosphere in THF containing the complex under study (0.14 mM) and the supporting electrolyte [n-Bu4N][PF6] (0.13 mM). Data were recorded with Autolab NOVA software (v. 1.11). Thermogravimetric analysis was performed by the Microanalysis Laboratory at the University of Manchester. Mass spectrometry data were obtained by the Mass Spectrometry Laboratory at the University of Manchester.
Synthesis of 4BGIPN. 5H-Benzo[d]benzo[4,5]imidazo[1,2-a]imidazole (benzoguanidine, [10]) (700 mg, 3.38 mmol) was added to a suspension of NaH (81.0 mg, 3.38 mmol) in anhydrous DMF (40 mL) at 0 °C under a stream of N2. The reaction mixture was stirred for 1 h at room temperature. 2,4,5,6-Tetrafluoroisophthalonitrile (135 mg, 676 μmol) was added to the reaction mixture under N2. The reaction mixture was heated to 140 °C and left to stir overnight. The reaction mixture was dried under vacuum to remove DMF. The crude product was extracted with DCM and washed with water. The organic phase was collected and dried with MgSO4, filtered and concentrated under vacuo. The product was further purified by column chromatography (ethyl acetate/hexane 1:4) to give the pure product as a yellow powder in 70% yield (450 mg, 474 μmol). Single crystals suitable for X-ray diffraction were grown by layering a concentrated solution in DCM with hexane which was left for slow evaporation. 1H NMR (700 MHz, DMSO-d6) δ 8.46–8.35 (m), 8.23–8.22 (m), 8.16–8.06 (m), 7.94–6.86 (m), 6.82–6.80 (m), 6.59–6.54 (m), 6.47–6.41 (m), 6.35–6.29 (m), 6.24–6.23 (d, J = 7.9 Hz); 13C{1H} NMR (126 MHz, CD2Cl2) δ 151.17, 149.67, 146.84, 146.65, 146.27, 142.44, 142.29, 142.01, 134.45, 133.78, 133.00, 132.63, 131.91, 130.98, 128.91, 128.50, 127.82, 126.78, 126.58, 126.41, 126.24, 125.84, 124.85, 124.51, 124.39, 124.22, 124.11, 124.01, 123.82, 123.71, 123.53, 123.38, 123.30, 123.23, 122.97, 122.76, 121.99, 121.91, 121.61, 121.41, 121.28, 120.01, 119.91, 119.68, 119.43, 119.16, 117.13, 112.60, 112.41, 112.06, 112.00, 111.69, 111.33, 111.21, 111.11, 110.88, 110.82, 110.74, 110.44, 110.17 ppm; Anal. calcd. for C60H32N14 (948.29): C, 75.94; H, 3.40; N, 20.66; found: C, 75.59; H, 3.54; N, 20.28; HRESIMS m/z: [M + Na]+ calcd. for C60H32N14Na, 971.2827; found, 971.2854.
X-ray crystallography
Crystals suitable for X-ray diffraction study were obtained by slow layer diffusion of hexanes/petroleum ether into dichloromethane solution for 4BGIPN at room temperature. Crystals were mounted in oil on a MiTeGen loop and fixed on the diffractometer in a cold nitrogen stream. Data were collected using dual wavelength Rigaku FR-X rotating anode diffractometer using Cu Kα (λ = 1.54146 Å) radiation, equipped with an AFC-11 4-circle kappa goniometer, VariMAXTM microfocus optics, a Hypix-6000HE detector and an Oxford Cryosystems 800 plus nitrogen flow gas system, at a temperature of 100 K. Data were collected and reduced using CrysAlisPro v42 [21,22]. Absorption correction was performed using empirical methods (SCALE3 ABSPACK) based upon symmetry-equivalent reflections combined with measurements at different azimuthal angles.
For the final refinement, the contribution of severely disordered CH2Cl2 molecules in the crystals of 4BGIPN was accounted for by applying a solvent void mask calculated using BYPASS, implemented through Olex2 [23]. Structures were solved by direct method/intrinsic phasing and refined by the full-matrix least-squares against F2. All non-hydrogen atoms were refined with anisotropic atomic displacement parameters. All hydrogen atoms were positioned geometrically and constrained to ride on their parent atoms with C–H = 0.95–1.00 Å, and Uiso = 1.2–1.5 Ueq (parent atom). All calculations were performed using the SHELXL software and Olex2 graphical user interface [22,23].
4BGIPN (monoclinic P21), CCDC number 2243340, C60H32N14, monoclinic, space group P21 (no. 4), a = 17.4217(4) Å, b = 15.2552(3) Å, c = 20.8314(6) Å, β = 114.583(3)°, V = 5034.6(2) Å3, Z = 4, dcalc = 1.252 g cm−3, μ = 0.623 mm−1, yellow block, 33714 reflections measured (4.664° ≤ 2Θ ≤ 152.79°), 17837 unique (Rint = 0.0314, Rsigma = 0.0512) which were used in all calculations. The final R1 was 0.0462 (I > 2σ(I)) and wR2 was 0.1190 (all data). GOF = 1.042, Δρmin/Δρmax = 0.4/−0.2 e Å−3.
4BGIPN (triclinic P−1), CCDC number 2287367, C60H32N14·0.5CH2Cl2 (M = 991.46 g/mol): triclinic, space group P−1 (no. 2), a = 12.8390(16) Å, b = 21.536(3) Å, c = 23.149(5) Å, α = 65.321(17)°, β = 82.509(14)°, γ = 89.955(11)°, V = 5756.1(19) Å3, Z = 4, T = 100.00(13) K, μ(Cu Kα) = 0.981 mm−1, dcalc = 1.144 g/cm3, 68407 reflections measured (4.244° ≤ 2Θ ≤ 151.924°), 22637 unique (Rint = 0.1849, Rsigma = 0.1991) which were used in all calculations. The final R1 was 0.2870 (I > 2σ(I)) and wR2 was 0.6519 (all data).
Computational results
Computations were performed using density functional theory (DFT) for the ground state and time-dependent DFT (TD-DFT) with Tamm–Dancoff approximation [24,25] for the excited states calculations, using the global hybrid MN15 functional by Truhlar [26] in combination with the def2-TZVP basis set by Ahlrichs [27,28]. TD-DFT calculations were performed to elucidate the nature of the excited state in a crystalline and optimized molecular geometry of 4BGIPN with all data collected in Supporting Information File 1 (Tables S1–S4). All calculations were carried out by Gaussian 16 [29] and HOMO–LUMO overlap integrals were calculated using Multiwfn program [30].
Acknowledgements
We are grateful to ZEON EUROPE GmbH for providing ZEONEX® 480 Cyclo Olefin Polymer (COP) used in our studies. We are grateful to Dr. Louise Natrajan at the University of Manchester for providing access to FLS-1000 fluorometer.
Funding
This work was supported by the Royal Society and the Academy of Finland. A.S.R. acknowledges support from the Royal Society (grant nos. URF\R1\180288 and RGF\EA\181008). M.L. acknowledges the Academy of Finland Flagship Programme, Photonics Research and Innovation (PREIN), decision 320166. N.L.P. acknowledges the Doctoral Programme in Science, Technology and Computing (Sciteco, University of Eastern Finland). (TD)-DFT computations were made possible by use of the Finnish Grid and Cloud Infrastructure resources (urn:nbn:fi:research-infras-2016072533). A.S.R. acknowledges the support from the EPSRC (grant code EP/K039547/1).
References
-
Uoyama, H.; Goushi, K.; Shizu, K.; Nomura, H.; Adachi, C. Nature 2012, 492, 234–238. doi:10.1038/nature11687
Return to citation in text: [1] [2] [3] [4] -
Endo, A.; Ogasawara, M.; Takahashi, A.; Yokoyama, D.; Kato, Y.; Adachi, C. Adv. Mater. (Weinheim, Ger.) 2009, 21, 4802–4806. doi:10.1002/adma.200900983
Return to citation in text: [1] -
Parker, C. A.; Hatchard, C. G. Trans. Faraday Soc. 1961, 57, 1894–1904. doi:10.1039/tf9615701894
Return to citation in text: [1] -
Yang, Z.; Mao, Z.; Xie, Z.; Zhang, Y.; Liu, S.; Zhao, J.; Xu, J.; Chi, Z.; Aldred, M. P. Chem. Soc. Rev. 2017, 46, 915–1016. doi:10.1039/c6cs00368k
Return to citation in text: [1] -
Liu, Y.; Li, C.; Ren, Z.; Yan, S.; Bryce, M. R. Nat. Rev. Mater. 2018, 3, 18020. doi:10.1038/natrevmats.2018.20
Return to citation in text: [1] -
Bryden, M. A.; Zysman-Colman, E. Chem. Soc. Rev. 2021, 50, 7587–7680. doi:10.1039/d1cs00198a
Return to citation in text: [1] -
Wong, M. Y.; Zysman-Colman, E. Adv. Mater. (Weinheim, Ger.) 2017, 29, 1605444. doi:10.1002/adma.201605444
Return to citation in text: [1] -
Lee, Y. H.; Park, S.; Oh, J.; Shin, J. W.; Jung, J.; Yoo, S.; Lee, M. H. ACS Appl. Mater. Interfaces 2017, 9, 24035–24042. doi:10.1021/acsami.7b05615
Return to citation in text: [1] -
Kaji, H.; Suzuki, H.; Fukushima, T.; Shizu, K.; Suzuki, K.; Kubo, S.; Komino, T.; Oiwa, H.; Suzuki, F.; Wakamiya, A.; Murata, Y.; Adachi, C. Nat. Commun. 2015, 6, 8476. doi:10.1038/ncomms9476
Return to citation in text: [1] -
Muniz, C. N.; Schaab, J.; Razgoniaev, A.; Djurovich, P. I.; Thompson, M. E. J. Am. Chem. Soc. 2022, 144, 17916–17928. doi:10.1021/jacs.2c06948
Return to citation in text: [1] [2] [3] -
Kim, H. S.; Lee, J. Y.; Shin, S.; Jeong, W.; Lee, S. H.; Kim, S.; Lee, J.; Suh, M. C.; Yoo, S. Adv. Funct. Mater. 2021, 31, 2104646. doi:10.1002/adfm.202104646
Return to citation in text: [1] -
Etherington, M. K.; Kukhta, N. A.; Higginbotham, H. F.; Danos, A.; Bismillah, A. N.; Graves, D. R.; McGonigal, P. R.; Haase, N.; Morherr, A.; Batsanov, A. S.; Pflumm, C.; Bhalla, V.; Bryce, M. R.; Monkman, A. P. J. Phys. Chem. C 2019, 123, 11109–11117. doi:10.1021/acs.jpcc.9b01458
Return to citation in text: [1] [2] [3] [4] [5] [6] -
Tsuchiya, Y.; Nakamura, N.; Kakumachi, S.; Kusuhara, K.; Chan, C.-Y.; Adachi, C. Chem. Commun. 2022, 58, 11292–11295. doi:10.1039/d2cc01467j
Return to citation in text: [1] -
Chen, X.-K.; Tsuchiya, Y.; Ishikawa, Y.; Zhong, C.; Adachi, C.; Brédas, J.-L. Adv. Mater. (Weinheim, Ger.) 2017, 29, 1702767. doi:10.1002/adma.201702767
Return to citation in text: [1] [2] -
Bryden, M. A.; Millward, F.; Matulaitis, T.; Chen, D.; Villa, M.; Fermi, A.; Cetin, S.; Ceroni, P.; Zysman-Colman, E. J. Org. Chem. 2023, 88, 6364–6373. doi:10.1021/acs.joc.2c01137
Return to citation in text: [1] [2] [3] [4] [5] [6] [7] [8] -
Gritzner, G.; Kůta, J. Electrochim. Acta 1984, 29, 869–873. doi:10.1016/0013-4686(84)80027-4
Return to citation in text: [1] [2] -
Ishimatsu, R.; Matsunami, S.; Shizu, K.; Adachi, C.; Nakano, K.; Imato, T. J. Phys. Chem. A 2013, 117, 5607–5612. doi:10.1021/jp404120s
Return to citation in text: [1] [2] [3] [4] -
Etherington, M. K.; Gibson, J.; Higginbotham, H. F.; Penfold, T. J.; Monkman, A. P. Nat. Commun. 2016, 7, 13680. doi:10.1038/ncomms13680
Return to citation in text: [1] -
Cui, L.-S.; Gillett, A. J.; Zhang, S.-F.; Ye, H.; Liu, Y.; Chen, X.-K.; Lin, Z.-S.; Evans, E. W.; Myers, W. K.; Ronson, T. K.; Nakanotani, H.; Reineke, S.; Bredas, J.-L.; Adachi, C.; Friend, R. H. Nat. Photonics 2020, 14, 636–642. doi:10.1038/s41566-020-0668-z
Return to citation in text: [1] [2] -
dos Santos, P. L.; Ward, J. S.; Congrave, D. G.; Batsanov, A. S.; Eng, J.; Stacey, J. E.; Penfold, T. J.; Monkman, A. P.; Bryce, M. R. Adv. Sci. 2018, 5, 1700989. doi:10.1002/advs.201700989
Return to citation in text: [1] -
Programs CrysAlisPro; Oxford Diffraction Ltd.: Abingdon, UK, 2010.
Return to citation in text: [1] -
Sheldrick, G. M. Acta Crystallogr., Sect. C: Struct. Chem. 2015, 71, 3–8. doi:10.1107/s2053229614024218
Return to citation in text: [1] [2] -
Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. J. Appl. Crystallogr. 2009, 42, 339–341. doi:10.1107/s0021889808042726
Return to citation in text: [1] [2] -
Furche, F.; Rappoport, D. Density Functional Methods for Excited States: Equilibrium Structure and Electronic Spectra. In Computational Photochemistry, 1st ed.; Olivucci, M., Ed.; Elsevier: Amsterdam, Netherlands, 2005; Vol. 16, pp 93–128.
Return to citation in text: [1] -
Hirata, S.; Head-Gordon, M. Chem. Phys. Lett. 1999, 314, 291–299. doi:10.1016/s0009-2614(99)01149-5
Return to citation in text: [1] -
Yu, H. S.; He, X.; Li, S. L.; Truhlar, D. G. Chem. Sci. 2016, 7, 5032–5051. doi:10.1039/c6sc00705h
Return to citation in text: [1] -
Weigend, F.; Häser, M.; Patzelt, H.; Ahlrichs, R. Chem. Phys. Lett. 1998, 294, 143–152. doi:10.1016/s0009-2614(98)00862-8
Return to citation in text: [1] -
Weigend, F.; Ahlrichs, R. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. doi:10.1039/b508541a
Return to citation in text: [1] -
Gaussian 16, Revision A. 03; Gaussian Inc.: Wallingford, CT, USA, 2016.
Return to citation in text: [1] -
Lu, T.; Chen, F. J. Comput. Chem. 2012, 33, 580–592. doi:10.1002/jcc.22885
Return to citation in text: [1]
| 19. | Cui, L.-S.; Gillett, A. J.; Zhang, S.-F.; Ye, H.; Liu, Y.; Chen, X.-K.; Lin, Z.-S.; Evans, E. W.; Myers, W. K.; Ronson, T. K.; Nakanotani, H.; Reineke, S.; Bredas, J.-L.; Adachi, C.; Friend, R. H. Nat. Photonics 2020, 14, 636–642. doi:10.1038/s41566-020-0668-z |
| 20. | dos Santos, P. L.; Ward, J. S.; Congrave, D. G.; Batsanov, A. S.; Eng, J.; Stacey, J. E.; Penfold, T. J.; Monkman, A. P.; Bryce, M. R. Adv. Sci. 2018, 5, 1700989. doi:10.1002/advs.201700989 |
| 12. | Etherington, M. K.; Kukhta, N. A.; Higginbotham, H. F.; Danos, A.; Bismillah, A. N.; Graves, D. R.; McGonigal, P. R.; Haase, N.; Morherr, A.; Batsanov, A. S.; Pflumm, C.; Bhalla, V.; Bryce, M. R.; Monkman, A. P. J. Phys. Chem. C 2019, 123, 11109–11117. doi:10.1021/acs.jpcc.9b01458 |
| 1. | Uoyama, H.; Goushi, K.; Shizu, K.; Nomura, H.; Adachi, C. Nature 2012, 492, 234–238. doi:10.1038/nature11687 |
| 2. | Endo, A.; Ogasawara, M.; Takahashi, A.; Yokoyama, D.; Kato, Y.; Adachi, C. Adv. Mater. (Weinheim, Ger.) 2009, 21, 4802–4806. doi:10.1002/adma.200900983 |
| 3. | Parker, C. A.; Hatchard, C. G. Trans. Faraday Soc. 1961, 57, 1894–1904. doi:10.1039/tf9615701894 |
| 10. | Muniz, C. N.; Schaab, J.; Razgoniaev, A.; Djurovich, P. I.; Thompson, M. E. J. Am. Chem. Soc. 2022, 144, 17916–17928. doi:10.1021/jacs.2c06948 |
| 16. | Gritzner, G.; Kůta, J. Electrochim. Acta 1984, 29, 869–873. doi:10.1016/0013-4686(84)80027-4 |
| 22. | Sheldrick, G. M. Acta Crystallogr., Sect. C: Struct. Chem. 2015, 71, 3–8. doi:10.1107/s2053229614024218 |
| 23. | Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. J. Appl. Crystallogr. 2009, 42, 339–341. doi:10.1107/s0021889808042726 |
| 1. | Uoyama, H.; Goushi, K.; Shizu, K.; Nomura, H.; Adachi, C. Nature 2012, 492, 234–238. doi:10.1038/nature11687 |
| 15. | Bryden, M. A.; Millward, F.; Matulaitis, T.; Chen, D.; Villa, M.; Fermi, A.; Cetin, S.; Ceroni, P.; Zysman-Colman, E. J. Org. Chem. 2023, 88, 6364–6373. doi:10.1021/acs.joc.2c01137 |
| 17. | Ishimatsu, R.; Matsunami, S.; Shizu, K.; Adachi, C.; Nakano, K.; Imato, T. J. Phys. Chem. A 2013, 117, 5607–5612. doi:10.1021/jp404120s |
| 24. | Furche, F.; Rappoport, D. Density Functional Methods for Excited States: Equilibrium Structure and Electronic Spectra. In Computational Photochemistry, 1st ed.; Olivucci, M., Ed.; Elsevier: Amsterdam, Netherlands, 2005; Vol. 16, pp 93–128. |
| 25. | Hirata, S.; Head-Gordon, M. Chem. Phys. Lett. 1999, 314, 291–299. doi:10.1016/s0009-2614(99)01149-5 |
| 7. | Wong, M. Y.; Zysman-Colman, E. Adv. Mater. (Weinheim, Ger.) 2017, 29, 1605444. doi:10.1002/adma.201605444 |
| 8. | Lee, Y. H.; Park, S.; Oh, J.; Shin, J. W.; Jung, J.; Yoo, S.; Lee, M. H. ACS Appl. Mater. Interfaces 2017, 9, 24035–24042. doi:10.1021/acsami.7b05615 |
| 9. | Kaji, H.; Suzuki, H.; Fukushima, T.; Shizu, K.; Suzuki, K.; Kubo, S.; Komino, T.; Oiwa, H.; Suzuki, F.; Wakamiya, A.; Murata, Y.; Adachi, C. Nat. Commun. 2015, 6, 8476. doi:10.1038/ncomms9476 |
| 15. | Bryden, M. A.; Millward, F.; Matulaitis, T.; Chen, D.; Villa, M.; Fermi, A.; Cetin, S.; Ceroni, P.; Zysman-Colman, E. J. Org. Chem. 2023, 88, 6364–6373. doi:10.1021/acs.joc.2c01137 |
| 21. | Programs CrysAlisPro; Oxford Diffraction Ltd.: Abingdon, UK, 2010. |
| 22. | Sheldrick, G. M. Acta Crystallogr., Sect. C: Struct. Chem. 2015, 71, 3–8. doi:10.1107/s2053229614024218 |
| 4. | Yang, Z.; Mao, Z.; Xie, Z.; Zhang, Y.; Liu, S.; Zhao, J.; Xu, J.; Chi, Z.; Aldred, M. P. Chem. Soc. Rev. 2017, 46, 915–1016. doi:10.1039/c6cs00368k |
| 5. | Liu, Y.; Li, C.; Ren, Z.; Yan, S.; Bryce, M. R. Nat. Rev. Mater. 2018, 3, 18020. doi:10.1038/natrevmats.2018.20 |
| 6. | Bryden, M. A.; Zysman-Colman, E. Chem. Soc. Rev. 2021, 50, 7587–7680. doi:10.1039/d1cs00198a |
| 14. | Chen, X.-K.; Tsuchiya, Y.; Ishikawa, Y.; Zhong, C.; Adachi, C.; Brédas, J.-L. Adv. Mater. (Weinheim, Ger.) 2017, 29, 1702767. doi:10.1002/adma.201702767 |
| 15. | Bryden, M. A.; Millward, F.; Matulaitis, T.; Chen, D.; Villa, M.; Fermi, A.; Cetin, S.; Ceroni, P.; Zysman-Colman, E. J. Org. Chem. 2023, 88, 6364–6373. doi:10.1021/acs.joc.2c01137 |
| 23. | Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. J. Appl. Crystallogr. 2009, 42, 339–341. doi:10.1107/s0021889808042726 |
| 14. | Chen, X.-K.; Tsuchiya, Y.; Ishikawa, Y.; Zhong, C.; Adachi, C.; Brédas, J.-L. Adv. Mater. (Weinheim, Ger.) 2017, 29, 1702767. doi:10.1002/adma.201702767 |
| 15. | Bryden, M. A.; Millward, F.; Matulaitis, T.; Chen, D.; Villa, M.; Fermi, A.; Cetin, S.; Ceroni, P.; Zysman-Colman, E. J. Org. Chem. 2023, 88, 6364–6373. doi:10.1021/acs.joc.2c01137 |
| 16. | Gritzner, G.; Kůta, J. Electrochim. Acta 1984, 29, 869–873. doi:10.1016/0013-4686(84)80027-4 |
| 12. | Etherington, M. K.; Kukhta, N. A.; Higginbotham, H. F.; Danos, A.; Bismillah, A. N.; Graves, D. R.; McGonigal, P. R.; Haase, N.; Morherr, A.; Batsanov, A. S.; Pflumm, C.; Bhalla, V.; Bryce, M. R.; Monkman, A. P. J. Phys. Chem. C 2019, 123, 11109–11117. doi:10.1021/acs.jpcc.9b01458 |
| 15. | Bryden, M. A.; Millward, F.; Matulaitis, T.; Chen, D.; Villa, M.; Fermi, A.; Cetin, S.; Ceroni, P.; Zysman-Colman, E. J. Org. Chem. 2023, 88, 6364–6373. doi:10.1021/acs.joc.2c01137 |
| 10. | Muniz, C. N.; Schaab, J.; Razgoniaev, A.; Djurovich, P. I.; Thompson, M. E. J. Am. Chem. Soc. 2022, 144, 17916–17928. doi:10.1021/jacs.2c06948 |
| 13. | Tsuchiya, Y.; Nakamura, N.; Kakumachi, S.; Kusuhara, K.; Chan, C.-Y.; Adachi, C. Chem. Commun. 2022, 58, 11292–11295. doi:10.1039/d2cc01467j |
| 19. | Cui, L.-S.; Gillett, A. J.; Zhang, S.-F.; Ye, H.; Liu, Y.; Chen, X.-K.; Lin, Z.-S.; Evans, E. W.; Myers, W. K.; Ronson, T. K.; Nakanotani, H.; Reineke, S.; Bredas, J.-L.; Adachi, C.; Friend, R. H. Nat. Photonics 2020, 14, 636–642. doi:10.1038/s41566-020-0668-z |
| 11. | Kim, H. S.; Lee, J. Y.; Shin, S.; Jeong, W.; Lee, S. H.; Kim, S.; Lee, J.; Suh, M. C.; Yoo, S. Adv. Funct. Mater. 2021, 31, 2104646. doi:10.1002/adfm.202104646 |
| 12. | Etherington, M. K.; Kukhta, N. A.; Higginbotham, H. F.; Danos, A.; Bismillah, A. N.; Graves, D. R.; McGonigal, P. R.; Haase, N.; Morherr, A.; Batsanov, A. S.; Pflumm, C.; Bhalla, V.; Bryce, M. R.; Monkman, A. P. J. Phys. Chem. C 2019, 123, 11109–11117. doi:10.1021/acs.jpcc.9b01458 |
| 12. | Etherington, M. K.; Kukhta, N. A.; Higginbotham, H. F.; Danos, A.; Bismillah, A. N.; Graves, D. R.; McGonigal, P. R.; Haase, N.; Morherr, A.; Batsanov, A. S.; Pflumm, C.; Bhalla, V.; Bryce, M. R.; Monkman, A. P. J. Phys. Chem. C 2019, 123, 11109–11117. doi:10.1021/acs.jpcc.9b01458 |
| 10. | Muniz, C. N.; Schaab, J.; Razgoniaev, A.; Djurovich, P. I.; Thompson, M. E. J. Am. Chem. Soc. 2022, 144, 17916–17928. doi:10.1021/jacs.2c06948 |
| 15. | Bryden, M. A.; Millward, F.; Matulaitis, T.; Chen, D.; Villa, M.; Fermi, A.; Cetin, S.; Ceroni, P.; Zysman-Colman, E. J. Org. Chem. 2023, 88, 6364–6373. doi:10.1021/acs.joc.2c01137 |
| 15. | Bryden, M. A.; Millward, F.; Matulaitis, T.; Chen, D.; Villa, M.; Fermi, A.; Cetin, S.; Ceroni, P.; Zysman-Colman, E. J. Org. Chem. 2023, 88, 6364–6373. doi:10.1021/acs.joc.2c01137 |
| 26. | Yu, H. S.; He, X.; Li, S. L.; Truhlar, D. G. Chem. Sci. 2016, 7, 5032–5051. doi:10.1039/c6sc00705h |
| 17. | Ishimatsu, R.; Matsunami, S.; Shizu, K.; Adachi, C.; Nakano, K.; Imato, T. J. Phys. Chem. A 2013, 117, 5607–5612. doi:10.1021/jp404120s |
| 27. | Weigend, F.; Häser, M.; Patzelt, H.; Ahlrichs, R. Chem. Phys. Lett. 1998, 294, 143–152. doi:10.1016/s0009-2614(98)00862-8 |
| 28. | Weigend, F.; Ahlrichs, R. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. doi:10.1039/b508541a |
| 1. | Uoyama, H.; Goushi, K.; Shizu, K.; Nomura, H.; Adachi, C. Nature 2012, 492, 234–238. doi:10.1038/nature11687 |
| 18. | Etherington, M. K.; Gibson, J.; Higginbotham, H. F.; Penfold, T. J.; Monkman, A. P. Nat. Commun. 2016, 7, 13680. doi:10.1038/ncomms13680 |
| 1. | Uoyama, H.; Goushi, K.; Shizu, K.; Nomura, H.; Adachi, C. Nature 2012, 492, 234–238. doi:10.1038/nature11687 |
| 12. | Etherington, M. K.; Kukhta, N. A.; Higginbotham, H. F.; Danos, A.; Bismillah, A. N.; Graves, D. R.; McGonigal, P. R.; Haase, N.; Morherr, A.; Batsanov, A. S.; Pflumm, C.; Bhalla, V.; Bryce, M. R.; Monkman, A. P. J. Phys. Chem. C 2019, 123, 11109–11117. doi:10.1021/acs.jpcc.9b01458 |
| 12. | Etherington, M. K.; Kukhta, N. A.; Higginbotham, H. F.; Danos, A.; Bismillah, A. N.; Graves, D. R.; McGonigal, P. R.; Haase, N.; Morherr, A.; Batsanov, A. S.; Pflumm, C.; Bhalla, V.; Bryce, M. R.; Monkman, A. P. J. Phys. Chem. C 2019, 123, 11109–11117. doi:10.1021/acs.jpcc.9b01458 |
| 17. | Ishimatsu, R.; Matsunami, S.; Shizu, K.; Adachi, C.; Nakano, K.; Imato, T. J. Phys. Chem. A 2013, 117, 5607–5612. doi:10.1021/jp404120s |
| 15. | Bryden, M. A.; Millward, F.; Matulaitis, T.; Chen, D.; Villa, M.; Fermi, A.; Cetin, S.; Ceroni, P.; Zysman-Colman, E. J. Org. Chem. 2023, 88, 6364–6373. doi:10.1021/acs.joc.2c01137 |
| 17. | Ishimatsu, R.; Matsunami, S.; Shizu, K.; Adachi, C.; Nakano, K.; Imato, T. J. Phys. Chem. A 2013, 117, 5607–5612. doi:10.1021/jp404120s |
© 2023 Brannan et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.