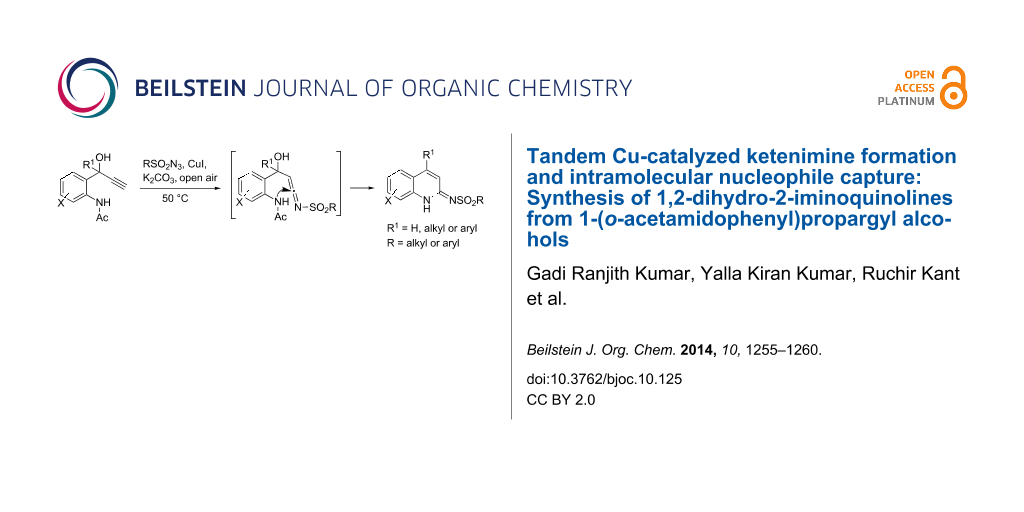
Abstract
The copper-catalyzed ketenimine formation reaction of 1-(o-acetamidophenyl)propargyl alcohols with various sulfonyl azides is found to undergo a concomitant intramolecular nucleophile attack to generate 1,2-dihydro-2-iminoquinolines after aromatization (via elimination of acetyl and hydroxy groups) and tautomerization. The reaction produces 4-substituted and 3,4-unsubstituted title compounds in moderate to good yields under mild reaction conditions.
Graphical Abstract
Introduction
The synthesis of N-sulfonylketenimines via CuAAC (copper-catalyzed azide–alkyne cycloaddition) between terminal alkynes and sulfonyl azides has staged for a conceptually novel class of approaches for the synthesis of various nitrogen-containing cyclic and acyclic motifs of pharmaceutical and material interests [1-23]. After its discovery by Chang and co-workers [1-6], various groups (including the discoverers) have utilized the reaction for the synthesis of diverse chemical compounds by trapping the thus formed ketenimine inter- and intramolecularly. Various multicomponent reactions using external nucleophiles like H2O, alcohols, amines, imines, etc., have been reported while very few recent methods [16-23], including ours [16], disclosed the utilization of internal nucleophiles for trapping the ketenimines successfully. In continuation of our interest in the functionalization of alkynes through electrophilic activation [24-29], we herein report a synthesis of 1,2-dihydro-2-iminoquinolines (tauromerized form of 2-sulfonylaminoquinolines) from 1-(o-acetamidophenyl)propargyl alcohols via copper-catalyzed ketenimine formation with various sulfonyl azides.
2-Aminoquinolines and their derivatives have been found to be important constituents in pharmaceutical chemistry [30-42] and also found to play a crucial role in molecular recognition processes [43-46]. Due to its vast-spread importance, several methods have been developed to construct this useful framework. Apart from the classical Chichibabin reaction [47], there has been considerable interest shown for the synthesis of these compounds with varying substitution patterns [48-64]. Most of the methods used presynthesized quinolines for the 2-amination via either creating initially a leaving group and its subsequent substitution or via activation of the nitrogen atom and direct amination at C-2 while few methods afforded the direct 2-aminoquinoline synthesis from the acyclic precursors.
Most recently, we have disclosed a method for the synthesis of E-α,β-unsaturated amides from propargyl acetates via copper catalyzed ketenimine formation with sulfonyl azides (Scheme 1) [16]. The reaction was thought to proceed through intramolecular nucleophilic attack on the in situ generated ketenimine. We became curious to know the fate of the reaction in the presence of another internal nucleophile in the same distance (6-membered) as a competitor.
Scheme 1: Synthesis of unsaturated amides via ketenimine formation.
Scheme 1: Synthesis of unsaturated amides via ketenimine formation.
Results and Discussion
Thus, we synthesized a substrate 3 (from 1 via 2a) through Grignard reaction followed by acetylation (Scheme 2). Substrate 3 contains two nucleophiles in the form of acetyl and acetamide groups at equal distance. When we treated 3 with similar reaction conditions (TsN3, 10 mol % CuI, 1.2 equiv K2CO3, 0.1 M CH3CN) as used in the previous method, surprisingly, only α,β-unsaturated amide 5 was obtained as a single product through acetyl migration on the intermediate 4. No trace of product through acetamide attack was obtained, which was probably due to the weaker nucleophilic nature of the latter nucleophile. We then wanted to test the method on 2a which contains a hydroxy group as a nucleophile for a relatively strained 4-membered attack and the acetamide group for the 6-membered attack. In our earlier study, the hydroxy group was found to be a less effective nucleophile compared to the acetyl group in such a reaction, as the earlier requires higher energy for its strained 4-membered cyclization. In light of this, we speculated that the acetamide group might have more chances for the cyclization in the tentative intermediate 6. As expected, the reaction on 2a proceeded through a 6-membered cyclization to form 7 which underwent aromatization via elimination of acetic acid to produce 8. Product 8 exists in tautomerized form of 9 (vide infra, X-ray structure of 9j). In fact, an elegant synthesis of such compounds was earlier achieved by Wang et al. through three component coupling reaction under similar conditions [13]. The method was shown to be not working for the synthesis of 4-unsubstituted adducts as the 2-aminobenzaldehyde was found to be unreactive in the given conditions and, moreover, the reaction is not possible with acetylene as the alkyne partner to produce 3,4-unsubstituted adducts. We herein report the intramolecular version of Wang's protocol for the synthesis of differently substituted products.
Scheme 2: Intramolecular nucleophilic capture by ketenimines.
Scheme 2: Intramolecular nucleophilic capture by ketenimines.
To explore the practicality of the above reaction, a series of starting materials were selected with the variation in substitution on propargyl alcohols as well as sulfonyl azides. It was found that the cascade process was applicable for a wide range of substrates providing the dihydroquinoline derivatives 9 in yields ranging from 36% to 77% (Scheme 3). Initially, we screened 2-propargyl alcohols prepared from aminobenzaldehydes as they were unreactive in Wang's protocol. Thus, 2a was treated with various sulfonyl azides. Trifluoromethanesulfonyl azides reacted similar to toluenesulfonyl azide to afford the products 9b and 9c in 71% and 55% yields. The lower yield in case of 9c can be attributed to the steric hindrance created by the bulky triflruoromethyl group at the ortho position. Benzene- and methanesulfonyl azides produced the corresponding products (9d and 9e) in somewhat lower yields (46% and 36%, respectively). Fluorinated 2o-propargyl alcohol 2b was then reacted with various aryl sulfonyl azides to get the corresponding products 9f–i in yields ranging from 45–50%.
Scheme 3: Synthesis of 1,2-dihydro-2-tosyliminoquinolines. Reaction conditions: Sulfonyl azide (1.2 mmol), 2 (1.0 mmol), base (1.5 mmol), CuI (0.1 mmol), DCE (5 mL, 0.2 M solution), 50 °C, open air. aIsolated yield.
Scheme 3: Synthesis of 1,2-dihydro-2-tosyliminoquinolines. Reaction conditions: Sulfonyl azide (1.2 mmol), 2 ...
Next, 3o-propargyl alcohols prepared from aminoacetophenones and aminobenzophenones were subjected to the title reaction for the synthesis of 4-methyl/phenyl-1,2-dihydro-2-iminoquinolines. It was found that these 3o-propargyl alcohols were more productive compared to the above 2o-propargyl alcohols. Thus 2c–f were subjected to the standard reaction conditions with various sulfonyl azides to get the corresponding 4-alkyl-substituted quinoline derivatives 9j-o in 48–77% yields. In further exploration, 4-phenyl-substituted adducts 9p and 9q were synthesized from 2g and 2h in 61% and 72% yields, respectively. Halogen groups (chloro and fluoro) on the core part as well as the pendant phenyl ring survived well in the reaction to produce the products (9r–t) in yields ranging from 55% to 69%. Electron rich phenyl substrate 2l produced the corresponding product 9u in 65% yield while electron poor substrate 2m was found to be unreactive in the reaction.
The structures of the products were confirmed by 1H NMR, 13C NMR and High Resolution Mass Spectrometry (HRMS). X-ray crystallography of one of the compounds, 9j, gave unambiguous structure confirmation (Figure 1). See also Supporting Information File 1, pages S49–S57 and Supporting Information File 2.
![[1860-5397-10-125-1]](/bjoc/content/figures/1860-5397-10-125-1.png?scale=2.5&max-width=1024&background=FFFFFF)
Figure 1: X-ray Structure of 9j (CCDC number 971729).
Figure 1: X-ray Structure of 9j (CCDC number 971729).
Conclusion
We have described a method for the synthesis of 1,2-dihydro-2-iminoquinolines, tautomerized products of 2-tosylaminoquinolines, from 1-(o-acetamidophenyl)propargyl alcohols through copper-catalyzed ketenimine formation with various sulfonyl azides and concomitant intramolecular nucleophilic attack. The method is overall an intramolecular version of the Wang's protocol for the synthesis of differently substituted 1,2-dihydro-2-tosyliminoquinolines which cannot be synthesized by the latter method. The reaction was performed under mild conditions to obtain the products in good yields and with a broad spectrum of substitution patterns.
Experimental
General information: All reagents and solvents were purchased from commercial sources and used without purification. NMR spectra were recorded with a 300 or 400 MHz spectrometer for 1H NMR, 75 or 100 MHz for 13C NMR spectroscopy. Chemical shifts are reported relative to tetramethylsilane in CDCl3 or to residual signals of undeuterasted solvent CDCl3/DMSO-d6 for 1H and 13C NMR spectroscopy. Multiplicities are reported as follows: singlet (s), doublet (d), broad singlet (bs), doublet of doublets (dd), doublet of triplets (dt), triplet (t), quartet (q), multiplet (m). HRMS spectra were recorded by using a QTof mass spectrometer. Column chromatography was performed with silica gel (100–200 mesh) as the stationary phase. All reactions were monitored by using TLC. The purity and characterization of compounds were further established by using HRMS.
General procedure for the synthesis of 9 from 2 taking synthesis of 9a as an example: To the substrate 2a (190 mg, 1 mmol) dissolved in dichloroethane (5 mL) was added tosyl azide (236 mg, 1.2 mmol), copper iodide (19 mg, 0.1 mmol), and K2CO3 (207 mg, 1.5 mmol). The mixture was stirred at 50 °C for 12 h. The reaction mixture was then added water (5 mL) followed by brine solution (5 mL) and extracted with ethyl acetate (3 × 10 mL). The combined extracts were dried over Na2SO4, filtered and concentrated. The crude solid product was purified by column chromatography (silicagel, 20–30% EtOAc in hexanes) to get the pure product 9 (180 mg, 62% yield).
Acknowledgements
GRK and YKK thank CSIR for the PhD fellowships. We thank SAIF division CSIR-CDRI for the analytical support. We thank Dr. Tejender S. Thakur of Molecular and Structural Biology Division, CSIR-Central Drug Research Institute for supervising the X-ray data collection and structure determination of 9j reported in this paper. We gratefully acknowledge the financial support by CSIR-Network project 'BSC0102' (CSIR-CDRI-THUNDER) and by DST under the 'Fast Track Proposal Scheme for Young Scientists'. CDRI Communication No. 8678.
References
-
Kim, S. H.; Park, S. H.; Choi, J. H.; Chang, S. Chem.–Asian J. 2011, 6, 2618–2634. doi:10.1002/asia.201100340
Return to citation in text: [1] [2] -
Yoo, E. J.; Chang, S. Curr. Org. Chem. 2009, 13, 1766–1776. doi:10.2174/138527209789630497
Return to citation in text: [1] [2] -
Yoo, E. J.; Park, S. H.; Lee, S. H.; Chang, S. Org. Lett. 2009, 11, 1155–1158. doi:10.1021/ol900023t
Return to citation in text: [1] [2] -
Cho, S. H.; Chang, S. Angew. Chem., Int. Ed. 2007, 46, 1897–1900. doi:10.1002/anie.200604358
Return to citation in text: [1] [2] -
Kim, J.; Lee, S. Y.; Lee, J.; Do, Y.; Chang, S. J. Org. Chem. 2008, 73, 9454–9457. doi:10.1021/jo802014g
Return to citation in text: [1] [2] -
Bae, I.; Han, H.; Chang, S. J. Am. Chem. Soc. 2005, 127, 2038–2039. doi:10.1021/ja0432968
Return to citation in text: [1] [2] -
Sun, L.; Zhu, Y.; Lu, P.; Wang, Y. Org. Lett. 2013, 15, 5894–5897. doi:10.1021/ol402996x
Return to citation in text: [1] -
Xing, Y.; Zhao, H.; Shang, Q.; Wang, J.; Lu, P.; Wang, Y. Org. Lett. 2013, 15, 2668–2671. doi:10.1021/ol4010323
Return to citation in text: [1] -
Zhou, F.; Liu, X.; Zhang, N.; Liang, Y.; Zhang, R.; Xin, X.; Dong, D. Org. Lett. 2013, 15, 5786–5789. doi:10.1021/ol4028368
Return to citation in text: [1] -
Jiang, Z.; Lu, P.; Wang, Y. Org. Lett. 2012, 14, 6266–6269. doi:10.1021/ol303023y
Return to citation in text: [1] -
Lu, P.; Wang, Y. Chem. Soc. Rev. 2012, 41, 5687–5705. doi:10.1039/c2cs35159e
Return to citation in text: [1] -
Lu, P.; Wang, Y. Synlett 2010, 165–173. doi:10.1055/s-0029-1218558
Return to citation in text: [1] -
Cui, S.-L.; Wang, J.; Wang, Y.-G. Tetrahedron 2008, 64, 487–495. doi:10.1016/j.tet.2007.11.025
Return to citation in text: [1] [2] -
Yao, W.; Pan, L.; Zhang, Y.; Wang, G.; Wang, X.; Ma, C. Angew. Chem., Int. Ed. 2010, 49, 9210–9214. doi:10.1002/anie.201004685
Return to citation in text: [1] -
Cassidy, M. P.; Raushel, J.; Fokin, V. V. Angew. Chem., Int. Ed. 2006, 45, 3154–3157. doi:10.1002/anie.200503805
Return to citation in text: [1] -
Kumar, Y. K.; Kumar, G. R.; Reddy, M. S. J. Org. Chem. 2014, 79, 823–828. doi:10.1021/jo402570t
Return to citation in text: [1] [2] [3] [4] -
Chauhan, D. P.; Varma, S. J.; Vijeta, A.; Banerjee, P.; Talukdar, P. Chem. Commun. 2014, 50, 323–325. doi:10.1039/c3cc47182a
Return to citation in text: [1] [2] -
Wang, J.; Wang, J.; Lu, P.; Wang, Y. J. Org. Chem. 2013, 78, 8816–8820. doi:10.1021/jo401094j
Return to citation in text: [1] [2] -
Cheng, G.; Cui, X. Org. Lett. 2013, 15, 1480–1483. doi:10.1021/ol400219n
Return to citation in text: [1] [2] -
Nagaraj, M.; Boominathan, M.; Perumal, D.; Muthusubramanian, S.; Bhuvanesh, N. J. Org. Chem. 2012, 77, 6319–6326. doi:10.1021/jo300855f
Return to citation in text: [1] [2] -
Li, S.; Luo, Y.; Wu, J. Org. Lett. 2011, 13, 3190–3193. doi:10.1021/ol2011067
Return to citation in text: [1] [2] -
Yoo, E. J.; Chang, S. Org. Lett. 2008, 10, 1163–1166. doi:10.1021/ol800049b
Return to citation in text: [1] [2] -
Chen, Z.; Zheng, D.; Wu, J. Org. Lett. 2011, 13, 848–851. doi:10.1021/ol102775s
Return to citation in text: [1] [2] -
Reddy, M. S.; Thirupathi, N.; Babu, M. H.; Puri, S. J. Org. Chem. 2013, 78, 5878–5888. doi:10.1021/jo400499r
Return to citation in text: [1] -
Reddy, M. S.; Kumar, Y. K.; Thirupathi, N. Org. Lett. 2012, 14, 824–827. doi:10.1021/ol2033493
Return to citation in text: [1] -
Reddy, M. S.; Thirupathi, N.; Babu, M. H. Eur. J. Org. Chem. 2012, 5803–5809. doi:10.1002/ejoc.201200782
Return to citation in text: [1] -
Reddy, M. S.; Thirupathi, N.; Kumar, Y. K. RSC Adv. 2012, 2, 3986–3992. doi:10.1039/c2ra20213a
Return to citation in text: [1] -
Ravindar, K.; Reddy, M. S.; Deslongchamps, P. Org. Lett. 2011, 13, 3178–3181. doi:10.1021/ol201102x
Return to citation in text: [1] -
Reddy, M. S.; Thirupathi, N.; Haribabu, M. Beilstein J. Org. Chem. 2013, 9, 180–184. doi:10.3762/bjoc.9.21
Return to citation in text: [1] -
Cheng, Y.; Judd, T. C.; Bartberger, M. D.; Brown, J.; Chen, K.; Fremeau, R. T., Jr.; Hickman, D.; Hitchcock, S. A.; Jordan, B.; Li, V.; Lopez, P.; Louie, S. W.; Luo, Y.; Michelsen, K.; Nixey, T.; Powers, T. S.; Rattan, C.; Sickmier, E. A.; St. Jean, D. J.; Wahl, R. C., Jr.; Wen, P. H.; Wood, S. J. Med. Chem. 2011, 54, 5836–5857. doi:10.1021/jm200544q
Return to citation in text: [1] -
Inglis, S.; Jones, R.; Fritz, D.; Stojkoski, C.; Booker, G.; Pyke, S. Org. Biomol. Chem. 2005, 3, 2543–2557. doi:10.1039/b504498g
Return to citation in text: [1] -
Gerster, J. F.; Lindstrom, K. J.; Miller, R. L.; Tomai, M. A.; Birmachu, W.; Bomersine, S. N.; Gibson, S. J.; Imbertson, L. M.; Jacobson, J. R.; Knafla, R. T.; Maye, P. V.; Nikolaides, N.; Oneyemi, F. Y.; Parkhurst, G. J.; Pecore, S. E.; Reiter, M. J.; Scribner, L. S.; Testerman, T. L.; Thompson, N. J.; Wagner, T. L.; Weeks, C. E.; Andre, J.-D.; Lagain, D.; Bastard, Y.; Lupu, M. J. Med. Chem. 2005, 48, 3481–3491. doi:10.1021/jm049211v
Return to citation in text: [1] -
Inglis, S. R.; Stojkoski, C.; Branson, K. M.; Cawthray, J. F.; Fritz, D.; Wiadrowski, E.; Pyke, S. M.; Booker, G. W. J. Med. Chem. 2004, 47, 5405–5417. doi:10.1021/jm049533z
Return to citation in text: [1] -
Tavares, F. X.; Boncek, V.; Deaton, D. N.; Hassell, A. M.; Long, S. T.; Miller, A. B.; Payne, A. A.; Miller, L. R.; Shewchuk, L. M.; Wells-Knecht, K.; Willard, D. H., Jr.; Wright, L. L.; Zhou, H.-Q. J. Med. Chem. 2004, 47, 588–599. doi:10.1021/jm030373l
Return to citation in text: [1] -
Webb, T. R.; Lvovskiy, D.; Kim, S.-A.; Ji, X.-d.; Melman, N.; Linden, J.; Jacobson, K. A. Bioorg. Med. Chem. 2003, 11, 77–85. doi:10.1016/S0968-0896(02)00323-1
Return to citation in text: [1] -
Renau, T. E.; Léger, R.; Yen, R.; She, M. W.; Flamme, E. M.; Sangalang, J.; Gannon, C. L.; Chamberland, S.; Lomovskaya, O.; Lee, V. J. Bioorg. Med. Chem. Lett. 2002, 12, 763–766. doi:10.1016/S0960-894X(02)00006-9
Return to citation in text: [1] -
Brzozowski, Z.; Saçzewski, F. J. Med. Chem. 2002, 45, 430–437. doi:10.1021/jm010953n
Return to citation in text: [1] -
Selwood, D. L.; Brummell, D. G.; Glen, R. C.; Goggin, M. C.; Reynolds, K.; Tatlock, M. A.; Wishart, G. Bioorg. Med. Chem. Lett. 2001, 11, 1089–1092. doi:10.1016/S0960-894X(01)00141-X
Return to citation in text: [1] -
Colotta, V.; Catarzi, D.; Varano, F.; Cecchi, L.; Filacchioni, G.; Martini, C.; Trincavelli, L.; Lucacchini, A. J. Med. Chem. 2000, 43, 3118–3124. doi:10.1021/jm000936i
Return to citation in text: [1] -
Pfister, J. R. J. Nat. Prod. 1988, 51, 969–970. doi:10.1021/np50059a027
Return to citation in text: [1] -
Campbell, S. F.; Hardstone, J. D.; Palmer, M. J. J. Med. Chem. 1988, 31, 1031–1035. doi:10.1021/jm00400a025
Return to citation in text: [1] -
Alhaider, A. A.; Abdelkader, M. A.; Lien, E. J. J. Med. Chem. 1985, 28, 1394–1398. doi:10.1021/jm00148a004
Return to citation in text: [1] -
Li, J.-S.; Gold, B. J. Org. Chem. 2005, 70, 8764–8771. doi:10.1021/jo0511445
Return to citation in text: [1] -
Li, J.-S.; Chen, F.-X.; Shikiya, R.; Marky, L. A.; Gold, B. J. Am. Chem. Soc. 2005, 127, 12657–12665. doi:10.1021/ja0530218
Return to citation in text: [1] -
Nakatani, K.; Sando, S.; Kumasawa, H.; Kikuchi, J.; Saito, I. J. Am. Chem. Soc. 2001, 123, 12650–12657. doi:10.1021/ja0109186
Return to citation in text: [1] -
Nakatani, K.; Sando, S.; Saito, I. J. Am. Chem. Soc. 2000, 122, 2172–2177. doi:10.1021/ja992956j
Return to citation in text: [1] -
Mcgill, C. K.; Rappa, A. Adv. Heterocycl. Chem. 1988, 44, 1–79. doi:10.1016/S0065-2725(08)60261-5
Return to citation in text: [1] -
Li, G.; Jia, C.; Sun, K. Org. Lett. 2013, 15, 5198–5201. doi:10.1021/ol402324v
Return to citation in text: [1] -
Liu, B.; Gao, H.; Yu, Y.; Wu, W.; Jiang, H. J. Org. Chem. 2013, 78, 10319–10328. doi:10.1021/jo401707j
Return to citation in text: [1] -
Tomioka, T.; Takahashi, Y.; Maejima, T. Org. Biomol. Chem. 2012, 10, 5113–5118. doi:10.1039/c2ob25709b
Return to citation in text: [1] -
Shelar, D. P.; Birari, D. R.; Rote, R. V.; Patil, S. R.; Toche, R. B.; Jachak, M. N. J. Phys. Org. Chem. 2011, 24, 203–211. doi:10.1002/poc.1727
Return to citation in text: [1] -
Yin, J.; Xiang, B.; Huffman, M. A.; Raab, C. E.; Davies, I. W. J. Org. Chem. 2007, 72, 4554–4557. doi:10.1021/jo070189y
Return to citation in text: [1] -
Couturier, M.; Caron, L.; Tumidajski, S.; Jones, K.; White, T. D. Org. Lett. 2006, 8, 1929–1932. doi:10.1021/ol060473w
Return to citation in text: [1] -
Arienzo, R.; Clark, D. E.; Cramp, S.; Daly, S.; Dyke, H. J.; Lockey, P.; Norman, D.; Roach, A. G.; Stuttle, K.; Tomlinson, M.; Wong, M.; Wren, S. P. Bioorg. Med. Chem. Lett. 2004, 14, 4099–4102. doi:10.1016/j.bmcl.2004.05.051
Return to citation in text: [1] -
Storz, T.; Marti, R.; Meier, R.; Nury, P.; Roeder, M.; Zhang, K. Org. Process Res. Dev. 2004, 8, 663–665. doi:10.1021/op049944p
Return to citation in text: [1] -
Manley, P. J.; Bilodeau, M. T. Org. Lett. 2002, 4, 3127–3129. doi:10.1021/ol0264556
Return to citation in text: [1] -
Schmittel, M.; Steffen, J.-P.; Engels, B.; Lennartz, C.; Hanrath, M. Angew. Chem., Int. Ed. 1998, 37, 2371–2373. doi:10.1002/(SICI)1521-3773(19980918)37:17<2371::AID-ANIE2371>3.0.CO;2-N
Return to citation in text: [1] -
Zhou, L.; Tu, S.; Shi, D.; Dai, G. J. Chem. Res., Synop. 1998, 398–399. doi:10.1039/A800588E
Return to citation in text: [1] -
Glennon, R. A.; Slusher, R. M.; Lyon, R. A.; Titeler, M.; McKenney, J. D. J. Med. Chem. 1986, 29, 2375–2380. doi:10.1021/jm00161a038
Return to citation in text: [1] -
Abramovitch, R. A.; Pilski, J.; Konitz, A.; Tomasik, P. J. Org. Chem. 1983, 48, 4391–4393. doi:10.1021/jo00171a048
Return to citation in text: [1] -
Kurbatov, Y. V.; Solekhova, M. A. Zh. Org. Khim. 1981, 17, 1121.
Return to citation in text: [1] -
Abramovich, R. A.; Singer, G. M. J. Org. Chem. 1974, 39, 1795–1802. doi:10.1021/jo00927a001
Return to citation in text: [1] -
Abramovich, R. A.; Rogers, R. B. J. Org. Chem. 1974, 39, 1802–1807. doi:10.1021/jo00927a002
Return to citation in text: [1] -
Abramovich, R. A.; Singer, G. M. J. Am. Chem. Soc. 1969, 91, 5672–5673. doi:10.1021/ja01048a059
Return to citation in text: [1]
| 1. | Kim, S. H.; Park, S. H.; Choi, J. H.; Chang, S. Chem.–Asian J. 2011, 6, 2618–2634. doi:10.1002/asia.201100340 |
| 2. | Yoo, E. J.; Chang, S. Curr. Org. Chem. 2009, 13, 1766–1776. doi:10.2174/138527209789630497 |
| 3. | Yoo, E. J.; Park, S. H.; Lee, S. H.; Chang, S. Org. Lett. 2009, 11, 1155–1158. doi:10.1021/ol900023t |
| 4. | Cho, S. H.; Chang, S. Angew. Chem., Int. Ed. 2007, 46, 1897–1900. doi:10.1002/anie.200604358 |
| 5. | Kim, J.; Lee, S. Y.; Lee, J.; Do, Y.; Chang, S. J. Org. Chem. 2008, 73, 9454–9457. doi:10.1021/jo802014g |
| 6. | Bae, I.; Han, H.; Chang, S. J. Am. Chem. Soc. 2005, 127, 2038–2039. doi:10.1021/ja0432968 |
| 7. | Sun, L.; Zhu, Y.; Lu, P.; Wang, Y. Org. Lett. 2013, 15, 5894–5897. doi:10.1021/ol402996x |
| 8. | Xing, Y.; Zhao, H.; Shang, Q.; Wang, J.; Lu, P.; Wang, Y. Org. Lett. 2013, 15, 2668–2671. doi:10.1021/ol4010323 |
| 9. | Zhou, F.; Liu, X.; Zhang, N.; Liang, Y.; Zhang, R.; Xin, X.; Dong, D. Org. Lett. 2013, 15, 5786–5789. doi:10.1021/ol4028368 |
| 10. | Jiang, Z.; Lu, P.; Wang, Y. Org. Lett. 2012, 14, 6266–6269. doi:10.1021/ol303023y |
| 11. | Lu, P.; Wang, Y. Chem. Soc. Rev. 2012, 41, 5687–5705. doi:10.1039/c2cs35159e |
| 12. | Lu, P.; Wang, Y. Synlett 2010, 165–173. doi:10.1055/s-0029-1218558 |
| 13. | Cui, S.-L.; Wang, J.; Wang, Y.-G. Tetrahedron 2008, 64, 487–495. doi:10.1016/j.tet.2007.11.025 |
| 14. | Yao, W.; Pan, L.; Zhang, Y.; Wang, G.; Wang, X.; Ma, C. Angew. Chem., Int. Ed. 2010, 49, 9210–9214. doi:10.1002/anie.201004685 |
| 15. | Cassidy, M. P.; Raushel, J.; Fokin, V. V. Angew. Chem., Int. Ed. 2006, 45, 3154–3157. doi:10.1002/anie.200503805 |
| 16. | Kumar, Y. K.; Kumar, G. R.; Reddy, M. S. J. Org. Chem. 2014, 79, 823–828. doi:10.1021/jo402570t |
| 17. | Chauhan, D. P.; Varma, S. J.; Vijeta, A.; Banerjee, P.; Talukdar, P. Chem. Commun. 2014, 50, 323–325. doi:10.1039/c3cc47182a |
| 18. | Wang, J.; Wang, J.; Lu, P.; Wang, Y. J. Org. Chem. 2013, 78, 8816–8820. doi:10.1021/jo401094j |
| 19. | Cheng, G.; Cui, X. Org. Lett. 2013, 15, 1480–1483. doi:10.1021/ol400219n |
| 20. | Nagaraj, M.; Boominathan, M.; Perumal, D.; Muthusubramanian, S.; Bhuvanesh, N. J. Org. Chem. 2012, 77, 6319–6326. doi:10.1021/jo300855f |
| 21. | Li, S.; Luo, Y.; Wu, J. Org. Lett. 2011, 13, 3190–3193. doi:10.1021/ol2011067 |
| 22. | Yoo, E. J.; Chang, S. Org. Lett. 2008, 10, 1163–1166. doi:10.1021/ol800049b |
| 23. | Chen, Z.; Zheng, D.; Wu, J. Org. Lett. 2011, 13, 848–851. doi:10.1021/ol102775s |
| 24. | Reddy, M. S.; Thirupathi, N.; Babu, M. H.; Puri, S. J. Org. Chem. 2013, 78, 5878–5888. doi:10.1021/jo400499r |
| 25. | Reddy, M. S.; Kumar, Y. K.; Thirupathi, N. Org. Lett. 2012, 14, 824–827. doi:10.1021/ol2033493 |
| 26. | Reddy, M. S.; Thirupathi, N.; Babu, M. H. Eur. J. Org. Chem. 2012, 5803–5809. doi:10.1002/ejoc.201200782 |
| 27. | Reddy, M. S.; Thirupathi, N.; Kumar, Y. K. RSC Adv. 2012, 2, 3986–3992. doi:10.1039/c2ra20213a |
| 28. | Ravindar, K.; Reddy, M. S.; Deslongchamps, P. Org. Lett. 2011, 13, 3178–3181. doi:10.1021/ol201102x |
| 29. | Reddy, M. S.; Thirupathi, N.; Haribabu, M. Beilstein J. Org. Chem. 2013, 9, 180–184. doi:10.3762/bjoc.9.21 |
| 16. | Kumar, Y. K.; Kumar, G. R.; Reddy, M. S. J. Org. Chem. 2014, 79, 823–828. doi:10.1021/jo402570t |
| 16. | Kumar, Y. K.; Kumar, G. R.; Reddy, M. S. J. Org. Chem. 2014, 79, 823–828. doi:10.1021/jo402570t |
| 17. | Chauhan, D. P.; Varma, S. J.; Vijeta, A.; Banerjee, P.; Talukdar, P. Chem. Commun. 2014, 50, 323–325. doi:10.1039/c3cc47182a |
| 18. | Wang, J.; Wang, J.; Lu, P.; Wang, Y. J. Org. Chem. 2013, 78, 8816–8820. doi:10.1021/jo401094j |
| 19. | Cheng, G.; Cui, X. Org. Lett. 2013, 15, 1480–1483. doi:10.1021/ol400219n |
| 20. | Nagaraj, M.; Boominathan, M.; Perumal, D.; Muthusubramanian, S.; Bhuvanesh, N. J. Org. Chem. 2012, 77, 6319–6326. doi:10.1021/jo300855f |
| 21. | Li, S.; Luo, Y.; Wu, J. Org. Lett. 2011, 13, 3190–3193. doi:10.1021/ol2011067 |
| 22. | Yoo, E. J.; Chang, S. Org. Lett. 2008, 10, 1163–1166. doi:10.1021/ol800049b |
| 23. | Chen, Z.; Zheng, D.; Wu, J. Org. Lett. 2011, 13, 848–851. doi:10.1021/ol102775s |
| 1. | Kim, S. H.; Park, S. H.; Choi, J. H.; Chang, S. Chem.–Asian J. 2011, 6, 2618–2634. doi:10.1002/asia.201100340 |
| 2. | Yoo, E. J.; Chang, S. Curr. Org. Chem. 2009, 13, 1766–1776. doi:10.2174/138527209789630497 |
| 3. | Yoo, E. J.; Park, S. H.; Lee, S. H.; Chang, S. Org. Lett. 2009, 11, 1155–1158. doi:10.1021/ol900023t |
| 4. | Cho, S. H.; Chang, S. Angew. Chem., Int. Ed. 2007, 46, 1897–1900. doi:10.1002/anie.200604358 |
| 5. | Kim, J.; Lee, S. Y.; Lee, J.; Do, Y.; Chang, S. J. Org. Chem. 2008, 73, 9454–9457. doi:10.1021/jo802014g |
| 6. | Bae, I.; Han, H.; Chang, S. J. Am. Chem. Soc. 2005, 127, 2038–2039. doi:10.1021/ja0432968 |
| 48. | Li, G.; Jia, C.; Sun, K. Org. Lett. 2013, 15, 5198–5201. doi:10.1021/ol402324v |
| 49. | Liu, B.; Gao, H.; Yu, Y.; Wu, W.; Jiang, H. J. Org. Chem. 2013, 78, 10319–10328. doi:10.1021/jo401707j |
| 50. | Tomioka, T.; Takahashi, Y.; Maejima, T. Org. Biomol. Chem. 2012, 10, 5113–5118. doi:10.1039/c2ob25709b |
| 51. | Shelar, D. P.; Birari, D. R.; Rote, R. V.; Patil, S. R.; Toche, R. B.; Jachak, M. N. J. Phys. Org. Chem. 2011, 24, 203–211. doi:10.1002/poc.1727 |
| 52. | Yin, J.; Xiang, B.; Huffman, M. A.; Raab, C. E.; Davies, I. W. J. Org. Chem. 2007, 72, 4554–4557. doi:10.1021/jo070189y |
| 53. | Couturier, M.; Caron, L.; Tumidajski, S.; Jones, K.; White, T. D. Org. Lett. 2006, 8, 1929–1932. doi:10.1021/ol060473w |
| 54. | Arienzo, R.; Clark, D. E.; Cramp, S.; Daly, S.; Dyke, H. J.; Lockey, P.; Norman, D.; Roach, A. G.; Stuttle, K.; Tomlinson, M.; Wong, M.; Wren, S. P. Bioorg. Med. Chem. Lett. 2004, 14, 4099–4102. doi:10.1016/j.bmcl.2004.05.051 |
| 55. | Storz, T.; Marti, R.; Meier, R.; Nury, P.; Roeder, M.; Zhang, K. Org. Process Res. Dev. 2004, 8, 663–665. doi:10.1021/op049944p |
| 56. | Manley, P. J.; Bilodeau, M. T. Org. Lett. 2002, 4, 3127–3129. doi:10.1021/ol0264556 |
| 57. | Schmittel, M.; Steffen, J.-P.; Engels, B.; Lennartz, C.; Hanrath, M. Angew. Chem., Int. Ed. 1998, 37, 2371–2373. doi:10.1002/(SICI)1521-3773(19980918)37:17<2371::AID-ANIE2371>3.0.CO;2-N |
| 58. | Zhou, L.; Tu, S.; Shi, D.; Dai, G. J. Chem. Res., Synop. 1998, 398–399. doi:10.1039/A800588E |
| 59. | Glennon, R. A.; Slusher, R. M.; Lyon, R. A.; Titeler, M.; McKenney, J. D. J. Med. Chem. 1986, 29, 2375–2380. doi:10.1021/jm00161a038 |
| 60. | Abramovitch, R. A.; Pilski, J.; Konitz, A.; Tomasik, P. J. Org. Chem. 1983, 48, 4391–4393. doi:10.1021/jo00171a048 |
| 61. | Kurbatov, Y. V.; Solekhova, M. A. Zh. Org. Khim. 1981, 17, 1121. |
| 62. | Abramovich, R. A.; Singer, G. M. J. Org. Chem. 1974, 39, 1795–1802. doi:10.1021/jo00927a001 |
| 63. | Abramovich, R. A.; Rogers, R. B. J. Org. Chem. 1974, 39, 1802–1807. doi:10.1021/jo00927a002 |
| 64. | Abramovich, R. A.; Singer, G. M. J. Am. Chem. Soc. 1969, 91, 5672–5673. doi:10.1021/ja01048a059 |
| 13. | Cui, S.-L.; Wang, J.; Wang, Y.-G. Tetrahedron 2008, 64, 487–495. doi:10.1016/j.tet.2007.11.025 |
| 47. | Mcgill, C. K.; Rappa, A. Adv. Heterocycl. Chem. 1988, 44, 1–79. doi:10.1016/S0065-2725(08)60261-5 |
| 43. | Li, J.-S.; Gold, B. J. Org. Chem. 2005, 70, 8764–8771. doi:10.1021/jo0511445 |
| 44. | Li, J.-S.; Chen, F.-X.; Shikiya, R.; Marky, L. A.; Gold, B. J. Am. Chem. Soc. 2005, 127, 12657–12665. doi:10.1021/ja0530218 |
| 45. | Nakatani, K.; Sando, S.; Kumasawa, H.; Kikuchi, J.; Saito, I. J. Am. Chem. Soc. 2001, 123, 12650–12657. doi:10.1021/ja0109186 |
| 46. | Nakatani, K.; Sando, S.; Saito, I. J. Am. Chem. Soc. 2000, 122, 2172–2177. doi:10.1021/ja992956j |
| 30. | Cheng, Y.; Judd, T. C.; Bartberger, M. D.; Brown, J.; Chen, K.; Fremeau, R. T., Jr.; Hickman, D.; Hitchcock, S. A.; Jordan, B.; Li, V.; Lopez, P.; Louie, S. W.; Luo, Y.; Michelsen, K.; Nixey, T.; Powers, T. S.; Rattan, C.; Sickmier, E. A.; St. Jean, D. J.; Wahl, R. C., Jr.; Wen, P. H.; Wood, S. J. Med. Chem. 2011, 54, 5836–5857. doi:10.1021/jm200544q |
| 31. | Inglis, S.; Jones, R.; Fritz, D.; Stojkoski, C.; Booker, G.; Pyke, S. Org. Biomol. Chem. 2005, 3, 2543–2557. doi:10.1039/b504498g |
| 32. | Gerster, J. F.; Lindstrom, K. J.; Miller, R. L.; Tomai, M. A.; Birmachu, W.; Bomersine, S. N.; Gibson, S. J.; Imbertson, L. M.; Jacobson, J. R.; Knafla, R. T.; Maye, P. V.; Nikolaides, N.; Oneyemi, F. Y.; Parkhurst, G. J.; Pecore, S. E.; Reiter, M. J.; Scribner, L. S.; Testerman, T. L.; Thompson, N. J.; Wagner, T. L.; Weeks, C. E.; Andre, J.-D.; Lagain, D.; Bastard, Y.; Lupu, M. J. Med. Chem. 2005, 48, 3481–3491. doi:10.1021/jm049211v |
| 33. | Inglis, S. R.; Stojkoski, C.; Branson, K. M.; Cawthray, J. F.; Fritz, D.; Wiadrowski, E.; Pyke, S. M.; Booker, G. W. J. Med. Chem. 2004, 47, 5405–5417. doi:10.1021/jm049533z |
| 34. | Tavares, F. X.; Boncek, V.; Deaton, D. N.; Hassell, A. M.; Long, S. T.; Miller, A. B.; Payne, A. A.; Miller, L. R.; Shewchuk, L. M.; Wells-Knecht, K.; Willard, D. H., Jr.; Wright, L. L.; Zhou, H.-Q. J. Med. Chem. 2004, 47, 588–599. doi:10.1021/jm030373l |
| 35. | Webb, T. R.; Lvovskiy, D.; Kim, S.-A.; Ji, X.-d.; Melman, N.; Linden, J.; Jacobson, K. A. Bioorg. Med. Chem. 2003, 11, 77–85. doi:10.1016/S0968-0896(02)00323-1 |
| 36. | Renau, T. E.; Léger, R.; Yen, R.; She, M. W.; Flamme, E. M.; Sangalang, J.; Gannon, C. L.; Chamberland, S.; Lomovskaya, O.; Lee, V. J. Bioorg. Med. Chem. Lett. 2002, 12, 763–766. doi:10.1016/S0960-894X(02)00006-9 |
| 37. | Brzozowski, Z.; Saçzewski, F. J. Med. Chem. 2002, 45, 430–437. doi:10.1021/jm010953n |
| 38. | Selwood, D. L.; Brummell, D. G.; Glen, R. C.; Goggin, M. C.; Reynolds, K.; Tatlock, M. A.; Wishart, G. Bioorg. Med. Chem. Lett. 2001, 11, 1089–1092. doi:10.1016/S0960-894X(01)00141-X |
| 39. | Colotta, V.; Catarzi, D.; Varano, F.; Cecchi, L.; Filacchioni, G.; Martini, C.; Trincavelli, L.; Lucacchini, A. J. Med. Chem. 2000, 43, 3118–3124. doi:10.1021/jm000936i |
| 40. | Pfister, J. R. J. Nat. Prod. 1988, 51, 969–970. doi:10.1021/np50059a027 |
| 41. | Campbell, S. F.; Hardstone, J. D.; Palmer, M. J. J. Med. Chem. 1988, 31, 1031–1035. doi:10.1021/jm00400a025 |
| 42. | Alhaider, A. A.; Abdelkader, M. A.; Lien, E. J. J. Med. Chem. 1985, 28, 1394–1398. doi:10.1021/jm00148a004 |
| 16. | Kumar, Y. K.; Kumar, G. R.; Reddy, M. S. J. Org. Chem. 2014, 79, 823–828. doi:10.1021/jo402570t |
© 2014 Kumar et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)