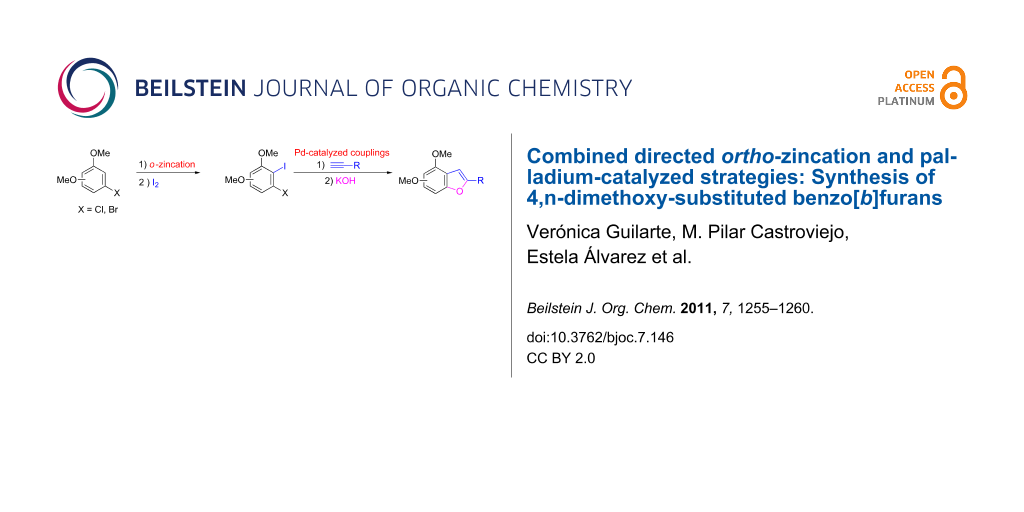
Abstract
A new route to regioselectively dialkoxy-functionalized benzo[b]furan derivatives has been developed from 3-halo-2-iodoanisoles bearing an additional methoxy group, which have been accessed through an ortho-zincation/iodination reaction. Two palladium-catalyzed processes, namely a Sonogashira coupling followed by a tandem hydroxylation/cyclization sequence, give rise to new and interesting dimethoxy-substituted benzo[b]furans.
Graphical Abstract
Introduction
The directed ortho-metallation (DoM) reaction has been widely used as a powerful and efficient method for regioselective functionalization of aromatic compounds and different directing groups have been used to facilitate the deprotonation reaction [1-5]. Various strong bases such as alkyl lithiums and their derivatives (for instance, TMEDA-activated complexes [6] and heavier alkali metal tert-butoxide-complexed alkyl lithium reagents, known as superbases and introduced by Schlosser [7]), as well as lithium dialkylamides, have usually been employed to perform deprotonative metallations. Whereas the use of these strong bases has several limitations regarding the presence of certain functional groups (mainly carboxylic acid derivatives and halogens), the introduction in the last years of new organometallic “ate” complexes [8,9], which combine an alkali metal with either magnesium, zinc, aluminium, or copper, has allowed more selective metallation reactions. The milder reaction conditions required make these deprotonation reactions tolerant to the coexistence of a wider range of functional groups. In this field, the work of Kondo and Uchiyama is remarkable as they described highly chemo- and regioselective deprotonative zincation [10-12], alumination [13], and cupration [14] reactions of some functionalized aromatic and heteroaromatic compounds, as well as of meta-functionalized haloaromatics. In particular, the alkali metal mediated zincation reactions have turned out to be very useful processes and the structures and reaction pathways of TMP-zincates (TMP = 2,2,6,6-tetramethylpiperidine) have been studied in detail [15-19].
On the other hand, benzo[b]furan is a basic skeleton found in a variety of significant natural products [20], and some derivatives are also used as organic materials, due to their optical and electronic properties [21]. Thus, many synthetic efforts have been devoted to the synthesis of this type of compound [22,23]. In particular, several benzo[b]furan derivatives with oxygen-bearing substituents, such as hydroxy, or alkoxy, at the benzene moiety are known to be biologically active compounds [24-28] (Figure 1).
Figure 1: Some representative dihydroxybenzofuran derived natural products.
Figure 1: Some representative dihydroxybenzofuran derived natural products.
Among the various approaches developed for the synthesis of the benzofuran ring system, the cyclodehydration of α-aryloxy ketones [29], the Claisen rearrangement of an allyl aryl ether followed by Pd-catalyzed intramolecular oxidative cyclization [30], and the tandem Sonogashira coupling/heterocyclization of 2-halophenols with terminal alkynes [31], are some of the most used. However, their application to the synthesis of 4-substituted benzo[b]furans is especially challenging, because the meta-substituted starting materials tend to cyclize at the less hindered ortho-position, leading to the formation of 6-substituted heterocycles or a mixture of 6- and 4-substituted ones [32].
The DoM strategy, when linked with different cross-coupling Pd-catalyzed reactions, could provide a superior approach for the construction of polysubstituted aromatic and heteroaromatic compounds [33-38]. In this context, we studied the o-lithiation of 3-halophenols and the resulting 2,3-difunctionalized phenol derivatives were applied to the synthesis of 4-functionalized benzo[b]furans [39], 4- or 7-alkoxyindoles [40], and 7-oxy-substituted benzo[b]thiophenes [41] by employing Pd-catalyzed cross-coupling reactions or halocyclization processes. Following our interest in the development of strategies for the synthesis of functionalized benzo[b]furan derivatives [39], we envisaged that 4,n-dimethoxybenzo[b]furans could be regioselectively synthesized from 3-halo-2-iodoanisoles bearing an additional methoxy group, by combining two palladium-catalyzed processes, that is a selective Sonogashira coupling and a tandem hydroxylation/heterocyclization reaction. The required o-dihaloanisole derivatives could be prepared by a selective ortho-metallation reaction and subsequent electrophilic quenching with iodine (Scheme 1).
Scheme 1: Retrosynthetic analysis of 4,n-dimethoxy-substituted benzo[b]furans.
Scheme 1: Retrosynthetic analysis of 4,n-dimethoxy-substituted benzo[b]furans.
Results and Discussion
As established in our proposed retrosynthetic analysis (Scheme 1), we needed to develop a convenient synthesis of 3-halo-2-iodoanisoles bearing an additional methoxy group. Taking advantage of the deprotonative ortho-zincation of meta-functionalized haloaromatics by using TMP-zincates, described by Uchiyama and co-workers [11,12], we previously developed efficient syntheses of 3-chloro-2-iodoanisole (2a) and 3-bromo-2-iodoanisole (2b) from the corresponding 3-haloanisoles 1, through their treatment with lithium di-tert-butyltetramethylpiperidinozincate, followed by electrophilic trapping of the intermediate arylzincate with iodine (Scheme 2) [40].
Scheme 2: Deprotonative zincation of 3-haloanisoles 1.
Scheme 2: Deprotonative zincation of 3-haloanisoles 1.
Thus, we decided to test this deprotonative metallation on the commercially available dimethoxyhalobenzene derivatives 3 with the same t-Bu2Zn(TMP)Li, easily prepared by reaction of preformed di-tert-butylzinc with lithium tetramethylpiperidide. Under these reaction conditions the zincation reactions took place regioselectively and, after treatment with iodine, afforded the corresponding 3-halo-2-iodoanisole derivatives 4 in good yields (Table 1). It is interesting to note that substrates 3c and 3d bearing the two methoxy groups in a meta relationship selectively undergo metalation at the position between the methoxy and the halide groups, irrespective of the nature of the halogen atom (chloro or bromo) (Table 1, entries 3 and 4).
Table 1: Synthesis of methoxy-substituted 3-halo-2-iodoanisoles 4.
|
|
|||||
| Entry | Starting material | X | OMea | Product | Yield (%)b |
|---|---|---|---|---|---|
| 1 | 3a | Cl | ortho | 4a | 70 |
| 2 | 3b | Br | ortho | 4b | 73 |
| 3 | 3c | Cl | meta | 4c | 88 |
| 4 | 3d | Br | meta | 4d | 75 |
| 5 | 3e | Cl | para | 4e | 78 |
| 6 | 3f | Br | para | 4f | 80 |
aPosition of the additional methoxy group relative to the existing one. bIsolated yield based on the starting material 3.
Having an efficient protocol for the synthesis of dimethoxyhaloiodobenzene derivatives 4, and with the retrosynthetic analysis outlined in Scheme 1 in mind, we tackled the selective introduction of an alkynyl moiety at the iodinated position. The presence of two different halogen atoms in compounds 4 implies that a selective Sonogashira coupling reaction should occur (Table 2). This has been achieved in two different ways. Considering the steric hindrance of the required position (o,o-disubstituted) we employed a copper- and solvent-free methodology for the Sonogashira coupling that uses tetrabutylammonium fluoride as base [42] (Table 2, method A). Subsequently, we checked that the selective coupling could be carried out under standard Pd–Cu catalysis by controlling the reaction temperature [43,44] (Table 2, method B).
Table 2: Synthesis of dimethoxy-substituted o-alkynylhaloarenes 5–7.
|
|
|||||||
| Entry | Starting material | X | OMea | R | Product | Methodb | Yield (%)c |
|---|---|---|---|---|---|---|---|
| 1 | 4a | Cl | ortho | n-Bu | 5a | A | 65 |
| 2 | 4a | Cl | ortho | c-C6H9d | 5b | A | 79 |
| 3 | 4b | Br | ortho | n-C6H13 | 5c | A | 91 |
| 4 | 4b | Br | ortho | n-C6H13 | 5c | B | 90 |
| 5 | 4b | Br | ortho | Ph | 5d | A | 83 |
| 6 | 4b | Br | ortho | Ph | 5d | B | 87 |
| 7 | 4c | Cl | meta | n-Bu | 6a | A | 57 |
| 8 | 4c | Cl | meta | c-C6H9d | 6b | A | 69 |
| 9 | 4d | Br | meta | n-C5H11 | 6c | A | 63 |
| 10 | 4d | Br | meta | n-C5H11 | 6c | B | 79 |
| 11 | 4d | Br | meta | Ph | 6d | A | 52 |
| 12 | 4d | Br | meta | Ph | 6d | B | 80 |
| 13 | 4d | Br | meta | 4-MeC6H4 | 6e | A | 75 |
| 14 | 4e | Cl | para | n-Bu | 7a | A | 69 |
| 15 | 4f | Br | para | n-C5H11 | 7b | A | 55 |
| 16 | 4f | Br | para | n-C5H11 | 7b | B | 95 |
| 17 | 4f | Br | para | Ph | 7c | A | 79 |
| 18 | 4f | Br | para | Ph | 7c | B | 92 |
| 19 | 4f | Br | para | 3-FC6H4 | 7d | A | 93 |
aPosition of the additional methoxy group referred to the existing one. bMethod A: alkyne (1.5–2 equiv), PdCl2(PPh3)2 (6 mol %), TBAF·3H2O (3 equiv), 50–60 °C. Method B: alkyne (1.2 equiv), PdCl2(PPh3)2 (3 mol %), CuI (5 mol %), Et2NH (1.5 equiv), DMF, 50 °C. cIsolated yield based on the starting material 4. d1-Cyclohexenyl.
By either of these procedures, A or B, o-alkynylhaloarenes 5–7 were prepared usually in high yields from the corresponding dimethoxyhaloiodobenzenes 4 (Table 2). Starting from chloroiodo derivatives 4a,c and e good yields were obtained for the corresponding coupled products 5–7 by using the Pd/TBAF method A, with no significant side-products (Table 2, entries 1,2,7,8 and 14). However, under these conditions bromine-containing compounds 4b,d and f afforded variable amounts of dialkynylation products in some cases, and so the Pd/Cu catalyzed procedure B gave rise to better selectivities and yields of the desired alkynes 5–7 (Table 2, entries 4,6,10,12,16 and 18).
According to our retrosynthetic analysis (Scheme 1), the final step to achieve the benzofuran derivatives should be the incorporation of the hydroxy group followed by in situ heterocyclization. In recent years, the direct hydroxylation of aryl halides has been developed by several groups by using palladium- or copper-catalyzed protocols. Whereas the reactions under copper catalysis work well for aryl iodides [45-48], the palladium-catalyzed hydroxylation also takes place with aryl bromides and chlorides [49-51]. Thus, in our case we employed the Pd-catalyzed Buchwald protocol [49] in an attempt to synthesise the desired dimethoxybenzo[b]furan derivatives. Thus, reaction of the previously prepared o-haloaryl alkynes 5–7 with KOH, in the presence of catalytic amounts of Pd2(dba)3 and t-BuXPhos (2-di-tert-butylphosphino-2',4',6'-triisopropylbiphenyl) at 100 °C in a 1:1 mixture of H2O:1,4-dioxane, gave rise to the corresponding benzo[b]furan derivatives 8–10 in moderate to good yields (Table 3, method C). In general, slightly better results were obtained starting from aryl bromides instead of aryl chlorides. In addition, we also observed better yields for the corresponding benzofuran derivatives 9 (Table 3, entries 5–9) derived from the starting o-alkynylhalobenzene derivatives 6, bearing the two methoxy groups in a 3,5-relationship relative to the halide. Moreover, because of the extended reaction times needed for complete consumption of the starting materials, we developed an alternative protocol under microwave irradiation that shortens the time required for the coupling to a few minutes (method D), and the final products were obtained in similar yields to those obtained by the conventional coupling method C.
Table 3: Synthesis of dimethoxy-substituted benzo[b]furans 8–10.
|
|
|||||||
| Entry | Starting material | X | R | Product | OMea | Methodb | Yield (%) |
|---|---|---|---|---|---|---|---|
| 1 | 5a | Cl | n-Bu | 8a | 4,5-(MeO)2 | D | 55 |
| 2 | 5b | Cl | c-C6H9c | 8b | 4,5-(MeO)2 | C | 57 |
| 3 | 5c | Br | n-C6H13 | 8c | 4,5-(MeO)2 | D | 55 |
| 4 | 5d | Br | Ph | 8d | 4,5-(MeO)2 | C | 50 |
| 5 | 6a | Cl | n-Bu | 9a | 4,6-(MeO)2 | C | 62 |
| 6 | 6b | Cl | c-C6H9c | 9b | 4,6-(MeO)2 | C | 73 |
| 7 | 6c | Br | n-C5H11 | 9c | 4,6-(MeO)2 | C | 70 |
| 8 | 6d | Br | Ph | 9d | 4,6-(MeO)2 | C | 81 |
| 9 | 6e | Br | 4-MeC6H4 | 9e | 4,6-(MeO)2 | C | 75 |
| 10 | 7a | Cl | n-Bu | 10a | 4,7-(MeO)2 | C | 60 |
| 11 | 7b | Br | n-C5H11 | 10b | 4,7-(MeO)2 | C | 64 |
| 12 | 7c | Br | Ph | 10c | 4,7-(MeO)2 | D | 71 |
| 13 | 7d | Br | 3-FC6H4 | 10d | 4,7-(MeO)2 | D | 65 |
aPosition of the methoxy groups referred to benzo[b]furan moiety. bMethod C: conventional heating (100 °C, overnight). Method D: MW (50 W, 150 °C, 12 min). c1-Cyclohexenyl.
Conclusion
We have developed a synthetic route to access regioselectively functionalized 4,n-dimethoxybenzo[b]furans through combined directed ortho-metallation (DoM)/palladium-catalyzed reactions. The deprotonative ortho-zincation of meta-functionalized haloanisoles bearing an additional methoxy group, followed by electrophilic quenching with iodine allows the regioselective and straightforward synthesis of highly functionalized dihalodimethoxybenzene derivatives. A subsequent selective Sonogashira coupling with terminal alkynes, followed by direct hydroxylation with KOH of the resulting o-haloaryl alkyne and in situ heterocyclization, afforded the benzo[b]furan derivatives. In addition, we have developed a new procedure for the Pd-catalyzed hydroxylation reaction that allows the coupling to take place within in a few minutes.
Supporting Information
Experimental procedures and spectroscopic data for all new compounds. Copies of 1H NMR and 13C NMR spectra for new compounds.
| Supporting Information File 1: Experimental and analytical data. | ||
| Format: PDF | Size: 310.3 KB | Download |
| Supporting Information File 2: NMR spectra. | ||
| Format: PDF | Size: 1.7 MB | Download |
References
-
Hartung, C. G.; Snieckus, V. The Directed ortho Metalation Reaction – A Point of Departure for New Synthetic Aromatic Chemistry. In Modern Arene Chemistry; Astruc, D., Ed.; Wiley-VCH: New York, 2002; pp 330–367. doi:10.1002/3527601767.ch10
Return to citation in text: [1] -
Clayden, J. Regioselective Synthesis of Organolithiums by Deprotonation. In Organolithiums: Selectivity for Synthesis; Tetrahedron Organic Chemistry Series, Vol. 23; Pergamon: Oxford, 2002; pp 9–109.
Return to citation in text: [1] -
Whisler, M. C.; MacNeil, S.; Snieckus, V.; Beak, P. Angew. Chem., Int. Ed. 2004, 43, 2206–2225. doi:10.1002/anie.200300590
Return to citation in text: [1] -
Anctil, E. J.-G.; Snieckus, V. In Metal-Catalyzed Cross-Coupling Reactions, 2nd ed.; de Meijere, A.; Diederich, F., Eds.; Wiley-VCH: Weinheim, 2004; pp 761–813.
Return to citation in text: [1] -
Macklin, T.; Snieckus, V. In Handbook of C–H Transformations; Dyker, G., Ed.; Wiley-VCH: New York, 2005; pp 106–119.
Return to citation in text: [1] -
Eberhardt, G. G.; Butte, W. A. J. Org. Chem. 1964, 29, 2928–2932. doi:10.1021/jo01033a029
Return to citation in text: [1] -
Schlosser, M.; Choi, J. H.; Takagishi, S. Tetrahedron 1990, 46, 5633–5648. doi:10.1016/S0040-4020(01)87763-2
Return to citation in text: [1] -
Mulvey, R. E.; Mongin, F.; Uchiyama, M.; Kondo, Y. Angew. Chem., Int. Ed. 2007, 46, 3802–3824. doi:10.1002/anie.200604369
Return to citation in text: [1] -
Mulvey, R. E. Acc. Chem. Res. 2009, 42, 743–755. doi:10.1021/ar800254y
Return to citation in text: [1] -
Kondo, Y.; Shilai, M.; Uchiyama, M.; Sakamoto, T. J. Am. Chem. Soc. 1999, 121, 3539–3540. doi:10.1021/ja984263t
Return to citation in text: [1] -
Uchiyama, M.; Miyoshi, T.; Kajihara, Y.; Sakamoto, T.; Otani, Y.; Ohwada, T.; Kondo, Y. J. Am. Chem. Soc. 2002, 124, 8514–8515. doi:10.1021/ja0202199
Return to citation in text: [1] [2] -
Uchiyama, M.; Kobayashi, Y.; Furuyama, T.; Nakamura, S.; Kajihara, Y.; Miyoshi, T.; Sakamoto, T.; Kondo, Y.; Morokuma, K. J. Am. Chem. Soc. 2008, 130, 472–480. doi:10.1021/ja071268u
Return to citation in text: [1] [2] -
Naka, H.; Uchiyama, M.; Matsumoto, Y.; Wheatley, A. E. H.; McPartlin, M.; Morey, J. V.; Kondo, Y. J. Am. Chem. Soc. 2007, 129, 1921–1930. doi:10.1021/ja064601n
Return to citation in text: [1] -
Usui, S.; Hashimoto, Y.; Morey, J. V.; Wheatley, A. E. H.; Uchiyama, M. J. Am. Chem. Soc. 2007, 129, 15102–15103. doi:10.1021/ja074669i
Return to citation in text: [1] -
Clegg, W.; Dale, S. H.; Drummond, A. M.; Hevia, E.; Honeyman, G. W.; Mulvey, R. E. J. Am. Chem. Soc. 2006, 128, 7434–7435. doi:10.1021/ja061898g
Return to citation in text: [1] -
Uchiyama, M.; Matsumoto, Y.; Nobuto, D.; Furuyama, T.; Yamaguchi, K.; Morokuma, K. J. Am. Chem. Soc. 2006, 128, 8748–8750. doi:10.1021/ja060489h
Return to citation in text: [1] -
Uchiyama, M.; Matsumoto, Y.; Usui, S.; Hashimoto, Y.; Morokuma, K. Angew. Chem., Int. Ed. 2007, 46, 926–929. doi:10.1002/anie.200602664
Return to citation in text: [1] -
Kondo, Y.; Morey, J. V.; Morgan, J. C.; Naka, H.; Nobuto, D.; Raithby, P. R.; Uchiyama, M.; Wheatley, A. E. H. J. Am. Chem. Soc. 2007, 129, 12734–12738. doi:10.1021/ja072118m
Return to citation in text: [1] -
Hevia, E.; Kennedy, A. R.; Klett, J.; McCall, M. D. Chem. Commun. 2009, 3240–3242. doi:10.1039/b903592c
Return to citation in text: [1] -
De Luca, L.; Nieddu, G.; Porcheddu, A.; Giacomelli, G. Curr. Med. Chem. 2009, 16, 1–20. doi:10.2174/092986709787002826
Return to citation in text: [1] -
Hwu, J. R.; Chuang, K.-S.; Chuang, S. H.; Tsay, S.-C. Org. Lett. 2005, 7, 1545–1548. doi:10.1021/ol050196d
Return to citation in text: [1] -
Hou, X.-L.; Yang, Z.; Yeung, K.-S.; Wong, H. N. C. Prog. Heterocycl. Chem. 2009, 21, 179–223. doi:10.1016/S0959-6380(09)70034-0
Return to citation in text: [1] -
Yeung, K.-S.; Yang, Z.; Peng, X.-S.; Hou, X.-L. Prog. Heterocycl. Chem. 2011, 22, 181–216. doi:10.1016/S0959-6380(11)22007-5
Return to citation in text: [1] -
Ober, A. G.; Fronczek, F. R.; Fischer, N. H. J. Nat. Prod. 1985, 48, 242–248.
Return to citation in text: [1] -
Parodi, F. J.; Fischer, N. H.; Flores, H. E. J. Nat. Prod. 1988, 51, 594–595.
Return to citation in text: [1] -
Wang, L.-Q.; Zhao, Y.-X.; Hu, J. M.; Jia, A.-Q.; Zhou, J. Helv. Chim. Acta 2008, 91, 159–164.
Return to citation in text: [1] -
Stevenson, P. C.; Veitch, N. C. Phytochemistry 1998, 48, 947–951.
Return to citation in text: [1] -
Joshi, A. S.; Li, X.-C.; Nimrod, A. C.; ElSholy, H. N.; Walker, L. A.; Clark, A. M. Planta Med. 2001, 67, 186–188.
Return to citation in text: [1] -
Kim, I.; Lee, S.-H.; Lee, S. Tetrahedron Lett. 2008, 49, 6579–6584. doi:10.1016/j.tetlet.2008.09.034
Return to citation in text: [1] -
Youn, S. W.; Eom, J. I. Org. Lett. 2005, 7, 3355–3358. doi:10.1021/ol051264z
Return to citation in text: [1] -
Heravi, M. M.; Sadjadi, S. Tetrahedron 2009, 65, 7761–7775. doi:10.1016/j.tet.2009.06.028
Return to citation in text: [1] -
Tsuchikama, K.; Hashimoto, Y.-k.; Endo, K.; Shibata, T. Adv. Synth. Catal. 2009, 351, 2850–2854.
Return to citation in text: [1] -
Alessi, M.; Larkin, A. L.; Ogilvie, K. A.; Green, L. A.; Lai, S.; López, S.; Snieckus, V. J. Org. Chem. 2007, 72, 1588–1594. doi:10.1021/jo0620359
Return to citation in text: [1] -
Blanchet, J.; Macklin, T.; Ang, P.; Metallinos, C.; Snieckus, V. J. Org. Chem. 2007, 72, 3199–3206. doi:10.1021/jo062385v
Return to citation in text: [1] -
Nerdinger, S.; Kendall, C.; Cai, X.; Marchart, R.; Riebel, P.; Johnson, M. R.; Yin, C.-F.; Hénaff, N.; Eltis, L. D.; Snieckus, V. J. Org. Chem. 2007, 72, 5960–5967. doi:10.1021/jo062543i
Return to citation in text: [1] -
MacNeil, S. L.; Gray, M.; Gusev, D. G.; Briggs, L. E.; Snieckus, V. J. Org. Chem. 2008, 73, 9710–9719. doi:10.1021/jo801856n
Return to citation in text: [1] -
James, C. A.; Snieckus, V. J. Org. Chem. 2009, 74, 4080–4093. doi:10.1021/jo9001454
Return to citation in text: [1] -
James, C. A.; Coelho, A. L.; Gevaert, M.; Forgione, P.; Snieckus, V. J. Org. Chem. 2009, 74, 4094–4103. doi:10.1021/jo900146d
Return to citation in text: [1] -
Sanz, R.; Castroviejo, M. P.; Fernández, Y.; Fañanás, F. J. J. Org. Chem. 2005, 70, 6548–6551. doi:10.1021/jo0508402
Return to citation in text: [1] [2] -
Sanz, R.; Castroviejo, M. P.; Guilarte, V.; Pérez, A.; Fañanás, F. J. J. Org. Chem. 2007, 72, 5113–5118. doi:10.1021/jo070643y
Return to citation in text: [1] [2] -
Sanz, R.; Guilarte, V.; Hernando, E.; Sanjuán, A. M. J. Org. Chem. 2010, 75, 7443–7446. doi:10.1021/jo101436f
Return to citation in text: [1] -
Liang, Y.; Xie, Y.-X.; Li, J.-H. J. Org. Chem. 2006, 71, 2535–2537. doi:10.1021/jo051882t
Return to citation in text: [1] -
Sanz, R.; Guilarte, V.; García, N. Org. Biomol. Chem. 2010, 8, 3860–3864. doi:10.1039/c004360e
Return to citation in text: [1] -
Guilarte, V.; Castroviejo, M. P.; García-García, P.; Fernández-Rodríguez, M. A.; Sanz, R. J. Org. Chem. 2011, 76, 3416–3437. doi:10.1021/jo200406f
Return to citation in text: [1] -
Tlili, A.; Xia, N.; Monnier, F.; Taillefer, M. Angew. Chem., Int. Ed. 2009, 48, 8725–8728. doi:10.1002/anie.200903639
Return to citation in text: [1] -
Zhao, D.; Wu, N.; Zhang, S.; Xi, P.; Su, X.; Lan, J.; You, J. Angew. Chem., Int. Ed. 2009, 48, 8729–8732. doi:10.1002/anie.200903923
Return to citation in text: [1] -
Jing, L.; Wei, J.; Zhou, L.; Huang, Z.; Li, Z.; Zhou, X. Chem. Commun. 2010, 46, 4767–4769. doi:10.1039/c0cc00434k
Return to citation in text: [1] -
Yang, D.; Fu, H. Chem.–Eur. J. 2010, 16, 2366–2370. doi:10.1002/chem.200903468
Return to citation in text: [1] -
Anderson, K. W.; Ikawa, T.; Tundel, R. E.; Buchwald, S. L. J. Am. Chem. Soc. 2006, 128, 10694–10695. doi:10.1021/ja0639719
Return to citation in text: [1] [2] -
Schulz, T.; Torborg, C.; Schäffner, B.; Huang, J.; Zapf, A.; Kadyrov, R.; Börner, A.; Beller, M. Angew. Chem., Int. Ed. 2009, 48, 918–921. doi:10.1002/anie.200804898
Return to citation in text: [1] -
Sergeev, A. G.; Schulz, T.; Torborg, C.; Spannenberg, A.; Neumann, H.; Beller, M. Angew. Chem., Int. Ed. 2009, 48, 7595–7599. doi:10.1002/anie.200902148
Return to citation in text: [1]
| 49. | Anderson, K. W.; Ikawa, T.; Tundel, R. E.; Buchwald, S. L. J. Am. Chem. Soc. 2006, 128, 10694–10695. doi:10.1021/ja0639719 |
| 1. | Hartung, C. G.; Snieckus, V. The Directed ortho Metalation Reaction – A Point of Departure for New Synthetic Aromatic Chemistry. In Modern Arene Chemistry; Astruc, D., Ed.; Wiley-VCH: New York, 2002; pp 330–367. doi:10.1002/3527601767.ch10 |
| 2. | Clayden, J. Regioselective Synthesis of Organolithiums by Deprotonation. In Organolithiums: Selectivity for Synthesis; Tetrahedron Organic Chemistry Series, Vol. 23; Pergamon: Oxford, 2002; pp 9–109. |
| 3. | Whisler, M. C.; MacNeil, S.; Snieckus, V.; Beak, P. Angew. Chem., Int. Ed. 2004, 43, 2206–2225. doi:10.1002/anie.200300590 |
| 4. | Anctil, E. J.-G.; Snieckus, V. In Metal-Catalyzed Cross-Coupling Reactions, 2nd ed.; de Meijere, A.; Diederich, F., Eds.; Wiley-VCH: Weinheim, 2004; pp 761–813. |
| 5. | Macklin, T.; Snieckus, V. In Handbook of C–H Transformations; Dyker, G., Ed.; Wiley-VCH: New York, 2005; pp 106–119. |
| 10. | Kondo, Y.; Shilai, M.; Uchiyama, M.; Sakamoto, T. J. Am. Chem. Soc. 1999, 121, 3539–3540. doi:10.1021/ja984263t |
| 11. | Uchiyama, M.; Miyoshi, T.; Kajihara, Y.; Sakamoto, T.; Otani, Y.; Ohwada, T.; Kondo, Y. J. Am. Chem. Soc. 2002, 124, 8514–8515. doi:10.1021/ja0202199 |
| 12. | Uchiyama, M.; Kobayashi, Y.; Furuyama, T.; Nakamura, S.; Kajihara, Y.; Miyoshi, T.; Sakamoto, T.; Kondo, Y.; Morokuma, K. J. Am. Chem. Soc. 2008, 130, 472–480. doi:10.1021/ja071268u |
| 31. | Heravi, M. M.; Sadjadi, S. Tetrahedron 2009, 65, 7761–7775. doi:10.1016/j.tet.2009.06.028 |
| 8. | Mulvey, R. E.; Mongin, F.; Uchiyama, M.; Kondo, Y. Angew. Chem., Int. Ed. 2007, 46, 3802–3824. doi:10.1002/anie.200604369 |
| 9. | Mulvey, R. E. Acc. Chem. Res. 2009, 42, 743–755. doi:10.1021/ar800254y |
| 32. | Tsuchikama, K.; Hashimoto, Y.-k.; Endo, K.; Shibata, T. Adv. Synth. Catal. 2009, 351, 2850–2854. |
| 7. | Schlosser, M.; Choi, J. H.; Takagishi, S. Tetrahedron 1990, 46, 5633–5648. doi:10.1016/S0040-4020(01)87763-2 |
| 29. | Kim, I.; Lee, S.-H.; Lee, S. Tetrahedron Lett. 2008, 49, 6579–6584. doi:10.1016/j.tetlet.2008.09.034 |
| 6. | Eberhardt, G. G.; Butte, W. A. J. Org. Chem. 1964, 29, 2928–2932. doi:10.1021/jo01033a029 |
| 20. | De Luca, L.; Nieddu, G.; Porcheddu, A.; Giacomelli, G. Curr. Med. Chem. 2009, 16, 1–20. doi:10.2174/092986709787002826 |
| 22. | Hou, X.-L.; Yang, Z.; Yeung, K.-S.; Wong, H. N. C. Prog. Heterocycl. Chem. 2009, 21, 179–223. doi:10.1016/S0959-6380(09)70034-0 |
| 23. | Yeung, K.-S.; Yang, Z.; Peng, X.-S.; Hou, X.-L. Prog. Heterocycl. Chem. 2011, 22, 181–216. doi:10.1016/S0959-6380(11)22007-5 |
| 15. | Clegg, W.; Dale, S. H.; Drummond, A. M.; Hevia, E.; Honeyman, G. W.; Mulvey, R. E. J. Am. Chem. Soc. 2006, 128, 7434–7435. doi:10.1021/ja061898g |
| 16. | Uchiyama, M.; Matsumoto, Y.; Nobuto, D.; Furuyama, T.; Yamaguchi, K.; Morokuma, K. J. Am. Chem. Soc. 2006, 128, 8748–8750. doi:10.1021/ja060489h |
| 17. | Uchiyama, M.; Matsumoto, Y.; Usui, S.; Hashimoto, Y.; Morokuma, K. Angew. Chem., Int. Ed. 2007, 46, 926–929. doi:10.1002/anie.200602664 |
| 18. | Kondo, Y.; Morey, J. V.; Morgan, J. C.; Naka, H.; Nobuto, D.; Raithby, P. R.; Uchiyama, M.; Wheatley, A. E. H. J. Am. Chem. Soc. 2007, 129, 12734–12738. doi:10.1021/ja072118m |
| 19. | Hevia, E.; Kennedy, A. R.; Klett, J.; McCall, M. D. Chem. Commun. 2009, 3240–3242. doi:10.1039/b903592c |
| 24. | Ober, A. G.; Fronczek, F. R.; Fischer, N. H. J. Nat. Prod. 1985, 48, 242–248. |
| 25. | Parodi, F. J.; Fischer, N. H.; Flores, H. E. J. Nat. Prod. 1988, 51, 594–595. |
| 26. | Wang, L.-Q.; Zhao, Y.-X.; Hu, J. M.; Jia, A.-Q.; Zhou, J. Helv. Chim. Acta 2008, 91, 159–164. |
| 27. | Stevenson, P. C.; Veitch, N. C. Phytochemistry 1998, 48, 947–951. |
| 28. | Joshi, A. S.; Li, X.-C.; Nimrod, A. C.; ElSholy, H. N.; Walker, L. A.; Clark, A. M. Planta Med. 2001, 67, 186–188. |
| 14. | Usui, S.; Hashimoto, Y.; Morey, J. V.; Wheatley, A. E. H.; Uchiyama, M. J. Am. Chem. Soc. 2007, 129, 15102–15103. doi:10.1021/ja074669i |
| 13. | Naka, H.; Uchiyama, M.; Matsumoto, Y.; Wheatley, A. E. H.; McPartlin, M.; Morey, J. V.; Kondo, Y. J. Am. Chem. Soc. 2007, 129, 1921–1930. doi:10.1021/ja064601n |
| 21. | Hwu, J. R.; Chuang, K.-S.; Chuang, S. H.; Tsay, S.-C. Org. Lett. 2005, 7, 1545–1548. doi:10.1021/ol050196d |
| 40. | Sanz, R.; Castroviejo, M. P.; Guilarte, V.; Pérez, A.; Fañanás, F. J. J. Org. Chem. 2007, 72, 5113–5118. doi:10.1021/jo070643y |
| 33. | Alessi, M.; Larkin, A. L.; Ogilvie, K. A.; Green, L. A.; Lai, S.; López, S.; Snieckus, V. J. Org. Chem. 2007, 72, 1588–1594. doi:10.1021/jo0620359 |
| 34. | Blanchet, J.; Macklin, T.; Ang, P.; Metallinos, C.; Snieckus, V. J. Org. Chem. 2007, 72, 3199–3206. doi:10.1021/jo062385v |
| 35. | Nerdinger, S.; Kendall, C.; Cai, X.; Marchart, R.; Riebel, P.; Johnson, M. R.; Yin, C.-F.; Hénaff, N.; Eltis, L. D.; Snieckus, V. J. Org. Chem. 2007, 72, 5960–5967. doi:10.1021/jo062543i |
| 36. | MacNeil, S. L.; Gray, M.; Gusev, D. G.; Briggs, L. E.; Snieckus, V. J. Org. Chem. 2008, 73, 9710–9719. doi:10.1021/jo801856n |
| 37. | James, C. A.; Snieckus, V. J. Org. Chem. 2009, 74, 4080–4093. doi:10.1021/jo9001454 |
| 38. | James, C. A.; Coelho, A. L.; Gevaert, M.; Forgione, P.; Snieckus, V. J. Org. Chem. 2009, 74, 4094–4103. doi:10.1021/jo900146d |
| 39. | Sanz, R.; Castroviejo, M. P.; Fernández, Y.; Fañanás, F. J. J. Org. Chem. 2005, 70, 6548–6551. doi:10.1021/jo0508402 |
| 45. | Tlili, A.; Xia, N.; Monnier, F.; Taillefer, M. Angew. Chem., Int. Ed. 2009, 48, 8725–8728. doi:10.1002/anie.200903639 |
| 46. | Zhao, D.; Wu, N.; Zhang, S.; Xi, P.; Su, X.; Lan, J.; You, J. Angew. Chem., Int. Ed. 2009, 48, 8729–8732. doi:10.1002/anie.200903923 |
| 47. | Jing, L.; Wei, J.; Zhou, L.; Huang, Z.; Li, Z.; Zhou, X. Chem. Commun. 2010, 46, 4767–4769. doi:10.1039/c0cc00434k |
| 48. | Yang, D.; Fu, H. Chem.–Eur. J. 2010, 16, 2366–2370. doi:10.1002/chem.200903468 |
| 49. | Anderson, K. W.; Ikawa, T.; Tundel, R. E.; Buchwald, S. L. J. Am. Chem. Soc. 2006, 128, 10694–10695. doi:10.1021/ja0639719 |
| 50. | Schulz, T.; Torborg, C.; Schäffner, B.; Huang, J.; Zapf, A.; Kadyrov, R.; Börner, A.; Beller, M. Angew. Chem., Int. Ed. 2009, 48, 918–921. doi:10.1002/anie.200804898 |
| 51. | Sergeev, A. G.; Schulz, T.; Torborg, C.; Spannenberg, A.; Neumann, H.; Beller, M. Angew. Chem., Int. Ed. 2009, 48, 7595–7599. doi:10.1002/anie.200902148 |
| 42. | Liang, Y.; Xie, Y.-X.; Li, J.-H. J. Org. Chem. 2006, 71, 2535–2537. doi:10.1021/jo051882t |
| 43. | Sanz, R.; Guilarte, V.; García, N. Org. Biomol. Chem. 2010, 8, 3860–3864. doi:10.1039/c004360e |
| 44. | Guilarte, V.; Castroviejo, M. P.; García-García, P.; Fernández-Rodríguez, M. A.; Sanz, R. J. Org. Chem. 2011, 76, 3416–3437. doi:10.1021/jo200406f |
| 11. | Uchiyama, M.; Miyoshi, T.; Kajihara, Y.; Sakamoto, T.; Otani, Y.; Ohwada, T.; Kondo, Y. J. Am. Chem. Soc. 2002, 124, 8514–8515. doi:10.1021/ja0202199 |
| 12. | Uchiyama, M.; Kobayashi, Y.; Furuyama, T.; Nakamura, S.; Kajihara, Y.; Miyoshi, T.; Sakamoto, T.; Kondo, Y.; Morokuma, K. J. Am. Chem. Soc. 2008, 130, 472–480. doi:10.1021/ja071268u |
| 40. | Sanz, R.; Castroviejo, M. P.; Guilarte, V.; Pérez, A.; Fañanás, F. J. J. Org. Chem. 2007, 72, 5113–5118. doi:10.1021/jo070643y |
| 41. | Sanz, R.; Guilarte, V.; Hernando, E.; Sanjuán, A. M. J. Org. Chem. 2010, 75, 7443–7446. doi:10.1021/jo101436f |
| 39. | Sanz, R.; Castroviejo, M. P.; Fernández, Y.; Fañanás, F. J. J. Org. Chem. 2005, 70, 6548–6551. doi:10.1021/jo0508402 |
© 2011 Guilarte et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)