Abstract
Tin-free Giese reactions, employing primary, secondary, and tertiary alkyl iodides as radical precursors, ethyl acrylate as a radical trap, and sodium cyanoborohydride as a radical mediator, were examined in a continuous flow system. With the use of an automated flow microreactor, flow reaction conditions for the Giese reaction were quickly optimized, and it was found that a reaction temperature of 70 °C in combination with a residence time of 10–15 minutes gave good yields of the desired addition products.
Graphical Abstract
Introduction
Organo halides are among the most useful precursors to access carbon radical species, and they have found numerous applications in chemical synthesis [1-5]. Alkyl radicals are classified as nucleophilic radicals, and therefore they are able to add preferentially to alkenes possessing an electron-withdrawing substituent [6,7]. This type of reductive radical addition reaction, better known as the Giese reaction, was historically carried out most by using tributyltin hydride as the radical mediator [8,9]. Recently borane derivatives such as borohydride reagents [10-13] or NHC-boranes [14-18] can be used in simple radical C–C bond forming reactions or radical reduction as efficient substitutes for tin hydride reagents, whose toxicity is of great concern to organic chemists. Thus far we have demonstrated the borohydride-based tin-free Giese reactions [10] and the related radical carbonylation and hydroxymethylation reaction [11-13,18] employing this methodology. In Scheme 1, a general mechanism of a borohydride-based Giese reaction with the possible products is shown.
Scheme 1: Giese reaction using borohydride-based radical mediators.
Scheme 1: Giese reaction using borohydride-based radical mediators.
In recent years, microreaction technologies have made a significant impact on chemical synthesis and production in terms of their advantageous characteristics, which include efficient mixing, efficient mass and heat transfer, and high operational safety [19-23]. Radical reactions also benefit from these advantages, and we have reported both photo- [24-26] and thermally-induced [27-30] radical reactions that are facilitated by flow reaction technology [31]. In this study, we report that cyanoborohydride-based Giese reactions of primary, secondary, and tertiary iodoalkanes with ethyl acrylate can be carried out efficiently using a microflow system. Optimal conditions for each substrate were quickly determined by the use of an automated microflow reactor [32], which revealed that running the continuous flow reactions at 70 °C for 10–15 min gave good yields of Giese addition products with effective suppression of the byproducts.
Results and Discussion
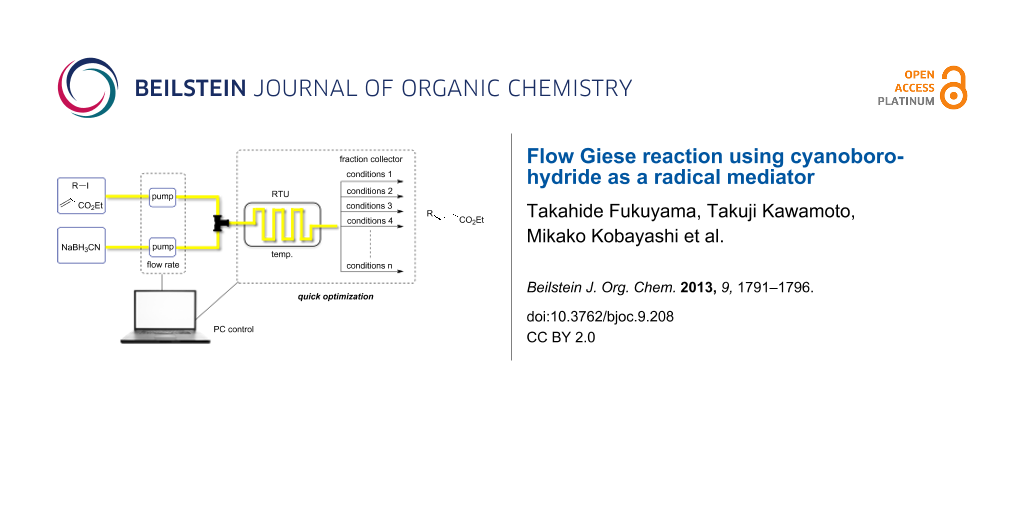
We employed an automated microflow reactor system, MiChS® System X-1 [33], equipped with a fraction collector, which allows screening of up to 20 reaction conditions in one operation through the programming of temperature and flow rates (Figure 1).
![[1860-5397-9-208-1]](/bjoc/content/figures/1860-5397-9-208-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: Pictures of the flow microreactor system (MiChS® System X-1), a micromixer (MiChS β-150, channel width: 150 μm), and a fraction collector used for this study.
Figure 1: Pictures of the flow microreactor system (MiChS® System X-1), a micromixer (MiChS β-150, channel wi...
Initially, the reaction of 1-iodooctane (1a) with ethyl acrylate in the presence of NaBH3CN (2 equiv) and 10 mol % AIBN (2,2’-azobisisobutyronitrile) was investigated. A variety of different temperatures (90–110 °C) and residence times (2–10 min) were screened. The reaction of 1a with ethyl acrylate was found to give the desired Giese reaction product 3a together with two main byproducts, octane (2a) and the 1:2 addition adduct 4a. As shown in Scheme 2, higher reaction temperatures tended to result in the formation of increased amounts of octane (2a). Under the same reaction conditions, the radical mediator Bu4NBH3CN gave similar results, whereas the reaction with Bu4NBH4 was found not to be suitable, since the competing reduction leading to 2a became the dominant product from the reaction.
Scheme 2: First screening for the reaction of 1a at different temperatures (90–110 °C) and residence times (2–10 min) in the presence of AIBN.
Scheme 2: First screening for the reaction of 1a at different temperatures (90–110 °C) and residence times (2...
To check the background hydride reduction of 1a with NaBH3CN, we treated 1a with 2 equiv of NaBH3CN at various temperatures (70–100 °C) for 10 min in the absence of a radical initiator and ethyl acrylate (Scheme 3). The reduction product 2a was not formed in large amounts and we found that its formation was effectively suppressed by lowering the temperature to 70 °C.
Scheme 3: Background reduction of 1a with NaBH3CN.
Scheme 3: Background reduction of 1a with NaBH3CN.
Setting the reaction temperature to 70 °C, we then further optimize the other reaction conditions. Consequently we found that the desired Giese product 3a could be obtained in 75% yield (Scheme 4) when the reaction was carried out with 1.6 equiv of ethyl acrylate and 3 equiv of NaBH3CN and 10 min residence time in the presence of V-65 (2,2’-azobis(2,4-dimethylvaleronitrile)) as the radical initiator, which decomposes at a lower temperature than AIBN (Figure 2). For comparison, we also carried out a batch reaction using a 20 mL test tube on 0.5 mmol scale under similar reaction conditions (70 °C (bath temp.), 10 min), which gave only 34% yield of 3a and a large amount of recovered 1a. We assume that excellent thermal efficiency inherent to tiny reaction channels would ensure efficient reaction in the microreactors.
Scheme 4: Second screening at 70 °C and residence time (5–20 min) in the presence of V-65.
Scheme 4: Second screening at 70 °C and residence time (5–20 min) in the presence of V-65.
Figure 2: Structures of V-65 and AIBN and their ten hour half-life decomposition temperature.
Figure 2: Structures of V-65 and AIBN and their ten hour half-life decomposition temperature.
We then carried out the optimization of the reaction conditions for the secondary and tertiary alkyl iodides, 2-iodooctane (1b) and 1-iodoadamantane (1c), reacting with ethyl acrylate. We were pleased to find that under similar reaction conditions (70 °C, 10–15 min) these two flow Giese reactions worked well to give the corresponding addition products 3b and 3c in 88 and 81% yield, respectively (Scheme 5). It should be noted that for these secondary and tertiary substrates, simple reduction to give octane (2b) or adamantane (2c) was hardly observed.
Scheme 5: Cyanoborohydride mediated Giese reaction of 1b and 1c with ethyl acrylate.
Scheme 5: Cyanoborohydride mediated Giese reaction of 1b and 1c with ethyl acrylate.
Conclusion
The cyanoborohydride-mediated Giese reaction of alkyl iodides 1a, 1b, and 1c with ethyl acrylate was studied in a continuous microflow reaction system. Optimized conditions with minimum formation of byproducts for the conversion of 1a to 3a were rapidly located by the use of an automated microflow system, MiChS® X-1, equipped with a static mixer having 150 μm width and an automated fraction collector. Using the optimized flow conditions (70 °C, 10–15 min), high yielding conversions of 1b to 3b and 1c to 3c were also obtained.
Supporting Information
| Supporting Information File 1: Typical experimental procedure and supplementary experimental data. | ||
| Format: PDF | Size: 257.0 KB | Download |
References
-
Renaud, P.; Sibi, M. P., Eds. Radicals in Organic Synthesis; Wiley-VCH: Weinheim, 2001.
Return to citation in text: [1] -
Togo, H., Ed. Advanced Free Radical Reactions for Organic Synthesis; Elsevier: Oxford, 2004.
Return to citation in text: [1] -
Heinrich, M. R.; Gansäuer, A., Eds. Radicals in Synthesis III; Topics Current Chemistry, Vol. 230; Springer: Berlin, 2012.
Return to citation in text: [1] -
Chatgilialoglu, C.; Studer, A., Eds. Encyclopedia of Radicals in Chemistry, Biology and Materials; Wiley: Chichester, U.K., 2012.
Return to citation in text: [1] -
Rowlands, G. J. Tetrahedron 2009, 65, 8603. doi:10.1016/j.tet.2009.07.001
Return to citation in text: [1] -
Giese, B. Angew. Chem., Int. Ed. 1983, 22, 753. doi:10.1002/anie.198307531
Return to citation in text: [1] -
Fisher, H.; Radom, L. Angew. Chem., Int. Ed. 2001, 40, 1340. doi:10.1002/1521-3773(20010417)40:8<1340::AID-ANIE1340>3.0.CO;2-#
Return to citation in text: [1] -
Giese, B.; González-Gómez, J. A.; Witzel, T. Angew. Chem., Int. Ed. 1984, 23, 69. doi:10.1002/anie.198400691
Return to citation in text: [1] -
Gerth, D. B.; Giese, B. J. Org. Chem. 1986, 51, 3726. doi:10.1021/jo00369a039
Return to citation in text: [1] -
Ryu, I.; Uehara, S.; Hirao, H.; Fukuyama, T. Org. Lett. 2008, 10, 1005. doi:10.1021/ol7031043
Return to citation in text: [1] [2] -
Kobayashi, S.; Kawamoto, T.; Uehara, S.; Fukuyama, T.; Ryu, I. Org. Lett. 2010, 12, 1548. doi:10.1021/ol1002847
Return to citation in text: [1] [2] -
Kawamoto, T.; Fukuyama, T.; Ryu, I. J. Am. Chem. Soc. 2012, 134, 875. doi:10.1021/ja210585n
Return to citation in text: [1] [2] -
Kawamoto, T.; Ryu, I. Chimia 2012, 66, 372.
Return to citation in text: [1] [2] -
Curran, D. P.; Solovyev, A.; Makhlouf Brahmi, M.; Fensterbank, L.; Malacria, M.; Lacote, E. Angew. Chem., Int. Ed. 2011, 50, 10294. doi:10.1002/anie.201102717
Return to citation in text: [1] -
Ueng, S.-H.; Fensterbank, L.; Lacôte, E.; Malacria, M.; Curran, D. P. Org. Lett. 2010, 12, 3002. doi:10.1021/ol101015m
Return to citation in text: [1] -
Ueng, S.-H.; Fensterbank, L.; Lacôte, E.; Malacria, M.; Curran, D. P. Org. Biomol. Chem. 2011, 9, 3415. doi:10.1039/c0ob01075h
Return to citation in text: [1] -
Pan, X.; Lacôte, E.; Lalevée, J.; Curran, D. P. J. Am. Chem. Soc. 2012, 134, 5669. doi:10.1021/ja300416f
Return to citation in text: [1] -
Kawamoto, T.; Okada, T.; Curran, D. P.; Ryu, I. Org. Lett. 2013, 15, 2144. doi:10.1021/ol4006294
Return to citation in text: [1] [2] -
Hessel, V.; Renken, A.; Schouten, J. C.; Yoshida, J., Eds. Micro Process Engineering; Wiley-VCH, 2009.
Return to citation in text: [1] -
Wirth, T., Ed. Microreactors in Organic Synthesis and Catalysis, 2nd ed.; Wiley-VCH: Weinheim, 2013.
Return to citation in text: [1] -
Jas, G.; Kirschning, A. Chem.–Eur. J. 2003, 9, 5708. doi:10.1002/chem.200305212
Return to citation in text: [1] -
Fukuyama, T.; Rahman, M. T.; Sato, M.; Ryu, I. Synlett 2008, 151. doi:10.1055/s-2007-1000884
Return to citation in text: [1] -
Yoshida, J.; Saito, K.; Nokami, T.; Nagaki, A. Synlett 2011, 1189. doi:10.1055/s-0030-1259946
Return to citation in text: [1] -
Sugimoto, A.; Sumino, Y.; Takagi, M.; Fukuyama, T.; Ryu, I. Tetrahedron Lett. 2006, 47, 6197. doi:10.1016/j.tetlet.2006.06.153
Return to citation in text: [1] -
Sugimoto, A.; Fukuyama, T.; Sumino, Y.; Takagi, M.; Ryu, I. Tetrahedron 2009, 65, 1593. doi:10.1016/j.tet.2008.12.063
Return to citation in text: [1] -
Matsubara, H.; Hino, Y.; Tokizane, M.; Ryu, I. Chem. Eng. J. 2011, 167, 567. doi:10.1016/j.cej.2010.08.086
Return to citation in text: [1] -
Fukuyama, T.; Kobayashi, M.; Rahman, M. T.; Kamata, N.; Ryu, I. Org. Lett. 2008, 10, 533. doi:10.1021/ol702718z
Return to citation in text: [1] -
Wienhöfer, I. C.; Studer, A.; Rahman, M. T.; Fukuyama, T.; Ryu, I. Org. Lett. 2009, 11, 2457. doi:10.1021/ol900713d
Return to citation in text: [1] -
Fukuyama, T.; Rahman, M. T.; Kamata, N.; Ryu, I. Beilstein J. Org. Chem. 2009, 5, No. 34. doi:10.3762/bjoc.5.34
Return to citation in text: [1] -
Fukuyama, T.; Kajihara, Y.; Ryu, I.; Studer, A. Synthesis 2012, 2555. doi:10.1055/s-0031-1290780
Return to citation in text: [1] -
Fukuyama, T.; Ryu, I. Radical Chemistry by Using Flow Microreactor Technology. In Encyclopedia of Radicals in Chemistry, Biology and Materials; Chatgilialoglu, C.; Studer, A., Eds.; Wiley: Chichester, U.K., 2012; Vol. 21, pp 1243–1258. doi:10.1002/9781119953678.rad035
Return to citation in text: [1] -
Sugimoto, A.; Fukuyama, T.; Rahman, M. T.; Ryu, I. Tetrahedron Lett. 2009, 50, 6364. doi:10.1016/j.tetlet.2009.08.089
Return to citation in text: [1] -
http://www.michs.jp/index_en.html.
Return to citation in text: [1]
| 1. | Renaud, P.; Sibi, M. P., Eds. Radicals in Organic Synthesis; Wiley-VCH: Weinheim, 2001. |
| 2. | Togo, H., Ed. Advanced Free Radical Reactions for Organic Synthesis; Elsevier: Oxford, 2004. |
| 3. | Heinrich, M. R.; Gansäuer, A., Eds. Radicals in Synthesis III; Topics Current Chemistry, Vol. 230; Springer: Berlin, 2012. |
| 4. | Chatgilialoglu, C.; Studer, A., Eds. Encyclopedia of Radicals in Chemistry, Biology and Materials; Wiley: Chichester, U.K., 2012. |
| 5. | Rowlands, G. J. Tetrahedron 2009, 65, 8603. doi:10.1016/j.tet.2009.07.001 |
| 14. | Curran, D. P.; Solovyev, A.; Makhlouf Brahmi, M.; Fensterbank, L.; Malacria, M.; Lacote, E. Angew. Chem., Int. Ed. 2011, 50, 10294. doi:10.1002/anie.201102717 |
| 15. | Ueng, S.-H.; Fensterbank, L.; Lacôte, E.; Malacria, M.; Curran, D. P. Org. Lett. 2010, 12, 3002. doi:10.1021/ol101015m |
| 16. | Ueng, S.-H.; Fensterbank, L.; Lacôte, E.; Malacria, M.; Curran, D. P. Org. Biomol. Chem. 2011, 9, 3415. doi:10.1039/c0ob01075h |
| 17. | Pan, X.; Lacôte, E.; Lalevée, J.; Curran, D. P. J. Am. Chem. Soc. 2012, 134, 5669. doi:10.1021/ja300416f |
| 18. | Kawamoto, T.; Okada, T.; Curran, D. P.; Ryu, I. Org. Lett. 2013, 15, 2144. doi:10.1021/ol4006294 |
| 10. | Ryu, I.; Uehara, S.; Hirao, H.; Fukuyama, T. Org. Lett. 2008, 10, 1005. doi:10.1021/ol7031043 |
| 11. | Kobayashi, S.; Kawamoto, T.; Uehara, S.; Fukuyama, T.; Ryu, I. Org. Lett. 2010, 12, 1548. doi:10.1021/ol1002847 |
| 12. | Kawamoto, T.; Fukuyama, T.; Ryu, I. J. Am. Chem. Soc. 2012, 134, 875. doi:10.1021/ja210585n |
| 13. | Kawamoto, T.; Ryu, I. Chimia 2012, 66, 372. |
| 8. | Giese, B.; González-Gómez, J. A.; Witzel, T. Angew. Chem., Int. Ed. 1984, 23, 69. doi:10.1002/anie.198400691 |
| 9. | Gerth, D. B.; Giese, B. J. Org. Chem. 1986, 51, 3726. doi:10.1021/jo00369a039 |
| 6. | Giese, B. Angew. Chem., Int. Ed. 1983, 22, 753. doi:10.1002/anie.198307531 |
| 7. | Fisher, H.; Radom, L. Angew. Chem., Int. Ed. 2001, 40, 1340. doi:10.1002/1521-3773(20010417)40:8<1340::AID-ANIE1340>3.0.CO;2-# |
| 24. | Sugimoto, A.; Sumino, Y.; Takagi, M.; Fukuyama, T.; Ryu, I. Tetrahedron Lett. 2006, 47, 6197. doi:10.1016/j.tetlet.2006.06.153 |
| 25. | Sugimoto, A.; Fukuyama, T.; Sumino, Y.; Takagi, M.; Ryu, I. Tetrahedron 2009, 65, 1593. doi:10.1016/j.tet.2008.12.063 |
| 26. | Matsubara, H.; Hino, Y.; Tokizane, M.; Ryu, I. Chem. Eng. J. 2011, 167, 567. doi:10.1016/j.cej.2010.08.086 |
| 31. | Fukuyama, T.; Ryu, I. Radical Chemistry by Using Flow Microreactor Technology. In Encyclopedia of Radicals in Chemistry, Biology and Materials; Chatgilialoglu, C.; Studer, A., Eds.; Wiley: Chichester, U.K., 2012; Vol. 21, pp 1243–1258. doi:10.1002/9781119953678.rad035 |
| 19. | Hessel, V.; Renken, A.; Schouten, J. C.; Yoshida, J., Eds. Micro Process Engineering; Wiley-VCH, 2009. |
| 20. | Wirth, T., Ed. Microreactors in Organic Synthesis and Catalysis, 2nd ed.; Wiley-VCH: Weinheim, 2013. |
| 21. | Jas, G.; Kirschning, A. Chem.–Eur. J. 2003, 9, 5708. doi:10.1002/chem.200305212 |
| 22. | Fukuyama, T.; Rahman, M. T.; Sato, M.; Ryu, I. Synlett 2008, 151. doi:10.1055/s-2007-1000884 |
| 23. | Yoshida, J.; Saito, K.; Nokami, T.; Nagaki, A. Synlett 2011, 1189. doi:10.1055/s-0030-1259946 |
| 32. | Sugimoto, A.; Fukuyama, T.; Rahman, M. T.; Ryu, I. Tetrahedron Lett. 2009, 50, 6364. doi:10.1016/j.tetlet.2009.08.089 |
| 11. | Kobayashi, S.; Kawamoto, T.; Uehara, S.; Fukuyama, T.; Ryu, I. Org. Lett. 2010, 12, 1548. doi:10.1021/ol1002847 |
| 12. | Kawamoto, T.; Fukuyama, T.; Ryu, I. J. Am. Chem. Soc. 2012, 134, 875. doi:10.1021/ja210585n |
| 13. | Kawamoto, T.; Ryu, I. Chimia 2012, 66, 372. |
| 18. | Kawamoto, T.; Okada, T.; Curran, D. P.; Ryu, I. Org. Lett. 2013, 15, 2144. doi:10.1021/ol4006294 |
| 10. | Ryu, I.; Uehara, S.; Hirao, H.; Fukuyama, T. Org. Lett. 2008, 10, 1005. doi:10.1021/ol7031043 |
| 27. | Fukuyama, T.; Kobayashi, M.; Rahman, M. T.; Kamata, N.; Ryu, I. Org. Lett. 2008, 10, 533. doi:10.1021/ol702718z |
| 28. | Wienhöfer, I. C.; Studer, A.; Rahman, M. T.; Fukuyama, T.; Ryu, I. Org. Lett. 2009, 11, 2457. doi:10.1021/ol900713d |
| 29. | Fukuyama, T.; Rahman, M. T.; Kamata, N.; Ryu, I. Beilstein J. Org. Chem. 2009, 5, No. 34. doi:10.3762/bjoc.5.34 |
| 30. | Fukuyama, T.; Kajihara, Y.; Ryu, I.; Studer, A. Synthesis 2012, 2555. doi:10.1055/s-0031-1290780 |
© 2013 Fukuyama et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)