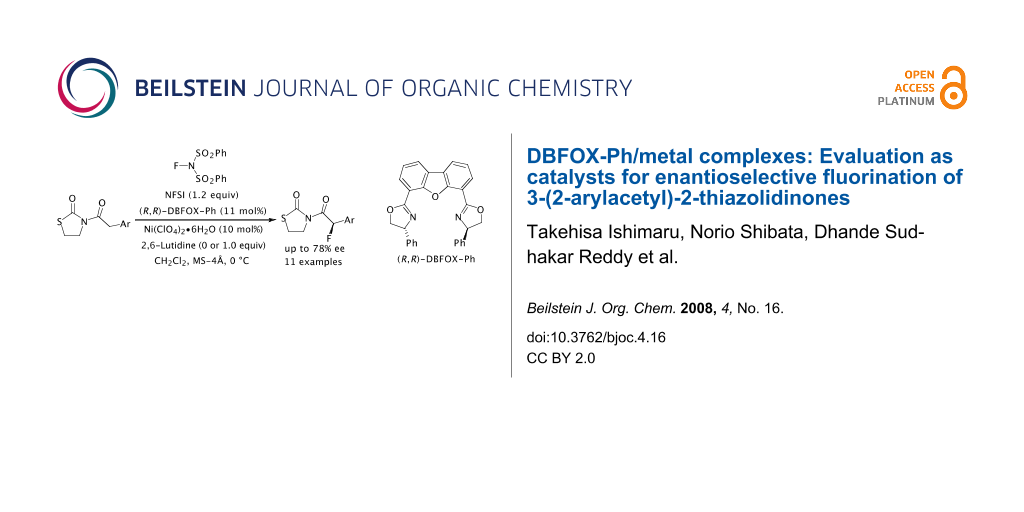
Abstract
We examined the catalytic enantioselective fluorination of 3-(2-arylacetyl)-2-thiazolidinones 1 with N-fluorobenzenesulfonimide (NFSI) by DBFOX-Ph/metal complexes under a variety of conditions. After optimization of the metal salts, solvents and additives, we found that the fluoro-2-thiazolidinones 2 were obtained in good to high yields with moderate to good enantioselectivities (up to 78% ee) when the reaction was carried out in the presence of DBFOX-Ph (11 mol%), Ni(ClO4)2·6H2O (10 mol%) and 2,6-lutidine (0 or 1.0 equiv) in CH2Cl2.
Graphical Abstract
Background
Enantioselective electrophilic fluorination represents an important and straightforward strategy for C-F bond formation at a carbon stereocenter, providing easy access to chiral fluoro-organic compounds [1,2]. Due to the significance of chiral fluoro-organic compounds, such as fluorinated quinolones [3,4] and liquid crystals [5], in pharmaceutical and material sciences considerable effort has been dedicated to this issue for decades [6-17]. As a consequence, a variety of procedures have been developed to increase the yields and enantioselectivities of electrophilic fluorination reactions. Stoichiometric approaches based on cinchona alkaloid/Selectfluor® combinations [18-32], chiral ligand/metal-catalyzed [33-57] or organocatalytic [58-64] procedures for enantioselective fluorination are major advances in recent years. The discovery that chiral ligands/metals can catalyze electrophilic fluorination with conventional fluorinating reagents has had a large impact on synthetic organic chemistry, because of the availability of commonly used classes of ligands for asymmetric catalysis, such as, TADDOLs [37,39,41,47], BINAPs [38,40,43,44,46,49,51,53,55-57] and bis(oxazoline) [33,34,36,42,45]. Of particular importance are BINAP ligands. Sodeoka et al. have used the latter ligands in asymmetric fluorination of a wide range of substrates, including β-keto esters, β-keto phosphonates, oxindoles [38,40,43,51,53,56,57]. They have also recently reported the enantioselective fluorination of 3-(2-arylacetyl)-2-thiazolidinones with their extended catalytic system, NiCl2-BINAP/R3SiOTf-lutidine with high enantioselectivities [57]. This study is useful because, up until now, the fluorinated products obtained by Sodeoka's method have been prepared by diastereoselective methods [65-67]. Independently, our group has focused on the development of enantioselective fluorination and related reactions using bis(oxazoline) ligands, Box-Ph [(S,S)-2,2'-isopropylidene-bis(4-phenyl-2-oxazoline)] and DBFOX-Ph [(R,R)-4,6-dibenzofurandiyl-2,2'-bis(4-phenyloxazoline)] [33,34,36]. As an extension of this study, we herein evaluate our DBFOX-Ph/metal catalysis for the enantioselective fluorination of 3-(2-arylacetyl)-2-thiazolidinones with N-fluorobenzenesulfonimide (NFSI) (Figure 1).
Figure 1: Structures of DBFOX-Ph, Box-Ph and NFSI.
Figure 1: Structures of DBFOX-Ph, Box-Ph and NFSI.
Results and Discussion
Our previous studies of the DBFOX-Ph/Ni(II)-catalyzed enantioselective fluorination of β-keto esters have shown that the optimal reaction conditions require NFSI as the fluorine source and a catalytic amount of Ni(ClO4)2·6H2O in CH2Cl2 at room temperature. Therefore, we first attempted the reaction of 1a with the same conditions and found that the desired fluorinated product 2a was obtained in 42% yield with 69% ee (Table 1, entry 1). The reaction at higher temperature (40 °C) improved the yield to 62% with slightly lower enantioselectivity (63% ee, entry 2). The reaction time in these experiments was shortened by the addition of 1 equiv of 2,6-lutidine and 2a was obtained in 87% yield with 66% ee at room temperature (entry 3). Both yield and selectivity were improved to 90% and 74% ee when the reaction was performed at 0 °C (entry 4). The highest ee value of 2a was obtained at −20 °C, but resulted in a decrease in yield (24%, 79% ee, entry 5). Changing the metal salts did not improve the results (entries 6 and 7). The absolute stereochemistry of 2a was determined by comparing the optical rotation and HPLC analysis with the literature values [57]. Although the enantioselectivities are moderate to good in these examples (63–79% ee), the results are quite impressive because the fluorination proceeds even in the absence of base (entries 1 and 2). That is, both Ni(ClO4)2-DBFOX-Ph (unary system, entries 1 and 2) and Ni(ClO4)2-DBFOX-Ph/lutidine (binary system, entries 3–6) are moderately effective in the enantioselective fluorination of 1a. According to the report by Sodeoka using their NiCl2-BINAP/R3SiOTf-lutidine (trinary system, up to 88% ee obtained), the reaction requires both R3SiOTf and lutidine [57]. They mentioned in the paper that a binary system consisting of Ni(OTf)2–binap complex and 2,6-lutidine failed to promote asymmetric fluorination. We also briefly attempted the fluorination of 1a using the (S,S)-Box-Ph ligand instead of DBFOX-Ph. While the Box-Ph/Cu(OTf)2 catalyst was not effective (run 8), the Box-Ph/Ni(ClO4)2·6H2O catalyst gave the desired product 2a in 33% yield with low enantioselectivity (15% ee, entry 9).
Table 1: Optimisation of the Conditions for DBFOX-Ph/Ni(II)-Catalysed Enantioselective Fluorination of 3-(2-Phenylacetyl)-2-thiazolidinone (1a)a.
|
|
||||||
| Run | Metal salt | 2,6- Lutidine (equiv) | Temp (°C) | Time | Yield (%) | ee (%) |
|---|---|---|---|---|---|---|
| 1 | Ni(ClO4)2·6H2O | none | rt | 6 d | 42 | 69 |
| 2 | Ni(ClO4)2·6H2O | none | 40 | 4 d | 62 | 63 |
| 3 | Ni(ClO4)2·6H2O | 1.0 | rt | 17 h | 87 | 66 |
| 4 | Ni(ClO4)2·6H2O | 1.0 | 0 | 20 h | 90 | 74 |
| 5 | Ni(ClO4)2·6H2O | 1.0 | −20 | 4 d | 24 | 79 |
| 6 | Ni(OAc)2·4H2O | 1.0 | rt | 4 d | 55 | 72 |
| 7 | Zn(OAc)2 | 1.0 | rt | 3 d | NR | - |
| 8b,c | Cu(OTf)2 | 1.0 | 0 | 2 d | NR | - |
| 9b | Ni(ClO4)2·6H2O | 1.0 | 0 | 2 d | 33 | 15d |
aFor detailed reaction conditions, see Supporting Information File 1. Enantioselectivity was determined by chiral HPLC analysis. The absolute configuration of 2a was determined by comparison with the optical rotation and HPLC analysis in the literature [57]. NR: No reaction. b(S,S)-Box-Ph (11 mol%) was used instead of (R,R)-DBFOX-Ph. cEther was used as solvent. d(S)-2a was obtained.
The DBFOX-Ph/Ni(ClO4)2·6H2O catalysis for fluorination showed high generality for various 3-(2-arylacetyl)-2-thiazolidinones 1a–k in good to high yields with moderate to good enantioselectivities. The results are summarized in Table 2. The fluorination reaction was not very sensitive to substitution in the position of the phenyl group and the desired products with methoxy or methyl groups at the o-, m-, or p-position of the benzene ring were obtained in 65–78% ee (entries 2–7). The reactions of fluoro or bromo-substituted 1h, i and bulky-substituted 1j, k afforded the desired products 2h–k in good yields with slightly lower enantioselectivities (56–62% ee, entries 8–11).
Table 2: Enantioselective Fluorination Reaction of 3-(2-Arylacetyl)-2-thiazolidinones with NFSI Catalyzed by DBFOX-Ph/Ni(II)a.
|
|
||||||
| Entry | 1 | Ar | 2 | Time (h) | Yield (%) | ee (%) |
|---|---|---|---|---|---|---|
| 1 | 1a | Ph | 2a | 20 | 90 | 74 |
| 2 | 1b | C6H4-o-OMe | 2b | 48 | 96 | 78 |
| 3 | 1c | C6H4-m-OMe | 2c | 24 | 94 | 66 |
| 4 | 1d | C6H4-p-OMe | 2d | 24 | 90 | 65 |
| 5 | 1e | C6H4-o-Me | 2e | 48 | 69 | 76 |
| 6 | 1f | C6H4-m-Me | 2f | 48 | 75 | 73 |
| 7 | 1g | C6H4-p-Me | 2g | 48 | 75 | 77 |
| 8 | 1h | C6H4-p-F | 2h | 48 | 60 | 62 |
| 9 | 1i | C6H4-p-Br | 2i | 48 | 77 | 56 |
| 10 | 1j | 1-Naphthyl | 2j | 48 | 85 | 59 |
| 11 | 1k | 2-Naphthyl | 2k | 48 | 90 | 60 |
aFor detailed reaction conditions, see Supporting Information File 1. Enantioselectivity was determined by chiral HPLC analysis. The absolute configuration of 2a was determined by comparison with the optical rotation and HPLC analysis in the literature [57]. Others were tentatively assigned by comparing the signs of their optical rotations to that of 2a.
The R-enantioselection of 2 can be explained by assuming an octahedral complex coordinated with a water molecule for DBFOX-Ph/Ni(II)/1 as shown in Scheme 1. In the complex, the Si face is shielded by one of the phenyl groups of DBFOX-Ph so that NFSI approaches from the Re face of the substrates (Scheme 1). Since a major difference in ee values of 2 was not observed for the fluorination reaction of 1 with NFSI in the presence or absence of 2,6-lutidine (entries 1–3, Table 1), 2,6-lutidine presumably just accelerates the tautomerization of 1 to its enol form.
Scheme 1: Transition-State Structure for the DBFOX-Ph/Ni(II) Catalyzed Enantioselective Fluorination of 1 to 2.
Scheme 1: Transition-State Structure for the DBFOX-Ph/Ni(II) Catalyzed Enantioselective Fluorination of 1 to 2...
Conclusion
This research has demonstrated that DBFOX-Ph/Ni(II) catalysis can be used for the catalytic enantioselective fluorination of 3-(2-arylacetyl)-2-thiazolidinones with or without 2,6-lutidine to afford chiral 2-fluoro-2-arylacetate derivatives in good to high yields with moderate to good enantioselectivities of up to 78% ee. The Box-Ph ligand was not effective for this reaction. Our best ee value is slightly lower than that of Sodeoka's report [57]; this is presumably due to the low activity of our catalyst system which requires higher reaction temperature conditions (0 °C vs. −20 °C [57]). Racemization of the products 2 during the fluorination reaction was ruled out since no racemization was observed when 2a was stirred overnight under the same fluorination conditions. Further studies to improve the enantioselectivity of DBFOX-Ph/metal catalysis in enantioselective fluorination are under way.
Supporting Information
| Supporting Information File 1: Experimental methods. General methods, general procedure for the enantioselective catalytic fluorination, spectral data of 2, copies of 1H, 13C and 19F-NMRs and HPLC charts of 2 | ||
| Format: DOC | Size: 7.2 MB | Download |
References
-
Soloshonok, V. A., Ed. Enantiocontrolled Synthesis of Fluoro-Organic Compounds: Stereochemical Challenges and Biomedical Targets; John Wiley & Sons: Chichester, 1999.
Return to citation in text: [1] -
Kirsch, P. Modern Fluoroorganic Chemistry; Wiley-VCH: Weinheim, 2004.
Return to citation in text: [1] -
Kimura, Y.; Atarashi, S.; Kawakami, K.; Sato, K.; Hayakawa, I. J. Med. Chem. 1994, 37, 3344–3352. doi:10.1021/jm00046a019
Return to citation in text: [1] -
Takemura, M.; Takahashi, H.; Kawakami, K.; Namba, K.; Tanaka, M.; Miyauchi, R. (Daiichi Pharmaceutical Co., Ltd., Tokyo, Japan) Anti-acid fast bacterial agents containing pyridonecarboxylic acids as the active ingredient. European Patent Application EP 1262477A1, Dec 4, 2002.
Return to citation in text: [1] -
Kusumoto, T.; Hiyama, T. Fluorine-Containing Chiral Liquid Crystals: Syntheses and Properties. In Enantiocontrolled Synthesis of Fluoro-Organic Compounds: Stereochemical Challenges and Biomedical Targets; Soloshonok, V. A., Ed.; John Wiley & Sons: Chichester, 1999; pp 535–556.
Return to citation in text: [1] -
Ma, J.-A.; Cahard, D. Chem. Rev. 2004, 104, 6119–6146. doi:10.1021/cr030143e
Return to citation in text: [1] -
Ibrahim, H.; Togni, A. Chem. Commun. 2004, 1147–1155. doi:10.1039/b317004g
Return to citation in text: [1] -
Oestreich, M. Angew. Chem., Int. Ed. 2005, 44, 2324–2327. doi:10.1002/anie.200500478
Return to citation in text: [1] -
Pihko, P. M. Angew. Chem., Int. Ed. 2006, 45, 544–547. doi:10.1002/anie.200502425
Return to citation in text: [1] -
Bobbio, C.; Gouverneur, V. Org. Biomol. Chem. 2006, 4, 2065–2075. doi:10.1039/b603163c
Return to citation in text: [1] -
Hamashima, Y.; Sodeoka, M. Synlett 2006, 1467–1478. doi:10.1055/s-2006-941578
Return to citation in text: [1] -
Shibata, N. J. Synth. Org. Chem., Jpn. 2006, 64, 14–24.
Return to citation in text: [1] -
Shibata, N.; Ishimaru, T.; Nakamura, S.; Toru, T. J. Fluorine Chem. 2007, 128, 469–483. doi:10.1016/j.jfluchem.2006.12.014
Return to citation in text: [1] -
Hamashima, Y.; Sodeoka, M. J. Synth. Org. Chem., Jpn. 2007, 64, 1099–1107.
Return to citation in text: [1] -
Brunet, V. A.; O'Hagan, D. Angew. Chem., Int. Ed. 2008, 47, 1179–1182. doi:10.1002/anie.200704700
Return to citation in text: [1] -
O'Hagan, D. Chem. Soc. Rev. 2008, 37, 308–319. doi:10.1039/b711844a
Return to citation in text: [1] -
Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320–330. doi:10.1039/b610213c
Return to citation in text: [1] -
Shibata, N.; Suzuki, E.; Takeuchi, Y. J. Am. Chem. Soc. 2000, 122, 10728–10729. doi:10.1021/ja002732x
Return to citation in text: [1] -
Shibata, N.; Suzuki, E.; Asahi, T.; Shiro, M. J. Am. Chem. Soc. 2001, 123, 7001–7009. doi:10.1021/ja010789t
Return to citation in text: [1] -
Shibata, N.; Ishimaru, T.; Suzuki, E.; Kirk, K. L. J. Org. Chem. 2003, 68, 2494–2497. doi:10.1021/jo026792s
Return to citation in text: [1] -
Shibata, N.; Ishimaru, T.; Nakamura, M.; Toru, T. Synlett 2004, 2509–2512. doi:10.1055/s-2004-834810
Return to citation in text: [1] -
Fukuzumi, T.; Shibata, N.; Sugiura, M.; Nakamura, S.; Toru, T. J. Fluorine Chem. 2006, 127, 548–551. doi:10.1016/j.jfluchem.2006.01.004
Return to citation in text: [1] -
Cahard, D.; Audouard, C.; Plaquevent, J.-C.; Roques, N. Org. Lett. 2000, 2, 3699–3701. doi:10.1021/ol006610l
Return to citation in text: [1] -
Mohar, B.; Baudoux, J.; Plaquevent, J.-C.; Cahard, D. Angew. Chem., Int. Ed. 2001, 40, 4214–4216. doi:10.1002/1521-3773(20011119)40:22<4214::AID-ANIE4214>3.0.CO;2-B
Return to citation in text: [1] -
Cahard, D.; Audouard, C.; Plaquevent, J.-C.; Toupet, L.; Roques, N. Tetrahedron Lett. 2001, 42, 1867–1869. doi:10.1016/S0040-4039(01)00017-X
Return to citation in text: [1] -
Baudequin, C.; Plaquevent, J.-C.; Audouard, C.; Cahard, D. Green Chem. 2002, 4, 584–586. doi:10.1039/b208817g
Return to citation in text: [1] -
Baudequin, C.; Loubassou, J.-F.; Plaquevent, J.-C.; Cahard, D. J. Fluorine Chem. 2003, 122, 189–193. doi:10.1016/S0022-1139(03)00085-X
Return to citation in text: [1] -
Zoute, L.; Audouard, C.; Plaquevent, J.-C.; Cahard, D. Org. Biomol. Chem. 2003, 1, 1833–1834. doi:10.1039/b303113f
Return to citation in text: [1] -
Mohar, B.; Sterk, D.; Ferron, L.; Cahard, D. Tetrahedron Lett. 2005, 46, 5029–5031. doi:10.1016/j.tetlet.2005.05.074
Return to citation in text: [1] -
Greedy, B.; Paris, J.-M.; Vidal, T.; Gouverneur, V. Angew. Chem., Int. Ed. 2003, 42, 3291–3294. doi:10.1002/anie.200351405
Return to citation in text: [1] -
Wang, M.; Wang, B. M.; Shi, L.; Tu, Y. Q.; Fan, C.-A.; Wang, S. H.; Hu, X. D.; Zhang, S. Y. Chem. Commun. 2005, 5580–5582. doi:10.1039/b510004f
Return to citation in text: [1] -
Ramírez, J.; Huber, D. P.; Togni, A. Synlett 2007, 1143–1147. doi:10.1055/s-2007-973897
Return to citation in text: [1] -
Shibata, N.; Ishimaru, T.; Nagai, T.; Kohno, J.; Toru, T. Synlett 2004, 1703–1706. doi:10.1055/s-2004-829571
Return to citation in text: [1] [2] [3] -
Shibata, N.; Kohno, J.; Takai, K.; Ishimaru, T.; Nakamura, S.; Toru, T.; Kanemasa, S. Angew. Chem., Int. Ed. 2005, 44, 4204–4207. doi:10.1002/anie.200501041
Return to citation in text: [1] [2] [3] -
Shibata, N.; Yasui, H.; Nakamura, S.; Toru, T. Synlett 2007, 1153–1157. doi:10.1055/s-2007-977429
Return to citation in text: [1] -
Reddy, D. S.; Shibata, N.; Nagai, J.; Nakamura, S.; Toru, T.; Kanemasa, S. Angew. Chem., Int. Ed. 2008, 47, 164–168. doi:10.1002/anie.200704093
Return to citation in text: [1] [2] [3] -
Hintermann, L.; Togni, A. Angew. Chem., Int. Ed. 2000, 39, 4359–4362. doi:10.1002/1521-3773(20001201)39:23<4359::AID-ANIE4359>3.0.CO;2-P
Return to citation in text: [1] [2] -
Hamashima, Y.; Yagi, K.; Takano, H.; Tamás, L.; Sodeoka, M. J. Am. Chem. Soc. 2002, 124, 14530–14531. doi:10.1021/ja028464f
Return to citation in text: [1] [2] [3] -
Frantz, R.; Hintermann, L.; Perseghini, M.; Broggini, D.; Togni, A. Org. Lett. 2003, 5, 1709–1712. doi:10.1021/ol0343459
Return to citation in text: [1] [2] -
Hamashima, Y.; Takano, H.; Hotta, D.; Sodeoka, M. Org. Lett. 2003, 5, 3225–3228. doi:10.1021/ol035053a
Return to citation in text: [1] [2] [3] -
Toullec, P. Y.; Bonaccorsi, C.; Mezzetti, A.; Togni, A. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 5810–5814. doi:10.1073/pnas.0307716101
Return to citation in text: [1] [2] -
Ma, J.-A.; Cahard, D. Tetrahedron: Asymmetry 2004, 15, 1007–1011. doi:10.1016/j.tetasy.2004.01.014
Return to citation in text: [1] [2] -
Hamashima, Y.; Suzuki, T.; Takano, H.; Shimura, Y.; Sodeoka, M. J. Am. Chem. Soc. 2005, 127, 10164–10165. doi:10.1021/ja0513077
Return to citation in text: [1] [2] [3] -
Kim, H. R.; Kim, D. Y. Tetrahedron Lett. 2005, 46, 3115–3117. doi:10.1016/j.tetlet.2005.02.164
Return to citation in text: [1] [2] -
Bernardi, L.; Jørgensen, K. A. Chem. Commun. 2005, 1324–1326. doi:10.1039/b415568h
Return to citation in text: [1] [2] -
Kim, S. M.; Kim, H. R.; Kim, D. Y. Org. Lett. 2005, 7, 2309–2311. doi:10.1021/ol050413a
Return to citation in text: [1] [2] -
Perseghini, M.; Massaccesi, M.; Liu, Y.; Togni, A. Tetrahedron 2006, 62, 7180–7190. doi:10.1016/j.tet.2005.12.071
Return to citation in text: [1] [2] -
Bonaccorsi, C.; Althaus, M.; Becker, C.; Togni, A.; Mezzetti, A. Pure Appl. Chem. 2006, 78, 391–396. doi:10.1351/pac200678020391
Return to citation in text: [1] -
Kim, S. M.; Kang, Y. K.; Lee, K. S.; Mang, J. Y.; Kim, D. Y. Bull. Korean Chem. Soc. 2006, 27, 423–425.
Return to citation in text: [1] [2] -
Suzuki, S.; Furuno, H.; Yokoyama, Y.; Inanaga, J. Tetrahedron: Asymmetry 2006, 17, 504–507. doi:10.1016/j.tetasy.2005.12.029
Return to citation in text: [1] -
Hamashima, Y.; Suzuki, T.; Takano, H.; Shimura, Y.; Tsuchiya, Y.; Moriya, K.; Goto, T.; Sodeoka, M. Tetrahedron 2006, 62, 7168–7179. doi:10.1016/j.tet.2005.12.070
Return to citation in text: [1] [2] [3] -
Althaus, M.; Becker, C.; Togni, A.; Mezzetti, A. Organometallics 2007, 26, 5902–5911. doi:10.1021/om700714u
Return to citation in text: [1] -
Suzuki, T.; Goto, T.; Hamashima, Y.; Sodeoka, M. J. Org. Chem. 2007, 72, 246–250. doi:10.1021/jo062048m
Return to citation in text: [1] [2] [3] -
Shibatomi, K.; Tsuzuki, Y.; Nakata, S.; Sumikawa, Y.; Iwasa, S. Synlett 2007, 551–554. doi:10.1055/s-2007-970746
Return to citation in text: [1] -
Kang, Y. K.; Cho, M. J.; Kim, S. M.; Kim, D. Y. Synlett 2007, 1135–1138. doi:10.1055/s-2007-977436
Return to citation in text: [1] [2] -
Moriya, K.; Hamashima, Y.; Sodeoka, M. Synlett 2007, 1139–1142. doi:10.1055/s-2007-977437
Return to citation in text: [1] [2] [3] -
Suzuki, T.; Hamashima, Y.; Sodeoka, M. Angew. Chem., Int. Ed. 2007, 46, 5435–5439. doi:10.1002/anie.200701071
Return to citation in text: [1] [2] [3] [4] [5] [6] [7] [8] [9] [10] -
Kim, D. Y.; Park, E. J. Org. Lett. 2002, 4, 545–547. doi:10.1021/ol010281v
Return to citation in text: [1] -
Park, E. J.; Kim, H. R.; Joung, C. U.; Kim, D. Y. Bull. Korean Chem. Soc. 2004, 25, 1451–1452.
Return to citation in text: [1] -
Enders, D.; Hüttl, M. R. M. Synlett 2005, 991–993. doi:10.1055/s-2005-864813
Return to citation in text: [1] -
Marigo, M.; Fielenbach, D.; Braunton, A.; Kjærsgaard, A.; Jørgensen, K. A. Angew. Chem., Int. Ed. 2005, 44, 3703–3706. doi:10.1002/anie.200500395
Return to citation in text: [1] -
Steiner, D. D.; Mase, N.; Barbas, C. F., III. Angew. Chem., Int. Ed. 2005, 44, 3706–3710. doi:10.1002/anie.200500571
Return to citation in text: [1] -
Beeson, T. D.; MacMillan, D. W. C. J. Am. Chem. Soc. 2005, 127, 8826–8828. doi:10.1021/ja051805f
Return to citation in text: [1] -
Brandes, S.; Niess, B.; Bella, M.; Prieto, A.; Overgaard, J.; Jørgensen, K. A. Chem.–Eur. J. 2006, 12, 6039–6052. doi:10.1002/chem.200600495
Return to citation in text: [1] -
Davis, F. A.; Han, W. Tetrahedron Lett. 1992, 33, 1153–1156. doi:10.1016/S0040-4039(00)91883-5
Return to citation in text: [1] -
Davis, F. A.; Qi, H. Tetrahedron Lett. 1996, 37, 4345–4348. doi:10.1016/0040-4039(96)00825-8
Return to citation in text: [1] -
Davis, F. A.; Kasu, P. V. N. Tetrahedron Lett. 1998, 39, 6135–6138. doi:10.1016/S0040-4039(98)01296-9
Return to citation in text: [1]
| 57. | Suzuki, T.; Hamashima, Y.; Sodeoka, M. Angew. Chem., Int. Ed. 2007, 46, 5435–5439. doi:10.1002/anie.200701071 |
| 57. | Suzuki, T.; Hamashima, Y.; Sodeoka, M. Angew. Chem., Int. Ed. 2007, 46, 5435–5439. doi:10.1002/anie.200701071 |
| 57. | Suzuki, T.; Hamashima, Y.; Sodeoka, M. Angew. Chem., Int. Ed. 2007, 46, 5435–5439. doi:10.1002/anie.200701071 |
| 1. | Soloshonok, V. A., Ed. Enantiocontrolled Synthesis of Fluoro-Organic Compounds: Stereochemical Challenges and Biomedical Targets; John Wiley & Sons: Chichester, 1999. |
| 2. | Kirsch, P. Modern Fluoroorganic Chemistry; Wiley-VCH: Weinheim, 2004. |
| 18. | Shibata, N.; Suzuki, E.; Takeuchi, Y. J. Am. Chem. Soc. 2000, 122, 10728–10729. doi:10.1021/ja002732x |
| 19. | Shibata, N.; Suzuki, E.; Asahi, T.; Shiro, M. J. Am. Chem. Soc. 2001, 123, 7001–7009. doi:10.1021/ja010789t |
| 20. | Shibata, N.; Ishimaru, T.; Suzuki, E.; Kirk, K. L. J. Org. Chem. 2003, 68, 2494–2497. doi:10.1021/jo026792s |
| 21. | Shibata, N.; Ishimaru, T.; Nakamura, M.; Toru, T. Synlett 2004, 2509–2512. doi:10.1055/s-2004-834810 |
| 22. | Fukuzumi, T.; Shibata, N.; Sugiura, M.; Nakamura, S.; Toru, T. J. Fluorine Chem. 2006, 127, 548–551. doi:10.1016/j.jfluchem.2006.01.004 |
| 23. | Cahard, D.; Audouard, C.; Plaquevent, J.-C.; Roques, N. Org. Lett. 2000, 2, 3699–3701. doi:10.1021/ol006610l |
| 24. | Mohar, B.; Baudoux, J.; Plaquevent, J.-C.; Cahard, D. Angew. Chem., Int. Ed. 2001, 40, 4214–4216. doi:10.1002/1521-3773(20011119)40:22<4214::AID-ANIE4214>3.0.CO;2-B |
| 25. | Cahard, D.; Audouard, C.; Plaquevent, J.-C.; Toupet, L.; Roques, N. Tetrahedron Lett. 2001, 42, 1867–1869. doi:10.1016/S0040-4039(01)00017-X |
| 26. | Baudequin, C.; Plaquevent, J.-C.; Audouard, C.; Cahard, D. Green Chem. 2002, 4, 584–586. doi:10.1039/b208817g |
| 27. | Baudequin, C.; Loubassou, J.-F.; Plaquevent, J.-C.; Cahard, D. J. Fluorine Chem. 2003, 122, 189–193. doi:10.1016/S0022-1139(03)00085-X |
| 28. | Zoute, L.; Audouard, C.; Plaquevent, J.-C.; Cahard, D. Org. Biomol. Chem. 2003, 1, 1833–1834. doi:10.1039/b303113f |
| 29. | Mohar, B.; Sterk, D.; Ferron, L.; Cahard, D. Tetrahedron Lett. 2005, 46, 5029–5031. doi:10.1016/j.tetlet.2005.05.074 |
| 30. | Greedy, B.; Paris, J.-M.; Vidal, T.; Gouverneur, V. Angew. Chem., Int. Ed. 2003, 42, 3291–3294. doi:10.1002/anie.200351405 |
| 31. | Wang, M.; Wang, B. M.; Shi, L.; Tu, Y. Q.; Fan, C.-A.; Wang, S. H.; Hu, X. D.; Zhang, S. Y. Chem. Commun. 2005, 5580–5582. doi:10.1039/b510004f |
| 32. | Ramírez, J.; Huber, D. P.; Togni, A. Synlett 2007, 1143–1147. doi:10.1055/s-2007-973897 |
| 57. | Suzuki, T.; Hamashima, Y.; Sodeoka, M. Angew. Chem., Int. Ed. 2007, 46, 5435–5439. doi:10.1002/anie.200701071 |
| 6. | Ma, J.-A.; Cahard, D. Chem. Rev. 2004, 104, 6119–6146. doi:10.1021/cr030143e |
| 7. | Ibrahim, H.; Togni, A. Chem. Commun. 2004, 1147–1155. doi:10.1039/b317004g |
| 8. | Oestreich, M. Angew. Chem., Int. Ed. 2005, 44, 2324–2327. doi:10.1002/anie.200500478 |
| 9. | Pihko, P. M. Angew. Chem., Int. Ed. 2006, 45, 544–547. doi:10.1002/anie.200502425 |
| 10. | Bobbio, C.; Gouverneur, V. Org. Biomol. Chem. 2006, 4, 2065–2075. doi:10.1039/b603163c |
| 11. | Hamashima, Y.; Sodeoka, M. Synlett 2006, 1467–1478. doi:10.1055/s-2006-941578 |
| 12. | Shibata, N. J. Synth. Org. Chem., Jpn. 2006, 64, 14–24. |
| 13. | Shibata, N.; Ishimaru, T.; Nakamura, S.; Toru, T. J. Fluorine Chem. 2007, 128, 469–483. doi:10.1016/j.jfluchem.2006.12.014 |
| 14. | Hamashima, Y.; Sodeoka, M. J. Synth. Org. Chem., Jpn. 2007, 64, 1099–1107. |
| 15. | Brunet, V. A.; O'Hagan, D. Angew. Chem., Int. Ed. 2008, 47, 1179–1182. doi:10.1002/anie.200704700 |
| 16. | O'Hagan, D. Chem. Soc. Rev. 2008, 37, 308–319. doi:10.1039/b711844a |
| 17. | Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320–330. doi:10.1039/b610213c |
| 57. | Suzuki, T.; Hamashima, Y.; Sodeoka, M. Angew. Chem., Int. Ed. 2007, 46, 5435–5439. doi:10.1002/anie.200701071 |
| 5. | Kusumoto, T.; Hiyama, T. Fluorine-Containing Chiral Liquid Crystals: Syntheses and Properties. In Enantiocontrolled Synthesis of Fluoro-Organic Compounds: Stereochemical Challenges and Biomedical Targets; Soloshonok, V. A., Ed.; John Wiley & Sons: Chichester, 1999; pp 535–556. |
| 65. | Davis, F. A.; Han, W. Tetrahedron Lett. 1992, 33, 1153–1156. doi:10.1016/S0040-4039(00)91883-5 |
| 66. | Davis, F. A.; Qi, H. Tetrahedron Lett. 1996, 37, 4345–4348. doi:10.1016/0040-4039(96)00825-8 |
| 67. | Davis, F. A.; Kasu, P. V. N. Tetrahedron Lett. 1998, 39, 6135–6138. doi:10.1016/S0040-4039(98)01296-9 |
| 3. | Kimura, Y.; Atarashi, S.; Kawakami, K.; Sato, K.; Hayakawa, I. J. Med. Chem. 1994, 37, 3344–3352. doi:10.1021/jm00046a019 |
| 4. | Takemura, M.; Takahashi, H.; Kawakami, K.; Namba, K.; Tanaka, M.; Miyauchi, R. (Daiichi Pharmaceutical Co., Ltd., Tokyo, Japan) Anti-acid fast bacterial agents containing pyridonecarboxylic acids as the active ingredient. European Patent Application EP 1262477A1, Dec 4, 2002. |
| 33. | Shibata, N.; Ishimaru, T.; Nagai, T.; Kohno, J.; Toru, T. Synlett 2004, 1703–1706. doi:10.1055/s-2004-829571 |
| 34. | Shibata, N.; Kohno, J.; Takai, K.; Ishimaru, T.; Nakamura, S.; Toru, T.; Kanemasa, S. Angew. Chem., Int. Ed. 2005, 44, 4204–4207. doi:10.1002/anie.200501041 |
| 36. | Reddy, D. S.; Shibata, N.; Nagai, J.; Nakamura, S.; Toru, T.; Kanemasa, S. Angew. Chem., Int. Ed. 2008, 47, 164–168. doi:10.1002/anie.200704093 |
| 38. | Hamashima, Y.; Yagi, K.; Takano, H.; Tamás, L.; Sodeoka, M. J. Am. Chem. Soc. 2002, 124, 14530–14531. doi:10.1021/ja028464f |
| 40. | Hamashima, Y.; Takano, H.; Hotta, D.; Sodeoka, M. Org. Lett. 2003, 5, 3225–3228. doi:10.1021/ol035053a |
| 43. | Hamashima, Y.; Suzuki, T.; Takano, H.; Shimura, Y.; Sodeoka, M. J. Am. Chem. Soc. 2005, 127, 10164–10165. doi:10.1021/ja0513077 |
| 44. | Kim, H. R.; Kim, D. Y. Tetrahedron Lett. 2005, 46, 3115–3117. doi:10.1016/j.tetlet.2005.02.164 |
| 46. | Kim, S. M.; Kim, H. R.; Kim, D. Y. Org. Lett. 2005, 7, 2309–2311. doi:10.1021/ol050413a |
| 49. | Kim, S. M.; Kang, Y. K.; Lee, K. S.; Mang, J. Y.; Kim, D. Y. Bull. Korean Chem. Soc. 2006, 27, 423–425. |
| 51. | Hamashima, Y.; Suzuki, T.; Takano, H.; Shimura, Y.; Tsuchiya, Y.; Moriya, K.; Goto, T.; Sodeoka, M. Tetrahedron 2006, 62, 7168–7179. doi:10.1016/j.tet.2005.12.070 |
| 53. | Suzuki, T.; Goto, T.; Hamashima, Y.; Sodeoka, M. J. Org. Chem. 2007, 72, 246–250. doi:10.1021/jo062048m |
| 55. | Kang, Y. K.; Cho, M. J.; Kim, S. M.; Kim, D. Y. Synlett 2007, 1135–1138. doi:10.1055/s-2007-977436 |
| 56. | Moriya, K.; Hamashima, Y.; Sodeoka, M. Synlett 2007, 1139–1142. doi:10.1055/s-2007-977437 |
| 57. | Suzuki, T.; Hamashima, Y.; Sodeoka, M. Angew. Chem., Int. Ed. 2007, 46, 5435–5439. doi:10.1002/anie.200701071 |
| 38. | Hamashima, Y.; Yagi, K.; Takano, H.; Tamás, L.; Sodeoka, M. J. Am. Chem. Soc. 2002, 124, 14530–14531. doi:10.1021/ja028464f |
| 40. | Hamashima, Y.; Takano, H.; Hotta, D.; Sodeoka, M. Org. Lett. 2003, 5, 3225–3228. doi:10.1021/ol035053a |
| 43. | Hamashima, Y.; Suzuki, T.; Takano, H.; Shimura, Y.; Sodeoka, M. J. Am. Chem. Soc. 2005, 127, 10164–10165. doi:10.1021/ja0513077 |
| 51. | Hamashima, Y.; Suzuki, T.; Takano, H.; Shimura, Y.; Tsuchiya, Y.; Moriya, K.; Goto, T.; Sodeoka, M. Tetrahedron 2006, 62, 7168–7179. doi:10.1016/j.tet.2005.12.070 |
| 53. | Suzuki, T.; Goto, T.; Hamashima, Y.; Sodeoka, M. J. Org. Chem. 2007, 72, 246–250. doi:10.1021/jo062048m |
| 56. | Moriya, K.; Hamashima, Y.; Sodeoka, M. Synlett 2007, 1139–1142. doi:10.1055/s-2007-977437 |
| 57. | Suzuki, T.; Hamashima, Y.; Sodeoka, M. Angew. Chem., Int. Ed. 2007, 46, 5435–5439. doi:10.1002/anie.200701071 |
| 37. | Hintermann, L.; Togni, A. Angew. Chem., Int. Ed. 2000, 39, 4359–4362. doi:10.1002/1521-3773(20001201)39:23<4359::AID-ANIE4359>3.0.CO;2-P |
| 39. | Frantz, R.; Hintermann, L.; Perseghini, M.; Broggini, D.; Togni, A. Org. Lett. 2003, 5, 1709–1712. doi:10.1021/ol0343459 |
| 41. | Toullec, P. Y.; Bonaccorsi, C.; Mezzetti, A.; Togni, A. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 5810–5814. doi:10.1073/pnas.0307716101 |
| 47. | Perseghini, M.; Massaccesi, M.; Liu, Y.; Togni, A. Tetrahedron 2006, 62, 7180–7190. doi:10.1016/j.tet.2005.12.071 |
| 57. | Suzuki, T.; Hamashima, Y.; Sodeoka, M. Angew. Chem., Int. Ed. 2007, 46, 5435–5439. doi:10.1002/anie.200701071 |
| 58. | Kim, D. Y.; Park, E. J. Org. Lett. 2002, 4, 545–547. doi:10.1021/ol010281v |
| 59. | Park, E. J.; Kim, H. R.; Joung, C. U.; Kim, D. Y. Bull. Korean Chem. Soc. 2004, 25, 1451–1452. |
| 60. | Enders, D.; Hüttl, M. R. M. Synlett 2005, 991–993. doi:10.1055/s-2005-864813 |
| 61. | Marigo, M.; Fielenbach, D.; Braunton, A.; Kjærsgaard, A.; Jørgensen, K. A. Angew. Chem., Int. Ed. 2005, 44, 3703–3706. doi:10.1002/anie.200500395 |
| 62. | Steiner, D. D.; Mase, N.; Barbas, C. F., III. Angew. Chem., Int. Ed. 2005, 44, 3706–3710. doi:10.1002/anie.200500571 |
| 63. | Beeson, T. D.; MacMillan, D. W. C. J. Am. Chem. Soc. 2005, 127, 8826–8828. doi:10.1021/ja051805f |
| 64. | Brandes, S.; Niess, B.; Bella, M.; Prieto, A.; Overgaard, J.; Jørgensen, K. A. Chem.–Eur. J. 2006, 12, 6039–6052. doi:10.1002/chem.200600495 |
| 57. | Suzuki, T.; Hamashima, Y.; Sodeoka, M. Angew. Chem., Int. Ed. 2007, 46, 5435–5439. doi:10.1002/anie.200701071 |
| 33. | Shibata, N.; Ishimaru, T.; Nagai, T.; Kohno, J.; Toru, T. Synlett 2004, 1703–1706. doi:10.1055/s-2004-829571 |
| 34. | Shibata, N.; Kohno, J.; Takai, K.; Ishimaru, T.; Nakamura, S.; Toru, T.; Kanemasa, S. Angew. Chem., Int. Ed. 2005, 44, 4204–4207. doi:10.1002/anie.200501041 |
| 35. | Shibata, N.; Yasui, H.; Nakamura, S.; Toru, T. Synlett 2007, 1153–1157. doi:10.1055/s-2007-977429 |
| 36. | Reddy, D. S.; Shibata, N.; Nagai, J.; Nakamura, S.; Toru, T.; Kanemasa, S. Angew. Chem., Int. Ed. 2008, 47, 164–168. doi:10.1002/anie.200704093 |
| 37. | Hintermann, L.; Togni, A. Angew. Chem., Int. Ed. 2000, 39, 4359–4362. doi:10.1002/1521-3773(20001201)39:23<4359::AID-ANIE4359>3.0.CO;2-P |
| 38. | Hamashima, Y.; Yagi, K.; Takano, H.; Tamás, L.; Sodeoka, M. J. Am. Chem. Soc. 2002, 124, 14530–14531. doi:10.1021/ja028464f |
| 39. | Frantz, R.; Hintermann, L.; Perseghini, M.; Broggini, D.; Togni, A. Org. Lett. 2003, 5, 1709–1712. doi:10.1021/ol0343459 |
| 40. | Hamashima, Y.; Takano, H.; Hotta, D.; Sodeoka, M. Org. Lett. 2003, 5, 3225–3228. doi:10.1021/ol035053a |
| 41. | Toullec, P. Y.; Bonaccorsi, C.; Mezzetti, A.; Togni, A. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 5810–5814. doi:10.1073/pnas.0307716101 |
| 42. | Ma, J.-A.; Cahard, D. Tetrahedron: Asymmetry 2004, 15, 1007–1011. doi:10.1016/j.tetasy.2004.01.014 |
| 43. | Hamashima, Y.; Suzuki, T.; Takano, H.; Shimura, Y.; Sodeoka, M. J. Am. Chem. Soc. 2005, 127, 10164–10165. doi:10.1021/ja0513077 |
| 44. | Kim, H. R.; Kim, D. Y. Tetrahedron Lett. 2005, 46, 3115–3117. doi:10.1016/j.tetlet.2005.02.164 |
| 45. | Bernardi, L.; Jørgensen, K. A. Chem. Commun. 2005, 1324–1326. doi:10.1039/b415568h |
| 46. | Kim, S. M.; Kim, H. R.; Kim, D. Y. Org. Lett. 2005, 7, 2309–2311. doi:10.1021/ol050413a |
| 47. | Perseghini, M.; Massaccesi, M.; Liu, Y.; Togni, A. Tetrahedron 2006, 62, 7180–7190. doi:10.1016/j.tet.2005.12.071 |
| 48. | Bonaccorsi, C.; Althaus, M.; Becker, C.; Togni, A.; Mezzetti, A. Pure Appl. Chem. 2006, 78, 391–396. doi:10.1351/pac200678020391 |
| 49. | Kim, S. M.; Kang, Y. K.; Lee, K. S.; Mang, J. Y.; Kim, D. Y. Bull. Korean Chem. Soc. 2006, 27, 423–425. |
| 50. | Suzuki, S.; Furuno, H.; Yokoyama, Y.; Inanaga, J. Tetrahedron: Asymmetry 2006, 17, 504–507. doi:10.1016/j.tetasy.2005.12.029 |
| 51. | Hamashima, Y.; Suzuki, T.; Takano, H.; Shimura, Y.; Tsuchiya, Y.; Moriya, K.; Goto, T.; Sodeoka, M. Tetrahedron 2006, 62, 7168–7179. doi:10.1016/j.tet.2005.12.070 |
| 52. | Althaus, M.; Becker, C.; Togni, A.; Mezzetti, A. Organometallics 2007, 26, 5902–5911. doi:10.1021/om700714u |
| 53. | Suzuki, T.; Goto, T.; Hamashima, Y.; Sodeoka, M. J. Org. Chem. 2007, 72, 246–250. doi:10.1021/jo062048m |
| 54. | Shibatomi, K.; Tsuzuki, Y.; Nakata, S.; Sumikawa, Y.; Iwasa, S. Synlett 2007, 551–554. doi:10.1055/s-2007-970746 |
| 55. | Kang, Y. K.; Cho, M. J.; Kim, S. M.; Kim, D. Y. Synlett 2007, 1135–1138. doi:10.1055/s-2007-977436 |
| 56. | Moriya, K.; Hamashima, Y.; Sodeoka, M. Synlett 2007, 1139–1142. doi:10.1055/s-2007-977437 |
| 57. | Suzuki, T.; Hamashima, Y.; Sodeoka, M. Angew. Chem., Int. Ed. 2007, 46, 5435–5439. doi:10.1002/anie.200701071 |
| 33. | Shibata, N.; Ishimaru, T.; Nagai, T.; Kohno, J.; Toru, T. Synlett 2004, 1703–1706. doi:10.1055/s-2004-829571 |
| 34. | Shibata, N.; Kohno, J.; Takai, K.; Ishimaru, T.; Nakamura, S.; Toru, T.; Kanemasa, S. Angew. Chem., Int. Ed. 2005, 44, 4204–4207. doi:10.1002/anie.200501041 |
| 36. | Reddy, D. S.; Shibata, N.; Nagai, J.; Nakamura, S.; Toru, T.; Kanemasa, S. Angew. Chem., Int. Ed. 2008, 47, 164–168. doi:10.1002/anie.200704093 |
| 42. | Ma, J.-A.; Cahard, D. Tetrahedron: Asymmetry 2004, 15, 1007–1011. doi:10.1016/j.tetasy.2004.01.014 |
| 45. | Bernardi, L.; Jørgensen, K. A. Chem. Commun. 2005, 1324–1326. doi:10.1039/b415568h |
© 2008 Ishimaru et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)