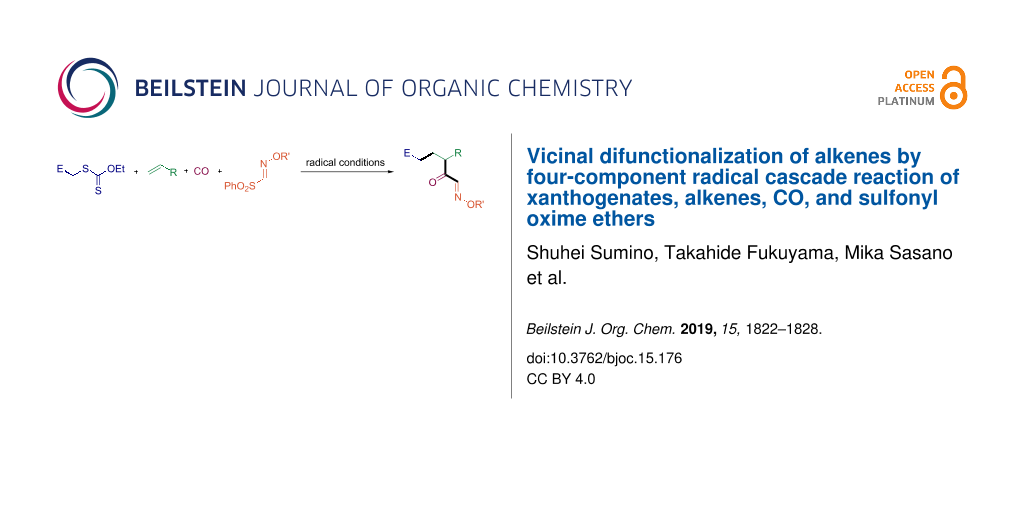
Abstract
Four-component coupling reactions between xanthogenates, alkenes, CO, and sulfonyl oxime ethers were studied. In the presence of hexabutylditin, working as a propagating radical reagent, the chain reaction proceeds, as expected, taking into account reagents polarities, affording the corresponding functionalized α-keto oximes. Although yields are modest, this rare one-pot four-component process is easy to carry out and the resulting compounds, bearing multiple functionalities, have the potential for further elaboration.
Graphical Abstract
Introduction
Multicomponent reactions constitute a powerful and highly efficient tool in organic synthesis to build up intricate compounds from simple molecules in a single operation [1-5]. Needless to say, the contribution by radical chemistry is not trivial [5-7]. While alkenes and alkynes have served as efficient radical donor/acceptor type C2 synthons in multicomponent radical reactions, CO and isonitriles were shown to react as donor/acceptor type C1 synthons [6-15]. In this context, sulfonyl oxime ethers are powerful acceptors of type C1 synthon [8,16,17], which terminates the multicomponent reaction by a β-scission of RSO2 radicals [18-20]. Recently, one of us reported on a three-component radical reaction using xanthogenates, alkenes, and sulfonyl oxime ethers (Scheme 1, reaction 1) [21,22]. The reaction proceeds efficiently to provide good yields of α-alkoxyimino esters, potential precursors of lactams, lactones and β-keto esters. Since the three-component radical reaction involving alkyl halides and two radical C1 synthons, CO and sulfonyl oxime ethers, is known to be feasible [23-25], we were tempted to explore a novel class of four-component radical reaction [26-28] incorporating a xanthogenate, an alkene, CO, and a sulfonyl oxime ether (Scheme 1, reaction 2). This paper reports on the synthesis of functionalized α-keto oximes through such a one-pot, four-component procedure.
Scheme 1: Concept: Alkene difuctionalization by four-component radical reaction using xanthates, alkenes, CO and sulfonyl oxime ethers.
Scheme 1: Concept: Alkene difuctionalization by four-component radical reaction using xanthates, alkenes, CO ...
Results and Discussion
We first investigated the reaction of xanthate 1a [29], 1-octene (2a), CO, and sulfonyl oxime ether 3a as a model reaction. When the mixture of 1a, 2a (5 equiv), and 3a (1.2 equiv) in C6H6 (16 mL) in the presence of hexabutylditin as a radical mediator, and DTBHN (di-tert-butyl hyponitrite) as a radical initiator was heated under CO (130 atm) at 45 °C for 16 h, the envisaged four-component coupling product, keto oxime 5a, was obtained in 43% yield, along with the three-component product 4a (4a/5a = 9:91) (Table 1, entry 1). In this reaction, several unidentified byproducts were also formed. Since the conversion of 1a (ca. 70%) was incomplete, a higher concentration ([1a] = 0.05 M) using 8 mL of C6H6 was employed, resulting in a higher conversion (ca. 80%), affording 5a in 47% yield (Table 1, entry 2). The use of DCE (1,2-dichloroethane) as a solvent gave a 50% yield of 5a (Table 1, entry 3). The present four-component product also proceeded under photoirradiation conditions in the absence of costly DTBHN (Table 1, entry 4). The reaction with 10 equivalents of 2a together with 1.5 equivalents of 3a led to a higher conversion (ca. 90%), affording acceptable yield and selectivity (Table 1, entry 5).
Table 1: Four-component coupling reaction of ethyl 2-((ethoxycarbonothioyl)thio)acetate (1a), 1-octene (2a), CO, and sulfonyl oxime ether 3a under radical conditionsa.
|
|
|||||
| entry | solvent | 2a (equiv) | 3a (equiv) | ratiob (4a/5a) | 5ac (%) |
| 1d | C6H6 | 5.0 | 1.2 | 9:91 | 43 |
| 2 | C6H6 | 5.0 | 1.2 | 13:87 | 47 (39) |
| 3 | DCE | 5.0 | 1.2 | 9:91 | 50 (43) |
| 4e | DCE | 5.0 | 1.2 | 8:92 | 41 (38) |
| 5 | DCE | 10.0 | 1.5 | 13:87 | 56 (52) |
aReaction conditions: 1a (0.4 mmol), 2a (2 or 4 mmol), CO (130 atm), 3a (0.48 or 0.6 mmol), DTBHN (0.12 mmol), (Bu3Sn)2 (0.8 mmol), C6H6 or DCE (8 mL), 45 °C, 16 h. bDetermined by GC. cGC yields determined by using nonane as an internal standard. Isolated yields by silica gel chromatography are given in the parenthesis. dC6H6 (16 mL). Conversion of 1a = ca. 70%. eIrradiation by Xe lamp was carried out in the absence of DTBHN.
With optimized reaction conditions in hand (Table 1, entry 5), we then examined the generality of this four-component radical cascade reaction using xanthates 1, olefins 2, CO, and oxime esters 3, leading to 5a–l (Figure 1). The xanthate 1b, bearing a phenyl ester, gave similarly to 1a, α-keto oxime 5b in moderate yield. The reaction of 1a or 1b with vinylcyclohexane (2b) in the presence of CO and 3a afforded the corresponding α-keto oximes 5c and 5d in 54 or 32% yield, respectively. The conditions were shown to be compatible with the presence of nitriles, ethers and halogens. Alkenes having a tert-butyldimethylsilyl ether such as 6-siloxy-1-hexene 2c thus participated to the reaction to give 5e in 57% yield. Alkenes having a chlorine atom, as in 2d, were also competent substrates in the present four-component coupling reaction, affording 5f, albeit in modest yield. The reaction of 1a with 6-heptenenitrile (2e) and 5-hexen-2-one (2f) gave the corresponding four-component coupling products 5g and 5h, in 34 and 41% yield, respectively. The reaction with cyano-substituted sulfonyl oxime ester 3b also worked well to provide cyano-functionalized α-keto oximes. 5i, 5j, and 5k were thus accessible through the four-component coupling reaction between xanthogenates, alkenes, CO, and 3b in acceptable isolated yields (39–50%). Finally, the reaction between acetophenone xanthate 1c, 2b and 3a gave the corresponding keto oxime 5l in 39% yield. The functionalized α-keto oximes obtained herein should be useful scaffolds for further functionalization. Indeed, the α-keto oximes were reported to be used for the synthesis of a variety of synthetic intermediates, including functionalized keto-aldehydes [22], aminoalcohols [30], triazoles [31], just to name a few.
Figure 1: Vicinal difunctionalization of alkenes by four-component radical cascade reaction using xanthogenate 1, alkenes 2, CO, and sulfonyl oxime ethers 3 leading to 5a–l. Reaction conditions: 1 (0.4 mmol), 2 (4 mmol), CO (130 atm), 3 (0.5 mmol), DTBHN (30 mol %), (Bu3Sn)2 (0.8 mmol), DCE (8 mL), 45 °C, 16 h.
Figure 1: Vicinal difunctionalization of alkenes by four-component radical cascade reaction using xanthogenat...
A reaction mechanism is finally proposed for the four-component cascade reaction, which is depicted in Figure 2 [22-24,32-34]. Initially, α-carbonyl radical A [8] was generated by the reaction of the tributyltin radical with 1a. The electrophilic α-carbonyl radical A does not react with CO even at high CO pressure [6], and therefore selectively adds to electron-rich olefin 2a to form a carbon-centered radical B. The radical B, regarded as a nucleophilic radical, then undergoes radical carbonylation with CO to give an acyl radical C [35], which then adds to electron-deficient sulfonyl oxime ether 3a to afford 5a. The resulting radical D then undergoes β-fragmentation providing 5a along with the phenylsulfonyl radical E. SH2 reaction between radical E and hexabutylditin regenerates the tributyltin radical which sustains the radical chain. Since radical B can also add to sulfonyl oxime ether 3a, we used high CO pressure conditions to encourage the radical carbonylation to form acyl radical C.
Figure 2: Proposed radical chain mechanism.
Figure 2: Proposed radical chain mechanism.
Conclusion
In summary, we demonstrated that a four-component radical cascade reaction, between xanthogenates, alkenes, CO, and sulfonyl oxime ethers, can proceed under radical mediated conditions, using hexabutylditin as a radical chain carrier, to give the corresponding keto-oximes in moderate yields. A variety of functional groups are tolerated under the high CO pressure and temperature conditions. Among multicomponent reactions, specific four-component reactions are still rare [26-28]. The present procedure, which is easy to carry out using an autoclave in a single operation, shows that a fine tuning of the reaction conditions (pressure and temperature) and reagents polarities offer a straightforward access to polyfunctionalized substrates from readily available starting materials.
Experimental
General information
1H NMR spectra were recorded on a JEOL ECP-500 (500 MHz) and JEOL ECS-400 (400 MHz) spectrometers in CDCl3 and referenced at 0.00 ppm for TMS. 13C NMR spectra were recorded on a JEOL ECP-500 (125 MHz) and JEOL ECS-400 (100 MHz) spectrometers in CDCl3 and referenced at 77.00 ppm for CHCl3. Chemical shifts are reported in parts per million (δ). Splitting patterns are indicated as follows: br, broad; s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet. Infrared spectra were obtained on a JASCO FT/IR-4100 spectrometer; absorptions were reported in reciprocal centimeters. High-resolution mass spectra were recorded on a JEOL MS700 spectrometer or Exactive Plus EMR (Thermo Fisher Scientific). The products were purified by flash chromatography on silica gel (Kanto Chem. Co. Silica Gel 60N (spherical, neutral, 40–50 μm)) and, if necessary, were further purified by recycling preparative HPLC (Japan Analytical Industry Co. Ltd., LC-918) equipped with GPC columns (JAIGEL-1H + JAIGEL-2H columns) using CHCl3 as eluent. Xanthogenate 1a,b [20], 1c [36], alkene 2c [37], and oxime ester 3a,b [20] were prepared according to reported procedures. Photoirradiation was carried out using a 500 W Xenon short arc lamp (Ushio Co. Ltd., lamp house: SX-UI500XQ, Xenon short arc lamp: UXL-500SX, power supply: BA-X500).
Typical procedure for the synthesis of 5a under thermal conditions
A magnetic stirring bar, 1a (90.1 mg, 0.4 mmol), 2a (455.3 mg, 4.0 mmol), 3a (141.4 mg, 0.51 mmol), (Bu3Sn)2 (474.7 mg, 0.82 mmol), DTBHN (21.4 mg, 0.12 mmol), and 1,2-dichloroethane (8 mL) were placed in a stainless steel autoclave. The autoclave was closed, purged three times with carbon monoxide, pressurized with 130 atm of CO and then stirred at 45 °C for 16 h. Excess CO was discharged at room temperature after the reaction. The reaction mixture was then filtered and concentrated in vacuo to give a residue, which was subjected to silica gel column chromatography using hexane/EtOAc 10:1 as eluent, affording 5a (83.7 mg, 0.22 mmol, 52%).
Procedure for the synthesis of 5a under photoirradiation conditions
A magnetic stirring bar, 1a (82.0 mg, 0.39 mmol), 2a (225.8 mg, 2.0 mmol), 3a (133.1 mg, 0.48 mmol), (Bu3Sn)2 (452.6 mg, 0.78 mmol), and 1,2-dichloroethane (8 mL) were placed in a stainless-steel autoclave equipped with two sapphire glass windows and an inserted Pyrex glass liner. The autoclave was closed, purged three times with carbon monoxide, pressurized with 130 atm of CO and then irradiated by Xenon lamp (500 W) with stirring for 16 h. Excess CO was discharged after the reaction. The reaction mixture was then filtered and concentrated in vacuo to give a residue, which was subjected to silica gel column chromatography using hexane/EtOAc 10:1 as eluent, affording 5a (52.9 mg, 0.15 mmol, 38%).
Ethyl (E)-4-(2-((benzyloxy)imino)acetyl)decanoate (5a): IR (neat, ZnSe) νmax (cm−1): 3065, 2955, 1732, 1584, 1455, 1303, 1210; 1H NMR (CDCl3, 400 MHz) δ 7.49 (s, 1H), 7.38–7.33 (m, 5H), 5.25 (s, 2H), 4.10–4.08 (m, 2H), 3.35–3.33 (m, 1H), 2.21–2.17 (m, 2H), 1.25–1.21 (m, 11H), 0.87 (t, J = 6.8 Hz, 3H); 13C NMR (CDCl3, 100 MHz) δ 201.7, 173.0, 148.0, 136.1, 128.6, 128.6, 128.5, 77.9, 60.3, 45.3, 32.1, 32.0, 31.6, 29.2, 27.1, 26.4, 22.6, 14.2, 14.1; EIMS m/z (relative intensity): 316 (4), 227 (8), 199 (2), 91 (100); HRMS–EI (m/z): [M − C2H5O]+ calcd for C19H26NO3, 316.1913; found, 316.1916.
Phenyl (E)-4-(2-((benzyloxy)imino)acetyl)decanoate (5b): IR (neat, ZnSe) νmax (cm−1): 2954, 2928, 1760, 1685, 1196, 1188; 1H NMR (CDCl3, 500 MHz) δ 7.54 (s, 1H), 7.39–7.34 (m, 8H), 7.24–7.21 (t, J = 7.4 Hz, 1H), 7.07–7.06 (d, J = 9.5 Hz, 11H), 5.25 (s, 2H), 3.49–3.44 (m, 1H), 2.57–2.45 (m, 2H), 2.13–2.04 (m, 1H), 1.98–1.89 (m, 1H), 1.72–1.62 (m, 1H), 1.48–1.40 (m, 1H), 1.29–1.22 (m, 10H), 0.89–0.86 (t, J = 7.5 Hz, 3H); 13C NMR (CDCl3, 125 MHz) δ 201.7, 171.7, 150.8, 148.2, 136.3, 129.5, 128.7, 128.6, 125.9, 121.7, 78.1, 45.4, 32.3, 32.2, 31.7, 29.4, 27.3, 26.5, 22.7, 14.2; EIMS m/z (relative intensity): 316 (1), 91 (100); HRMS–EI (m/z): [M − C6H5O]+ calcd for C19H26NO3, 316.1913; found, 316.1910.
Ethyl (E)-6-((benzyloxy)imino)-4-cyclohexyl-5-oxohexanoate (5c): IR (neat, ZnSe) νmax (cm−1): 3065, 2978, 1681, 1497, 1370, 1251, 1210; 1H NMR (CDCl3, 500 MHz) δ 7,48 (s, 1H), 7.38–7.33 (m, 5H), 5.25 (s, 2H), 4.12–4.06 (m, 2H), 3.27–3.23 (m, 1H), 2.22–2.17 (m, 1H), 2,13–2.06 (m, 1H), 1.96–1.92 (m, 1H), 1.86–1.85 (m, 1H), 1.71–1.59 (m, 5H), 1.25–1.22 (t, J = 7 Hz, 3H), 1.17–0.90 (m, 5H); 13C NMR (CDCl3, 125 MHz) δ 201.1, 173.1, 148.7, 148.6, 136.2, 128.6, 128.5, 77.9, 60.3, 51.0, 50.9, 40.3, 32.2, 26.3, 23.4, 14.3, 14.1; EIMS m/z (relative intensity): 359 (1), 125 (2), 109 (6), 91 (100); HRMS–EI (m/z): [M]+ calcd for C21H29NO4, 359.2097; found, 359.2067.
Phenyl (E)-6-((benzyloxy)imino)-4-cyclohexyl-5-oxohexanoate (5d): IR (neat, ZnSe) νmax (cm−1): 2927, 2852, 1759, 1682, 1492, 1197, 1135; 1H NMR (CDCl3, 500 MHz) δ 7.52 (s, 1H), 7.39–7.34 (m, 7H), 7.26–7.21 (m, 2H), 7.07–7.05 (m, 2H), 5.24 (s, 2H), 3.36–3.33 (m, 1H), 2.51–2.30 (m, 2H), 2.12–1.95 (m, 2H), 1.82–1.50 (m, 7H), 1.23–0.89 (m, 6H); 13C NMR (CDCl3, 100 MHz) δ 202.0, 171.8, 148.9, 136.4, 129.6, 128.9, 128.8, 128.8, 128.7, 126.0, 121.7, 78.1, 51.1, 40.5, 32.5, 31.6, 30.2, 26.5, 26.5, 23.5; EIMS m/z (relative intensity): 314 (53), 232 (8), 91 (100); HRMS–EI (m/z): [M − C6H5O]+ calcd for C19H26NO3, 314.1756, found, 314.1760.
Phenyl (E)-4-(2-((benzyloxy)imino)acetyl)-8-((tert-butyldimethylsilyl)oxy)octanoate (5e): IR (neat, ZnSe) νmax (cm−1): 2952, 2854, 1760, 1686, 1595, 1493. 1H NMR (CDCl3, 500 MHz) δ 7.49 (s, 1H), 7.35–7.33 (m, 8H), 7.20–7.19 (m, 1H), 7.04–7.02 (m, 2H), 5.21 (s, 2H), 3.54–3.50 (m, 2H), 3.50–3.40 (m, 1H), 2.45–2.42 (m, 2H), 2.08–2.03 (m, 1H), 1.93–1.87 (m, 1H), 1.71–1.61 (m, 1H), 1.56–1.40 (m, 3H), 0.85 (s, 9H), 0.06 (s, 6H); 13C NMR (CDCl3, 125 MHz) δ 201.4, 171.5, 150.6, 148.0, 136.0, 129.4, 128.6, 125.8, 121.5, 78.6, 62.7, 44.9, 31.9, 30.3, 28.3, 26.7, 26.4, 25.9, 18.3, −5.1, −5.3; EIMS m/z (relative intensity): 440 (20), 404 (24), 263 (20), 91 (100); HRMS–EI (m/z): [M − OCH2Ph]+ calcd for C22H34NO4Si, 404.2257; found, 404.2260.
Ethyl (E)-4-(2-((benzyloxy)imino)acetyl)-12-chlorododecanoate (5f): IR (neat, ZnSe) νmax (cm−1): 2930, 2856, 1731, 1682, 1455, 1371, 699; 1H NMR (CDCl3, 500 MHz) δ 7.49 (s, 1H), 7.38–7.34 (m, 5H), 5.25 (s, 2H), 4.12–4.07 (m, 2H), 3.54–3.51 (t, J = 6.5 Hz, 2H), 3.37–3.35 (m, 1H), 2.24–2.15 (m, 2H), 1.96–1.92 (m, 1H), 1.83–1.73 (m, 3H), 1.65–1.58 (m,1H), 1.42–1.37 (m, 3H), 1.25–1.22 (m, 11H); 13C NMR (CDCl3, 125 MHz) 201.6, 173.1, 148.1, 128.6, 128.5, 77.9, 60.3, 45.4, 45.1, 32.6, 32.1, 32.0, 29.5, 29.2, 28.8, 27.1, 26.8, 26.5, 14.2; EIMS m/z (relative intensity): 378 (1), 289 (3), 105 (1), 91 (100); HRMS–EI (m/z): [M − C2H5O]+ calcd for C21H29NO3Cl, 379.1419; found, 378.1842.
Ethyl (E)-4-(2-((benzyloxy)imino)acetyl)-8-cyanooctanoate (5g): IR (neat, ZnSe) νmax (cm−1): 2938, 2246, 1683, 1731; 1H NMR (CDCl3, 400 MHz) δ 7.50 (s, 1H), 7.35–7.41 (m, 5H), 5.26 (s, 2H), 4.08–4.14 (m, 2H), 3.34–3.40 (m, 1H), 2.14–2.27 (m, 5H), 1.90–1.97 (m, 1H), 1.77–1.82 (m, 1H), 1.54–1.68 (m, 3H), 1.29–1.47 (m, 2H), 1.24 (t, J = 7.6 Hz, 3H); 13C NMR (CDCl3, 100 MHz) δ 201.0, 172.8, 148.0, 136.0, 128.6, 128.5, 119.5, 77.9, 60.4, 44.7, 31.8, 31.0, 26.5, 26.2, 25.2, 16.9, 14.2; EIMS m/z (relative intensity): 313 (1), 91 (100), 77 (6), 55 (7); HRMS–EI (m/z): [M − C2H5O]+ calcd for C18H21N2O3, 313.1552; found, 313.1556.
Ethyl (E)-4-(2-((benzyloxy)imino)acetyl)-7-oxooctanoate (5h): IR (neat, ZnSe) νmax (cm−1): 3510, 2936, 1732, 1715, 1684; 1H NMR (CDCl3, 400 MHz) δ 7.49 (s, 1H), 7.38–7.26 (m, 5H), 5.25 (s, 2H), 4.12–4.07 (m, 2H), 3.39–3.35 (m, 1H), 2.40–2.27 (m, 2H), 2.27–2.20 (m, 2H), 2.06 (s, 3H), 2.00–1.85 (m, 2H), 1.82–1.73 (m, 2H), 1.25–1.22 (t, J = 7.2 Hz, 3H); 13C NMR (CDCl3, 100 MHz,) δ 207.7, 200.9, 172.9, 147.9, 136.0, 128.6, 128.6, 128.5, 78.0, 60.4, 44.3, 40.6, 31.7, 29.9, 26.5, 25.2, 14.2; EIMS m/z (relative intensity): 302 (2), 91 (100), HRMS–EI (m/z): [M − C2H5O]+ calcd for C17H20NO4, 302.1392; found, 302.1390.
Ethyl (E)-4-(2-((benzyloxy)imino)-2-cyanoacetyl)decanoate (5i): IR (neat, ZnSe) νmax (cm−1): 2930, 2857, 1733, 1698, 1455, 1035; 1H NMR (CDCl3, 500 MHz) δ 7.43–7.38 (m, 5H), 5.50 (s, 2H), 4.12–4.06 (m, 2H), 3.37–3.32 (m, 1H), 2.26–2.14 (m, 2H), 2.01–1.94 (m, 1H), 1.85–1.80 (m, 1H), 1.66–1.60 (m, 1H), 1.44–1.33 (m, 1H), 1.28–1.19 (m, 11H), 0.89–0.86 (t, J = 6.5 Hz, 3H); 13C NMR (CDCl3, 125 MHz) δ 195.4, 172.7, 134.4, 132.4, 129.2, 128.9, 128.8, 107.4, 80.6, 60.4, 45.4, 31.9 31.6, 31.5, 29.1, 27.0, 26.1, 22.5, 14.2, 14.1; EIMS m/z (relative intensity): 341 (2), 200 (6), 131 (8), 91 (100); HRMS–EI (m/z): [M − C2H5O]+ calcd for C20H26N2O3, 341.1865; found, 341.1867.
Ethyl (E)-4-(2-((benzyloxy)imino)-2-cyanoacetyl)-7-((tert-butyldimethylsilyl)oxy)heptanoate (5j): IR (neat, ZnSe) νmax (cm−1): 2929, 2857, 1732, 1698, 1255; 1H NMR (CDCl3, 500 MHz) δ 7.34 (m, 5H), 5.47 (s, 2H), 4.12–4.05 (m, 2H), 3.53–3.48 (m, 2H), 3.39–3.36 (m, 1H), 2.28–2.18 (m, 2H), 2.06–1.93 (m, 1H), 1.85–1.78 (m, 1H), 1.72–1.61 (m, 1H), 1.59–1.50 (m, 1H), 1.41–1.38 (m, 2H), 1.26–1.21 (t, 3H), 0.88 (s, 9H), 0.02 (s, 6H); 13C NMR (CDCl3, 125 MHz) δ 195.2, 172.6, 134.3, 132.4, 129.2, 129.0, 128.8, 107.3, 80.7, 62.5, 60.5, 41.1, 31.5, 30.1, 28.0, 26.2, 25.9, 18.3, 14.2, −5.2; EIMS m/z (relative intensity): 417 (13), 215 (4), 131 (5), 91 (100); HRMS–EI (m/z): [M − C4H9]+ calcd for C21H29N2O5Si, 417.1846; found, 417.1853.
Ethyl (E)-4-(2-((benzyloxy)imino)-2-cyanoacetyl)-12-chlorododecanoate (5k): IR (neat, ZnSe) νmax (cm−1): 2932, 2857, 1731, 1698, 1035; 1H NMR (CDCl3, 500 MHz) δ 7.40 (m, 5H), 5.48 (s, 2H), 4.12–4.07 (m, 2H), 3.55–3.53 (t, J = 6.5 Hz, 2H) 3.36–3.33 (m, 1H), 2.29–2.18 (m, 2H), 2.00–1.92 (m, 1H), 1.85–1.74 (m, 3H), 1.67–1.62 (m, 1H), 1.42–1.38 (m, 3H), 1.25–1.12 (m, 12H); 13C NMR (CDCl3, 125 MHz) δ 195.4, 172.7, 134.3, 132.4, 129.2, 129.0, 128.8, 107.3, 80.6, 60.4, 45.4, 45.1, 32.5, 31.8, 31.6, 29.3, 29.1, 28.7, 27.0, 26.7, 26.1, 14.2; EIMS m/z (relative intensity): 404 (1), 181 (16), 169 (14), 131 (23), 119 (17), 91 (100); HRMS–EI (m/z): [M − C2H5O]+ calcd for C22H29N2O3Cl, 404.1867; found, 404.1858.
(E)-3-Cyclohexyl-2,6-dioxo-6-phenylhexanal O-benzyl oxime (5l): IR (neat, ZnSe) νmax (cm−1): 2926, 2852, 1682, 1449, 1208; 1H NMR (CDCl3, 400 MHz) δ 7.89–7.88 (m, 2H), 7.54–7.32 (m, 8H), 5.17 (s, 2H), 3.34–3.30 (m, 1H), 2.89–2.69 (m, 2H), 2.02–2.00 (m, 2H), 1.69–1.51 (m, 6H), 1.16–0.88 (m, 6H); 13C NMR (CDCl3, 100 MHz) 202.3, 199.5, 148.6, 136.7, 136.1, 132.9, 128.7, 128.5, 128.5, 128.4, 128.0, 77.8, 51.1, 40.2, 36.1, 31.4, 29.9, 26.3, 22.6; EIMS m/z (relative intensity): 300 (6), 284 (9), 257 (5), 91 (100); HRMS–EI (m/z): [M − OCH2Ph]+ calcd for C18H22NO2, 284.1651; found, 284.1645.
Supporting Information
| Supporting Information File 1: Copies of NMR spectra. | ||
| Format: PDF | Size: 2.9 MB | Download |
Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research (B) (no. 19H02722) from the JSPS and Scientific Research on Innovative Areas 2707 Middle molecular strategy (no. JP15H05850) from the MEXT. AJ thanks the Région Aquitaine for a mobility grant. The French Agence Nationale de la Recherche (ANR-11-BS07-010-01), University of Bordeaux, CNRS, and the Hubert Curien Sakura program are gratefully acknowledged for financial support.
References
-
Bienaymé, H.; Hulme, C.; Oddon, G.; Schmitt, P. Chem. – Eur. J. 2000, 6, 3321–3329. doi:10.1002/1521-3765(20000915)6:18<3321::aid-chem3321>3.0.co;2-a
Return to citation in text: [1] -
Tojino, M.; Ryu, I. Free‐Radical‐Mediated Multicomponent Coupling Reactions. In Multicomponent Reactions, 1st ed.; Zhu, J.; Bienayme, H., Eds.; Wiley-VC: Weinheim, 2005; pp 169 ff. doi:10.1002/3527605118.ch6
Return to citation in text: [1] -
Müller, T. J. J., Ed. Science of Synthesis: Multicomponent Reactions 1 and 2; Georg Thieme Verlag KG: Stuttgart.
Return to citation in text: [1] -
Liautard, V.; Landais, Y. Free‐Radical Multicomponent Processes. In Multicomponent Reactions, 2nd ed.; Zhu, J.; Wang, Q.; Wang, M. X., Eds.; Wiley-VCH: Weinheim. doi:10.1002/9783527678174.ch14
Return to citation in text: [1] -
Fusano, A.; Ryu, I. In Science of Synthesis: Multicomponent Reactions; Müller, T. J. J., Ed.; Georg Thieme Verlag KG: Stuttgart, 2014; Vol. 2, pp 409 ff.
Return to citation in text: [1] [2] -
Ryu, I.; Sonoda, N.; Curran, D. P. Chem. Rev. 1996, 96, 177–194. doi:10.1021/cr9400626
Return to citation in text: [1] [2] [3] -
Malacria, M. Chem. Rev. 1996, 96, 289–306. doi:10.1021/cr9500186
Return to citation in text: [1] [2] -
Godineau, E.; Landais, Y. Chem. – Eur. J. 2009, 15, 3044–3055. doi:10.1002/chem.200802415
Return to citation in text: [1] [2] [3] -
Sumino, S.; Ui, T.; Hamada, Y.; Fukuyama, T.; Ryu, I. Org. Lett. 2015, 17, 4952–4955. doi:10.1021/acs.orglett.5b02302
Return to citation in text: [1] -
Fukuyama, T.; Nakashima, N.; Okada, T.; Ryu, I. J. Am. Chem. Soc. 2013, 135, 1006–1008. doi:10.1021/ja312654q
Return to citation in text: [1] -
Kippo, T.; Hamaoka, K.; Ryu, I. J. Am. Chem. Soc. 2013, 135, 632–635. doi:10.1021/ja311821h
Return to citation in text: [1] -
Kawamoto, T.; Fukuyama, T.; Ryu, I. J. Am. Chem. Soc. 2012, 134, 875–877. doi:10.1021/ja210585n
Return to citation in text: [1] -
Sumino, S.; Fusano, A.; Fukuyama, T.; Ryu, I. Acc. Chem. Res. 2014, 47, 1563–1574. doi:10.1021/ar500035q
Return to citation in text: [1] -
Zhang, B.; Studer, A. Chem. Soc. Rev. 2015, 44, 3505–3521. doi:10.1039/c5cs00083a
Return to citation in text: [1] -
Lei, J.; Li, D.; Zhu, Q. Top. Heterocycl. Chem. 2018, 54, 285–320. doi:10.1007/7081_2017_9
Return to citation in text: [1] -
Kim, S.; Lee, I. Y.; Yoon, J.-Y.; Oh, D. H. J. Am. Chem. Soc. 1996, 118, 5138–5139. doi:10.1021/ja9600993
Return to citation in text: [1] -
Kim, S.; Kim, S. Bull. Chem. Soc. Jpn. 2007, 80, 809–822. doi:10.1246/bcsj.80.809
Return to citation in text: [1] -
Bertrand, F.; Le Guyader, F.; Liguori, L.; Ouvry, G.; Quiclet-Sire, B.; Seguin, S.; Zard, S. Z. C. R. Acad. Sci., Ser. IIc: Chim. 2001, 4, 547–555. doi:10.1016/s1387-1609(01)01270-1
Return to citation in text: [1] -
Ovadia, B.; Robert, F.; Landais, Y. Chimia 2016, 70, 34–42. doi:10.2533/chimia.2016.34
Return to citation in text: [1] -
Kim, S.; Song, H.-J.; Choi, T.-L.; Yoon, J.-Y. Angew. Chem., Int. Ed. 2001, 40, 2524–2526. doi:10.1002/1521-3773(20010702)40:13<2524::aid-anie2524>3.3.co;2-w
Return to citation in text: [1] [2] [3] -
Godineau, E.; Landais, Y. J. Am. Chem. Soc. 2007, 129, 12662–12663. doi:10.1021/ja075755l
Return to citation in text: [1] -
Landais, Y.; Robert, F.; Godineau, E.; Huet, L.; Likhite, N. Tetrahedron 2013, 69, 10073–10080. doi:10.1016/j.tet.2013.09.051
Return to citation in text: [1] [2] [3] -
Ryu, I.; Kuriyama, H.; Minakata, S.; Komatsu, M.; Yoon, J.-Y.; Kim, S. J. Am. Chem. Soc. 1999, 121, 12190–12191. doi:10.1021/ja992125d
Return to citation in text: [1] [2] -
Ryu, I.; Kuriyama, H.; Miyazato, H.; Minakata, S.; Komatsu, M.; Yoon, J.-Y.; Kim, S. Bull. Chem. Soc. Jpn. 2004, 77, 1407–1408. doi:10.1246/bcsj.77.1407
Return to citation in text: [1] [2] -
Kim, S.; Lim, K.-C.; Kim, S.; Ryu, I. Adv. Synth. Catal. 2007, 349, 527–530. doi:10.1002/adsc.200600500
Return to citation in text: [1] -
Ryu, I.; Yamazaki, H.; Ogawa, A.; Kambe, N.; Sonoda, N. J. Am. Chem. Soc. 1993, 115, 1187–1189. doi:10.1021/ja00056a076
Return to citation in text: [1] [2] -
Miura, K.; Tojino, M.; Fujisawa, N.; Hosomi, A.; Ryu, I. Angew. Chem., Int. Ed. 2004, 43, 2423–2425. doi:10.1002/anie.200453702
Return to citation in text: [1] [2] -
Uenoyama, Y.; Fukuyama, T.; Ryu, I. Synlett 2006, 2342–2344. doi:10.1055/s-2006-949643
Return to citation in text: [1] [2] -
Quiclet-Sire, B.; Zard, S. Z. Top. Curr. Chem. 2006, 264, 201–236. doi:10.1007/128_029
Return to citation in text: [1] -
Bosiak, M. J.; Pakulski, M. M. Synthesis 2011, 316–324. doi:10.1055/s-0030-1258355
Return to citation in text: [1] -
Stensbøl, T. B.; Uhlmann, P.; Morel, S.; Eriksen, B. L.; Felding, J.; Kromann, H.; Hermit, M. B.; Greenwood, J. R.; Braüner-Osborne, H.; Madsen, U.; Junager, F.; Krogsgaard-Larsen, P.; Begtrup, M.; Vedsø, P. J. Med. Chem. 2002, 45, 19–31. doi:10.1021/jm010303j
Return to citation in text: [1] -
Renaud, P.; Ollivier, C.; Panchaud, P. Angew. Chem., Int. Ed. 2002, 41, 3460–3462. doi:10.1002/1521-3773(20020916)41:18<3460::aid-anie3460>3.0.co;2-6
Return to citation in text: [1] -
Panchaud, P.; Chabaud, L.; Landais, Y.; Ollivier, C.; Renaud, P.; Zigmantas, S. Chem. – Eur. J. 2004, 10, 3606–3614. doi:10.1002/chem.200400027
Return to citation in text: [1] -
Beniazza, R.; Liautard, V.; Poittevin, C.; Ovadia, B.; Mohammed, S.; Robert, F.; Landais, Y. Chem. – Eur. J. 2017, 23, 2439–2447. doi:10.1002/chem.201605043
Return to citation in text: [1] -
Chatgilialoglu, C.; Crich, D.; Komatsu, M.; Ryu, I. Chem. Rev. 1999, 99, 1991–2070. doi:10.1021/cr9601425
Return to citation in text: [1] -
Liautard, V.; Robert, F.; Landais, Y. Org. Lett. 2011, 13, 2658–2661. doi:10.1021/ol2007633
Return to citation in text: [1] -
Tasker, S. Z.; Gutierrez, A. C.; Jamison, T. F. Angew. Chem., Int. Ed. 2014, 53, 1858–1861. doi:10.1002/anie.201308391
Return to citation in text: [1]
| 36. | Liautard, V.; Robert, F.; Landais, Y. Org. Lett. 2011, 13, 2658–2661. doi:10.1021/ol2007633 |
| 26. | Ryu, I.; Yamazaki, H.; Ogawa, A.; Kambe, N.; Sonoda, N. J. Am. Chem. Soc. 1993, 115, 1187–1189. doi:10.1021/ja00056a076 |
| 27. | Miura, K.; Tojino, M.; Fujisawa, N.; Hosomi, A.; Ryu, I. Angew. Chem., Int. Ed. 2004, 43, 2423–2425. doi:10.1002/anie.200453702 |
| 28. | Uenoyama, Y.; Fukuyama, T.; Ryu, I. Synlett 2006, 2342–2344. doi:10.1055/s-2006-949643 |
| 20. | Kim, S.; Song, H.-J.; Choi, T.-L.; Yoon, J.-Y. Angew. Chem., Int. Ed. 2001, 40, 2524–2526. doi:10.1002/1521-3773(20010702)40:13<2524::aid-anie2524>3.3.co;2-w |
| 1. | Bienaymé, H.; Hulme, C.; Oddon, G.; Schmitt, P. Chem. – Eur. J. 2000, 6, 3321–3329. doi:10.1002/1521-3765(20000915)6:18<3321::aid-chem3321>3.0.co;2-a |
| 2. | Tojino, M.; Ryu, I. Free‐Radical‐Mediated Multicomponent Coupling Reactions. In Multicomponent Reactions, 1st ed.; Zhu, J.; Bienayme, H., Eds.; Wiley-VC: Weinheim, 2005; pp 169 ff. doi:10.1002/3527605118.ch6 |
| 3. | Müller, T. J. J., Ed. Science of Synthesis: Multicomponent Reactions 1 and 2; Georg Thieme Verlag KG: Stuttgart. |
| 4. | Liautard, V.; Landais, Y. Free‐Radical Multicomponent Processes. In Multicomponent Reactions, 2nd ed.; Zhu, J.; Wang, Q.; Wang, M. X., Eds.; Wiley-VCH: Weinheim. doi:10.1002/9783527678174.ch14 |
| 5. | Fusano, A.; Ryu, I. In Science of Synthesis: Multicomponent Reactions; Müller, T. J. J., Ed.; Georg Thieme Verlag KG: Stuttgart, 2014; Vol. 2, pp 409 ff. |
| 18. | Bertrand, F.; Le Guyader, F.; Liguori, L.; Ouvry, G.; Quiclet-Sire, B.; Seguin, S.; Zard, S. Z. C. R. Acad. Sci., Ser. IIc: Chim. 2001, 4, 547–555. doi:10.1016/s1387-1609(01)01270-1 |
| 19. | Ovadia, B.; Robert, F.; Landais, Y. Chimia 2016, 70, 34–42. doi:10.2533/chimia.2016.34 |
| 20. | Kim, S.; Song, H.-J.; Choi, T.-L.; Yoon, J.-Y. Angew. Chem., Int. Ed. 2001, 40, 2524–2526. doi:10.1002/1521-3773(20010702)40:13<2524::aid-anie2524>3.3.co;2-w |
| 6. | Ryu, I.; Sonoda, N.; Curran, D. P. Chem. Rev. 1996, 96, 177–194. doi:10.1021/cr9400626 |
| 8. | Godineau, E.; Landais, Y. Chem. – Eur. J. 2009, 15, 3044–3055. doi:10.1002/chem.200802415 |
| 16. | Kim, S.; Lee, I. Y.; Yoon, J.-Y.; Oh, D. H. J. Am. Chem. Soc. 1996, 118, 5138–5139. doi:10.1021/ja9600993 |
| 17. | Kim, S.; Kim, S. Bull. Chem. Soc. Jpn. 2007, 80, 809–822. doi:10.1246/bcsj.80.809 |
| 35. | Chatgilialoglu, C.; Crich, D.; Komatsu, M.; Ryu, I. Chem. Rev. 1999, 99, 1991–2070. doi:10.1021/cr9601425 |
| 6. | Ryu, I.; Sonoda, N.; Curran, D. P. Chem. Rev. 1996, 96, 177–194. doi:10.1021/cr9400626 |
| 7. | Malacria, M. Chem. Rev. 1996, 96, 289–306. doi:10.1021/cr9500186 |
| 8. | Godineau, E.; Landais, Y. Chem. – Eur. J. 2009, 15, 3044–3055. doi:10.1002/chem.200802415 |
| 9. | Sumino, S.; Ui, T.; Hamada, Y.; Fukuyama, T.; Ryu, I. Org. Lett. 2015, 17, 4952–4955. doi:10.1021/acs.orglett.5b02302 |
| 10. | Fukuyama, T.; Nakashima, N.; Okada, T.; Ryu, I. J. Am. Chem. Soc. 2013, 135, 1006–1008. doi:10.1021/ja312654q |
| 11. | Kippo, T.; Hamaoka, K.; Ryu, I. J. Am. Chem. Soc. 2013, 135, 632–635. doi:10.1021/ja311821h |
| 12. | Kawamoto, T.; Fukuyama, T.; Ryu, I. J. Am. Chem. Soc. 2012, 134, 875–877. doi:10.1021/ja210585n |
| 13. | Sumino, S.; Fusano, A.; Fukuyama, T.; Ryu, I. Acc. Chem. Res. 2014, 47, 1563–1574. doi:10.1021/ar500035q |
| 14. | Zhang, B.; Studer, A. Chem. Soc. Rev. 2015, 44, 3505–3521. doi:10.1039/c5cs00083a |
| 15. | Lei, J.; Li, D.; Zhu, Q. Top. Heterocycl. Chem. 2018, 54, 285–320. doi:10.1007/7081_2017_9 |
| 22. | Landais, Y.; Robert, F.; Godineau, E.; Huet, L.; Likhite, N. Tetrahedron 2013, 69, 10073–10080. doi:10.1016/j.tet.2013.09.051 |
| 23. | Ryu, I.; Kuriyama, H.; Minakata, S.; Komatsu, M.; Yoon, J.-Y.; Kim, S. J. Am. Chem. Soc. 1999, 121, 12190–12191. doi:10.1021/ja992125d |
| 24. | Ryu, I.; Kuriyama, H.; Miyazato, H.; Minakata, S.; Komatsu, M.; Yoon, J.-Y.; Kim, S. Bull. Chem. Soc. Jpn. 2004, 77, 1407–1408. doi:10.1246/bcsj.77.1407 |
| 32. | Renaud, P.; Ollivier, C.; Panchaud, P. Angew. Chem., Int. Ed. 2002, 41, 3460–3462. doi:10.1002/1521-3773(20020916)41:18<3460::aid-anie3460>3.0.co;2-6 |
| 33. | Panchaud, P.; Chabaud, L.; Landais, Y.; Ollivier, C.; Renaud, P.; Zigmantas, S. Chem. – Eur. J. 2004, 10, 3606–3614. doi:10.1002/chem.200400027 |
| 34. | Beniazza, R.; Liautard, V.; Poittevin, C.; Ovadia, B.; Mohammed, S.; Robert, F.; Landais, Y. Chem. – Eur. J. 2017, 23, 2439–2447. doi:10.1002/chem.201605043 |
| 5. | Fusano, A.; Ryu, I. In Science of Synthesis: Multicomponent Reactions; Müller, T. J. J., Ed.; Georg Thieme Verlag KG: Stuttgart, 2014; Vol. 2, pp 409 ff. |
| 6. | Ryu, I.; Sonoda, N.; Curran, D. P. Chem. Rev. 1996, 96, 177–194. doi:10.1021/cr9400626 |
| 7. | Malacria, M. Chem. Rev. 1996, 96, 289–306. doi:10.1021/cr9500186 |
| 8. | Godineau, E.; Landais, Y. Chem. – Eur. J. 2009, 15, 3044–3055. doi:10.1002/chem.200802415 |
| 29. | Quiclet-Sire, B.; Zard, S. Z. Top. Curr. Chem. 2006, 264, 201–236. doi:10.1007/128_029 |
| 30. | Bosiak, M. J.; Pakulski, M. M. Synthesis 2011, 316–324. doi:10.1055/s-0030-1258355 |
| 26. | Ryu, I.; Yamazaki, H.; Ogawa, A.; Kambe, N.; Sonoda, N. J. Am. Chem. Soc. 1993, 115, 1187–1189. doi:10.1021/ja00056a076 |
| 27. | Miura, K.; Tojino, M.; Fujisawa, N.; Hosomi, A.; Ryu, I. Angew. Chem., Int. Ed. 2004, 43, 2423–2425. doi:10.1002/anie.200453702 |
| 28. | Uenoyama, Y.; Fukuyama, T.; Ryu, I. Synlett 2006, 2342–2344. doi:10.1055/s-2006-949643 |
| 31. | Stensbøl, T. B.; Uhlmann, P.; Morel, S.; Eriksen, B. L.; Felding, J.; Kromann, H.; Hermit, M. B.; Greenwood, J. R.; Braüner-Osborne, H.; Madsen, U.; Junager, F.; Krogsgaard-Larsen, P.; Begtrup, M.; Vedsø, P. J. Med. Chem. 2002, 45, 19–31. doi:10.1021/jm010303j |
| 23. | Ryu, I.; Kuriyama, H.; Minakata, S.; Komatsu, M.; Yoon, J.-Y.; Kim, S. J. Am. Chem. Soc. 1999, 121, 12190–12191. doi:10.1021/ja992125d |
| 24. | Ryu, I.; Kuriyama, H.; Miyazato, H.; Minakata, S.; Komatsu, M.; Yoon, J.-Y.; Kim, S. Bull. Chem. Soc. Jpn. 2004, 77, 1407–1408. doi:10.1246/bcsj.77.1407 |
| 25. | Kim, S.; Lim, K.-C.; Kim, S.; Ryu, I. Adv. Synth. Catal. 2007, 349, 527–530. doi:10.1002/adsc.200600500 |
| 37. | Tasker, S. Z.; Gutierrez, A. C.; Jamison, T. F. Angew. Chem., Int. Ed. 2014, 53, 1858–1861. doi:10.1002/anie.201308391 |
| 21. | Godineau, E.; Landais, Y. J. Am. Chem. Soc. 2007, 129, 12662–12663. doi:10.1021/ja075755l |
| 22. | Landais, Y.; Robert, F.; Godineau, E.; Huet, L.; Likhite, N. Tetrahedron 2013, 69, 10073–10080. doi:10.1016/j.tet.2013.09.051 |
| 22. | Landais, Y.; Robert, F.; Godineau, E.; Huet, L.; Likhite, N. Tetrahedron 2013, 69, 10073–10080. doi:10.1016/j.tet.2013.09.051 |
| 20. | Kim, S.; Song, H.-J.; Choi, T.-L.; Yoon, J.-Y. Angew. Chem., Int. Ed. 2001, 40, 2524–2526. doi:10.1002/1521-3773(20010702)40:13<2524::aid-anie2524>3.3.co;2-w |
© 2019 Sumino et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the authors and source are credited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)