Abstract
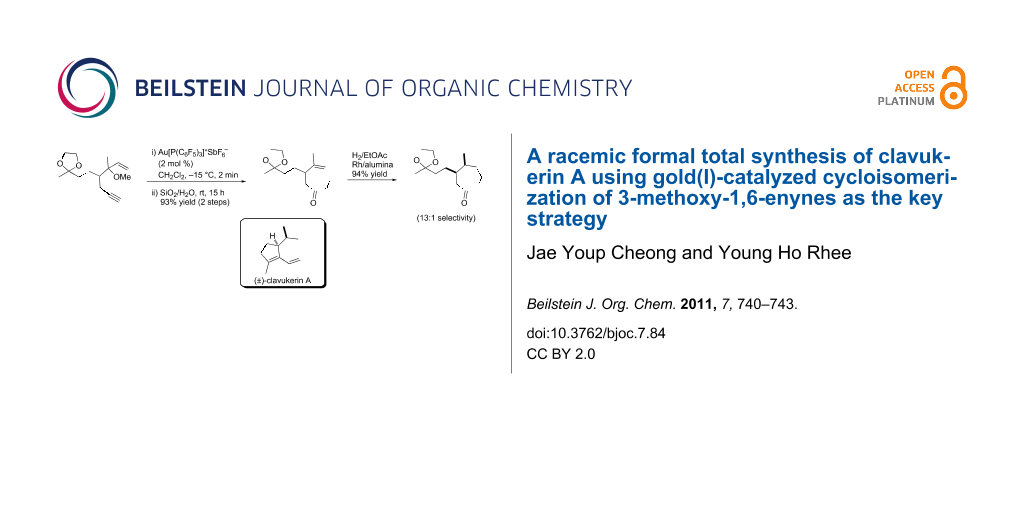
An efficient formal total synthesis of (±)-clavukerin A was accomplished via a gold-catalyzed cycloisomerization of a 3-methoxy-1,6-enyne 5 as the key strategy followed by Rh-catalyzed stereoselective hydrogenation of the cycloheptenone 4.
Graphical Abstract
Findings
Clavukerin A is a member of marine trinorguaiane sesquiterpene natural products. It was first isolated in 1983, by the group of Kitawara, from the Okinawa soft coral Clavularia koellikeri. The structure of clavukerin A was established by CD spectra and X-ray diffraction [1]. The first total synthesis of clavukerin A was reported by Asaoka in 1991, which was followed by several other racemic and enantioselective syntheses [2-14]. Herein, we report a short formal total synthesis of racemic clavukerin A employing the gold(I)-catalyzed cycloisomerization of a 3-methoxy-1,6-enyne as the key strategy, which was recently developed by us [15]. This reaction provides cycloheptane frameworks in a unique manner and illustrates the utility of the gold-catalyzed reactions [16-23].
From a retrosynthetic point of view, we envisioned two different approaches to the key enone intermediate 1 [3] to clavukerin A, starting from the cycloheptenone 4 (Scheme 1). In the first approach, enone 1 could be prepared by the sequential cyclization and the chemo- and stereoselective hydrogenation from cycloheptenone 4 (path A). Alternatively, enone 1 could be accessed by the hydrogenation of 4 and the subsequent cyclization (path B). The cycloheptenone 4 could then be synthesized from the enyne substrate 5 by gold(I)-catalyzed cycloisomerization.
The synthesis of enyne substrate 5 commenced with the alkylation of methyl acetoacetate with the known bromide 6 [24] to provide compound 7 in 55% yield (Scheme 2). Propargylation of 7 followed by the decarbomethoxylation with LiCl [25] gave the ketone 8 in 51% yield (over two steps). Addition of the vinyl group to this ketone gave the alkynol 9 in 90% yield as an inseparable 3:1 mixture of diastereomers. The diastereomeric ratio was determined by integration of the 1H NMR spectrum of the crude reaction product. Subsequent methylation gave the 1,6-enyne 5 in 88% yield.
We then investigated the gold-catalyzed cycloisomerization of enyne 5 using the optimized conditions from our previous study [15]. The use of the pre-generated complex Au[P(C6F5)3]+SbF6− (2 mol %) provided the relatively unstable enol ether 12, which was then immediately treated with aqueous silica gel to give the ketone 4 in 93% yield over two steps. Formation of 12 was unambiguously confirmed by the analysis of 1H NMR data of the crude reaction mixture. From a mechanistic viewpoint, the reaction presumably proceeds via the initial heterocyclization intermediate 10 and the subsequently rearranged intermediate 11 (Scheme 3). Notably, when the gold(I)-catalyzed reaction was carried out on a multi-mmol scale, there was no decrease in the yield at the same catalyst loading.
Scheme 3: Synthesis of the cycloheptenone 4.
Scheme 3: Synthesis of the cycloheptenone 4.
With ketone 4 in hand, the final stage in the formal synthesis of clavukerin A was explored. We first investigated the cyclization–hydrogenation strategy (path A in Scheme 4). Deprotection of 4 and the aldol condensation of the resulting diketone under basic conditions proceeded smoothly to give the enone 2 in good yield. However, extensive attempts at the chemoselective hydrogenation of the trisubstituted olefin 2 gave only compound 1 with poor selectivity. For example, various metal (Pd or Rh)-catalyzed hydrogenations resulted in a mixture of 1 and 3. This problem was also noted in another work on the synthesis of clavukerin A [13].
Scheme 4: Completion of the formal synthesis of clavukerin A.
Scheme 4: Completion of the formal synthesis of clavukerin A.
Thus, we decided to investigate the alternative strategy that involved sequential hydrogenation–cyclization of 4. Initial efforts using various Pd catalysts or Wilkinson catalyst again showed poor stereoselectivity for the hydrogenation. However, with a Rh/alumina catalyst the selectivity was significantly improved and afforded the cis-ketone 3 in 94% yield with ~13:1 selectivity. The structure of 3 was unambiguously confirmed by comparison of the 1H and 13C data with those in the literature [3]. Because the ketone 3 was previously transformed into the enone 1 [3], synthesis of 3 represents the completion of the formal synthesis of clavukerin A.
In summary, a formal synthesis of racemic clavukerin A was accomplished via the gold(I)-catalyzed cycloisomerization of a 3-methoxy-1,6-enyne as the key strategy and stereoselective Rh-catalyzed hydrogenation. Notably, the gold(I)-catalyzed reaction was compatible with the acid-sensitive functional group. Further application of the gold(I)-catalyzed cycloisomerization reaction of 3-methoxy-1,6-enynes to the enantioselective synthesis of more structurally complex cycloheptane natural products is in progress, and will be reported in due course.
Supporting Information
| Supporting Information File 1: Experimental section for the preparation of compounds 2–12, and 1H and 13C NMR spectra for all new compounds. | ||
| Format: PDF | Size: 675.9 KB | Download |
References
-
Kobayashi, M.; Son, B. W.; Kido, M.; Kyogoku, Y.; Kitagawa, I. Chem. Pharm. Bull. 1983, 31, 2160.
Return to citation in text: [1] -
Asaoka, M.; Kosaka, T.; Itahana, H.; Takei, H. Chem. Lett. 1991, 20, 1295. doi:10.1246/cl.1991.1295
Return to citation in text: [1] -
Kim, S. K.; Park, C. S. J. Org. Chem. 1991, 56, 6829. doi:10.1021/jo00024a024
Return to citation in text: [1] [2] [3] [4] -
Shimizu, I.; Ishikawa, T. Tetrahedron Lett. 1994, 35, 1905. doi:10.1016/S0040-4039(00)73192-3
Return to citation in text: [1] -
Honda, T.; Ishige, H.; Nagase, H. J. Chem. Soc., Perkin Trans. 1 1994, 3305. doi:10.1039/P19940003305
Return to citation in text: [1] -
Trost, B. M.; Higuchi, R. I. J. Am. Chem. Soc. 1996, 118, 10094. doi:10.1021/ja961561m
Return to citation in text: [1] -
Lee, E.; Yoon, C. H. Tetrahedron Lett. 1996, 37, 5929. doi:10.1016/0040-4039(96)01279-8
Return to citation in text: [1] -
Friese, J. C.; Krause, S.; Schäfer, H. J. Tetrahedron Lett. 2002, 43, 2683. doi:10.1016/S0040-4039(02)00402-1
Return to citation in text: [1] -
Alexakis, A.; March, S. J. Org. Chem. 2002, 67, 8753. doi:10.1021/jo026262w
Return to citation in text: [1] -
Grimm, E. L.; Methot, J.-L.; Shamji, M. Pure Appl. Chem. 2003, 75, 231. doi:10.1351/pac200375020231
Return to citation in text: [1] -
Blay, G.; García, B.; Molina, E.; Pedro, J. R. J. Nat. Prod. 2006, 69, 1234. doi:10.1021/np060184g
Return to citation in text: [1] -
Li, W.; Liu, X.; Zhou, X.; Lee, C.-S. Org. Lett. 2010, 12, 548. doi:10.1021/ol902567e
Return to citation in text: [1] -
Srikrishna, A.; Pardeshi, V. H.; Satyanarayana, G. Tetrahedron: Asymmetry 2010, 21, 746. doi:10.1016/j.tetasy.2010.04.003
Return to citation in text: [1] [2] -
Knüppel, S.; Rogachev, V. O.; Metz, P. Eur. J. Org. Chem. 2010, 6145. doi:10.1002/ejoc.201001087
Return to citation in text: [1] -
Bae, H. J.; Baskar, B.; An, S. E.; Cheong, J. Y.; Thangadurai, D. T.; Hwang, I.-C.; Rhee, Y. H. Angew. Chem., Int. Ed. 2008, 47, 2263. doi:10.1002/anie.200705117
Return to citation in text: [1] [2] -
Fürstner, A.; Davies, P. W. Angew. Chem., Int. Ed. 2007, 46, 3410. doi:10.1002/anie.200604335
Return to citation in text: [1] -
Hashmi, A. S. K.; Hutchings, G. J. Angew. Chem., Int. Ed. 2006, 45, 7896. doi:10.1002/anie.200602454
Return to citation in text: [1] -
Gorin, D. J.; Toste, F. D. Nature 2007, 446, 395. doi:10.1038/nature05592
Return to citation in text: [1] -
Hashmi, A. S. K. Chem. Rev. 2007, 107, 3180. doi:10.1021/cr000436x
Return to citation in text: [1] -
Li, Z.; Brouwer, C.; He, C. Chem. Rev. 2008, 108, 3239. doi:10.1021/cr068434l
Return to citation in text: [1] -
Jiménez-Núñez, E.; Echavarren, A. M. Chem. Rev. 2008, 108, 3326. doi:10.1021/cr0684319
Return to citation in text: [1] -
Fürstner, A. Chem. Soc. Rev. 2009, 38, 3208. doi:10.1039/b816696j
Return to citation in text: [1] -
Shapiro, N. D.; Toste, F. D. Synlett 2010, 5, 675. doi:10.1055/s-0029-1219369
Return to citation in text: [1] -
Rigby, J. H.; Wilson, J. A. Z. J. Org. Chem. 1987, 52, 34. doi:10.1021/jo00377a006
Return to citation in text: [1] -
Saeki, M.; Toyota, M. Tetrahedron Lett. 2010, 51, 4620. doi:10.1016/j.tetlet.2010.06.114
Return to citation in text: [1]
| 1. | Kobayashi, M.; Son, B. W.; Kido, M.; Kyogoku, Y.; Kitagawa, I. Chem. Pharm. Bull. 1983, 31, 2160. |
| 16. | Fürstner, A.; Davies, P. W. Angew. Chem., Int. Ed. 2007, 46, 3410. doi:10.1002/anie.200604335 |
| 17. | Hashmi, A. S. K.; Hutchings, G. J. Angew. Chem., Int. Ed. 2006, 45, 7896. doi:10.1002/anie.200602454 |
| 18. | Gorin, D. J.; Toste, F. D. Nature 2007, 446, 395. doi:10.1038/nature05592 |
| 19. | Hashmi, A. S. K. Chem. Rev. 2007, 107, 3180. doi:10.1021/cr000436x |
| 20. | Li, Z.; Brouwer, C.; He, C. Chem. Rev. 2008, 108, 3239. doi:10.1021/cr068434l |
| 21. | Jiménez-Núñez, E.; Echavarren, A. M. Chem. Rev. 2008, 108, 3326. doi:10.1021/cr0684319 |
| 22. | Fürstner, A. Chem. Soc. Rev. 2009, 38, 3208. doi:10.1039/b816696j |
| 23. | Shapiro, N. D.; Toste, F. D. Synlett 2010, 5, 675. doi:10.1055/s-0029-1219369 |
| 15. | Bae, H. J.; Baskar, B.; An, S. E.; Cheong, J. Y.; Thangadurai, D. T.; Hwang, I.-C.; Rhee, Y. H. Angew. Chem., Int. Ed. 2008, 47, 2263. doi:10.1002/anie.200705117 |
| 2. | Asaoka, M.; Kosaka, T.; Itahana, H.; Takei, H. Chem. Lett. 1991, 20, 1295. doi:10.1246/cl.1991.1295 |
| 3. | Kim, S. K.; Park, C. S. J. Org. Chem. 1991, 56, 6829. doi:10.1021/jo00024a024 |
| 4. | Shimizu, I.; Ishikawa, T. Tetrahedron Lett. 1994, 35, 1905. doi:10.1016/S0040-4039(00)73192-3 |
| 5. | Honda, T.; Ishige, H.; Nagase, H. J. Chem. Soc., Perkin Trans. 1 1994, 3305. doi:10.1039/P19940003305 |
| 6. | Trost, B. M.; Higuchi, R. I. J. Am. Chem. Soc. 1996, 118, 10094. doi:10.1021/ja961561m |
| 7. | Lee, E.; Yoon, C. H. Tetrahedron Lett. 1996, 37, 5929. doi:10.1016/0040-4039(96)01279-8 |
| 8. | Friese, J. C.; Krause, S.; Schäfer, H. J. Tetrahedron Lett. 2002, 43, 2683. doi:10.1016/S0040-4039(02)00402-1 |
| 9. | Alexakis, A.; March, S. J. Org. Chem. 2002, 67, 8753. doi:10.1021/jo026262w |
| 10. | Grimm, E. L.; Methot, J.-L.; Shamji, M. Pure Appl. Chem. 2003, 75, 231. doi:10.1351/pac200375020231 |
| 11. | Blay, G.; García, B.; Molina, E.; Pedro, J. R. J. Nat. Prod. 2006, 69, 1234. doi:10.1021/np060184g |
| 12. | Li, W.; Liu, X.; Zhou, X.; Lee, C.-S. Org. Lett. 2010, 12, 548. doi:10.1021/ol902567e |
| 13. | Srikrishna, A.; Pardeshi, V. H.; Satyanarayana, G. Tetrahedron: Asymmetry 2010, 21, 746. doi:10.1016/j.tetasy.2010.04.003 |
| 14. | Knüppel, S.; Rogachev, V. O.; Metz, P. Eur. J. Org. Chem. 2010, 6145. doi:10.1002/ejoc.201001087 |
| 13. | Srikrishna, A.; Pardeshi, V. H.; Satyanarayana, G. Tetrahedron: Asymmetry 2010, 21, 746. doi:10.1016/j.tetasy.2010.04.003 |
| 15. | Bae, H. J.; Baskar, B.; An, S. E.; Cheong, J. Y.; Thangadurai, D. T.; Hwang, I.-C.; Rhee, Y. H. Angew. Chem., Int. Ed. 2008, 47, 2263. doi:10.1002/anie.200705117 |
| 25. | Saeki, M.; Toyota, M. Tetrahedron Lett. 2010, 51, 4620. doi:10.1016/j.tetlet.2010.06.114 |
| 24. | Rigby, J. H.; Wilson, J. A. Z. J. Org. Chem. 1987, 52, 34. doi:10.1021/jo00377a006 |
© 2011 Cheong and Rhee; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)