Abstract
This study investigates the fabrication of BiVO4 photoanodes using a controlled-intensity current electrodeposition method to improve their photoelectrochemical (PEC) performance. The impact of varying the deposition current density and VO(acac)2 concentration was systematically analyzed to optimize the crystallinity, surface morphology, and electronic properties of the films. Subsequently, an electrochemical deposition method was developed to facilitate the uniform distribution of V2O5 among Bi–O–I flakes to homogeneously enhance the conversion reaction. The XRD pattern confirms the monoclinic scheelite BiVO4 structure with dominant (121) and (004) peaks. FESEM imaging revealed that the different deposition conditions influenced the surface morphologies of the BiOI and BiVO4 films. Photocurrent density measurements showed that BiVO4(326) achieved 1.2 mA·cm−2 at 1.23 V vs RHE, representing a significant enhancement compared to the other samples. The surface hole injection efficiency was measured to be 47%, whereas the incident photon-to-current efficiency reached a peak of 18.1% at 420 nm. The applied bias photon-to-current efficiency of BiVO4(326) was also superior to that of the samples fabricated with lower current density, highlighting the benefits of the optimized electrodeposition conditions for the former.
Introduction
In the context of the increasing global energy demand, the development of renewable and sustainable energy sources has become a top priority in science and technology [1,2]. Photoelectrochemical (PEC) water-splitting systems hold significant promises for converting abundant solar energy into chemical fuels, such as hydrogen [3,4]. However, their widespread application is still limited by material challenges, including insufficient light absorption, high electron–hole recombination rates, and poor stability under operating conditions [5,6]. Among various semiconductor materials, bismuth vanadate (BiVO4) has attracted considerable interest due to its strong visible light absorption, moderate bandgap (≈2.4 eV), high theoretical photocurrent density (≈7.5 mA·cm−2), and chemical stability in aqueous environments [7-9]. Nevertheless, BiVO4 suffers from intrinsic drawbacks such as low charge carrier mobility, limited conductivity, and rapid recombination of photogenerated charge carriers, which severely restrict its PEC performance [10-12].
Various strategies have been explored to overcome these challenges and optimize the structural, electronic, and surface properties of BiVO4 [13,14]. Hydrothermal synthesis has been used to produce highly crystalline BiVO4 films with large surface areas; for instance, Yun He et al. [15] reported flower-like BiVO4 photoanodes achieving a photocurrent density of 0.81 mA·cm−2 at 1.23 V vs RHE. However, the hydrothermal method often requires high temperatures and prolonged reaction times, and offers limited control over film thickness. Alternatively, Liu et al. [16] employed RF sputtering with a single BiVO4 target, but the volatility of Bi in a vacuum environment often led to an imbalanced Bi/V ratio, requiring precise regulation of oxygen partial pressure. Gong et al. [17] utilized DC co-sputtering of Bi and V targets to produce BiVO4 thin films at high deposition rates; however, this method resulted in irregular grain structures and significant material defects, limiting the PEC performance improvements. Electrodeposition has emerged as a promising low-cost and scalable technique for BiVO4 film fabrication, offering better control over film morphology and crystallinity under mild conditions. Kim et al. [18] reported that BiVO4 films fabricated via electrodeposition achieved a maximum photocurrent density of 1.4 mA·cm−2 at 1.23 V vs RHE. These films exhibited a three-dimensional nanoporous structure that facilitated charge carrier transport; however, their uneven porosity and high charge recombination rates hindered their PEC performance improvement. McDonald and Choi [19] introduced a facile electrodeposition method based on p-benzoquinone reduction to fabricate ultrathin BiOI films, which could be thermally converted into porous BiVO4 photoanodes. This approach yielded electrodes with enhanced PEC activity, achieving a photocurrent density of approximately 1.25 mA·cm−2 at 0.5 V vs RHE in neutral phosphate buffer under AM1.5G illumination. This study highlighted the potential of BiOI-derived BiVO4 as a template-guided route for improving water oxidation performance. However, the use of a constant-potential deposition technique presents limitations in controlling the film thickness, morphology, and uniformity, which are crucial for consistent BiVO4 performance after conversion. Variability in the BiOI film quality remains a significant challenge, affecting the reproducibility and optimization of the final photoanodes.
Building on these limitations, in this study, we introduce a novel controlled-intensity current electrodeposition method to precisely tailor the deposition conditions of BiOI and subsequently optimize its conversion to BiVO4. By systematically adjusting the deposition current density and vanadium precursor concentration, we achieved fine control over the crystallinity, grain size, porosity, and optical properties of the resulting films. This level of tunability leads to substantial improvements in PEC performance. Our method offers a higher degree of control over both the intermediate BiOI layer and the final BiVO4 structure, thereby enabling enhanced charge separation and surface reaction kinetics. Furthermore, this approach provides a deeper understanding of the relationship between the synthesis parameters and PEC activity, while presenting a scalable and reproducible route for fabricating high-performance BiVO4 photoanodes.
Experimental
Material
Bismuth nitrate pentahydrate (Bi(NO3)3·5H2O, 99.9%, Sigma-Aldrich) and vanadyl acetylacetonate (VO(acac)2, 98%, Sigma-Aldrich) were used as Bi and V sources, respectively. Potassium iodide (KI, 99.5%, Sigma-Aldrich) was used for the initial deposition of BiOI. p-Benzoquinone (C6H4O2, 98%, Sigma-Aldrich) was used as a redox mediator in the electrodeposition process. Nitric acid (HNO₃, 65%, Merck) and sodium hydroxide (NaOH, 99%, Sigma-Aldrich) were used to adjust the pH during deposition. Ethanol (C2H5OH, 99.9%, Merck) and deionized (DI) water were used for cleaning and dilution, respectively. Fluorine-doped tin oxide (FTO) glass substrates (7 Ω·sq−1, Pilkington) served as the conductive support for electrodeposition.
Fabrication of BiVO4 photoanodes
The BiVO4 film was deposited using electrochemical deposition. A solution of 0.2962 g Bi(NO3)3 dissolved in 50 mL distilled water was ultrasonicated for 30 min. Subsequently, 400 mM KI and 5% HNO3 were added to adjust the pH to 2. Additionally, 50 mM p-benzoquinone (0.2 g) was dissolved in 10 mL of ethanol via ultrasonication for 30 min and added to the solution. The FTO glass substrates were cleaned with ethanol and distilled water via sequential ultrasonication. The BiOI film was electrochemically deposited onto the FTO substrate at various current deposition intensities (14, 22, and 32 mA) using a reference electrode saturated with Ag/AgCl and platinum foil at various potentials vs Ag/AgCl. This process was adapted from the p-benzoquinone-based method reported by McDonald and Choi [19], in which benzoquinone serves as a redox mediator for BiOI formation. However, unlike the original study, which applied constant potential conditions, our method employs a variable current-controlled deposition strategy. This approach enables the fine-tuning of the nucleation and growth behavior of BiOI flakes, resulting in enhanced control over the thickness, grain structure, and uniformity, which are key factors that influence the subsequent BiVO4 conversion and PEC performance. Then, a 0.2 M VO(acac)2 solution in ethanol was coated onto the BiOI film via spin coating with two different volumes of solution (0.4 µL and 0.6 µL). The BiOI film (1 cm × 1 cm) with the VO(acac)2 layer was annealed at 450 °C for 2 h. Finally, the BiVO4 electrode was rinsed with 1 M NaOH to remove excess V2O5 from the surface, followed by rinsing with distilled water and drying at room temperature. The photoanode BiVO4 was named BiVO4(xy), where x indicates the current intensity for BiOI deposition, and y denotes the vanadium precursor volume (x = 14, 22, 32; y = 4, 6).
Note on BiVO4(144) sample exclusion
The BiVO4(144) sample was excluded from the detailed photoelectrochemical (PEC) and comparative analyses because of its poor film uniformity and significantly lower performance metrics. Preliminary characterizations showed that the film exhibited inhomogeneous coverage and an inconsistent PEC response, which could lead to misleading interpretations when comparing the material trends. Therefore, these samples were not included in subsequent analyses to maintain the clarity and consistency of the dataset.
Characteristics of materials
X-ray diffraction (XRD, Bruker D8 Advance) and Raman spectroscopy (LabRAM Odyssey Semiconductor) were used to analyze the crystal structures of photoanodes. UV–vis absorption spectra were obtained using a Cary 60 spectrophotometer. X-ray photoelectron spectroscopy (XPS, VG ESCALAB250) was employed to determine the chemical states of each photoanode. All photoanode morphologies were examined using field-emission transmission electron microscopy (FESEM, Hitachi SU8010).
Photoelectrochemical measurements
PEC experiments were performed in a conventional three-electrode cell using an electrochemical workstation (CHI650E, CH Instruments, USA). The three electrodes included a working electrode (BiVO4 photoelectrode, 1.0 × 1.0 cm2), counter electrode (Pt plate), and reference electrode (Ag/AgCl). The electrolyte used in the photoelectrochemical measurements was 0.50 M Na2SO4 (pH 5.6), and the xenon lamp was 300 W (PLS-SXE 300C, 100 mW·cm−2) equipped with an AM1.5G filter to simulate solar light conditions. Linear sweep voltammetry (LSV) measurements were performed by scanning the potential from −0.6 to 1.2 V (vs Ag/AgCl), at a scan rate of 0.05 V·s−1. In the LSV test, the light source illuminated the sample from the back of the FTO glass. Under AM1.5G illumination, electrochemical impedance spectroscopy (EIS) measurements were performed at an open-circuit voltage, covering a frequency spectrum from 1 Hz to 10 kHz. Mott–Schottky curves were recorded at a frequency of 1 kHz in a dark light.
Applied bias photo to current efficiency
The applied bias photon-to-current efficiencies (ABPEs) of the different photoanodes were determined using [20]:
where Jp is the photocurrent density (mA·cm−2) obtained from the LSV curve, Io is the incident light intensity of the solar simulator (100 mW·cm−2), and is the standard reversible potential for the water-splitting reaction (1.23 V).
Incident photon-to-current conversion efficiency
From the excitation at wavelengths of 300–900 nm at 0.5 V, the incident photon-to-current conversion efficiency (IPCE) was evaluated using a chopped monochromator with a 150 W Xe lamp as the simulated light source (developed by HS Technologies, Korea).
where Plight is the power density of monochromatic light acquired at a given wavelength, and J is the photocurrent density (mA·cm−2) under illumination at a wavelength (mW·cm−2).
Results and Discussion
Structural analysis (XRD)
X-ray diffraction (XRD) measurements were conducted to investigate the crystal structures of the BiVO4 photoanodes under various deposition conditions (BiVO4(146), BiVO4(224), BiVO4(226), BiVO4(324), and BiVO4(326)), as shown Figure 1. The diffraction peaks of all photoanodes matched those of monoclinic BiVO4 (JCPDS PDF #14-0688) and fluorine-doped tin oxide (FTO) substrate (JCPDS PDF #46-1088) [21-23]. The peaks at approximately 28.9°, 30.6°, 34.6°, and 35.2° were assigned to the (110), (121), (040), (200), and (002) planes of monoclinic BiVO4, respectively. Notably, the (121) plane exhibited the highest intensity across all samples, which aligns with its high refractive index and superior photocatalytic properties owing to the enhanced adsorption and deionization of water molecules in the structure. Additionally, the (040) peak intensity exhibited a systematic increase at higher electrodeposition current densities, suggesting preferential growth along the crystallographic direction. Higher deposition currents influence ion migration rates and nucleation kinetics, potentially leading to a preferential orientation along the (040) plane. The intensity and sharpness of the peaks increased with increasing current density and VO(acac)2 concentration, indicating improved crystallinity and potentially larger grain sizes of the films. This variation in crystallographic properties is expected to influence PEC performance by affecting charge transport and surface reaction kinetics. The average crystallite size (grain size) of the BiVO4 films was estimated using the Scherrer equation based on the full width at half maximum (FWHM) of the (121) diffraction peak.
![[2190-4286-16-94-1]](/bjnano/content/figures/2190-4286-16-94-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: (a) XRD patterns of BiVO4(146), BiVO4(224), BiVO4(226), BiVO4(324), and BiVO4(326) photoanodes.
Figure 1: (a) XRD patterns of BiVO4(146), BiVO4(224), BiVO4(226), BiVO4(324), and BiVO4(326) photoanodes.
The Scherrer equation to calculate average crystallite size (grain size) [24,25] is
where λ is the X-ray wavelength, θ is the Bragg angle in radians, β is the full width at half maximum of the peak in radians, D is the particle size, and k is a constant with a value of 0.9.
As shown in Table 1, an increase in the deposition current and VO(acac)2 concentration led to narrower peak widths and larger crystallite sizes, indicating an improved crystalline quality. BiVO4(326) exhibited the largest average crystallite size (≈40 nm), consistent with the enhanced PEC performance and reduced lattice strain observed in the Raman analysis.
Table 1: Estimated crystallite sizes of BiVO4 photoanodes (from the Scherrer equation).
| Sample | 2θ– (121) Peak | FWHM (β) (°) | Crystallite size D (nm) | Estimated particle size (FESEM) (nm) | Notes |
| BiVO4(146) | 28.76 | 0.32 | 25.1 | ≈400–500 | large, irregular particles |
| BiVO4(224) | 28.82 | 0.29 | 27.6 | ≈300–400 | rougher morphology |
| BiVO4(226) | 28.88 | 0.26 | 30.8 | ≈250–350 | more uniform particles |
| BiVO4 (324) | 28.94 | 0.23 | 34.9 | ≈300–450 | larger, loosely packed |
| BiVO4(326) | 28.99 | 0.20 | 40.1 | ≈200–300 | densely packed, porous |
Morphological characterization (FESEM)
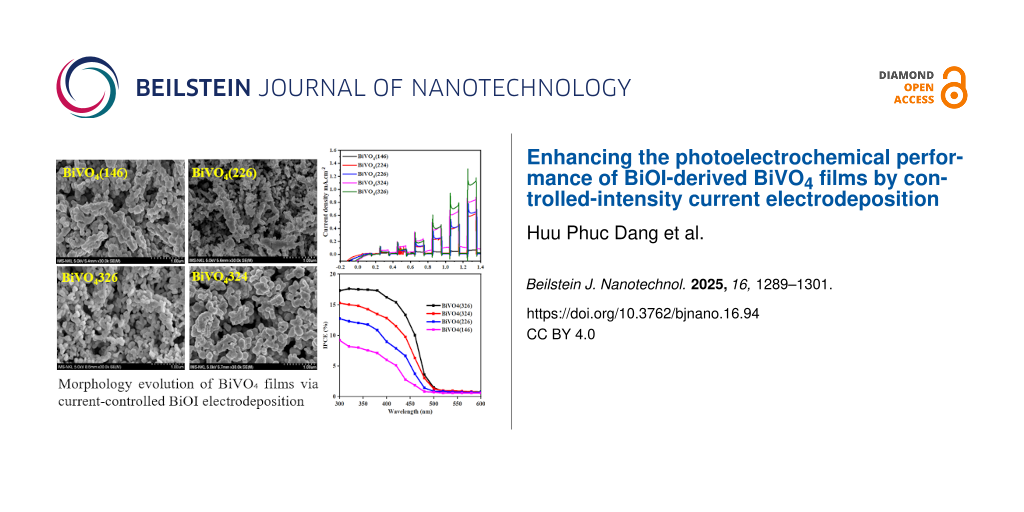
Field-emission scanning electron microscopy (FESEM) images highlighted the evolution of the surface morphology under different fabrication conditions. The transition from two-dimensional plate-like BiOI crystals to three-dimensional BiVO4 particles was accompanied by the formation of submicrometer-scale voids, indicative of grain growth and recrystallization during the annealing process (Figure 2a,c,e). At lower current densities (e.g., BiVO4(146)), the BiVO4 films exhibited larger and more irregular particles with relatively low surface coverage (Figure 2b). In contrast, higher current densities (e.g., BiVO4(324) and BiVO4(326)) resulted in more uniform and closely packed particles, along with visible submicrometer-scale voids (Figure 2f,g). This morphology maximizes the active surface area available for photoelectrochemical reactions. Additionally, the observed submicrometer voids, which were more prominent in films deposited with larger VO(acac)2 volumes, suggested improved charge separation and transport pathways. These voids facilitate the diffusion of the reactants and products, reducing the recombination rates and enhancing the water-splitting efficiency. The finer particle sizes and increased porosity, as observed for BiVO4(326), align with the optical and Raman results, highlighting the impact of the optimized deposition parameters on PEC performance. The FESEM images also revealed that the films prepared at higher current densities exhibited well-defined grain boundaries, which correlated with the reduced lattice strain observed in the Raman spectra. These structural features are critical for improving the electronic and catalytic properties of BiVO4 photoanodes.
![[2190-4286-16-94-2]](/bjnano/content/figures/2190-4286-16-94-2.jpg?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: FESEM images of (a), (c), and (e) BiOI film under various intensities of current deposition, and (b) BiVO4(146), (d) BiVO4(226), (f) BiVO4(326), and (g) BiVO4(324) films.
Figure 2: FESEM images of (a), (c), and (e) BiOI film under various intensities of current deposition, and (b...
To complement the XRD-derived crystallite sizes, the particle sizes were estimated from the FESEM images. As shown in Table 1, the particle size trends do not perfectly follow the crystallite size evolution. For instance, BiVO4(326) exhibits the largest crystallite size (≈40 nm) but has smaller surface particle aggregates (200–300 nm) than BiVO4(324). This mismatch arises because the particles observed via SEM are often composed of multiple crystalline grains. This distinction suggests that while the crystallite size governs the internal crystalline quality and carrier mobility, the particle size affects the surface area and interface kinetics. Ideally, a material such as BiVO4(326) with both large crystallites and small, porous particles offers superior PEC performance owing to improved charge transport and enhanced surface reaction sites.
Optical properties (UV–vis)
UV–vis absorption spectroscopy (Figure 3) showed that BiVO4 samples absorb visible light, with absorption edges between 502 and 541 nm and optical bandgaps between 2.46 and 2.30 eV (Figure 3b). Bandgap values were determined using Tauc plots for indirect allowed transitions, based on (αhν)2 ∝ (hν – Eg), where α is the absorption coefficient, h is Planck’s constant, ν is the frequency, and Eg is the bandgap energy. The (αhν)2 values plotted against the photon energy determined Eg at the absorption edge intersection, as shown Figure 3b. Samples prepared with higher electrodeposition currents and larger VO(acac)2 amounts exhibited redshifted absorption edges, indicating enhanced light harvesting due to improved crystallinity and reduced disorder. XRD and Raman spectroscopy confirmed these improvements through stronger peaks, suggesting fewer defects. The decrease in the bandgap (≈0.16 eV) is consistent with research linking oxygen vacancies to band tailing in BiVO4 films [26]. Besides, Figure 3a shows that the BiVO4(326) and BiVO4(324) samples have absorption that goes beyond 520 nm, with some absorption still measurable up to about 650 nm. This sub-bandgap absorption occurs because of the creation of mid-gap states, mainly caused by missing oxygen atoms and structural issues that arise during high-current electrodeposition or when using higher amounts of the VO(acac)2 precursor. The redshifted tails indicate that there are special energy states in the material that allow it to absorb light even at energies lower than those normally expected. The BiVO4(326) sample, in particular, exhibited the most pronounced tailing, consistent with its enhanced photoelectrochemical performance. The evidence suggests an optimal concentration of oxygen vacancies that broadens light absorption while avoiding excessive recombination of the charge carriers. In contrast, BiVO4(146) has a clear absorption edge and very little tailing, indicating that it has fewer defects but does not absorb light well beyond 520 nm. These findings match other studies that connect oxygen vacancies to the spread of light absorption and smaller optical bandgaps in BiVO4 [26].
![[2190-4286-16-94-3]](/bjnano/content/figures/2190-4286-16-94-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: (a) UV–vis spectrum, (b) bandgap energies of BiVO4(146), BiVO4(224), BiVO4(226), BiVO4(324), and BiVO4(326) photoanodes. Minor fluctuations above 500 nm are due to light scattering from porous films.
Figure 3: (a) UV–vis spectrum, (b) bandgap energies of BiVO4(146), BiVO4(224), BiVO4(226), BiVO4(324), and Bi...
Vibrational properties (Raman)
The Raman spectra (Figure 4) corroborated the XRD findings, displaying characteristic peaks of monoclinic BiVO4 at 219, 329, 370, 712, and 830 cm−1 [27]. These bands correspond to the vibrational modes associated with the VO4− tetrahedral structure, which directly affects the electronic properties of the material [28]. The band at 830 cm−1, assigned to the symmetric stretching of the V–O bond [29], appears in samples prepared at higher current densities and larger VO(acac)₂ volumes. This observation suggests that improved crystallinity enhances the structural uniformity of the VO4− tetrahedra, leading to more efficient charge-transfer pathways. Furthermore, the appearance of sharper and more intense Raman peaks with increasing deposition parameters indicates a reduction in the structural defects and lattice strain. The vibrational modes at 329 cm−1 and 370 cm−1, corresponding to the asymmetric and symmetric deformations of the V–O bond [30,31], respectively, showed a strong correlation with XRD-derived grain size variations. Larger grains typically result in fewer grain boundaries, reducing phonon scattering and enhancing vibrational coherence. Raman spectra also provide insights into the influence of fabrication conditions on surface chemistry. The relative intensities of the peaks suggest that higher deposition currents promote the formation of active crystal facets, which are critical for PEC performance. These results align with the enhanced photoelectrochemical activity observed for BiVO4(326), as the improved vibrational characteristics reflect more efficient light absorption and charge separation.
![[2190-4286-16-94-4]](/bjnano/content/figures/2190-4286-16-94-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: Raman spectrum of BiVO4(146), BiVO4(224), BiVO4(226), BiVO4(324), and BiVO4(326) photoanodes.
Figure 4: Raman spectrum of BiVO4(146), BiVO4(224), BiVO4(226), BiVO4(324), and BiVO4(326) photoanodes.
Photoelectrochemical performance
Linear sweep voltammetry (LSV) was used to evaluate the water-splitting efficiency of the photoelectrochemical (PEC) system in a 0.50 M Na₂SO4 solution (pH 5.6) under AM1.5G illumination (100 mW·cm−2). The optimal PEC performance was achieved for the BiVO4(326) sample, synthesized using a current intensity of 32 mA and VO(acac)2 precursor volume of 0.6 µL. As shown in Figure 5a, this sample exhibited the highest photocurrent density of 1.2 mA·cm−2 at 1.23 V vs RHE, indicating efficient photoelectrochemical activity. This superior performance can be attributed to the combined effects of enhanced crystallinity, film morphology, and preferential crystal orientation. As shown in the XRD patterns (Figure 1), BiVO4(326) displays the most intense (121) and (040) diffraction peaks among all the samples, suggesting preferred growth along these planes, which are known to facilitate efficient charge separation and transport. Previous studies have reported that the (121), (040), and (010) facets of monoclinic BiVO4 contribute to enhanced photocatalytic activity by serving as active sites for oxidation and reduction reactions [32,33]. In particular, the large surface area of the (010) facet is associated with the effective suppression of charge recombination. Although other samples also exhibited these orientations, the relatively higher (121)/(040) intensity ratio and sharper peaks for BiVO4(326) indicate improved structural ordering, which, together with its more porous and interconnected morphology (Figure 2), likely promotes better carrier mobility and PEC efficiency.
![[2190-4286-16-94-5]](/bjnano/content/figures/2190-4286-16-94-5.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: (a) LSV curves, (b) ABPE curves, (c) hole injection efficiency (ηsurface) curves, and (d) EIS plots for each photoanode.
Figure 5: (a) LSV curves, (b) ABPE curves, (c) hole injection efficiency (ηsurface) curves, and (d) EIS plots...
The ABPE curves (Figure 5b) demonstrate that BiVO4(326) achieved the highest efficiency of 0.4% at 0.8 V vs RHE, compared to the lower efficiencies of the other samples. This improvement is attributed to the synergistic effects of enhanced light absorption, reduced charge recombination, and increased charge transport efficiency, which are facilitated by the optimized fabrication parameters.
To investigate the influence of different chemical components on charge separation within the BiVO4 bulk and surface charge transfer, LSV experiments were conducted in a 0.50 M Na2SO4 (pH 5.6) solution with 1 M Na2SO3 as a hole scavenger (Figure 5c). The hole injection efficiency was calculated as the ratio of the photocurrent density values obtained with and without the Na2SO3 hole scavenger. The hole injection efficiency of the BiVO4(326) photoanode at 1.23 V vs RHE was 47%, which was higher than that under other conditions. This indicates that the formation of films with larger surface areas reduces the charge recombination rate and facilitates faster charge transfer rates.
AC impedance measurements were performed on the photoanodes to assess their charge transfer capabilities. As shown in Figure 5d, BiVO4(326) exhibited a smaller impedance arc under illumination, indicating a minimal charge-transfer resistance. This is favorable for the rapid utilization of photogenerated holes in water oxidation reactions. Overall, these results demonstrate that the optimized BiVO4(326) configuration provides improved photocatalytic activity by enhancing charge transport, reducing recombination, and maximizing the active surface area for efficient PEC water splitting.
The LSV results revealed that the photoanodes fabricated at higher current densities and larger VO(acac)2 volumes (e.g., BiVO4(326)) exhibited the highest photocurrent density at 1.23 V vs RHE, reaching 1.2 mA·cm−2. This is nearly 4.9 times higher than that of BiVO4(146). The enhanced photocurrent density correlated with the increased crystallinity, optimized grain size, and improved surface area observed in the XRD, Raman, and FESEM analyses, which collectively improved charge separation and transport. The chopped illumination data (Figure 6a) confirmed stable photoresponses with minimal decay over time, indicating the high photostability of the optimized photoanodes.
![[2190-4286-16-94-6]](/bjnano/content/figures/2190-4286-16-94-6.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 6: (a) LSV curves under chopped illumination, (b) IPCE curves for each photoanode, and (c) chronoamperometry curve of BiVO4(326) measured at 1.23 V vs RHE under continuous AM1.5G illumination in 0.50 M Na2SO4 for 600 min.
Figure 6: (a) LSV curves under chopped illumination, (b) IPCE curves for each photoanode, and (c) chronoamper...
The IPCE measurements (Figure 6b) over the wavelength range of 300–500 nm showed that BiVO4(326) exhibited a peak IPCE value of 18.1%, which was significantly higher than the 8.9% of BiVO4(146). This increase is consistent with the improved optical absorption and structural properties, as well as the reduced electron–hole recombination rates observed in the Raman and UV–vis analyses.
The long-term photoelectrochemical stability of the optimized BiVO4(326) photoanode was evaluated using chronoamperometry under continuous AM1.5G illumination at 1.23 V vs RHE in 0.50 M Na2SO4 electrolyte (Figure 6c). The photocurrent density initially peaked at approximately 1.6 mA·cm−2 and stabilized quickly at ≈1.2 mA·cm−2, maintaining this value consistently for 600 min (10 h) of operation. This stable behavior demonstrates the excellent PEC durability of the BiVO4(326) photoanode and confirms the robustness of its structural and surface properties under prolonged operational conditions. The stability is attributed to the optimized crystallinity, reduced recombination, and uniform morphology achieved through controlled-intensity current electrodeposition.
The PEC performance of BiVO4(326) was benchmarked against similar studies employing hydrothermal synthesis, direct electrodeposition, and BiOI-derived conversion methods. As summarized in Table 2, our sample achieved a photocurrent density of 1.2 mA·cm−2 at 1.23 V vs RHE, which is comparable to or better than the values reported for other BiVO4-based photoanodes. Notably, this performance was achieved under mild synthesis conditions and was supported by excellent long-term stability (600 min), which many previous reports do not provide. The enhanced performance in our study can be attributed to the optimized grain structure, controlled crystallinity, and superior film uniformity achieved via the controlled-intensity current electrodeposition of BiOI.
Table 2: Summary of recent single-layer BiVO4 photoanodes and their PEC performance.
| Study | Method | Photocurrent density (mA/cm2) | Stability (duration) | Notes | Ref |
| McDonald and Choi, (2012) | BiOI-derived BiVO4 (simple electrodeposition) | ≈1.25 @ 0.5 V | not reported | BiOI precursor, no stability test | [19] |
| Kim et al. (2014) | electrodeposition of BiVO4 | ≈1.4 @ 1.23 V | not reported | nanoporous BiVO4 | [18] |
| Yun He et al. (2017) | hydrothermal BiVO4 | ≈0.81 @ 1.23 V | few minutes | flower-like BiVO4, high recombination | [15] |
| Mohamed et al. (2021) | electrodeposition (needle-like nanoflower) | ≈0.32 | not reported | petal-like morphology, 7 min ED time | [21] |
| Fuentes-Camargo et al. (2020) | pulse plating of Bi, then conversion to BiVO4 | ≈0.35 | stable and reusable | focused on pollutant degradation under Xe lamp | [34] |
| Pelissari et al. (2021) | SILAR (5 cycles), annealed | 1.95 | not reported | highly optimized multilayer thin film | [29] |
| Qiuhang Lu et al. (2022) | RF magnetron sputtering (BiVO4 target) | ≈2.1 | not reported | precise thickness control, but lower crystallinity | [35] |
| this work | controlled-intensity BiOI → BiVO4 (electrodeposition) | 1.2 | 600 min stable | tuned crystallinity, porosity, and facet orientation | |
Surface chemical composition (XPS)
X-ray photoelectron spectroscopy (XPS) was employed to investigate the surface chemical states and composition of the BiVO4 photoanodes, focusing on the optimized BiVO4(326) sample. The survey spectrum (Figure 7a) confirmed the presence of Bi, V, and O, which is consistent with the BiVO4 structure. High-resolution Bi 4f spectra (Figure 7b) show a characteristic doublet at binding energies of 159.0 eV (Bi 4f7/2) and 164.2 eV (Bi 4f5/2), corresponding to Bi3+ in the monoclinic phase of BiVO4 [36,37]. The V 2p region (Figure 7d) exhibits peaks at 516.2 eV (V 2p3/2) and 524.1 eV (V 2p1/2), indicating the presence of V5+ species associated with the VO43− tetrahedra [38,39]. The Bi 4f and V 2p spectra remained unchanged after testing, confirming chemical and structural stability of the electrode during long-term PEC operation. The O 1s spectrum (Figure 7c) was deconvoluted into two main peaks: the dominant peak at 530.1 eV is attributed to lattice oxygen (O2−), while the broader shoulder around 531.8 eV corresponds to surface hydroxy groups and oxygen vacancies [40-42]. Notably, post-PEC testing revealed an increase in the intensity of the oxygen vacancy-related component, suggesting the formation or activation of surface defects during prolonged photoelectrochemical operation. These oxygen vacancies are known to play a crucial role in enhancing charge separation and facilitating surface water oxidation reactions, thereby contributing to improved catalytic performance [43]. The post-stability XPS analysis also showed that BiVO4(326) was chemically stable and that oxygen vacancies helped maintain its long-term photoelectrochemical activity.
![[2190-4286-16-94-7]](/bjnano/content/figures/2190-4286-16-94-7.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 7: X-ray photoelectron spectroscopy (XPS) analysis of the BiVO4(326) photoanode before and after PEC stability testing in 30 min (0.5 M Na2SO4). (a) XPS view scans of survey spectrum and high-resolution spectra of (b) Bi 4f, (c) O 1s, and (d) V 2p.
Figure 7: X-ray photoelectron spectroscopy (XPS) analysis of the BiVO4(326) photoanode before and after PEC s...
Conclusion
This study demonstrates the successful synthesis of high-performance BiVO4 photoanodes through controlled-intensity current electrodeposition, emphasizing the critical role of fabrication conditions on the structural, optical, and photoelectrochemical properties. XRD and Raman analyses confirmed the enhanced crystallinity and reduced lattice strain in the samples prepared under higher current densities and greater VO(acac)2 volumes, which correlated with improved charge transport and reduced recombination losses. UV–vis absorption spectroscopy and FESEM imaging revealed that the optimized conditions led to better light-harvesting capabilities and enhanced surface area owing to finer particle morphologies and increased porosity of the photocatalysts. XPS analysis highlighted the presence of oxygen vacancies and well-defined chemical states, further contributing to the improved catalytic activity and charge separation. Photochemical measurements demonstrated that the BiVO4(326) sample achieved the highest photocurrent density of 1.2 mA·cm−2 at 1.23 V vs RHE, a surface hole injection efficiency of 47%, and a peak IPCE of 18.1%, outperforming the other samples. These results highlight the synergistic effects of an improved crystalline structure, optimized morphology, and enhanced electronic properties. This study provides a comprehensive understanding of the interplay between fabrication parameters and PEC performance, paving the way for efficient BiVO-based photoanodes for solar-driven water-splitting applications.
Data Availability Statement
Data generated and analyzed during this study is available from the corresponding author upon reasonable request.
References
-
Haji Yassin, S. N.; Sim, A. S. L.; Jennings, J. R. Nano Mater. Sci. 2020, 2, 227–234. doi:10.1016/j.nanoms.2019.10.003
Return to citation in text: [1] -
Chong, M. N.; Jin, B.; Chow, C. W. K.; Saint, C. Water Res. 2010, 44, 2997–3027. doi:10.1016/j.watres.2010.02.039
Return to citation in text: [1] -
Galán-González, A.; Sivan, A. K.; Hernández-Ferrer, J.; Bowen, L.; Di Mario, L.; Martelli, F.; Benito, A. M.; Maser, W. K.; Chaudhry, M. U.; Gallant, A.; Zeze, D. A.; Atkinson, D. ACS Appl. Nano Mater. 2020, 3, 7781–7788. doi:10.1021/acsanm.0c01325
Return to citation in text: [1] -
Kim, K.; Moon, J. H. ACS Appl. Mater. Interfaces 2018, 10, 34238–34244. doi:10.1021/acsami.8b11241
Return to citation in text: [1] -
Han, H.; Choi, H.; Mhin, S.; Hong, Y.-R.; Kim, K. M.; Kwon, J.; Ali, G.; Chung, K. Y.; Je, M.; Umh, H. N.; Lim, D.-H.; Davey, K.; Qiao, S.-Z.; Paik, U.; Song, T. Energy Environ. Sci. 2019, 12, 2443–2454. doi:10.1039/c9ee00950g
Return to citation in text: [1] -
Eidsvåg, H.; Bentouba, S.; Vajeeston, P.; Yohi, S.; Velauthapillai, D. Molecules 2021, 26, 1687. doi:10.3390/molecules26061687
Return to citation in text: [1] -
Hajra, P.; Kundu, S.; Maity, A.; Bhattacharya, C. Chem. Eng. J. 2019, 374, 1221–1230. doi:10.1016/j.cej.2019.06.014
Return to citation in text: [1] -
Wang, J.; Ma, Q.; Wei, Y.; Guo, Y.; Li, H.; Song, H.; Sun, W.; Li, X. Mol. Catal. 2024, 561, 114154. doi:10.1016/j.mcat.2024.114154
Return to citation in text: [1] -
Tian, K.; Jin, L.; Mahmood, A.; Yang, H.; An, P.; Zhang, J.; Ji, Y.; Li, Y.; Li, D.; Liu, S.; Yan, J. Adv. Funct. Mater. 2024, 34, 2410548. doi:10.1002/adfm.202410548
Return to citation in text: [1] -
Trześniewski, B. J.; Smith, W. A. J. Mater. Chem. A 2016, 4, 2919–2926. doi:10.1039/c5ta04716a
Return to citation in text: [1] -
Wang, S.; He, T.; Yun, J.-H.; Hu, Y.; Xiao, M.; Du, A.; Wang, L. Adv. Funct. Mater. 2018, 28, 1802685. doi:10.1002/adfm.201802685
Return to citation in text: [1] -
Kang, B.; Bilal Hussain, M.; Cheng, X.; Peng, C.; Wang, Z. J. Colloid Interface Sci. 2022, 626, 146–155. doi:10.1016/j.jcis.2022.06.095
Return to citation in text: [1] -
Seabold, J. A.; Choi, K.-S. J. Am. Chem. Soc. 2012, 134, 2186–2192. doi:10.1021/ja209001d
Return to citation in text: [1] -
Smilyk, V. O.; Fomanyuk, S. S.; Kolbasov, G. Y.; Rusetskyi, I. A.; Vorobets, V. S. Res. Chem. Intermed. 2019, 45, 4149–4161. doi:10.1007/s11164-019-03897-y
Return to citation in text: [1] -
He, Y.; Li, L.; Fan, W.; Zhang, C.; Leung, M. K. H. J. Alloys Compd. 2018, 732, 593–602. doi:10.1016/j.jallcom.2017.10.153
Return to citation in text: [1] [2] -
Liu, J.; Tajima, K.; Abdellaoui, I.; Islam, M. M.; Ikeda, S.; Sakurai, T. Energies (Basel, Switz.) 2021, 14, 2122. doi:10.3390/en14082122
Return to citation in text: [1] -
Gong, H.; Freudenberg, N.; Nie, M.; van de Krol, R.; Ellmer, K. AIP Adv. 2016, 6, 045108. doi:10.1063/1.4947121
Return to citation in text: [1] -
Kim, T. W.; Choi, K.-S. Science 2014, 343, 990–994. doi:10.1126/science.1246913
Return to citation in text: [1] [2] -
McDonald, K. J.; Choi, K.-S. Energy Environ. Sci. 2012, 5, 8553. doi:10.1039/c2ee22608a
Return to citation in text: [1] [2] [3] -
Dolai, S.; Maiti, P.; Ghorai, A.; Bhunia, R.; Paul, P. K.; Ghosh, D. ACS Appl. Mater. Interfaces 2021, 13, 438–448. doi:10.1021/acsami.0c16972
Return to citation in text: [1] -
Mohamed, N. A.; Arzaee, N. A.; Mohamad Noh, M. F.; Ismail, A. F.; Safaei, J.; Sagu, J. S.; Johan, M. R.; Mat Teridi, M. A. Ceram. Int. 2021, 47, 24227–24239. doi:10.1016/j.ceramint.2021.05.134
Return to citation in text: [1] [2] -
Li, Y.-l.; Liu, Y.; Hao, Y.-j.; Wang, X.-j.; Liu, R.-h.; Li, F.-t. Mater. Des. 2020, 187, 108379. doi:10.1016/j.matdes.2019.108379
Return to citation in text: [1] -
Senasu, T.; Youngme, S.; Hemavibool, K.; Nanan, S. J. Solid State Chem. 2021, 297, 122088. doi:10.1016/j.jssc.2021.122088
Return to citation in text: [1] -
Sundaresan, A.; Bhargavi, R.; Rangarajan, N.; Siddesh, U.; Rao, C. N. R. Phys. Rev. B 2006, 74, 161306. doi:10.1103/physrevb.74.161306
Return to citation in text: [1] -
Arda, L. J. Magn. Magn. Mater. 2019, 475, 493–501. doi:10.1016/j.jmmm.2018.11.121
Return to citation in text: [1] -
Zafar, Z.; Yi, S.; Li, J.; Li, C.; Zhu, Y.; Zada, A.; Yao, W.; Liu, Z.; Yue, X. Energy Environ. Mater. 2022, 5, 68–114. doi:10.1002/eem2.12171
Return to citation in text: [1] [2] -
Lopes, O. F.; Carvalho, K. T. G.; Macedo, G. K.; de Mendonça, V. R.; Avansi, W.; Ribeiro, C. New J. Chem. 2015, 39, 6231–6237. doi:10.1039/c5nj00984g
Return to citation in text: [1] -
Peng, Z.; Su, Y.; Ennaji, I.; Khojastehnezhad, A.; Siaj, M. Chem. Eng. J. 2023, 477, 147082. doi:10.1016/j.cej.2023.147082
Return to citation in text: [1] -
Pelissari, M. R. d. S.; Azevedo Neto, N. F.; Camargo, L. P.; Dall’Antonia, L. H. Electrocatalysis 2021, 12, 211–224. doi:10.1007/s12678-021-00641-2
Return to citation in text: [1] [2] -
Gao, Y.; Zhao, W.; Tian, Z.; Zhang, L.; Teng, Z.; Li, N.; Ge, L. ACS Appl. Energy Mater. 2023, 6, 4342–4353. doi:10.1021/acsaem.3c00326
Return to citation in text: [1] -
Yang, J.; Tao, Z.; Zhao, Q.; Li, J.; Liu, G. J. Alloys Compd. 2025, 1010, 177461. doi:10.1016/j.jallcom.2024.177461
Return to citation in text: [1] -
Chen, S.; Huang, D.; Xu, P.; Gong, X.; Xue, W.; Lei, L.; Deng, R.; Li, J.; Li, Z. ACS Catal. 2020, 10, 1024–1059. doi:10.1021/acscatal.9b03411
Return to citation in text: [1] -
Tan, H. L.; Tahini, H. A.; Wen, X.; Wong, R. J.; Tan, X.; Iwase, A.; Kudo, A.; Amal, R.; Smith, S. C.; Ng, Y. H. Small 2016, 12, 5295–5302. doi:10.1002/smll.201601536
Return to citation in text: [1] -
Fuentes-Camargo, I.; Carrera-Crespo, J. E.; Vazquez-Arenas, J.; Romero-Ibarra, I.; Rodríguez, J. L.; Lartundo-Rojas, L.; Cardoso-Martínez, J. J. Phys. Chem. C 2020, 124, 1421–1428. doi:10.1021/acs.jpcc.9b09898
Return to citation in text: [1] -
Lu, Q.; Ding, L.; Li, J.; Wang, N.; Ji, M.; Wang, N.; Chang, K. Int. J. Hydrogen Energy 2024, 71, 1142–1150. doi:10.1016/j.ijhydene.2024.04.168
Return to citation in text: [1] -
Radzi, A. A. S. M.; Safaei, J.; Teridi, M. A. M. Nano-Struct. Nano-Objects 2019, 18, 100274. doi:10.1016/j.nanoso.2019.100274
Return to citation in text: [1] -
Bai, W.; Zhou, Y.; Peng, G.; Wang, J.; Li, A.; Corvini, P. F.-X. Appl. Catal., B 2022, 315, 121606. doi:10.1016/j.apcatb.2022.121606
Return to citation in text: [1] -
Mohamed, N. A.; Safaei, J.; Ismail, A. F.; Khalid, M. N.; Mohd Jailani, M. F. A.; Noh, M. F. M.; Arzaee, N. A.; Zhou, D.; Sagu, J. S.; Teridi, M. A. M. Mater. Res. Bull. 2020, 125, 110779. doi:10.1016/j.materresbull.2020.110779
Return to citation in text: [1] -
Guan, Y.; Gu, X.; Deng, Q.; Wang, S.; Li, Z.; Yan, S.; Zou, Z. Inorg. Chem. 2023, 62, 16919–16931. doi:10.1021/acs.inorgchem.3c02622
Return to citation in text: [1] -
Xu, H.; Fan, W.; Zhao, Y.; Chen, B.; Gao, Y.; Chen, X.; Xu, D.; Shi, W. Chem. Eng. J. 2021, 411, 128480. doi:10.1016/j.cej.2021.128480
Return to citation in text: [1] -
Wang, Y.; Zhang, J.; Balogun, M.-S.; Tong, Y.; Huang, Y. Mater. Today Sustainability 2022, 18, 100118. doi:10.1016/j.mtsust.2022.100118
Return to citation in text: [1] -
Wu, J.-M.; Chen, Y.; Pan, L.; Wang, P.; Cui, Y.; Kong, D.; Wang, L.; Zhang, X.; Zou, J.-J. Appl. Catal., B 2018, 221, 187–195. doi:10.1016/j.apcatb.2017.09.031
Return to citation in text: [1] -
Fernández-Climent, R.; Giménez, S.; García-Tecedor, M. Sustainable Energy Fuels 2020, 4, 5916–5926. doi:10.1039/d0se01305f
Return to citation in text: [1]
| 29. | Pelissari, M. R. d. S.; Azevedo Neto, N. F.; Camargo, L. P.; Dall’Antonia, L. H. Electrocatalysis 2021, 12, 211–224. doi:10.1007/s12678-021-00641-2 |
| 35. | Lu, Q.; Ding, L.; Li, J.; Wang, N.; Ji, M.; Wang, N.; Chang, K. Int. J. Hydrogen Energy 2024, 71, 1142–1150. doi:10.1016/j.ijhydene.2024.04.168 |
| 36. | Radzi, A. A. S. M.; Safaei, J.; Teridi, M. A. M. Nano-Struct. Nano-Objects 2019, 18, 100274. doi:10.1016/j.nanoso.2019.100274 |
| 37. | Bai, W.; Zhou, Y.; Peng, G.; Wang, J.; Li, A.; Corvini, P. F.-X. Appl. Catal., B 2022, 315, 121606. doi:10.1016/j.apcatb.2022.121606 |
| 1. | Haji Yassin, S. N.; Sim, A. S. L.; Jennings, J. R. Nano Mater. Sci. 2020, 2, 227–234. doi:10.1016/j.nanoms.2019.10.003 |
| 2. | Chong, M. N.; Jin, B.; Chow, C. W. K.; Saint, C. Water Res. 2010, 44, 2997–3027. doi:10.1016/j.watres.2010.02.039 |
| 10. | Trześniewski, B. J.; Smith, W. A. J. Mater. Chem. A 2016, 4, 2919–2926. doi:10.1039/c5ta04716a |
| 11. | Wang, S.; He, T.; Yun, J.-H.; Hu, Y.; Xiao, M.; Du, A.; Wang, L. Adv. Funct. Mater. 2018, 28, 1802685. doi:10.1002/adfm.201802685 |
| 12. | Kang, B.; Bilal Hussain, M.; Cheng, X.; Peng, C.; Wang, Z. J. Colloid Interface Sci. 2022, 626, 146–155. doi:10.1016/j.jcis.2022.06.095 |
| 24. | Sundaresan, A.; Bhargavi, R.; Rangarajan, N.; Siddesh, U.; Rao, C. N. R. Phys. Rev. B 2006, 74, 161306. doi:10.1103/physrevb.74.161306 |
| 25. | Arda, L. J. Magn. Magn. Mater. 2019, 475, 493–501. doi:10.1016/j.jmmm.2018.11.121 |
| 7. | Hajra, P.; Kundu, S.; Maity, A.; Bhattacharya, C. Chem. Eng. J. 2019, 374, 1221–1230. doi:10.1016/j.cej.2019.06.014 |
| 8. | Wang, J.; Ma, Q.; Wei, Y.; Guo, Y.; Li, H.; Song, H.; Sun, W.; Li, X. Mol. Catal. 2024, 561, 114154. doi:10.1016/j.mcat.2024.114154 |
| 9. | Tian, K.; Jin, L.; Mahmood, A.; Yang, H.; An, P.; Zhang, J.; Ji, Y.; Li, Y.; Li, D.; Liu, S.; Yan, J. Adv. Funct. Mater. 2024, 34, 2410548. doi:10.1002/adfm.202410548 |
| 26. | Zafar, Z.; Yi, S.; Li, J.; Li, C.; Zhu, Y.; Zada, A.; Yao, W.; Liu, Z.; Yue, X. Energy Environ. Mater. 2022, 5, 68–114. doi:10.1002/eem2.12171 |
| 5. | Han, H.; Choi, H.; Mhin, S.; Hong, Y.-R.; Kim, K. M.; Kwon, J.; Ali, G.; Chung, K. Y.; Je, M.; Umh, H. N.; Lim, D.-H.; Davey, K.; Qiao, S.-Z.; Paik, U.; Song, T. Energy Environ. Sci. 2019, 12, 2443–2454. doi:10.1039/c9ee00950g |
| 6. | Eidsvåg, H.; Bentouba, S.; Vajeeston, P.; Yohi, S.; Velauthapillai, D. Molecules 2021, 26, 1687. doi:10.3390/molecules26061687 |
| 20. | Dolai, S.; Maiti, P.; Ghorai, A.; Bhunia, R.; Paul, P. K.; Ghosh, D. ACS Appl. Mater. Interfaces 2021, 13, 438–448. doi:10.1021/acsami.0c16972 |
| 3. | Galán-González, A.; Sivan, A. K.; Hernández-Ferrer, J.; Bowen, L.; Di Mario, L.; Martelli, F.; Benito, A. M.; Maser, W. K.; Chaudhry, M. U.; Gallant, A.; Zeze, D. A.; Atkinson, D. ACS Appl. Nano Mater. 2020, 3, 7781–7788. doi:10.1021/acsanm.0c01325 |
| 4. | Kim, K.; Moon, J. H. ACS Appl. Mater. Interfaces 2018, 10, 34238–34244. doi:10.1021/acsami.8b11241 |
| 21. | Mohamed, N. A.; Arzaee, N. A.; Mohamad Noh, M. F.; Ismail, A. F.; Safaei, J.; Sagu, J. S.; Johan, M. R.; Mat Teridi, M. A. Ceram. Int. 2021, 47, 24227–24239. doi:10.1016/j.ceramint.2021.05.134 |
| 22. | Li, Y.-l.; Liu, Y.; Hao, Y.-j.; Wang, X.-j.; Liu, R.-h.; Li, F.-t. Mater. Des. 2020, 187, 108379. doi:10.1016/j.matdes.2019.108379 |
| 23. | Senasu, T.; Youngme, S.; Hemavibool, K.; Nanan, S. J. Solid State Chem. 2021, 297, 122088. doi:10.1016/j.jssc.2021.122088 |
| 17. | Gong, H.; Freudenberg, N.; Nie, M.; van de Krol, R.; Ellmer, K. AIP Adv. 2016, 6, 045108. doi:10.1063/1.4947121 |
| 19. | McDonald, K. J.; Choi, K.-S. Energy Environ. Sci. 2012, 5, 8553. doi:10.1039/c2ee22608a |
| 43. | Fernández-Climent, R.; Giménez, S.; García-Tecedor, M. Sustainable Energy Fuels 2020, 4, 5916–5926. doi:10.1039/d0se01305f |
| 16. | Liu, J.; Tajima, K.; Abdellaoui, I.; Islam, M. M.; Ikeda, S.; Sakurai, T. Energies (Basel, Switz.) 2021, 14, 2122. doi:10.3390/en14082122 |
| 19. | McDonald, K. J.; Choi, K.-S. Energy Environ. Sci. 2012, 5, 8553. doi:10.1039/c2ee22608a |
| 15. | He, Y.; Li, L.; Fan, W.; Zhang, C.; Leung, M. K. H. J. Alloys Compd. 2018, 732, 593–602. doi:10.1016/j.jallcom.2017.10.153 |
| 38. | Mohamed, N. A.; Safaei, J.; Ismail, A. F.; Khalid, M. N.; Mohd Jailani, M. F. A.; Noh, M. F. M.; Arzaee, N. A.; Zhou, D.; Sagu, J. S.; Teridi, M. A. M. Mater. Res. Bull. 2020, 125, 110779. doi:10.1016/j.materresbull.2020.110779 |
| 39. | Guan, Y.; Gu, X.; Deng, Q.; Wang, S.; Li, Z.; Yan, S.; Zou, Z. Inorg. Chem. 2023, 62, 16919–16931. doi:10.1021/acs.inorgchem.3c02622 |
| 13. | Seabold, J. A.; Choi, K.-S. J. Am. Chem. Soc. 2012, 134, 2186–2192. doi:10.1021/ja209001d |
| 14. | Smilyk, V. O.; Fomanyuk, S. S.; Kolbasov, G. Y.; Rusetskyi, I. A.; Vorobets, V. S. Res. Chem. Intermed. 2019, 45, 4149–4161. doi:10.1007/s11164-019-03897-y |
| 18. | Kim, T. W.; Choi, K.-S. Science 2014, 343, 990–994. doi:10.1126/science.1246913 |
| 40. | Xu, H.; Fan, W.; Zhao, Y.; Chen, B.; Gao, Y.; Chen, X.; Xu, D.; Shi, W. Chem. Eng. J. 2021, 411, 128480. doi:10.1016/j.cej.2021.128480 |
| 41. | Wang, Y.; Zhang, J.; Balogun, M.-S.; Tong, Y.; Huang, Y. Mater. Today Sustainability 2022, 18, 100118. doi:10.1016/j.mtsust.2022.100118 |
| 42. | Wu, J.-M.; Chen, Y.; Pan, L.; Wang, P.; Cui, Y.; Kong, D.; Wang, L.; Zhang, X.; Zou, J.-J. Appl. Catal., B 2018, 221, 187–195. doi:10.1016/j.apcatb.2017.09.031 |
| 28. | Peng, Z.; Su, Y.; Ennaji, I.; Khojastehnezhad, A.; Siaj, M. Chem. Eng. J. 2023, 477, 147082. doi:10.1016/j.cej.2023.147082 |
| 26. | Zafar, Z.; Yi, S.; Li, J.; Li, C.; Zhu, Y.; Zada, A.; Yao, W.; Liu, Z.; Yue, X. Energy Environ. Mater. 2022, 5, 68–114. doi:10.1002/eem2.12171 |
| 27. | Lopes, O. F.; Carvalho, K. T. G.; Macedo, G. K.; de Mendonça, V. R.; Avansi, W.; Ribeiro, C. New J. Chem. 2015, 39, 6231–6237. doi:10.1039/c5nj00984g |
| 21. | Mohamed, N. A.; Arzaee, N. A.; Mohamad Noh, M. F.; Ismail, A. F.; Safaei, J.; Sagu, J. S.; Johan, M. R.; Mat Teridi, M. A. Ceram. Int. 2021, 47, 24227–24239. doi:10.1016/j.ceramint.2021.05.134 |
| 34. | Fuentes-Camargo, I.; Carrera-Crespo, J. E.; Vazquez-Arenas, J.; Romero-Ibarra, I.; Rodríguez, J. L.; Lartundo-Rojas, L.; Cardoso-Martínez, J. J. Phys. Chem. C 2020, 124, 1421–1428. doi:10.1021/acs.jpcc.9b09898 |
| 18. | Kim, T. W.; Choi, K.-S. Science 2014, 343, 990–994. doi:10.1126/science.1246913 |
| 15. | He, Y.; Li, L.; Fan, W.; Zhang, C.; Leung, M. K. H. J. Alloys Compd. 2018, 732, 593–602. doi:10.1016/j.jallcom.2017.10.153 |
| 32. | Chen, S.; Huang, D.; Xu, P.; Gong, X.; Xue, W.; Lei, L.; Deng, R.; Li, J.; Li, Z. ACS Catal. 2020, 10, 1024–1059. doi:10.1021/acscatal.9b03411 |
| 33. | Tan, H. L.; Tahini, H. A.; Wen, X.; Wong, R. J.; Tan, X.; Iwase, A.; Kudo, A.; Amal, R.; Smith, S. C.; Ng, Y. H. Small 2016, 12, 5295–5302. doi:10.1002/smll.201601536 |
| 19. | McDonald, K. J.; Choi, K.-S. Energy Environ. Sci. 2012, 5, 8553. doi:10.1039/c2ee22608a |
| 29. | Pelissari, M. R. d. S.; Azevedo Neto, N. F.; Camargo, L. P.; Dall’Antonia, L. H. Electrocatalysis 2021, 12, 211–224. doi:10.1007/s12678-021-00641-2 |
| 30. | Gao, Y.; Zhao, W.; Tian, Z.; Zhang, L.; Teng, Z.; Li, N.; Ge, L. ACS Appl. Energy Mater. 2023, 6, 4342–4353. doi:10.1021/acsaem.3c00326 |
| 31. | Yang, J.; Tao, Z.; Zhao, Q.; Li, J.; Liu, G. J. Alloys Compd. 2025, 1010, 177461. doi:10.1016/j.jallcom.2024.177461 |
© 2025 Dang et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjnano/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.