Search results
Search for "solid phase" in Full Text gives 251 result(s) in Beilstein Journal of Organic Chemistry. Showing first 200.
Synthetic strategies of phosphonodepsipeptides
Beilstein J. Org. Chem. 2021, 17, 461–484, doi:10.3762/bjoc.17.41
- hydrogenolysis (Scheme 23) [37]. Following a similar strategy, phosphonodepsidipeptides 141 and phosphonodepsitripeptides 144 were synthesized as norleucine-derived phosphonopeptides (Scheme 24) [38]. The protected phosphonodepsipeptide 145 was applied in a solid-phase phosphonodepsipeptide synthesis. After
- phosphonodepsitripeptide with BOP as coupling reagent. Synthesis of norleucine-derived phosphonodepsipeptides 135 and 138. Synthesis of norleucine-derived phosphonodepsipeptides 141 and 144. Solid-phase synthesis of phosphonodepsipeptides. Synthesis of phosphonodepsidipeptides via the Mitsunobu reaction. Synthesis of γ
19F NMR as a tool in chemical biology
Beilstein J. Org. Chem. 2021, 17, 293–318, doi:10.3762/bjoc.17.28

- aliphatic amino acid, (E)-2-amino-5-(pentafluorosulfanyl)pent-4-enoic acid (14, Figure 1), SF5NVa [21]. Most recently, Cobb et al. [22] reported the synthesis of several pentafluorosulfanyl phenylalanine derivatives with suitable protecting groups to allow incorporation into peptides through common solid
- -phase peptide synthesis (SPPS) methods (15 and 16, Figure 1). A second well-established methodology for the 19F isotopic labelling of protein and peptides involves the post-translational chemical conjugation of an 19F probe to specific amino acids present within the protein, typically cysteine and
Supramolecular polymerization of sulfated dendritic peptide amphiphiles into multivalent L-selectin binders
Beilstein J. Org. Chem. 2021, 17, 97–104, doi:10.3762/bjoc.17.10

- intact. Commonly this heterofunctionalization is achieved using esterification of trimesic acid and subsequent partial hydrolysis, however, this route suffers from difficult purification steps [37][38]. Here, it was conveniently achieved in one step via a solid-phase supported approach, using trimesic
- yielding novel solid phase approach and will be further used for dendritic peptide amphiphile heterofunctionalization. By taking advantage of copper-catalyzed azide–alkyne cycloaddition chemistry, a highly soluble sulfated nonaphenylalanine peptide amphiphile was prepared via this route. The spectroscopic
Incorporation of a metal-mediated base pair into an ATP aptamer – using silver(I) ions to modulate aptamer function
Beilstein J. Org. Chem. 2020, 16, 2870–2879, doi:10.3762/bjoc.16.236

- quadruplex, so that metal-mediated base pairs can be introduced. 3) It is a short oligonucleotide, so the modified aptamers can easily be synthesized using automated solid-phase synthesis. 4) The target molecule of this aptamer is a small molecule, facilitating the binding assays. 5) The aptamer also binds
- phosphoramidite-protected imidazole nucleoside as required for automated DNA solid-phase synthesis was synthesized as previously reported [30]. The oligonucleotides were synthesized on a DNA/RNA synthesizer H-8 (K&A Laborgeräte) using standard protocols for automated solid-phase synthesis (coupling time: 1000 s
Changed reactivity of secondary hydroxy groups in C8-modified adenosine – lessons learned from silylation
Beilstein J. Org. Chem. 2020, 16, 2854–2861, doi:10.3762/bjoc.16.234

- synthesis strategy was re-designed, allowing the preparation of building block 9 (Scheme 2) ready for use in solid-phase RNA synthesis with excellent yield. Here, we report on the selectivity problem in 2’-O-silylation of adenosine derivative 7 (Scheme 1) and the optimized synthesis strategy for the
- building block 9 ready for use in solid-phase RNA synthesis. When starting the synthesis via this way, we were not sure, if the protected adenosine derivative 10 is a suitable substrate for iodination. The cyclic nature of the 3′,5′-O-di-tert-butylsilyl group is associated with a slight ring strain energy
On the mass spectrometric fragmentations of the bacterial sesterterpenes sestermobaraenes A–C
Beilstein J. Org. Chem. 2020, 16, 2807–2819, doi:10.3762/bjoc.16.231
- water, simple alcohols. These volatile compounds can efficiently be trapped by specialised methods including the closed-loop stripping apparatus (CLSA) [4] technique or solid-phase microextraction (SPME) [5][6], and then analysed by gas chromatography–mass spectrometry (GC–MS) [7]. Through these and
Enzyme-instructed morphological transition of the supramolecular assemblies of branched peptides
Beilstein J. Org. Chem. 2020, 16, 2709–2718, doi:10.3762/bjoc.16.221

- form the branched peptide 1. To investigate how the acetylation of aspartic acid affects the proteolysis and the formation of assemblies, the acetylation of the N-terminal of the branch in 1 would generate 2. Synthesis We used 2-chlorotrityl chloride resin for the typical Fmoc solid-phase peptide
pH- and concentration-dependent supramolecular self-assembly of a naturally occurring octapeptide
Beilstein J. Org. Chem. 2020, 16, 2017–2025, doi:10.3762/bjoc.16.168

- development of novel drug nanocarrier assemblies. Keywords: aqueous self-assembly; pH-responsive systems; secondary structure; self-assembled nanostructures; solid-phase peptide synthesis; Introduction The self-assembly of small molecules is a ubiquitous phenomenon in nature [1] and also has key
- nanostructures as well as the secondary structures. Results and Discussion Solid-phase peptide synthesis and purification The target octapeptide was synthesized in the solid phase following four steps, including: i) deprotection of the Fmoc protecting group, ii) coupling of an amino acid, iii) cleavage of the
Nonenzymatic synthesis of anomerically pure, mannosyl-based molecular probes for scramblase identification studies
Beilstein J. Org. Chem. 2020, 16, 1732–1739, doi:10.3762/bjoc.16.145
- into stereodefined, anomeric phosphoramidite derivatives. The method is based on adapted procedures developed for DNA solid-phase synthesis [17]. Phosphoramidite chemistry allows the connection of two molecular entities via a phosphodiester linkage and was found to be perfectly suited for this purpose
Rearrangement of o-(pivaloylaminomethyl)benzaldehydes: an experimental and computational study
Beilstein J. Org. Chem. 2020, 16, 1636–1648, doi:10.3762/bjoc.16.136
- ). According to the single-crystal X-ray experiments of compounds 3b, 8b, and 23a, the solid-phase structures are stabilized by a hydrogen bond between the amide NH and the carbonyl oxygen atom of the other amide group. The H–C–C–H dihedral angles between the two asymmetric centers are not far from 90° as
- possibility of a rotation of the ortho-formylphenyl group. Notably, conformer 23b1 is the more stable one, corresponding to the structure determined by solid-phase X-ray measurements (Figure 3, right). It should be mentioned that a few signals in the NMR spectra of 23b, e.g., δHC−N (5.71 dd), δHC= (131.1
Disposable cartridge concept for the on-demand synthesis of turbo Grignards, Knochel–Hauser amides, and magnesium alkoxides
Beilstein J. Org. Chem. 2020, 16, 1343–1356, doi:10.3762/bjoc.16.115

- construction, pressure tolerance, heat conduction, and diameter/particle size matching; (3) the column orientation and setup–filters, etc.; (4) activation of the solid phase. The activation issue is one of the most important factors when considering the metal packing. Although our team had success with zinc
A systematic review on silica-, carbon-, and magnetic materials-supported copper species as efficient heterogeneous nanocatalysts in “click” reactions
Beilstein J. Org. Chem. 2020, 16, 551–586, doi:10.3762/bjoc.16.52
- loss of catalytic activity. Using a multistep solid-phase procedure, Shantz et al. reported on SBA-15 functionalized with melamine-based dendrons (Scheme 3) [27]. The Cu and Cu/Au nanoparticles were then supported on this new composite to generate a nanocatalyst. The catalytic application of this
Photocontrolled DNA minor groove interactions of imidazole/pyrrole polyamides
Beilstein J. Org. Chem. 2020, 16, 60–70, doi:10.3762/bjoc.16.8
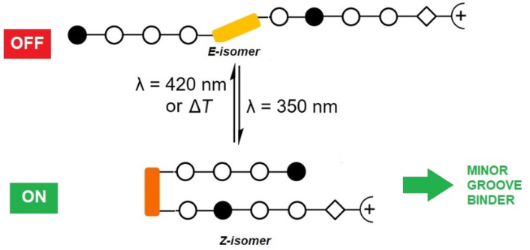
- experiments. The assembly of the photoswitchable pyrrole/imidazole polyamides (P1–P3) was performed stepwise on solid phase using the acid-labile 2-chlorotrityl resin and Fmoc as a temporary protecting group, according to the synthetic route published by the Dervan group [40]. The synthesis of the Fmoc
- -protected photoswitchable ω-amino acid 1 (Scheme 2A) was performed based on a procedure by Aemissegger et al. [35]. The ω-amino acid 1 was subsequently used in the solid-phase synthesis or for the synthesis of the dimer 7 in solution (Scheme 2B). The incorporation of β-alanine as a 'molecular spring' was
Emission and biosynthesis of volatile terpenoids from the plasmodial slime mold Physarum polycephalum
Beilstein J. Org. Chem. 2019, 15, 2872–2880, doi:10.3762/bjoc.15.281
- flakes as nutrient source and subjected to volatile profiling at two time points: 8 days and 18 days after the transfer of plasmodia to a fresh agar plate. Volatiles were collected from the headspace using a solid phase-microextraction fiber and analyzed using gas chromatography–mass spectrometry (GC–MS
- continuous darkness. Headspace collection and GC–MS analysis On the 8th day and 18th day after the transfer of plasmodia to a fresh agar plate, volatiles were collected from the headspace of plasmodia on the agar plate using solid phase microextraction (SPME) (https://www.sigmaaldrich.com). After collection
Skeletocutins M–Q: biologically active compounds from the fruiting bodies of the basidiomycete Skeletocutis sp. collected in Africa
Beilstein J. Org. Chem. 2019, 15, 2782–2789, doi:10.3762/bjoc.15.270
- . Isolation of compounds 1–6 The crude extract (vide supra) was filtered using solid-phase microextraction (SPME) with a Strata™-X 33 µm Polymeric Reversed Phase (RP) cartridge (Phenomenex). The extract was fractionated by preparative RP chromatography (RPC) using a PLC 2020 purification system (Gilson). A
Synthesis of novel sulfide-based cyclic peptidomimetic analogues to solonamides
Beilstein J. Org. Chem. 2019, 15, 2544–2551, doi:10.3762/bjoc.15.247
- have been synthesized via solid-phase peptide synthesis and SN2’ reaction on a Morita–Baylis–Hillman (MBH) residue introduced at the N-terminal of a tetrapeptide. This last step takes advantage of the electrophilic feature of the MBH residue and represents a new cyclization strategy occurring. The
In search of visible-light photoresponsive peptide nucleic acids (PNAs) for reversible control of DNA hybridization
Beilstein J. Org. Chem. 2019, 15, 2500–2508, doi:10.3762/bjoc.15.243

- improved mismatch discrimination under physiological conditions than natural ones. Furthermore, PNAs have a straightforward chemical synthesis by Fmoc-based PNA solid-phase synthesis and remarkable stability against nuclease- and protease-mediated degradation [32][33]. In regard to all these beneficial
- . Black lines controls: solid: FAM-ssDNA without PNA and under the same conditions; dashed: BHQ/FAM-dsDNA. Solid-phase synthesis of photoswitchable PNAs; Aeg = N-(2-aminoethyl)glycine, Bhoc = benzhydryloxycarbonyl. Isomerization conversions at the photostationary state (PSS). Melting temperatures (TM) of
Attempted synthesis of a meta-metalated calix[4]arene
Beilstein J. Org. Chem. 2019, 15, 1996–2002, doi:10.3762/bjoc.15.195
- resulted in applications in several fields, including, amongst others, the use as ligands in transition metal catalysis [2][3], in solid-phase gas sorption [4], as molecular sensors [5][6] and as biomimetic compounds [7][8]. Our own interest has been in the synthesis of inherently chiral calix[4]arenes
Analysis of sesquiterpene hydrocarbons in grape berry exocarp (Vitis vinifera L.) using in vivo-labeling and comprehensive two-dimensional gas chromatography–mass spectrometry (GC×GC–MS)
Beilstein J. Org. Chem. 2019, 15, 1945–1961, doi:10.3762/bjoc.15.190
- ) coupled to a time-of-flight mass spectrometer (TOF–MS) after headspace-solid phase microextraction (HS-SPME). The identification of structurally complex natural compounds, such as sesquiterpenes from fruits and vegetables, is often reported as “tentative”, as authentic standards are not commercially
- -solid phase microextraction (HS-SPME). The comprehensive two-dimensional gas chromatography first demonstrated on an oil sample in 1991 is suitable for the analysis of highly complex samples [13]. Using GC×GC, all analytes of a sample can be separated on two different capillary separation columns of
Synthesis and conformational preferences of short analogues of antifreeze glycopeptides (AFGP)
Beilstein J. Org. Chem. 2019, 15, 1581–1591, doi:10.3762/bjoc.15.162

- ., in food industry and biomedicine. Keywords: antifreeze glycopeptides; conformational preferences; NMR; PP II; solid phase synthesis; Introduction Certain species of polar and sub-polar fish created evolutionary strategies to survive in water at temperatures below the colligative freezing point
- with the aim of excluding pH-dependent charge effects and to simulate a protein environment. The introduction of a methyl amide function at the C-terminus of glycopeptides was carried out on solid phase. The N-methylation of the peptide terminus on solid support was an efficient four-step procedure
- -methylamide. The introduction of a methylamide function at the C-terminal end was performed on solid phase. In order to achieve this, the Fmoc-Sieber-PS resin was modified (Scheme 1A). This type of resin enabled cleavage of peptides under mild conditions, hence without destroying fragile O-glycosidic bond
Steroid diversification by multicomponent reactions
Beilstein J. Org. Chem. 2019, 15, 1236–1256, doi:10.3762/bjoc.15.121
- targeting steroid-fused pyrimidines, Boruah’s group developed a solid-phase MCR between 2-hydroxymethylene-3-ketosteroids, aromatic aldehydes and ammonium acetate [46]. As shown in Scheme 15, the reaction was carried out with steroid 49 and different aldehydes to furnish the set of compounds 50 in good to
- two different steroidal scaffolds [65], but it failed for the ligation of larger peptides to steroids due to the poor solubility of the former ones. In this sense, Rivera’s group introduced a solid-phase multicomponent procedure enabling the conjugation of steroids and lipids to peptides longer than
- solid-phase multicomponent methodologies, which opens a venue of possibilities for the production of more complex biomolecular conjugates. Nonetheless, there are still many MCRs that have not been implemented using steroidal substrates, therefore, there is still much to be done for an effective
Electrophilic oligodeoxynucleotide synthesis using dM-Dmoc for amino protection
Beilstein J. Org. Chem. 2019, 15, 1116–1128, doi:10.3762/bjoc.15.108
- Shahien Shahsavari Dhananjani N. A. M. Eriyagama Bhaskar Halami Vagarshak Begoyan Marina Tanasova Jinsen Chen Shiyue Fang Department of Chemistry, Michigan Technological University, 1400 Townsend Drive, Houghton, Michigan 49931, USA 10.3762/bjoc.15.108 Abstract Solid-phase synthesis of
- thioester. Using the technology, the sensitive groups can be installed at any location within the ODN sequences without using any sequence- or functionality-specific conditions and procedures. Keywords: Dmoc; electrophilic; oligonucleotides; protecting group; solid-phase synthesis; Introduction After over
- sequences were very close and in some cases even overlapped, which made HPLC purification of the products difficult. A typical RP HPLC profile of ODNs synthesized in that manner is given in Supporting Information File 2. We therefore tried to keep the 5'-DMTr group at the end of solid phase synthesis to
Synthesis of (macro)heterocycles by consecutive/repetitive isocyanide-based multicomponent reactions
Beilstein J. Org. Chem. 2019, 15, 906–930, doi:10.3762/bjoc.15.88
- and Constabel [17] who developed a solid phase strategy to obtain tetrazoles and hydantoinimide derivatives successfully (Scheme 2). In each case, the synthetic sequence began with a classical Ugi reaction between N-Fmoc glycine (1), isobutyraldehyde (2) and tert-butyl isocyanide (4) in the presence
Mechanochemistry of supramolecules
Beilstein J. Org. Chem. 2019, 15, 881–900, doi:10.3762/bjoc.15.86
- -bond formation in supramolecular chemistry which occurs in solution-phase synthesis is almost impossible in solid-state reactions. However, MacGillivray’s group demonstrated several examples of co-crystal formation or supramolecular synthesis in the solid phase through mechano-milling or dry grinding
Solid-phase synthesis of biaryl bicyclic peptides containing a 3-aryltyrosine or a 4-arylphenylalanine moiety
Beilstein J. Org. Chem. 2019, 15, 761–768, doi:10.3762/bjoc.15.72
- Iteng Ng-Choi Angel Oliveras Lidia Feliu Marta Planas LIPPSO, Departament de Química, University of Girona, Maria Aurèlia Capmany 69, Girona 17003, Spain 10.3762/bjoc.15.72 Abstract A methodology for the solid-phase synthesis of biaryl bicyclic peptides containing a Phe-Phe, a Phe-Tyr or a Tyr
- resulting biaryl monocyclic peptidyl resin leading to the formation of the expected biaryl bicyclic peptide. This study provides the first solid-phase synthesis of this type of bicyclic compounds being amenable to prepare a diversity of synthetic or natural biaryl bicyclic peptides. Keywords: borylation
- ; cross-coupling; cyclization; macrocycles; solid-phase synthesis; Introduction Monocyclic and bicyclic peptides are acquiring a relevant interest in current drug discovery. They display improved biological properties over their acyclic counterparts and, at the same time, they are suitable to modulate










































































































































































































































































































































































