Search results
Search for "mechanistic studies" in Full Text gives 205 result(s) in Beilstein Journal of Organic Chemistry. Showing first 200.
A comprehensive review of flow chemistry techniques tailored to the flavours and fragrances industries
- Guido Gambacorta,
- James S. Sharley and
- Ian R. Baxendale
Beilstein J. Org. Chem. 2021, 17, 1181–1312, doi:10.3762/bjoc.17.90

Graphical Abstract

Figure 1: Representative shares of the global F&F market (2018) segmented on their applications [1].

Figure 2: General structure of an international fragrance company [2].

Figure 3: The Michael Edwards fragrance wheel.

Figure 4: Examples of oriental (1–3), woody (4–7), fresh (8–10), and floral (11 and 12) notes.

Figure 5: A basic depiction of batch vs flow.

Scheme 1: Examples of reactions for which flow processing outperforms batch.

Scheme 2: Some industrially important aldol-based transformations.

Scheme 3: Biphasic continuous aldol reactions of acetone and various aldehydes.

Scheme 4: Aldol synthesis of 43 in flow using LiHMDS as the base.

Scheme 5: A semi-continuous synthesis of doravirine (49) involving a key aldol reaction.

Scheme 6: Enantioselective aldol reaction using 5-(pyrrolidin-2-yl)tetrazole (51) as catalyst in a microreact...

Scheme 7: Gröger's example of asymmetric aldol reaction in aqueous media.

Figure 6: Immobilised reagent column reactor types.

Scheme 8: Photoinduced thiol–ene coupling preparation of silica-supported 5-(pyrrolidin-2-yl)tetrazole 63 and...

Scheme 9: Continuous-flow approach for enantioselective aldol reactions using the supported catalyst 67.

Scheme 10: Ötvös’ employment of a solid-supported peptide aldol catalyst in flow.

Scheme 11: The use of proline tetrazole packed in a column for aldol reaction between cyclohexanone (65) and 2...

Scheme 12: Schematic diagram of an aminosilane-grafted Si-Zr-Ti/PAI-HF reactor for continuous-flow aldol and n...

Scheme 13: Continuous-flow condensation for the synthesis of the intermediate 76 to nabumetone (77) and Microi...

Scheme 14: Synthesis of ψ-Ionone (80) in continuous-flow via aldol condensation between citral (79) and aceton...

Scheme 15: Synthesis of β-methyl-ionones (83) from citral (79) in flow. The steps are separately described, an...

Scheme 16: Continuous-flow synthesis of 85 from 84 described by Gavriilidis et al.

Scheme 17: Continuous-flow scCO2 apparatus for the synthesis of 2-methylpentanal (87) and the self-condensed u...

Scheme 18: Chen’s two-step flow synthesis of coumarin (90).

Scheme 19: Pechmann condensation for the synthesis of 7-hydroxyxcoumarin (93) in flow. The setup extended to c...

Scheme 20: Synthesis of the dihydrojasmonate 35 exploiting nitro derivative proposed by Ballini et al.

Scheme 21: Silica-supported amines as heterogeneous catalyst for nitroaldol condensation in flow.

Scheme 22: Flow apparatus for the nitroaldol condensation of p-hydroxybenzaldehyde (102) to nitrostyrene 103 a...

Scheme 23: Nitroaldol reaction of 64 to 105 employing a quaternary ammonium functionalised PANF.

Scheme 24: Enantioselective nitroaldol condensation for the synthesis of 108 under flow conditions.

Scheme 25: Enatioselective synthesis of 1,2-aminoalcohol 110 via a copper-catalysed nitroaldol condensation.

Scheme 26: Examples of Knoevenagel condensations applied for fragrance components.

Scheme 27: Flow apparatus for Knoevenagel condensation described in 1989 by Venturello et al.

Scheme 28: Knoevenagel reaction using a coated multichannel membrane microreactor.

Scheme 29: Continuous-flow apparatus for Knoevenagel condensation employing sugar cane bagasse as support deve...

Scheme 30: Knoevenagel reaction for the synthesis of 131–135 in flow using an amine-functionalised silica gel. ...

Scheme 31: Continuous-flow synthesis of compound 137, a key intermediate for the synthesis of pregabalin (138)...

Scheme 32: Continuous solvent-free apparatus applied for the synthesis of compounds 140–143 using a TSE. Throu...

Scheme 33: Lewis et al. developed a spinning disc reactor for Darzens condensation of 144 and a ketone to furn...

Scheme 34: Some key industrial applications of conjugate additions in the F&F industry.

Scheme 35: Continuous-flow synthesis of 4-(2-hydroxyethyl)thiomorpholine 1,1-dioxide (156) via double conjugat...

Scheme 36: Continuous-flow system for Michael addition using CsF on alumina as the catalyst.

Scheme 37: Calcium chloride-catalysed asymmetric Michael addition using an immobilised chiral ligand.

Scheme 38: Continuous multistep synthesis for the preparation of (R)-rolipram (173). Si-NH2: primary amine-fun...

Scheme 39: Continuous-flow Michael addition using ion exchange resin Amberlyst® A26.

Scheme 40: Preparation of the heterogeneous catalyst 181 developed by Paixão et al. exploiting Ugi multicompon...

Scheme 41: Continuous-flow system developed by the Paixão’s group for the preparation of Michael asymmetric ad...

Scheme 42: Continuous-flow synthesis of nitroaldols catalysed by supported catalyst 184 developed by Wennemers...

Scheme 43: Heterogenous polystyrene-supported catalysts developed by Pericàs and co-workers.

Scheme 44: PANF-supported pyrrolidine catalyst for the conjugate addition of cyclohexanone (65) and trans-β-ni...

Scheme 45: Synthesis of (−)-paroxetine precursor 195 developed by Ötvös, Pericàs, and Kappe.

Scheme 46: Continuous-flow approach for the 5-step synthesis of (−)-oseltamivir (201) as devised by Hayashi an...

Scheme 47: Continuous-flow enzyme-catalysed Michael addition.

Scheme 48: Continuous-flow copper-catalysed 1,4 conjugate addition of Grignard reagents to enones. Reprinted w...

Scheme 49: A collection of commonly encountered hydrogenation reactions.

Figure 7: The ThalesNano H-Cube® continuous-flow hydrogenator.

Scheme 50: Chemoselective reduction of an α,β-unsaturated ketone using the H-Cube® reactor.

Scheme 51: Incorporation of Lindlar’s catalyst into the H-Cube® reactor for the reduction of an alkyne.

Scheme 52: Continuous-flow semi-hydrogenation of alkyne 208 to 209 using SACs with H-Cube® system.

Figure 8: The standard setups for tube-in-tube gas–liquid reactor units.

Scheme 53: Homogeneous hydrogenation of olefins using a tube-in-tube reactor setup.

Scheme 54: Recyclable heterogeneous flow hydrogenation system.

Scheme 55: Leadbeater’s reverse tube-in-tube hydrogenation system for olefin reductions.

Scheme 56: a) Hydrogenation using a Pd-immobilised microchannel reactor (MCR) and b) a representation of the i...

Scheme 57: Hydrogenation of alkyne 238 exploiting segmented flow in a Pd-immobilised capillary reactor.

Scheme 58: Continuous hydrogenation system for the preparation of cyrene (241) from (−)-levoglucosenone (240).

Scheme 59: Continuous hydrogenation system based on CSMs developed by Hornung et al.

Scheme 60: Chemoselective reduction of carbonyls (ketones over aldehydes) in flow.

Scheme 61: Continuous system for the semi-hydrogenation of 256 and 258, developed by Galarneau et al.

Scheme 62: Continuous synthesis of biodiesel fuel 261 from lignin-derived furfural acetone (260).

Scheme 63: Continuous synthesis of γ-valerolacetone (263) via CTH developed by Pineda et al.

Scheme 64: Continuous hydrogenation of lignin-derived biomass (products 265, 266, and 267) using a sustainable...

Scheme 65: Ru/C or Rh/C-catalysed hydrogenation of arene in flow as developed by Sajiki et al.

Scheme 66: Polysilane-immobilized Rh–Pt-catalysed hydrogenation of arenes in flow by Kobayashi et al.

Scheme 67: High-pressure in-line mixing of H2 for the asymmetric reduction of 278 at pilot scale with a 73 L p...

Figure 9: Picture of the PFR employed at Eli Lilly & Co. for the continuous hydrogenation of 278 [287]. Reprinted ...

Scheme 68: Continuous-flow asymmetric hydrogenation using Oppolzer's sultam 280 as chiral auxiliary.

Scheme 69: Some examples of industrially important oxidation reactions in the F&F industry. CFL: compact fluor...

Scheme 70: Gold-catalysed heterogeneous oxidation of alcohols in flow.

Scheme 71: Uozumi’s ARP-Pt flow oxidation protocol.

Scheme 72: High-throughput screening of aldehyde oxidation in flow using an in-line GC.

Scheme 73: Permanganate-mediated Nef oxidation of nitroalkanes in flow with the use of in-line sonication to p...

Scheme 74: Continuous-flow aerobic anti-Markovnikov Wacker oxidation.

Scheme 75: Continuous-flow oxidation of 2-benzylpyridine (312) using air as the oxidant.

Scheme 76: Continuous-flow photo-oxygenation of monoterpenes.

Scheme 77: A tubular reactor design for flow photo-oxygenation.

Scheme 78: Glucose oxidase (GOx)-mediated continuous oxidation of glucose using compressed air and the FFMR re...

Scheme 79: Schematic continuous-flow sodium hypochlorite/TEMPO oxidation of alcohols.

Scheme 80: Oxidation using immobilised TEMPO (344) was developed by McQuade et al.

Scheme 81: General protocol for the bleach/catalytic TBAB oxidation of aldehydes and alcohols.

Scheme 82: Continuous-flow PTC-assisted oxidation using hydrogen peroxide. The process was easily scaled up by...

Scheme 83: Continuous-flow epoxidation of cyclohexene (348) and in situ preparation of m-CPBA.

Scheme 84: Continuous-flow epoxidation using DMDO as oxidant.

Scheme 85: Mukayama aerobic epoxidation optimised in flow mode by the Favre-Réguillon group.

Scheme 86: Continuous-flow asymmetric epoxidation of derivatives of 359 exploiting a biomimetic iron catalyst.

Scheme 87: Continuous-flow enzymatic epoxidation of alkenes developed by Watts et al.

Scheme 88: Engineered multichannel microreactor for continuous-flow ozonolysis of 366.

Scheme 89: Continuous-flow synthesis of the vitamin D precursor 368 using multichannel microreactors. MFC: mas...

Scheme 90: Continuous ozonolysis setup used by Kappe et al. for the synthesis of various substrates employing ...

Scheme 91: Continuous-flow apparatus for ozonolysis as developed by Ley et al.

Scheme 92: Continuous-flow ozonolysis for synthesis of vanillin (2) using a film-shear flow reactor.

Scheme 93: Examples of preparative methods for ajoene (386) and allicin (388).

Scheme 94: Continuous-flow oxidation of thioanisole (389) using styrene-based polymer-supported peroxytungstat...

Scheme 95: Continuous oxidation of thiosulfinates using Oxone®-packed reactor.

Scheme 96: Continuous-flow electrochemical oxidation of thioethers.

Scheme 97: Continuous-flow oxidation of 400 to cinnamophenone (235).

Scheme 98: Continuous-flow synthesis of dehydrated material 401 via oxidation of methyl dihydrojasmonate (33).

Scheme 99: Some industrially important transformations involving Grignard reagents.

Scheme 100: Grachev et al. apparatus for continuous preparation of Grignard reagents.

Scheme 101: Example of fluidized Mg bed reactor with NMR spectrometer as on-line monitoring system.

Scheme 102: Continuous-flow synthesis of Grignard reagents and subsequent quenching reaction.

Figure 10: Membrane-based, liquid–liquid separator with integrated pressure control [52]. Adapted with permission ...

Scheme 103: Continuous-flow synthesis of 458, an intermediate to fluconazole (459).

Scheme 104: Continuous-flow synthesis of ketones starting from benzoyl chlorides.

Scheme 105: A Grignard alkylation combining CSTR and PFR technologies with in-line infrared reaction monitoring....

Scheme 106: Continuous-flow preparation of 469 from Grignard addition of methylmagnesium bromide.

Scheme 107: Continuous-flow synthesis of Grignard reagents 471.

Scheme 108: Preparation of the Grignard reagent 471 using CSTR and the continuous process for synthesis of the ...

Scheme 109: Continuous process for carboxylation of Grignard reagents in flow using tube-in-tube technology.

Scheme 110: Continuous synthesis of propargylic alcohols via ethynyl-Grignard reagent.

Scheme 111: Silica-supported catalysed enantioselective arylation of aldehydes using Grignard reagents in flow ...

Scheme 112: Acid-catalysed rearrangement of citral and dehydrolinalool derivatives.

Scheme 113: Continuous stilbene isomerisation with continuous recycling of photoredox catalyst.

Scheme 114: Continuous-flow synthesis of compound 494 as developed by Ley et al.

Scheme 115: Selected industrial applications of DA reaction.

Scheme 116: Multistep flow synthesis of the spirocyclic structure 505 via employing DA cycloaddition.

Scheme 117: Continuous-flow DA reaction developed in a plater flow reactor for the preparation of the adduct 508...

Scheme 118: Continuous-flow DA reaction using a silica-supported imidazolidinone organocatalyst.

Scheme 119: Batch vs flow for the DA reaction of (cyclohexa-1,5-dien-1-yloxy)trimethylsilane (513) with acrylon...

Scheme 120: Continuous-flow DA reaction between 510 and 515 using a shell-core droplet system.

Scheme 121: Continuous-flow synthesis of bicyclic systems from benzyne precursors.

Scheme 122: Continuous-flow synthesis of bicyclic scaffolds 527 and 528 for further development of potential ph...

Scheme 123: Continuous-flow inverse-electron hetero-DA reaction to pyridine derivatives such as 531.

Scheme 124: Comparison between batch and flow for the synthesis of pyrimidinones 532–536 via retro-DA reaction ...

Scheme 125: Continuous-flow coupled with ultrasonic system for preparation of ʟ-ascorbic acid derivatives 539 d...

Scheme 126: Two-step continuous-flow synthesis of triazole 543.

Scheme 127: Continuous-flow preparation of triazoles via CuAAC employing 546-based heterogeneous catalyst.

Scheme 128: Continuous-flow synthesis of compounds 558 through A3-coupling and 560 via AgAAC both employing the...

Scheme 129: Continuous-flow photoinduced [2 + 2] cycloaddition for the preparation of bicyclic derivatives of 5...

Scheme 130: Continuous-flow [2 + 2] and [5 + 2] cycloaddition on large scale employing a flow reactor developed...

Scheme 131: Continuous-flow preparation of the tricyclic structures 573 and 574 starting from pyrrole 570 via [...

Scheme 132: Continuous-flow [2 + 2] photocyclization of cinnamates.

Scheme 133: Continuous-flow preparation of cyclobutane 580 on a 5-plates photoreactor.

Scheme 134: Continuous-flow [2 + 2] photocycloaddition under white LED lamp using heterogeneous PCN as photocat...

Figure 11: Picture of the parallel tube flow reactor (PTFR) "The Firefly" developed by Booker-Milburn et al. a...

Scheme 135: Continuous-flow acid-catalysed [2 + 2] cycloaddition between silyl enol ethers and acrylic esters.

Scheme 136: Continuous synthesis of lactam 602 using glass column reactors.

Scheme 137: In situ generation of ketenes for the Staudinger lactam synthesis developed by Ley and Hafner.

Scheme 138: Application of [2 + 2 + 2] cycloadditions in flow employed by Ley et al.

Scheme 139: Examples of FC reactions applied in F&F industry.

Scheme 140: Continuous-flow synthesis of ibuprofen developed by McQuade et al.

Scheme 141: The FC acylation step of Jamison’s three-step ibuprofen synthesis.

Scheme 142: Synthesis of naphthalene derivative 629 via FC acylation in microreactors.

Scheme 143: Flow system for rapid screening of catalysts and reaction conditions developed by Weber et al.

Scheme 144: Continuous-flow system developed by Buorne, Muller et al. for DSD optimisation of the FC acylation ...

Scheme 145: Continuous-flow FC acylation of alkynes to yield β-chlorovinyl ketones such as 638.

Scheme 146: Continuous-flow synthesis of tonalide (619) developed by Wang et al.

Scheme 147: Continuous-flow preparation of acylated arene such as 290 employing Zr4+-β-zeolite developed by Kob...

Scheme 148: Flow system applied on an Aza-FC reaction catalysed by the thiourea catalyst 648.

Scheme 149: Continuous hydroformylation in scCO2.

Scheme 150: Two-step flow synthesis of aldehyde 655 through a sequential Heck reaction and subsequent hydroform...

Scheme 151: Single-droplet (above) and continuous (below) flow reactors developed by Abolhasani et al. for the ...

Scheme 152: Continuous hydroformylation of 1-dodecene (655) using a PFR-CSTR system developed by Sundmacher et ...

Scheme 153: Continuous-flow synthesis of the aldehyde 660 developed by Eli Lilly & Co. [32]. Adapted with permissio...

Scheme 154: Continuous asymmetric hydroformylation employing heterogenous catalst supported on carbon-based sup...

Scheme 155: Examples of acetylation in F&F industry: synthesis of bornyl (S,R,S-664) and isobornyl (S,S,S-664) ...

Scheme 156: Continuous-flow preparation of bornyl acetate (S,R,S-664) employing the oscillating flow reactor.

Scheme 157: Continuous-flow synthesis of geranyl acetate (666) from acetylation of geraniol (343) developed by ...

Scheme 158: 12-Ttungstosilicic acid-supported silica monolith-catalysed acetylation in flow.

Scheme 159: Continuous-flow preparation of cyclopentenone 676.

Scheme 160: Two-stage synthesis of coumarin (90) via acetylation of salicylaldehyde (88).

Scheme 161: Intensification process for acetylation of 5-methoxytryptamine (677) to melatonin (678) developed b...

Scheme 162: Examples of macrocyclic musky odorants both natural (679–681) and synthetic (682 and 683).

Scheme 163: Flow setup combined with microwave for the synthesis of macrocycle 686 via RCM.

Scheme 164: Continuous synthesis of 2,5-dihydro-1H-pyrroles via ring-closing metathesis.

Scheme 165: Continuous-flow metathesis of 485 developed by Leadbeater et al.

Figure 12: Comparison between RCM performed using different routes for the preparation of 696. On the left the...

Scheme 166: Continuous-flow RCM of 697 employed the solid-supported catalyst 698 developed by Grela, Kirschning...

Scheme 167: Continuous-flow RORCM of cyclooctene employing the silica-absorbed catalyst 700.

Scheme 168: Continuous-flow self-metathesis of methyl oleate (703) employing SILP catalyst 704.

Scheme 169: Flow apparatus for the RCM of 697 using a nanofiltration membrane for the recovery and reuse of the...

Scheme 170: Comparison of loadings between RCMs performed with different routes for the synthesis of 709.
Recent advances in palladium-catalysed asymmetric 1,4–additions of arylboronic acids to conjugated enones and chromones
- Jan Bartáček,
- Jan Svoboda,
- Martin Kocúrik,
- Jaroslav Pochobradský,
- Alexander Čegan,
- Miloš Sedlák and
- Jiří Váňa
Beilstein J. Org. Chem. 2021, 17, 1048–1085, doi:10.3762/bjoc.17.84
- chromone derivative for the total syntheses of (−)-caesalpinnone A and (−)-caesalpinflavan B (Scheme 12) [9]. Mechanistic studies of this catalytic system were also made by Stoltz’s group. A linear relationship between the ee of the catalyst and the product has been found [48]. That means that the
- catalytically relevant species is monomeric Pd–PyOx. This was further supported by a mass spectrometric study [52]. The catalytic cycle was also suggested in accordance with DFT calculations and mechanistic studies (Scheme 13) [48][49]. The key step for both, the enantioselectivity and turnover, is the

Graphical Abstract

Scheme 1: Synthesis of optically pure 4-phenylchroman-2-one [34].

Scheme 2: Synthesis of (R)-tolterodine [3].

Scheme 3: Catalytic cycle of the Pd(II)-catalysed 1,4-addition of organoboron reagents to enones [3,26,35].

Scheme 4: Enantioselective β-arylation of cyclohexanone [38].

Scheme 5: Application of L2/Pd(OAc)2 in the total synthesis of terpenes [8].

Scheme 6: Plausible catalytic cycle for the addition of phenylboronic acid to 2-cyclohexenone catalysed by L3...

Scheme 7: Microwave-assisted addition of phenylboronic acid to 2-cyclohexenone catalysed by L4/Pd2(dba)3·CHCl3...

Scheme 8: Plausible catalytic cycle of the addition of phenylboronic acid to 2-cyclohexenone catalysed by pal...

Scheme 9: Proposed catalytic cycle for the addition of phenylboronic acids to 2-cyclohexenone catalysed by Pd...

Scheme 10: Usage of addition reactions of boronic acids to various chromones in the syntheses of potentially a...

Scheme 11: Multigram-scale synthesis of ABBV-2222 [6].

Scheme 12: Application of the asymmetric addition of phenylboronic acid to a chromone derivative for the total...

Scheme 13: Plausible catalytic cycle for the addition of phenylboronic acid to 3-methyl-2-cyclohexenone cataly...

Scheme 14: Total syntheses of naturally occurring terpenoids [10,11].

Scheme 15: Use of the L9/Pd(TFA)2 catalytic system for the synthesis of intermediates of biologically active c...

Scheme 16: Usage of a Michael addition catalysed by L9/Pd(TFA)2 in the total synthesis of (–)-ar-tenuifolene [12].

Scheme 17: Synthesis of terpenoids by Michael addition to 3-methyl-2-cyclopentenone [13].

Scheme 18: Rh-catalysed isomerisation of 3-alkyl-3-arylcyclopentanones to 1-tetralones [53].

Scheme 19: Addition reaction of phenylboronic acid to 3-methyl-2-cyclohexenone catalysed by L9/Pd(TFA)2 in wat...

Scheme 20: Micellar nanoreactor PdL10c for the synthesis of flavanones [58].

Scheme 21: Plausible catalytic cycle for the desymmetrisation of polycyclic cyclohexenediones by the addition ...

Scheme 22: Attempt to use the catalytic system L2/Pd(TFA)2 for the addition of phenylboronic acid to 3-methyl-...

Scheme 23: Ring opening of an enantioenriched tetrahydropyran-2-one derivative as alternative strategy to line...

Scheme 24: Synthesis of biologically active compounds from addition products [14-16].

Scheme 25: Chiral 1,10-phenantroline derivative L15 as ligand for the Pd-catalysed addition reactions of pheny...

Scheme 26: The Rh-catalysed addition reaction of phenylboronic acid to a 3-substituted enone [20].

Scheme 27: Underdeveloped methodologies [14,15,65-67].

Scheme 28: Flowchart for the selection of the proper catalytic system.
Recent developments in enantioselective photocatalysis
- Callum Prentice,
- James Morrisson,
- Andrew D. Smith and
- Eli Zysman-Colman
Beilstein J. Org. Chem. 2020, 16, 2363–2441, doi:10.3762/bjoc.16.197

- carbonyl electrophilicity [105]. The use of chiral Lewis acids can induce asymmetry [106]. Yoon et al. applied this well-known form of catalysis to a photocatalytic system using enones 269 and 270 (Scheme 43a) [107]. Mechanistic studies of a closely related achiral reaction [20], showed this reaction

Graphical Abstract

Scheme 1: Amine/photoredox-catalysed α-alkylation of aldehydes with alkyl bromides bearing electron-withdrawi...

Scheme 2: Amine/HAT/photoredox-catalysed α-functionalisation of aldehydes using alkenes.

Scheme 3: Amine/cobalt/photoredox-catalysed α-functionalisation of ketones and THIQs.

Scheme 4: Amine/photoredox-catalysed α-functionalisation of aldehydes or ketones with imines. (a) Using keton...

Scheme 5: Bifunctional amine/photoredox-catalysed enantioselective α-functionalisation of aldehydes.

Scheme 6: Bifunctional amine/photoredox-catalysed α-functionalisation of aldehydes using amine catalysts via ...

Scheme 7: Amine/photoredox-catalysed RCA of iminium ion intermediates. (a) Synthesis of quaternary stereocent...

Scheme 8: Bifunctional amine/photoredox-catalysed RCA of enones in a radical chain reaction initiated by an i...

Scheme 9: Bifunctional amine/photoredox-catalysed RCA reactions of iminium ions with different radical precur...

Scheme 10: Bifunctional amine/photoredox-catalysed radical cascade reactions between enones and alkenes with a...

Scheme 11: Amine/photocatalysed photocycloadditions of iminium ion intermediates. (a) External photocatalyst u...

Scheme 12: Amine/photoredox-catalysed addition of acrolein (94) to iminium ions.

Scheme 13: Dual NHC/photoredox-catalysed acylation of THIQs.

Scheme 14: NHC/photocatalysed spirocyclisation via photoisomerisation of an extended Breslow intermediate.

Scheme 15: CPA/photoredox-catalysed aza-pinacol cyclisation.

Scheme 16: CPA/photoredox-catalysed Minisci-type reaction between azaarenes and α-amino radicals.

Scheme 17: CPA/photoredox-catalysed radical additions to azaarenes. (a) α-Amino radical or ketyl radical addit...

Scheme 18: CPA/photoredox-catalysed reduction of azaarene-derived substrates. (a) Reduction of ketones. (b) Ex...

Scheme 19: CPA/photoredox-catalysed radical coupling reactions of α-amino radicals with α-carbonyl radicals. (...

Scheme 20: CPA/photoredox-catalysed Povarov reaction.

Scheme 21: CPA/photoredox-catalysed reactions with imines. (a) Decarboxylative imine generation followed by Po...

Scheme 22: Bifunctional CPA/photocatalysed [2 + 2] photocycloadditions.

Scheme 23: PTC/photocatalysed oxygenation of 1-indanone-derived β-keto esters.

Scheme 24: PTC/photoredox-catalysed perfluoroalkylation of 1-indanone-derived β-keto esters via a radical chai...

Scheme 25: Bifunctional hydrogen bonding/photocatalysed intramolecular [2 + 2] photocycloadditions of quinolon...

Scheme 26: Bifunctional hydrogen bonding/photocatalysed intramolecular RCA cyclisation of a quinolone.

Scheme 27: Bifunctional hydrogen bonding/photocatalysed intramolecular [2 + 2] photocycloadditions of quinolon...

Scheme 28: Bifunctional hydrogen bonding/photocatalysed [2 + 2] photocycloaddition reactions. (a) First use of...

Scheme 29: Bifunctional hydrogen bonding/photocatalysed deracemisation of allenes.

Scheme 30: Bifunctional hydrogen bonding/photocatalysed deracemisation reactions. (a) Deracemisation of sulfox...

Scheme 31: Bifunctional hydrogen bonding/photocatalysed intramolecular [2 + 2] photocycloaddition of coumarins....

Scheme 32: Bifunctional hydrogen bonding/photocatalysed [2 + 2] photocycloadditions of quinolones. (a) Intramo...

Scheme 33: Hydrogen bonding/photocatalysed formal arylation of benzofuranones.

Scheme 34: Hydrogen bonding/photoredox-catalysed dehalogenative protonation of α,α-chlorofluoro ketones.

Scheme 35: Hydrogen bonding/photoredox-catalysed reductions. (a) Reduction of 1,2-diketones. (b) Reduction of ...

Scheme 36: Hydrogen bonding/HAT/photocatalysed deracemisation of cyclic ureas.

Scheme 37: Hydrogen bonding/HAT/photoredox-catalysed synthesis of cyclic sulfonamides.

Scheme 38: Hydrogen bonding/photoredox-catalysed reaction between imines and indoles.

Scheme 39: Chiral cation/photoredox-catalysed radical coupling of two α-amino radicals.

Scheme 40: Chiral phosphate/photoredox-catalysed hydroetherfication of alkenols.

Scheme 41: Chiral phosphate/photoredox-catalysed synthesis of pyrroloindolines.

Scheme 42: Chiral anion/photoredox-catalysed radical cation Diels–Alder reaction.

Scheme 43: Lewis acid/photoredox-catalysed cycloadditions of carbonyls. (a) Formal [2 + 2] cycloaddition of en...

Scheme 44: Lewis acid/photoredox-catalysed RCA reaction using a scandium Lewis acid between α-amino radicals a...

Scheme 45: Lewis acid/photoredox-catalysed RCA reaction using a copper Lewis acid between α-amino radicals and...

Scheme 46: Lewis acid/photoredox-catalysed synthesis of 1,2-amino alcohols from aldehydes and nitrones using a...

Scheme 47: Lewis acid/photocatalysed [2 + 2] photocycloadditions of enones and alkenes.

Scheme 48: Meggers’s chiral-at-metal catalysts.

Scheme 49: Lewis acid/photoredox-catalysed α-functionalisation of ketones with alkyl bromides bearing electron...

Scheme 50: Bifunctional Lewis acid/photoredox-catalysed radical coupling reaction using α-chloroketones and α-...

Scheme 51: Lewis acid/photocatalysed RCA of enones. (a) Using aldehydes as acyl radical precursors. (b) Other ...

Scheme 52: Bifunctional Lewis acid/photocatalysis for a photocycloaddition of enones.

Scheme 53: Lewis acid/photoredox-catalysed RCA reactions of enones using DHPs as radical precursors.

Scheme 54: Lewis acid/photoredox-catalysed functionalisation of β-ketoesters. (a) Hydroxylation reaction catal...

Scheme 55: Bifunctional copper-photocatalysed alkylation of imines.

Scheme 56: Copper/photocatalysed alkylation of imines. (a) Bifunctional copper catalysis using α-silyl amines....

Scheme 57: Bifunctional Lewis acid/photocatalysed intramolecular [2 + 2] photocycloaddition.

Scheme 58: Bifunctional Lewis acid/photocatalysed [2 + 2] photocycloadditions (a) Intramolecular cycloaddition...

Scheme 59: Bifunctional Lewis acid/photocatalysed rearrangement of 2,4-dieneones.

Scheme 60: Lewis acid/photocatalysed [2 + 2] cycloadditions of cinnamate esters and styrenes.

Scheme 61: Nickel/photoredox-catalysed arylation of α-amino acids using aryl bromides.

Scheme 62: Nickel/photoredox catalysis. (a) Desymmetrisation of cyclic meso-anhydrides using benzyl trifluorob...

Scheme 63: Nickel/photoredox catalysis for the acyl-carbamoylation of alkenes with aldehydes using TBADT as a ...

Scheme 64: Bifunctional copper/photoredox-catalysed C–N coupling between α-chloro amides and carbazoles or ind...

Scheme 65: Bifunctional copper/photoredox-catalysed difunctionalisation of alkenes with alkynes and alkyl or a...

Scheme 66: Copper/photoredox-catalysed decarboxylative cyanation of benzyl phthalimide esters.

Scheme 67: Copper/photoredox-catalysed cyanation reactions using TMSCN. (a) Propargylic cyanation (b) Ring ope...

Scheme 68: Palladium/photoredox-catalysed allylic alkylation reactions. (a) Using alkyl DHPs as radical precur...

Scheme 69: Manganese/photoredox-catalysed epoxidation of terminal alkenes.

Scheme 70: Chromium/photoredox-catalysed allylation of aldehydes.

Scheme 71: Enzyme/photoredox-catalysed dehalogenation of halolactones.

Scheme 72: Enzyme/photoredox-catalysed dehalogenative cyclisation.

Scheme 73: Enzyme/photoredox-catalysed reduction of cyclic imines.

Scheme 74: Enzyme/photocatalysed enantioselective reduction of electron-deficient alkenes as mixtures of (E)/(Z...

Scheme 75: Enzyme/photoredox catalysis. (a) Deacetoxylation of cyclic ketones. (b) Reduction of heteroaromatic...

Scheme 76: Enzyme/photoredox-catalysed synthesis of indole-3-ones from 2-arylindoles.

Scheme 77: Enzyme/HAT/photoredox catalysis for the DKR of primary amines.

Scheme 78: Bifunctional enzyme/photoredox-catalysed benzylic C–H hydroxylation of trifluoromethylated arenes.
Formation of an exceptionally stable ketene during phototransformations of bicyclo[2.2.2]oct-5-en-2-ones having mixed chromophores
- Asitanga Ghosh
Beilstein J. Org. Chem. 2020, 16, 2297–2303, doi:10.3762/bjoc.16.190

- position; ketene; mixed chromophores; Introduction Enones exhibit a rich and diverse photochemistry. The deep-seated photochemical rearrangements found in these systems have attracted numerous mechanistic studies. In this context, the photochemistry of α,β-enones A and β,γ-enones B (Figure 1) has become

Graphical Abstract

Figure 1: Model mixed enones.

Scheme 1: Quantitative photoisomerization of 1 to 2 in all types of solvents.

Scheme 2: Accepted mechanistic pathway for the photochemical transformations of 1.

Scheme 3: Photochemical reactions of 3a–g. Irradiation using a Hanovia medium pressure 450 W lamp with a pyre...

Figure 2: Enones used for this work.

Scheme 4: Synthesis of 7a,b.

Scheme 5: Photochemical reaction of 7a,b; a) solvent and conditions are given in Table 2.

Figure 3: Time-dependent absorption spectra of 10a,b in acetonitrile at rt.

Scheme 6: Conversion of ketene 10a to its methyl esters 11a,b.
Photosensitized direct C–H fluorination and trifluoromethylation in organic synthesis
- Shahboz Yakubov and
- Joshua P. Barham
Beilstein J. Org. Chem. 2020, 16, 2151–2192, doi:10.3762/bjoc.16.183
- : Here, we briefly summarize the importance of fluorine atoms in organic molecules in the context of medicinal chemistry, materials chemistry, analytical chemistry and in the mechanistic studies of synthetic reactions. The importance of fluorination reactions in organic synthesis is reviewed exhaustively
- (sp3)–H fluorination mechanistic studies: In order to unearth a greater understanding of their previously-reported photosensitized C(sp3)–H fluorination, Tan, Lu, Soo and co-workers employed a nanosecond-scale transient absorption (TA) spectroscopy and time-dependent density functional theory (TDDFT

Graphical Abstract

Figure 1: Fluorine-containing drugs.

Figure 2: Fluorinated agrochemicals.

Scheme 1: Selectivity of fluorination reactions.

Scheme 2: Different mechanisms of photocatalytic activation. Sub = substrate.

Figure 3: Jablonski diagram showing visible-light-induced energy transfer pathways: a) absorption, b) IC, c) ...

Figure 4: Schematic illustration of TTET.

Figure 5: Organic triplet PSCats.

Figure 6: Additional organic triplet PSCats.

Figure 7: A) Further organic triplet PSCats and B) transition metal triplet PSCats.

Figure 8: Different fluorination reagents grouped by generation.

Scheme 3: Synthesis of Selectfluor®.

Scheme 4: General mechanism of PS TTET C(sp3)–H fluorination.

Scheme 5: Selective benzylic mono- and difluorination using 9-fluorenone and xanthone PSCats, respectively.

Scheme 6: Chen’s photosensitized monofluorination: reaction scope.

Scheme 7: Chen’s photosensitized benzylic difluorination reaction scope.

Scheme 8: Photosensitized monofluorination of ethylbenzene on a gram scale.

Scheme 9: Substrate scope of Tan’s AQN-photosensitized C(sp3)–H fluorination.

Scheme 10: AQN-photosensitized C–H fluorination reaction on a gram scale.

Scheme 11: Reaction mechanism of the AQN-assisted fluorination.

Figure 9: 3D structures of the singlet ground and triplet excited states of Selectfluor®.

Scheme 12: Associated transitions for the activation of acetophenone by violet light.

Scheme 13: Ethylbenzene C–H fluorination with various PSCats and conditions.

Scheme 14: Effect of different PSCats on the C(sp3)–H fluorination of cyclohexane (39).

Scheme 15: Reaction scope of Chen’s acetophenone-photosensitized C(sp3)–H fluorination reaction.

Figure 10: a) Site-selectivity of Chen’s acetophenone-photosensitized C–H fluorination reaction [201]. b) Site-sele...

Scheme 16: Formation of the AQN–Selectfluor® exciplex Int1.

Scheme 17: Generation of the C3 2° pentane radical and the Selectfluor® N-radical cation from the exciplex.

Scheme 18: Hydrogen atom abstraction by the Selectfluor® N-radical cation from pentane to give the C3 2° penta...

Scheme 19: Fluorine atom transfer from Selectfluor® to the C3 2° pentane radical to yield 3-fluoropentane and ...

Scheme 20: Barrierless fluorine atom transfer from Int1 to the C3 2° pentane radical to yield 3-fluoropentane,...

Scheme 21: Ketone-directed C(sp3)–H fluorination.

Scheme 22: Ketone-directed fluorination through a 5- and a 6-membered transition state, respectively.

Scheme 23: Effect of different PSCats on the photosensitized C(sp3)–H fluorination of 47.

Scheme 24: Substrate scope of benzil-photoassisted C(sp3)–H fluorinations.

Scheme 25: A) Benzil-photoassisted enone-directed C(sp3)–H fluorination. B) Classification of the reaction mod...

Scheme 26: A) Xanthone-photoassisted ketal-directed C(sp3)–H fluorination. B) Substrate scope. C) C–H fluorina...

Scheme 27: Rationale for the selective HAT at the C2 C–H bond of galactose acetonide.

Scheme 28: Photosensitized C(sp3)–H benzylic fluorination of a peptide using different PSCats.

Scheme 29: Peptide scope of 5-benzosuberenone-photoassisted C(sp3)–H fluorinations.

Scheme 30: Continuous flow PS TTET monofluorination of 72.

Scheme 31: Photosensitized C–H fluorination of N-butylphthalimide as a PSX.

Scheme 32: Substrate scope and limitations of the PSX C(sp3)–H monofluorination.

Scheme 33: Substrate crossover monofluorination experiment.

Scheme 34: PS TTET mechanism proposed by Hamashima and co-workers.

Scheme 35: Photosensitized TFM of 78 to afford α-trifluoromethylated ketone 80.

Scheme 36: Substrate scope for photosensitized styrene TFM to give α-trifluoromethylated ketones.

Scheme 37: Control reactions for photosensitized TFM of styrenes.

Scheme 38: Reaction mechanism for photosensitized TFM of styrenes to afford α-trifluoromethylated ketones.

Scheme 39: Reaction conditions for TFMs to yield the cis- and the trans-product, respectively.

Scheme 40: Substrate scope of trifluoromethylated (E)-styrenes.

Scheme 41: Strategies toward trifluoromethylated (Z)-styrenes.

Scheme 42: Substrate scope of trifluoromethylated (Z)-styrenes.

Scheme 43: Reaction mechanism for photosensitized TFM of styrenes to afford E- or Z-products.
When metal-catalyzed C–H functionalization meets visible-light photocatalysis
- Lucas Guillemard and
- Joanna Wencel-Delord
Beilstein J. Org. Chem. 2020, 16, 1754–1804, doi:10.3762/bjoc.16.147

- photoredox catalysis (Figure 5) [73]. The coupling products were generally isolated in good yields, although this procedure required high temperature (120 °C). Remarkably, molecular oxygen was used as the terminal oxidizing agent of the overall C–H functionalization reaction. The mechanistic studies revealed
- synthetically meaningful σ-bromo esters. The mildness of the reaction conditions warranted tolerance of sensitive and/or biologically relevant sugar, menthol, and steroid motifs (Figure 37). The mechanistic studies conducted by Ackermann et al. clearly showed that the metallacyclic intermediates resulting from
- synthesis. The mechanistic studies suggested a unique mode of action of this catalytic system (Figure 45). In the initial step both aromatic coupling partners coordinate to the Mn catalyst, thus generating a photoactive complex. Visible-light photoexcitation of the Ar–Mn species and subsequent electron

Graphical Abstract

Figure 1: Concept of dual synergistic catalysis.

Figure 2: Classification of catalytic systems involving two catalysts.

Figure 3: General mechanism for the dual nickel/photoredox catalytic system.

Figure 4: General mechanisms for C–H activation catalysis involving different reoxidation strategies.

Figure 5: Indole synthesis via dual C–H activation/photoredox catalysis.

Figure 6: Proposed mechanism for the indole synthesis via dual catalysis.

Figure 7: Oxidative Heck reaction on arenes via the dual catalysis.

Figure 8: Proposed mechanism for the Heck reaction on arenes via dual catalysis.

Figure 9: Oxidative Heck reaction on phenols via the dual catalysis.

Figure 10: Proposed mechanism for the Heck reaction on phenols via dual catalysis.

Figure 11: Carbazole synthesis via dual C–H activation/photoredox catalysis.

Figure 12: Proposed mechanism for the carbazole synthesis via dual catalysis.

Figure 13: Carbonylation of enamides via the dual C–H activation/photoredox catalysis.

Figure 14: Proposed mechanism for carbonylation of enamides via dual catalysis.

Figure 15: Annulation of benzamides via the dual C–H activation/photoredox catalysis.

Figure 16: Proposed mechanism for the annulation of benzamides via dual catalysis.

Figure 17: Synthesis of indoles via the dual C–H activation/photoredox catalysis.

Figure 18: Proposed mechanism for the indole synthesis via dual catalysis.

Figure 19: General concept of dual catalysis merging C–H activation and photoredox catalysis.

Figure 20: The first example of dual catalysis merging C–H activation and photoredox catalysis.

Figure 21: Proposed mechanism for the C–H arylation with diazonium salts via dual catalysis.

Figure 22: Dual catalysis merging C–H activation/photoredox using diaryliodonium salts.

Figure 23: Direct arylation via the dual catalytic system reported by Xu.

Figure 24: Direct arylation via dual catalytic system reported by Balaraman.

Figure 25: Direct arylation via dual catalytic system reported by Guo.

Figure 26: C(sp3)–H bond arylation via the dual Pd/photoredox catalytic system.

Figure 27: Acetanilide derivatives acylation via the dual C–H activation/photoredox catalysis.

Figure 28: Proposed mechanism for the C–H acylation with α-ketoacids via dual catalysis.

Figure 29: Acylation of azobenzenes via the dual catalysis C–H activation/photoredox.

Figure 30: C2-acylation of indoles via the dual C–H activation/photoredox catalysis.

Figure 31: Proposed mechanism for the C2-acylation of indoles with aldehydes via dual catalysis.

Figure 32: C2-acylation of indoles via the dual C–H activation/photoredox catalysis.

Figure 33: Perfluoroalkylation of arenes via the dual C–H activation/photoredox catalysis.

Figure 34: Proposed mechanism for perfluoroalkylation of arenes via dual catalysis.

Figure 35: Sulfonylation of 1-naphthylamides via the dual C–H activation/photoredox catalysis.

Figure 36: Proposed mechanism for sulfonylation of 1-naphthylamides via dual catalysis.

Figure 37: meta-C–H Alkylation of arenes via visible-light metallaphotocatalysis.

Figure 38: Alternative procedure for meta-C–H alkylation of arenes via metallaphotocatalysis.

Figure 39: Proposed mechanism for meta-C–H alkylation of arenes via metallaphotocatalysis.

Figure 40: C–H borylation of arenes via visible-light metallaphotocatalysis.

Figure 41: Proposed mechanism for C–H borylation of arenes via visible-light metallaphotocatalysis.

Figure 42: Undirected C–H aryl–aryl cross coupling via dual gold/photoredox catalysis.

Figure 43: Proposed mechanism for the undirected C–H aryl–aryl cross-coupling via dual catalysis.

Figure 44: Undirected C–H arylation of (hetero)arenes via dual manganese/photoredox catalysis.

Figure 45: Proposed mechanism for the undirected arylation of (hetero)arenes via dual catalysis.

Figure 46: Photoinduced C–H arylation of azoles via copper catalysis.

Figure 47: Photo-induced C–H chalcogenation of azoles via copper catalysis.

Figure 48: Decarboxylative C–H adamantylation of azoles via dual cobalt/photoredox catalysis.

Figure 49: Proposed mechanism for the C–H adamantylation of azoles via dual catalysis.

Figure 50: General mechanisms for the “classical” (left) and Cu-free variant (right) Sonogoshira reaction.

Figure 51: First example of a dual palladium/photoredox catalysis for Sonogashira-type couplings.

Figure 52: Arylation of terminal alkynes with diazonium salts via dual gold/photoredox catalysis.

Figure 53: Proposed mechanism for the arylation of terminal alkynes via dual catalysis.

Figure 54: C–H Alkylation of alcohols promoted by H-atom transfer (HAT).

Figure 55: Proposed mechanism for the C–H alkylation of alcohols promoted by HAT.

Figure 56: C(sp3)–H arylation of latent nucleophiles promoted by H-atom transfer.

Figure 57: Proposed mechanism for the C(sp3)–H arylation of latent nucleophiles promoted by HAT.

Figure 58: Direct α-arylation of alcohols promoted by H-atom transfer.

Figure 59: Proposed mechanism for the direct α-arylation of alcohols promoted by HAT.

Figure 60: C–H arylation of amines via dual Ni/photoredox catalysis.

Figure 61: Proposed mechanism for the C–H arylation of amines via dual Ni/photoredox catalysis.

Figure 62: C–H functionalization of nucleophiles via excited ketone/nickel dual catalysis.

Figure 63: Proposed mechanism for the C–H functionalization enabled by excited ketones.

Figure 64: Selective sp3–sp3 cross-coupling promoted by H-atom transfer.

Figure 65: Proposed mechanism for the selective sp3–sp3 cross-coupling promoted by HAT.

Figure 66: Direct C(sp3)–H acylation of amines via dual Ni/photoredox catalysis.

Figure 67: Proposed mechanism for the C–H acylation of amines via dual Ni/photoredox catalysis.

Figure 68: C–H hydroalkylation of internal alkynes via dual Ni/photoredox catalysis.

Figure 69: Proposed mechanism for the C–H hydroalkylation of internal alkynes.

Figure 70: Alternative procedure for the C–H hydroalkylation of ynones, ynoates, and ynamides.

Figure 71: Allylic C(sp3)–H activation via dual Ni/photoredox catalysis.

Figure 72: Proposed mechanism for the allylic C(sp3)–H activation via dual Ni/photoredox catalysis.

Figure 73: Asymmetric allylation of aldehydes via dual Cr/photoredox catalysis.

Figure 74: Proposed mechanism for the asymmetric allylation of aldehydes via dual catalysis.

Figure 75: Aldehyde C–H functionalization promoted by H-atom transfer.

Figure 76: Proposed mechanism for the C–H functionalization of aldehydes promoted by HAT.

Figure 77: Direct C–H arylation of strong aliphatic bonds promoted by HAT.

Figure 78: Proposed mechanism for the C–H arylation of strong aliphatic bonds promoted by HAT.

Figure 79: Direct C–H trifluoromethylation of strong aliphatic bonds promoted by HAT.

Figure 80: Proposed mechanism for the C–H trifluoromethylation of strong aliphatic bonds.
In silico rationalisation of selectivity and reactivity in Pd-catalysed C–H activation reactions
- Liwei Cao,
- Mikhail Kabeshov,
- Steven V. Ley and
- Alexei A. Lapkin
Beilstein J. Org. Chem. 2020, 16, 1465–1475, doi:10.3762/bjoc.16.122
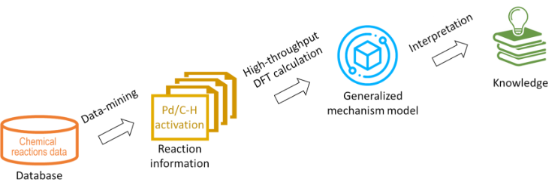
- transition metal coordination sphere; the energy of a new M–C bond formed and the thermodynamic stability of organometallic product. With new developments in computational chemistry, mechanistic studies using density functional theory (DFT) provide valuable insights into the reactivity of organometallic

Graphical Abstract

Figure 1: An approximate energy map for the electrophilic aromatic substitution mechanism.

Scheme 1: Schematic representation of the two mechanisms of Pd-catalysed C–H activation reaction considered i...
Recent synthesis of thietanes
- Jiaxi Xu
Beilstein J. Org. Chem. 2020, 16, 1357–1410, doi:10.3762/bjoc.16.116
- mechanistic studies. The reactivity of the substituted allenes towards triplet aromatic thiones was investigated. The product analysis revealed the formation of thietanes and occasionally of [4 + 2] cycloadducts (thiopyrans) generally in high overall yields. Steady-state measurements showed that electron

Graphical Abstract

Figure 1: Examples of biologically active thietane-containing molecules.

Figure 2: The diverse methods for the synthesis of thietanes.

Scheme 1: Synthesis of 1-(thietan-2-yl)ethan-1-ol (10) from 3,5-dichloropentan-2-ol (9).

Scheme 2: Synthesis of thietanose nucleosides 2,14 from 2,2-bis(bromomethyl)propane-1,3-diol (11).

Scheme 3: Synthesis of methyl 3-vinylthietane-3-carboxylate (19).

Scheme 4: Synthesis of 1,6-thiazaspiro[3.3]heptane (24).

Scheme 5: Synthesis of 6-amino-2-thiaspiro[3.3]heptane hydrochloride (28).

Scheme 6: Synthesis of optically active thietane 31 from vitamin C.

Scheme 7: Synthesis of an optically active thietane nucleoside from diethyl L-tartrate (32).

Scheme 8: Synthesis of thietane-containing spironucleoside 40 from 5-aldo-3-O-benzyl-1,2-O-isopropylidene-α-D...

Scheme 9: Synthesis of optically active 2-methylthietane-containing spironucleoside 43.

Scheme 10: Synthesis of a double-linked thietane-containing spironucleoside 48.

Scheme 11: Synthesis of two diastereomeric thietanose nucleosides via 2,4-di(benzyloxymethyl)thietane (49).

Scheme 12: Synthesis of the thietane-containing PI3k inhibitor candidate 54.

Scheme 13: Synthesis of the spirothietane 57 as the key intermediate to Nuphar sesquiterpene thioalkaloids.

Scheme 14: Synthesis of spirothietane 61 through a direct cyclic thioetherification of 3-mercaptopropan-1-ol.

Scheme 15: Synthesis of thietanes 66 from 1,3-diols 62.

Scheme 16: Synthesis of thietanylbenzimidazolone 75 from (iodomethyl)thiazolobenzimidazole 70.

Scheme 17: Synthesis of 2-oxa-6-thiaspiro[3.3]heptane (80) from bis(chloromethyl)oxetane 76 and thiourea.

Scheme 18: Synthesis of the thietane-containing glycoside, 2-O-p-toluenesulfonyl-4,6-thioanhydro-α-D-gulopyran...

Scheme 19: Synthesis of methyl 4,6-thioanhydro-α-D-glucopyranoside (89).

Scheme 20: Synthesis of thietane-fused α-D-galactopyranoside 93.

Scheme 21: Synthesis of thietane-fused α-D-gulopyranoside 100.

Scheme 22: Synthesis of 3,5-anhydro-3-thiopentofuranosides 104.

Scheme 23: Synthesis of anhydro-thiohexofuranosides 110, 112 and 113 from from 1,2:4,5-di-O-isopropylidene D-f...

Scheme 24: Synthesis of optically active thietanose nucleosides from D- and L-xyloses.

Scheme 25: Synthesis of thietane-fused nucleosides.

Scheme 26: Synthesis of 3,5-anhydro-3-thiopentofuranosides.

Scheme 27: Synthesis of 2-amino-3,5-anhydro-3-thiofuranoside 141.

Scheme 28: Synthesis of thietane-3-ols 145 from (1-chloromethyl)oxiranes 142 and hydrogen sulfide.

Scheme 29: Synthesis of thietane-3-ol 145a from chloromethyloxirane (142a).

Scheme 30: Synthesis of thietane-3-ols 145 from 2-(1-haloalkyl)oxiranes 142 and 147 with ammonium monothiocarb...

Scheme 31: Synthesis of 7-deoxy-5(20)thiapaclitaxel 154a, a thietane derivative of taxoids.

Scheme 32: Synthesis of 5(20)-thiadocetaxel 158 from 10-deacetylbaccatin III (155).

Scheme 33: Synthesis of thietane derivatives 162 as precursors for deoxythiataxoid synthesis through oxiraneme...

Scheme 34: Synthesis of 7-deoxy 5(20)-thiadocetaxel 154b.

Scheme 35: Mechanism for the formation of the thietane ring in 171 from oxiranes with vicinal leaving groups 1...

Scheme 36: Synthesis of cis-2,3-disubstituted thietane 175 from thiirane-2-methanol 172.

Scheme 37: Synthesis of a bridged thietane 183 from aziridine cyclohexyl tosylate 179 and ammonium tetrathiomo...

Scheme 38: Synthesis of thietanes via the photochemical [2 + 2] cycloaddition of thiobenzophenone 184a with va...

Scheme 39: Synthesis of spirothietanes through the photo [2 + 2] cycloaddition of cyclic thiocarbonyls with ol...

Scheme 40: Photochemical synthesis of spirothietane-thioxanthenes 210 from thioxanthenethione (208) and butatr...

Scheme 41: Synthesis of thietanes 213 from 2,4,6-tri(tert-butyl)thiobenzaldehyde (211) with substituted allene...

Scheme 42: Photochemical synthesis of spirothietanes 216 and 217 from N-methylthiophthalimide (214) with olefi...

Scheme 43: Synthesis of fused thietanes from quadricyclane with thiocarbonyl derivatives 219.

Scheme 44: Synthesis of tricyclic thietanes via the photo [2 + 2] cycloaddition of N-methyldithiosuccinimides ...

Scheme 45: Synthesis of tricyclic thietanes via the photo [2 + 2] cycloaddition of N-methylthiosuccinimide/thi...

Scheme 46: Synthesis of tricyclic thietanes via the photo [2 + 2] cycloaddition of N-alkylmonothiophthalimides...

Scheme 47: Synthesis of spirothietanes from dithiosuccinimides 223 with 2,3-dimethyl-2-butene (215a).

Scheme 48: Synthesis of thietanes 248a,b from diaryl thione 184b and ketene acetals 247a,b.

Scheme 49: Photocycloadditions of acridine-9-thiones 249 and pyridine-4(1H)-thione (250) with 2-methylacrynitr...

Scheme 50: Synthesis of thietanes via the photo [2 + 2] cycloaddition of mono-, di-, and trithiobarbiturates 2...

Scheme 51: Synthesis of spirothietanes via the photo [2 + 2] cycloaddition of 1,1,3-trimethyl-2-thioxo-1,2-dih...

Scheme 52: Synthesis of spirothietanes via the photo [2 + 2] cycloaddition of thiocoumarin 286 with olefins.

Scheme 53: Photochemical synthesis of thietanes 296–299 from semicyclic and acyclic thioimides 292–295 and 2,3...

Scheme 54: Photochemical synthesis of spirothietane 301 from 1,3,3-trimethylindoline-2-thione (300) and isobut...

Scheme 55: Synthesis of spirobenzoxazolethietanes 303 via the photo [2 + 2] cycloaddition of alkyl and aryl 2-...

Scheme 56: Synthesis of spirothietanes from tetrahydrothioxoisoquinolines 306 and 307 with olefins.

Scheme 57: Synthesis of spirothietanes from 1,3-dihydroisobenzofuran-1-thiones 311 and benzothiophene-1-thione...

Scheme 58: Synthesis of 2-triphenylsilylthietanes from phenyl triphenylsilyl thioketone (316) with electron-po...

Scheme 59: Diastereoselective synthesis of spiropyrrolidinonethietanes 320 via the photo [2 + 2] cycloaddition...

Scheme 60: Synthesis of bicyclic thietane 323 via the photo [2 + 2] cycloaddition of 2,4-dioxo-3,4-dihydropyri...

Scheme 61: Photo-induced synthesis of fused thietane-2-thiones 325 and 326 from silacyclopentadiene 324 and ca...

Scheme 62: Synthesis of highly strained tricyclic thietanes 328 via the intramolecular photo [2 + 2] cycloaddi...

Scheme 63: Synthesis of tri- and pentacyclic thietanes 330 and 332, respectively, through the intramolecular p...

Scheme 64: Synthesis of tricyclic thietanes 334 via the intramolecular photo [2 + 2] cycloaddition of N-vinylt...

Scheme 65: Synthesis of tricyclic thietanes 336 via the intramolecular photo [2 + 2] cycloaddition of N-but-3-...

Scheme 66: Synthesis of tricyclic thietanes via the intramolecular photo [2 + 2] cycloaddition of N-but-3-enyl...

Scheme 67: Synthesis of tetracyclic thietane 344 through the intramolecular photo [2 + 2] cycloaddition of N-[...

Scheme 68: Synthesis of tri- and tetracyclic thietanes 348, 350, and 351, through the intramolecular photo [2 ...

Scheme 69: Synthesis of tetracyclic fused thietane 354 via the photo [2 + 2] cycloaddition of vinyl 2-thioxo-3H...

Scheme 70: Synthesis of highly rigid thietane-fused β-lactams via the intramolecular photo [2 + 2] cycloadditi...

Scheme 71: Asymmetric synthesis of a highly rigid thietane-fused β-lactam 356a via the intramolecular photo [2...

Scheme 72: Diastereoselective synthesis of the thietane-fused β-lactams via the intramolecular photo [2 + 2] c...

Scheme 73: Asymmetric synthesis of thietane-fused β-lactams 356 via the intramolecular photo [2 + 2] cycloaddi...

Scheme 74: Synthesis of the bridged bis(trifluoromethyl)thietane from 2,2,4,4-tetrakis(trifluoromethyl)-1,3-di...

Scheme 75: Synthesis of the bridged-difluorothietane 368 from 2,2,4,4-tetrafluoro-1,3-dithietane (367) and qua...

Scheme 76: Synthesis of bis(trifluoromethyl)thietanes from 2,2,4,4-tetrakis(trifluoromethyl)-1,3-dithietane (3...

Scheme 77: Synthesis of 2,2-dimethylthio-4,4-di(trifluoromethyl)thietane (378) from 2,2,4,4-tetrakis(trifluoro...

Scheme 78: Formation of bis(trifluoromethyl)thioacetone (381) through nucleophilic attack of dithietane 363 by...

Scheme 79: Synthesis of 2,2-bis(trifluoromethyl)thietanes from 2,2,4,4-tetrakis(trifluoromethyl)-1,3-dithietan...

Scheme 80: Synthesis of the bridged bis(trifluoromethyl)thietane 364 from of 2,2,4,4-tetrakis(trifluoromethyl)...

Scheme 81: Synthesis of 2,4-diiminothietanes 390 from alkenimines and 4-methylbenzenesulfonyl isothiocyanate (...

Scheme 82: Synthesis of arylidene 2,4-diiminothietanes 393 starting from phosphonium ylides 391 and isothiocya...

Scheme 83: Synthesis of thietane-2-ylideneacetates 397 through a DABCO-catalyzed formal [2 + 2] cycloaddition ...

Scheme 84: Synthesis of 3-substituted thietanes 400 from (1-chloroalkyl)thiiranes 398.

Scheme 85: Synthesis of N-(thietane-3-yl)azaheterocycles 403 and 404 through reaction of chloromethylthiirane (...

Scheme 86: Synthesis of 3-sulfonamidothietanes 406 from sulfonamides and chloromethylthiirane (398a).

Scheme 87: Synthesis of N-(thietane-3-yl)isatins 408 from chloromethylthiirane (398a) and isatins 407.

Scheme 88: Synthesis of 3-(nitrophenyloxy)thietanes 410 from nitrophenols 409 and chloromethylthiirane (398a).

Scheme 89: Synthesis of N-aryl-N-(thietane-3-yl)cyanamides 412 from N-arylcyanamides 411 and chloromethylthiir...

Scheme 90: Synthesis of 1-(thietane-3-yl)pyrimidin-2,4(1H,3H)-diones 414 from chloromethylthiirane (398a) and ...

Scheme 91: Synthesis of 2,4-diiminothietanes 418 from 2-iminothiiranes 416 and isocyanoalkanes 415.

Scheme 92: Synthesis of 2-vinylthietanes 421 from thiiranes 419 and 3-chloroallyl lithium (420).

Scheme 93: Synthesis of thietanes from thiiranes 419 and trimethyloxosulfonium iodide 424.

Scheme 94: Mechanism for synthesis of thietanes 425 from thiiranes 419 and trimethyloxosulfonium iodide 424.

Scheme 95: Synthesis of functionalized thietanes from thiiranes and dimethylsulfonium acylmethylides.

Scheme 96: Mechanism for the rhodium-catalyzed synthesis of functionalized thietanes 429 from thiiranes 419 an...

Scheme 97: Synthesis of 3-iminothietanes 440 through thermal isomerization from 4,5-dihydro-1,3-oxazole-4-spir...

Scheme 98: Synthesis of thietanes 443 from 3-chloro-2-methylthiolane (441) through ring contraction.

Scheme 99: Synthesis of an optically active thietanose 447 from D-xylose involving a ring contraction.

Scheme 100: Synthesis of optically thietane 447 via the DAST-mediated ring contraction of 448.

Scheme 101: Synthesis of the optically thietane nucleoside 451 via the ring contraction of thiopentose in 450.

Scheme 102: Synthesis of spirothietane 456 from 3,3,5,5-tetramethylthiolane-2,4-dithione (452) and benzyne (453...

Scheme 103: Synthesis of thietanes 461 via photoisomerization of 2H,6H-thiin-3-ones 459.

Scheme 104: Phosphorodithioate-mediated synthesis of 1,4-diarylthietanes 465.

Scheme 105: Mechanism of the phosphorodithioate-mediated synthesis of 1,4-diarylthietanes 465.

Scheme 106: Phosphorodithioate-mediated synthesis of trisubstituted thietanes (±)-470.

Scheme 107: Mechanism on the phosphorodithioate-mediated synthesis of trisubstituted thietanes.

Scheme 108: Phosphorodithioate-mediated synthesis of thietanes (±)-475.

Scheme 109: Phosphorodithioate-mediated synthesis of 1,2-disubstituted thietanes from aldehydes 476 and acrylon...

Scheme 110: Phosphorodithioate-mediated synthesis of 1,2-disubstituted thietanes via a one-pot three-component ...

Scheme 111: Mechanism for the phosphorodithioate-mediated synthesis of 1,2-disubstituted thietanes via three-co...

Scheme 112: Phosphorodithioate-mediated synthesis of substituted 3-nitrothietanes.

Scheme 113: Mechanism on the phosphorodithioate-mediated synthesis of 1,2-disubstituted thietanes (±)-486.

Scheme 114: Asymmetric synthesis of (S)-2-phenylthietane (497).

Scheme 115: Asymmetric synthesis of optically active 2,4-diarylthietanes.

Scheme 116: Synthesis of 3-acetamidothietan-2-one 503 via the intramolecular thioesterification of 3-mercaptoal...

Scheme 117: Synthesis of 4-substituted thietan-2-one via the intramolecular thioesterification of 3-mercaptoalk...

Scheme 118: Synthesis of 4,4-disubstituted thietan-2-one 511 via the intramolecular thioesterification of the 3...

Scheme 119: Synthesis of a spirothietan-2-one 514 via the intramolecular thioesterification of 3-mercaptoalkano...

Scheme 120: Synthesis of thiatetrahydrolipstatin starting from (S)-(−)-epichlorohydrin ((S)-142a).

Scheme 121: Synthesis of 2-phenethyl-4-(propan-2-ylidene)thietane (520) from 5-bromo-6-methyl-1-phenylhept-5-en...

Scheme 122: Synthesis of 2-phenethyl-4-(propan-2-ylidene)thietane (520) directly from S-(5-bromo-6-methyl-1-phe...

Scheme 123: Synthesis of 2-alkylidenethietanes from S-(2-bromoalk-1-en-4-yl)thioacetates.

Scheme 124: Synthesis of 2-alkylidenethietanes from S-(2-bromo/chloroalk-1-en-4-yl)thiols.

Scheme 125: Synthesis of spirothietan-3-ol 548 from enone 545 and ammonium hydrosulfide.

Scheme 126: Asymmetric synthesis of the optically active thietanoside from cis-but-2-ene-1,4-diol (47).

Scheme 127: Synthesis of 2-alkylidenethietan-3-ols 557 via the fluoride-mediated cyclization of thioacylsilanes ...

Scheme 128: Synthesis of 2-iminothietanes via the reaction of propargylbenzene (558) and isothiocyanates 560 in...

Scheme 129: Synthesis of 2-benzylidenethietane 567 via the nickel complex-catalyzed electroreductive cyclizatio...

Scheme 130: Synthesis of 2-iminothietanes 569 via the photo-assisted electrocyclic reaction of N-monosubstitute...

Scheme 131: Synthesis of ethyl 3,4-diiminothietane-2-carboxylates from ethyl thioglycolate (570) and bis(imidoy...

Scheme 132: Synthesis of N-(thietan-3-yl)-α-oxoazaheterocycles from azaheterocyclethiones and chloromethyloxira...

Scheme 133: Synthesis of thietan-3-yl benzoate (590) via the nickel-catalyzed intramolecular reductive thiolati...

Scheme 134: Synthesis of 2,2-bis(trifluoromethyl)thietane from 3,3-bis(trifluoromethyl)-1,2-dithiolane.

Scheme 135: Synthesis of thietanes from enamines and sulfonyl chlorides.

Scheme 136: Synthesis of spirothietane 603 via the [2 + 3] cycloaddition of 2,2,4,4-tetramethylcyclobutane-1,3-...

Scheme 137: Synthesis of thietane (605) from 1-bromo-3-chloropropane and sulfur.
Oxime radicals: generation, properties and application in organic synthesis
- Igor B. Krylov,
- Stanislav A. Paveliev,
- Alexander S. Budnikov and
- Alexander O. Terent’ev
Beilstein J. Org. Chem. 2020, 16, 1234–1276, doi:10.3762/bjoc.16.107
- of the richest families of stable and persistent organic radicals with applications ranging from catalysis of selective oxidation processes and mechanistic studies to production of polymers, energy storage, magnetic materials design and spectroscopic studies of biological objects. Compared to other N

Graphical Abstract

Figure 1: Imine-N-oxyl radicals (IV) discussed in the present review and other classes of N-oxyl radicals (I–...

Figure 2: The products of decomposition of iminoxyl radicals generated from oximes by oxidation with Ag2O.

Scheme 1: Generation of oxime radicals and study of the kinetics of their decay by photolysis of the solution...

Scheme 2: Synthesis of di-tert-butyliminoxyl radical and its decomposition products.

Scheme 3: The proposed reaction pathway of the decomposition of di-tert-butyliminoxyl radical (experimentally...

Scheme 4: Monomolecular decomposition of the tert-butyl(triethylmethyl)oxime radical.

Scheme 5: The synthesis and stability of the most stable dialkyl oxime radicals – di-tert-butyliminoxyl and d...

Scheme 6: The formation of iminoxyl radicals from β-diketones under the action of NO2.

Scheme 7: Synthesis of the diacetyliminoxyl radical.

Scheme 8: Examples of long-living oxime radicals with electron-withdrawing groups and the conditions for thei...

Figure 3: The electronic structure iminoxyl radicals and their geometry compared to the corresponding oximes.

Figure 4: Bond dissociation enthalpies (kcal/mol) of oximes and N,N-disubstituted hydroxylamines calculated o...

Scheme 9: Examples demonstrating the low reactivity of the di-tert-butyliminoxyl radical towards the substrat...

Scheme 10: The reactions of di-tert-butyliminoxyl radical with unsaturated hydrocarbons involving hydrogen ato...

Scheme 11: Possible mechanisms of reaction of di-tert-butyliminoxyl radical with alkenes.

Scheme 12: Products of the reaction between di-tert-butyliminoxyl radical and phenol derivatives.

Scheme 13: The reaction of di-tert-butyliminoxyl radical with amines.

Scheme 14: Reaction of di-tert-butyliminoxyl radicals with organolithium reagents.

Scheme 15: Cross-dehydrogenative C–O coupling of 1,3-dicarbonyl compounds with oximes under the action of mang...

Scheme 16: Cross-dehydrogenative C–O coupling of 1,3-dicarbonyl compounds with oximes under the action of Cu(BF...

Scheme 17: Oxidative C–O coupling of benzylmalononitrile (47) with 3-(hydroxyimino)pentane-2,4-dione (19).

Scheme 18: The proposed mechanism of the oxidative coupling of benzylmalononitrile (47) with diacetyl oxime (19...

Scheme 19: Oxidative C–O coupling of pyrazolones with oximes under the action of Fe(ClO4)3.

Scheme 20: The reaction of diacetyliminoxyl radical with pyrazolones.

Scheme 21: Oxidative C–O coupling of oximes with acetonitrile, ketones, and esters.

Scheme 22: Intramolecular cyclizations of oxime radicals to form substituted isoxazolines or cyclic nitrones.

Scheme 23: TEMPO-mediated oxidative cyclization of oximes with C–H bond cleavage.

Scheme 24: Proposed reaction mechanism of oxidative cyclization of oximes with C–H bond cleavage.

Scheme 25: Selectfluor/Bu4NI-mediated C–H oxidative cyclization of oximes.

Scheme 26: Oxidative cyclization of N-benzyl amidoximes to 1,2,4-oxadiazoles.

Scheme 27: The formation of quinazolinone 73a from 5-phenyl-4,5-dihydro-1,2,4-oxadiazole 74 under air.

Scheme 28: DDQ-mediated oxidative cyclization of thiohydroximic acids.

Scheme 29: Plausible mechanism of the oxidative cyclization of thiohydroximic acids.

Scheme 30: Silver-mediated oxidative cyclization of α-halogenated ketoximes and 1,3-dicarbonyl compounds.

Scheme 31: Possible pathway of one-pot oxidative cyclization of α-halogenated ketoximes and 1,3-dicarbonyl com...

Scheme 32: T(p-F)PPT-catalyzed oxidative cyclization of oximes with the formation of 1,2,4-oxadiazolines.

Scheme 33: Intramolecular cyclization of iminoxyl radicals involving multiple C=C and N=N bonds.

Scheme 34: Oxidative cyclization of β,γ- and γ,δ-unsaturated oximes employing the DEAD or TEMPO/DEAD system wi...

Scheme 35: Cobalt-catalyzed aerobic oxidative cyclization of β,γ-unsaturated oximes.

Scheme 36: Manganese-catalyzed aerobic oxidative cyclization of β,γ-unsaturated oximes.

Scheme 37: Visible light photocatalytic oxidative cyclization of β,γ-unsaturated oximes.

Scheme 38: TBAI/TBHP-mediated radical cascade cyclization of the β,γ-unsaturated oximes.

Scheme 39: TBAI/TBHP-mediated radical cascade cyclization of vinyl isocyanides with β,γ-unsaturated oximes.

Scheme 40: tert-Butylnitrite-mediated oxidative cyclization of unsaturated oximes with the introduction of an ...

Scheme 41: Transformation of unsaturated oxime to oxyiminomethylisoxazoline via the confirmed dimeric nitroso ...

Scheme 42: tert-Butylnitrite-mediated oxidative cyclization of unsaturated oximes with the introduction of a n...

Scheme 43: Synthesis of cyano-substituted oxazolines from unsaturated oximes using the TBN/[RuCl2(p-cymene)]2 ...

Scheme 44: Synthesis of trifluoromethylthiolated isoxazolines from unsaturated oximes.

Scheme 45: Copper-сatalyzed oxidative cyclization of β,γ-unsaturated oximes with the introduction of an azido ...

Scheme 46: TBHP-mediated oxidative cascade cyclization of β,γ-unsaturated oximes and unsaturated N-arylamides.

Scheme 47: Copper-сatalyzed oxidative cyclization of unsaturated oximes with the introduction of an amino grou...

Scheme 48: TEMPO-mediated oxidative cyclization of unsaturated oximes followed by elimination.

Scheme 49: Oxidative cyclization of β,γ-unsaturated oximes with the introduction of a trifluoromethyl group.

Scheme 50: Oxidative cyclization of unsaturated oximes with the introduction of a nitrile group.

Scheme 51: Oxidative cyclization of β,γ-unsaturated oximes to isoxazolines with the introduction of a nitrile ...

Scheme 52: Oxidative cyclization of β,γ-unsaturated oximes to isoxazolines with the introduction of a sulfonyl...

Scheme 53: Oxidative cyclization of β,γ- and γ,δ-unsaturated oximes to isoxazolines with the introduction of a...

Scheme 54: Oxidative cyclization of β,γ-unsaturated oximes to isoxazolines with the introduction of a thiocyan...

Scheme 55: PhI(OAc)2-mediated oxidative cyclization of oximes with C–S and C–Se bond formation.

Scheme 56: PhI(OAc)2-mediated oxidative cyclization of unsaturated oximes accompanied by alkoxylation.

Scheme 57: PhI(OAc)2-mediated cyclization of unsaturated oximes to methylisoxazolines.

Scheme 58: Oxidative cyclization-alkynylation of unsaturated oximes.

Scheme 59: TEMPO-mediated oxidative cyclization of C-glycoside ketoximes to C-glycosylmethylisoxazoles.

Scheme 60: Silver-сatalyzed oxidative cyclization of β,γ-unsaturated oximes with formation of fluoroalkyl isox...

Scheme 61: Oxidative cyclization of β,γ-unsaturated oximes with the formation of haloalkyl isoxazolines.

Scheme 62: Cyclization of β,γ-unsaturated oximes into haloalkyl isoxazolines under the action of the halogenat...

Scheme 63: Synthesis of haloalkyl isoxazoles and cyclic nitrones via oxidative cyclization and 1,2-halogen shi...

Scheme 64: Electrochemical oxidative cyclization of diaryl oximes.

Scheme 65: Copper-сatalyzed cyclization and dioxygenation oximes containing a triple C≡C bond.

Scheme 66: Photoredox-catalyzed sulfonylation of β,γ-unsaturated oximes by sulfonyl hydrazides.

Scheme 67: Oxidative cyclization of β,γ-unsaturated oximes with introduction of sulfonate group.

Scheme 68: Ultrasound-promoted oxidative cyclization of β,γ-unsaturated oximes.
Photocatalysis with organic dyes: facile access to reactive intermediates for synthesis
- Stephanie G. E. Amos,
- Marion Garreau,
- Luca Buzzetti and
- Jerome Waser
Beilstein J. Org. Chem. 2020, 16, 1163–1187, doi:10.3762/bjoc.16.103
- converted the aryl and alkyl α-keto acids 17.1 to the 3-acylindoles 17.3 using rose bengal (OD15) as a photocatalyst under aerobic conditions. Mechanistic studies suggest that rose bengal (OD15) acts as an energy transfer sensitizer generating singlet oxygen. The authors proposed that the latter would be

Graphical Abstract

Figure 1: Selected examples of organic dyes. Mes-Acr+: 9-mesityl-10-methylacridinium, DCA: 9,10-dicyanoanthra...

Scheme 1: Activation modes in photocatalysis.

Scheme 2: Main strategies for the formation of C(sp3) radicals used in organophotocatalysis.

Scheme 3: Illustrative example for the photocatalytic oxidative generation of radicals from carboxylic acids:...

Scheme 4: Illustrative example for the photocatalytic reductive generation of C(sp3) radicals from redoxactiv...

Figure 2: Common substrates for the photocatalytic oxidative generation of C(sp3) radicals.

Scheme 5: Illustrative example for the photocatalytic oxidative generation of radicals from dihydropyridines ...

Scheme 6: Illustrative example for the photocatalytic oxidative generation of C(sp3) radicals from trifluorob...

Scheme 7: Illustrative example for the photocatalytic reductive generation of C(sp3) radicals from benzylic h...

Scheme 8: Illustrative example for the photocatalytic generation of C(sp3) radicals via direct HAT: the cross...

Scheme 9: Illustrative example for the photocatalytic generation of C(sp3) radicals via indirect HAT: the deu...

Scheme 10: Selected precursors for the generation of aryl radicals using organophotocatalysis.

Scheme 11: Illustrative example for the photocatalytic reductive generation of aryl radicals from aryl diazoni...

Scheme 12: Illustrative examples for the photocatalytic reductive generation of aryl radicals from haloarenes:...

Scheme 13: Illustrative example for the photocatalytic reductive generation of aryl radicals from aryl halides...

Scheme 14: Illustrative example for the photocatalytic reductive generation of aryl radicals from arylsulfonyl...

Scheme 15: Illustrative example for the reductive photocatalytic generation of aryl radicals from triaryl sulf...

Scheme 16: Main strategies towards acyl radicals used in organophotocatalysis.

Scheme 17: Illustrative example for the decarboxylative photocatalytic generation of acyl radicals from α-keto...

Scheme 18: Illustrative example for the oxidative photocatalytic generation of acyl radicals from acyl silanes...

Scheme 19: Illustrative example for the oxidative photocatalytic generation of carbamoyl radicals from 4-carba...

Scheme 20: Illustrative example of the photocatalytic HAT approach for the generation of acyl radicals from al...

Scheme 21: General reactivity of a) radical cations; b) radical anions; c) the main strategies towards aryl an...

Scheme 22: Illustrative example for the oxidative photocatalytic generation of alkene radical cations from alk...

Scheme 23: Illustrative example for the reductive photocatalytic generation of an alkene radical anion from al...

Figure 3: Structure of C–X radical anions and their neutral derivatives.

Scheme 24: Illustrative example for the photocatalytic reduction of imines and the generation of an α-amino C(...

Scheme 25: Illustrative example for the oxidative photocatalytic generation of aryl radical cations from arene...

Scheme 26: NCR classifications and generation.

Scheme 27: Illustrative example for the photocatalytic reductive generation of iminyl radicals from O-aryl oxi...

Scheme 28: Illustrative example for the photocatalytic oxidative generation of iminyl radicals from α-N-oxy ac...

Scheme 29: Illustrative example for the photocatalytic oxidative generation of iminyl radicals via an N–H bond...

Scheme 30: Illustrative example for the photocatalytic oxidative generation of amidyl radicals from Weinreb am...

Scheme 31: Illustrative example for the photocatalytic reductive generation of amidyl radicals from hydroxylam...

Scheme 32: Illustrative example for the photocatalytic reductive generation of amidyl radicals from N-aminopyr...

Scheme 33: Illustrative example for the photocatalytic oxidative generation of amidyl radicals from α-amido-ox...

Scheme 34: Illustrative example for the photocatalytic oxidative generation of aminium radicals: the N-aryltet...

Scheme 35: Illustrative example for the photocatalytic oxidative generation of nitrogen-centered radical catio...

Scheme 36: Illustrative example for the photocatalytic oxidative generation of nitrogen-centered radical catio...

Scheme 37: Illustrative example for the photocatalytic oxidative generation of hydrazonyl radical from hydrazo...

Scheme 38: Generation of O-radicals.

Scheme 39: Illustrative examples for the photocatalytic generation of O-radicals from N-alkoxypyridinium salts...

Scheme 40: Illustrative examples for the photocatalytic generation of O-radicals from alkyl hydroperoxides: th...

Scheme 41: Illustrative example for the oxidative photocatalytic generation of thiyl radicals from thiols: the...

Scheme 42: Main strategies and reagents for the generation of sulfonyl radicals used in organophotocatalysis.

Scheme 43: Illustrative example for the reductive photocatalytic generation of sulfonyl radicals from arylsulf...

Scheme 44: Illustrative example of a Cl atom abstraction strategy for the photocatalytic generation of sulfamo...

Scheme 45: Illustrative example for the oxidative photocatalytic generation of sulfonyl radicals from sulfinic...

Scheme 46: Illustrative example for the photocatalytic generation of electronically excited triplet states: th...

Scheme 47: Illustrative example for the photocatalytic generation of electronically excited triplet states: th...
Fluorinated phenylalanines: synthesis and pharmaceutical applications
- Laila F. Awad and
- Mohammed Salah Ayoup
Beilstein J. Org. Chem. 2020, 16, 1022–1050, doi:10.3762/bjoc.16.91

- catalytic amination of (E)-cinnamic acid The enzyme phenylalanine aminomutase (PAM) from Taxus chinensis catalyzes the stereoselective isomerization of α-phenylalanine to β-phenyalanine 111a–c. Mechanistic studies showed that (E)-cinnamic acid is an intermediate in this transformation [63]. Accordingly

Graphical Abstract

Figure 1: Categories I–V of fluorinated phenylalanines.

Scheme 1: Synthesis of fluorinated phenylalanines via Jackson’s method.

Scheme 2: Synthesis of all-cis-tetrafluorocyclohexylphenylalanines.

Scheme 3: Synthesis of ʟ-4-[sulfono(difluoromethyl)]phenylalanine (nPt: neopentyl, TCE: trichloroethyl).

Scheme 4: Synthesis of ʟ-4-[sulfono(difluoromethyl)]phenylalanine derivatives 17.

Scheme 5: Synthesis of fluorinated Phe analogues from Cbz-protected aminomalonates.

Scheme 6: Synthesis of tetrafluorophenylalanine analogues via the 3-methyl-4-imidazolidinone auxiliary 25.

Scheme 7: Synthesis of tetrafluoro-Phe derivatives via chiral auxiliary 31.

Scheme 8: Synthesis of 2,5-difluoro-Phe and 2,4,5-trifluoro-Phe via Schöllkopf reagent 34.

Scheme 9: Synthesis of 2-fluoro- and 2,6-difluoro Fmoc-Phe derivatives starting from chiral auxiliary 39.

Scheme 10: Synthesis of 2-[18F]FPhe via chiral auxiliary 43.

Scheme 11: Synthesis of FPhe 49a via photooxidative cyanation.

Scheme 12: Synthesis of FPhe derivatives via Erlenmeyer azalactone synthesis.

Scheme 13: Synthesis of (R)- and (S)-2,5-difluoro Phe via the azalactone method.

Scheme 14: Synthesis of 3-bromo-4-fluoro-(S)-Phe (65).

Scheme 15: Synthesis of [18F]FPhe via radiofluorination of phenylalanine with [18F]F2 or [18F]AcOF.

Scheme 16: Synthesis of 4-borono-2-[18F]FPhe.

Scheme 17: Synthesis of protected 4-[18F]FPhe via arylstannane derivatives.

Scheme 18: Synthesis of FPhe derivatives via intermediate imine formation.

Scheme 19: Synthesis of FPhe derivatives via Knoevenagel condensation.

Scheme 20: Synthesis of FPhe derivatives 88a,b from aspartic acid derivatives.

Scheme 21: Synthesis of 2-(2-fluoroethyl)phenylalanine derivatives 93 and 95.

Scheme 22: Synthesis of FPhe derivatives via Zn2+ complexes.

Scheme 23: Synthesis of FPhe derivatives via Ni2+ complexes.

Scheme 24: Synthesis of 3,4,5-trifluorophenylalanine hydrochloride (109).

Scheme 25: Synthesis of FPhe derivatives via phenylalanine aminomutase (PAM).

Scheme 26: Synthesis of (R)-2,5-difluorophenylalanine 115.

Scheme 27: Synthesis of β-fluorophenylalanine via 2-amino-1,3-diol derivatives.

Scheme 28: Synthesis of β-fluorophenylalanine derivatives via the oxazolidinone chiral auxiliary 122.

Scheme 29: Synthesis of β-fluorophenylalanine from pyruvate hemiketal 130.

Scheme 30: Synthesis of β-fluorophenylalanine (136) via fluorination of β-hydroxyphenylalanine (137).

Scheme 31: Synthesis of β-fluorophenylalanine from aziridine derivatives.

Scheme 32: Synthesis of β-fluorophenylalanine 136 via direct fluorination of pyruvate esters.

Scheme 33: Synthesis of β-fluorophenylalanine via fluorination of ethyl 3-phenylpyruvate enol using DAST.

Scheme 34: Synthesis of β-fluorophenylalanine derivatives using photosensitizer TCB.

Scheme 35: Synthesis of β-fluorophenylalanine derivatives using Selectflour and dibenzosuberenone.

Scheme 36: Synthesis of protected β-fluorophenylalanine via aziridinium intermediate 150.

Scheme 37: Synthesis of β-fluorophenylalanine derivatives via fluorination of α-hydroxy-β-aminophenylalanine d...

Scheme 38: Synthesis of β-fluorophenylalanine derivatives from α- or β-hydroxy esters 152a and 155.

Scheme 39: Synthesis of a series of β-fluoro-Phe derivatives via Pd-catalyzed direct fluorination of β-methyle...

Scheme 40: Synthesis of series of β-fluorinated Phe derivatives using quinoline-based ligand 162 in the Pd-cat...

Scheme 41: Synthesis of β,β-difluorophenylalanine derivatives from 2,2-difluoroacetaldehyde derivatives 164a,b....

Scheme 42: Synthesis of β,β-difluorophenylalanine derivatives via an imine chiral auxiliary.

Scheme 43: Synthesis of α-fluorophenylalanine derivatives via direct fluorination of protected Phe 174.

Figure 2: Structures of PET radiotracers of 18FPhe derivatives.

Figure 3: Structures of melfufen (179) and melphalan (180) anticancer drugs.

Figure 4: Structure of gastrazole (JB95008, 181), a CCK2 receptor antagonist.

Figure 5: Dual CCK1/CCK2 antagonist 182.

Figure 6: Structure of sitagliptin (183), an antidiabetic drug.

Figure 7: Structure of retaglpitin (184) and antidiabetic drug.

Figure 8: Structure of evogliptin (185), an antidiabetic drug.

Figure 9: Structure of LY2497282 (186) a DPP-4 inhibitor for the treatment of type II diabetes.

Figure 10: Structure of ulimorelin (187).

Figure 11: Structure of GLP1R (188).

Figure 12: Structures of Nav1.7 blockers 189 and 190.
Suzuki–Miyaura cross coupling is not an informative reaction to demonstrate the performance of new solvents
- James Sherwood
Beilstein J. Org. Chem. 2020, 16, 1001–1005, doi:10.3762/bjoc.16.89
- coupling. Specific mechanistic studies whereby the rate limiting step or mechanism changes according to the solvent remain a valid pursuit, as does measuring the palladium contamination in products [23]. However, the works of Watson [24], Denmark [25], and others [26][27], have already elucidated many of

Graphical Abstract

Scheme 1: (a) Case study 1, reaction of 4-bromotoluene; (b) case study 2, reaction of 4-bromophenylacetic aci...
Recent applications of porphyrins as photocatalysts in organic synthesis: batch and continuous flow approaches
- Rodrigo Costa e Silva,
- Luely Oliveira da Silva,
- Aloisio de Andrade Bartolomeu,
- Timothy John Brocksom and
- Kleber Thiago de Oliveira
Beilstein J. Org. Chem. 2020, 16, 917–955, doi:10.3762/bjoc.16.83
- -rich and electron-deficient groups in the arylsulfinic acid and alkyl sulfinates, such as sodium ethylsulfinate and sodium methylsulfinate (Scheme 19). The authors also showed that the methodology can be applied to the sulfonation of steroid drug arimistane in 45% yield (Scheme 20). The mechanistic
- studies on this reaction are ongoing with initial suggestions of the coupling between the sulfonyl radical and the radical alkene as a key step for this transformation. In 2019, Ghaffari-Moghaddam, Oveisi and co-workers reported the synthesis of a new multifunctional MOF, namely Fe@PCN-222(Fe), and its

Graphical Abstract

Figure 1: Chemical structures of the porphyrinoids and their absorption spectra: in bold are highlighted the ...

Figure 2: Photophysical and photochemical processes (Por = porphyrin). Adapted from [12,18].

Figure 3: Main dual photocatalysts and their oxidative/reductive excited state potentials, including porphyri...

Scheme 1: Photoredox alkylation of aldehydes with diazo acetates using porphyrins and a Ru complex. aUsing a ...

Scheme 2: Proposed mechanism for the alkylation of aldehydes with diazo acetates in the presence of TPP.

Scheme 3: Arylation of heteroarenes with aryldiazonium salts using TPFPP as photocatalyst, and corresponding ...

Scheme 4: A) Scope with different aryldiazonium salts and enol acetates. B) Photocatalytic cycles and compari...

Scheme 5: Photoarylation of isopropenyl acetate A) Comparison between batch and continuous-flow approaches an...

Scheme 6: Dehalogenation induced by red light using thiaporphyrin (STPP).

Scheme 7: Applications of NiTPP as both photoreductant and photooxidant.

Scheme 8: Proposed mechanism for obtaining tetrahydroquinolines by reductive quenching.

Scheme 9: Selenylation and thiolation of anilines.

Scheme 10: NiTPP as photoredox catalyst in oxidative and reductive quenching, in comparison with other photoca...

Scheme 11: C–O bond cleavage of 1-phenylethanol using a cobalt porphyrin (CoTMPP) under visible light.

Scheme 12: Hydration of terminal alkynes by RhIII(TSPP) under visible light irradiation.

Scheme 13: Regioselective photocatalytic hydro-defluorination of perfluoroarenes by RhIII(TSPP).

Scheme 14: Formation of 2-methyl-2,3-dihydrobenzofuran by intramolecular hydro-functionalization of allylpheno...

Scheme 15: Photocatalytic oxidative hydroxylation of arylboronic acids using UNLPF-12 as heterogeneous photoca...

Scheme 16: Photocatalytic oxidative hydroxylation of arylboronic acids using MOF-525 as heterogeneous photocat...

Scheme 17: Preparation of the heterogeneous photocatalyst CNH.

Scheme 18: Photoinduced sulfonation of alkenes with sulfinic acid using CNH as photocatalyst.

Scheme 19: Sulfonic acid scope of the sulfonation reactions.

Scheme 20: Regioselective sulfonation reaction of arimistane.

Scheme 21: Synthesis of quinazolin-4-(3H)-ones.

Scheme 22: Selective photooxidation of aromatic benzyl alcohols to benzaldehydes using Pt/PCN-224(Zn).

Scheme 23: Photooxidation of benzaldehydes to benzoic acids using Pt or Pd porphyrins.

Scheme 24: Photocatalytic reduction of various nitroaromatics using a Ni-MOF.

Scheme 25: Photoinduced cycloadditions of CO2 with epoxides by MOF1.

Figure 4: Electronic configurations of the species of oxygen. Adapted from [66].

Scheme 26: TPP-photocatalyzed generation of 1O2 and its application in organic synthesis. Adapted from [67-69].

Scheme 27: Pericyclic reactions involving singlet oxygen and their mechanisms. Adapted from [67].

Scheme 28: First scaled up ascaridole preparation from α-terpinene.

Scheme 29: Antimalarial drug synthesis using an endoperoxidation approach.

Scheme 30: Photooxygenation of colchicine.

Scheme 31: Synthesis of (−)-pinocarvone from abundant (+)-α-pinene.

Scheme 32: Seeberger’s semi-synthesis of artemisinin.

Scheme 33: Synthesis of artemisinin using TPP and supercritical CO2.

Scheme 34: Synthesis of artemisinin using chlorophyll a.

Scheme 35: Quercitol stereoisomer preparation.

Scheme 36: Photocatalyzed preparation of naphthoquinones.

Scheme 37: Continuous endoperoxidation of conjugated dienes and subsequent rearrangements leading to oxidized ...

Scheme 38: The Opatz group total synthesis of (–)-oxycodone.

Scheme 39: Biomimetic syntheses of rhodonoids A, B, E, and F.

Scheme 40: α-Photooxygenation of chiral aldehydes.

Scheme 41: Asymmetric photooxidation of indanone β-keto esters by singlet oxygen using PTC as a chiral inducer...

Scheme 42: Asymmetric photooxidation of both β-keto esters and β-keto amides by singlet oxygen using PTC-2 as ...

Scheme 43: Bifunctional photo-organocatalyst used for the asymmetric oxidation of β-keto esters and β-keto ami...

Scheme 44: Mechanism of singlet oxygen oxidation of sulfides to sulfoxides.

Scheme 45: Controlled oxidation of sulfides to sulfoxides using protonated porphyrins as photocatalysts. aIsol...

Scheme 46: Photochemical oxidation of sulfides to sulfoxides using PdTPFPP as photocatalyst.

Scheme 47: Controlled oxidation of sulfides to sulfoxides using SnPor@PAF as a photosensitizer.

Scheme 48: Syntheses of 2D-PdPor-COF and 3D-Pd-COF.

Scheme 49: Photocatalytic oxidation of A) thioanisole to methyl phenyl sulfoxide and B) various aryl sulfides,...

Scheme 50: General mechanism for oxidation of amines to imines.

Scheme 51: Oxidation of secondary amines to imines.

Scheme 52: Oxidation of secondary amines using Pd-TPFPP as photocatalyst.

Scheme 53: Oxidative amine coupling using UNLPF-12 as heterogeneous photocatalyst.

Scheme 54: Synthesis of Por-COF-1 and Por-COF-2.

Scheme 55: Photocatalytic oxidation of amines to imines by Por-COF-2.

Scheme 56: Photocyanation of primary amines.

Scheme 57: Synthesis of ᴅ,ʟ-tert-leucine hydrochloride.

Scheme 58: Photocyanation of catharanthine and 16-O-acetylvindoline using TPP.

Scheme 59: Photochemical α-functionalization of N-aryltetrahydroisoquinolines using Pd-TPFPP as photocatalyst.

Scheme 60: Ugi-type reaction with 1,2,3,4-tetrahydroisoquinoline using molecular oxygen and TPP.

Scheme 61: Ugi-type reaction with dibenzylamines using molecular oxygen and TPP.

Scheme 62: Mannich-type reaction of tertiary amines using PdTPFPP as photocatalyst.

Scheme 63: Oxidative Mannich reaction using UNLPF-12 as heterogeneous photocatalyst.

Scheme 64: Transformation of amines to α-cyanoepoxides and the proposed mechanism.
Cation-induced ring-opening and oxidation reaction of photoreluctant spirooxazine–quinolizinium conjugates
- Phil M. Pithan,
- Sören Steup and
- Heiko Ihmels
Beilstein J. Org. Chem. 2020, 16, 904–916, doi:10.3762/bjoc.16.82

- indolinonaphthoxazoles. Herein, we present the synthesis of, and investigation on the conjugates 3a and 3b, as well as mechanistic studies on the metal ion-induced formation of the derivatives 4a and 4b, along with the pertinent spectroscopic data of the products. Results and Discussion Synthesis The spirooxazine

Graphical Abstract

Scheme 1: Photo- or cation-induced ring-opening reaction of spirooxazine 1aSO; Mn+ = Pb2+, La3+, Eu3+, Tb3+ [17].

Scheme 2: Synthesis of the spirooxazine–quinolizinium conjugates 3a and 3b.

Figure 1: Colors of the solutions resulting from the addition of metal ions (c = 50 µM) to derivative 3a (c =...

Figure 2: Spectrophotometric titration of 3a (A) and 3b (B) (c = 20 µM) with Cu(BF4)2 (c = 2.44 mM) in MeCN. ...

Figure 3: Absorption (A) and fluorescence spectrum (B) of 3a in MeCN (c = 5 µM) in the absence (black) and in...

Figure 4: Emission colors of solutions resulting from the addition of metal ions (c = 50 µM) to derivative 3a...

Scheme 3: Cu2+-induced formation of the oxazole derivatives 4a and 4b.

Figure 5: 1H NMR spectra (600 MHz, 6.4–9.4 ppm) of 3a (c = 2.0 mM) in the absence (A) and in the presence (B–...

Figure 6: Spectral changes of 3a (c = 20 µM) upon the addition of Cu2+ (A) and Fe3+ (B) (cM+ = 60 µM) in MeCN...

Scheme 4: Proposed mechanism for the formation of the oxazole derivatives 4a and 4b (cf. Scheme 3); Mn+ = Cu2+, Fe3+,...
Copper catalysis with redox-active ligands
- Agnideep Das,
- Yufeng Ren,
- Cheriehan Hessin and
- Marine Desage-El Murr
Beilstein J. Org. Chem. 2020, 16, 858–870, doi:10.3762/bjoc.16.77

- to benzoxepines and 2,2’-biphenols through catalyst control (Scheme 5, top) [20]. Extensive mechanistic studies evidenced a biomimetic pathway involving a key CuII-semiquinone intermediate in which the substrate becomes activated as a redox-active ligand (Scheme 5, bottom) [21]. This radical CuII

Graphical Abstract

Scheme 1: Copper complexes with amidophenolate type benzoxazole ligands for alcohol oxidations.

Scheme 2: Copper-catalyzed aerobic oxidation of alcohols and representative substrate scope.

Scheme 3: Introduction of H-bonding network in the ligand coordination sphere.

Scheme 4: Well-defined isatin copper complexes.

Scheme 5: Catalyst control in the biomimetic phenol ortho-oxidation.

Scheme 6: Structural diversity accessible by direct functionalization.

Scheme 7: Copper-catalyzed trifluoromethylation of heteroaromatics with redox-active iminosemiquinone ligands....

Scheme 8: Reversal of helical chirality upon redox stimuli and enantioselective Michael addition with a redox...

Scheme 9: Interaction of guanidine-copper catalyst with oxygen and representative coupling products. a4 mol %...

Scheme 10: Access to 1,2-oxy-aminoarenes by copper-catalyzed phenol–amine coupling.

Scheme 11: Copper-catalyzed aziridination through molecular spin catalysis with redox-active iminosemiquinone ...

Scheme 12: Nitrogen-group and carbon-group transfer in copper-catalyzed aziridination and cyclopropanation thr...
Aldehydes as powerful initiators for photochemical transformations
- Maria A. Theodoropoulou,
- Nikolaos F. Nikitas and
- Christoforos G. Kokotos
Beilstein J. Org. Chem. 2020, 16, 833–857, doi:10.3762/bjoc.16.76
- irradiation, supporting the other two pathways. Also, mechanistic studies by Cui et al. showed that the energy levels of the reactions (1) and (2) in Scheme 5 were closely tied, so distinguishing them is not always trivial [32]. The triplet state quenching of 8 and related compounds, such as aryl ketones, has

Graphical Abstract

Scheme 1: Norrish type I and II dissociations.

Scheme 2: Proposed radical pair formation after the photolysis of benzaldehyde (8).

Scheme 3: Aldehydes in the Paterno–Büchi reaction.

Scheme 4: 2,3-Diazabicyclo[2.2.1]hept-2-ene (DBH).

Scheme 5: Dissociation pathways of benzaldehyde.

Scheme 6: Reactions that lead to polarized products detectable by CIDNP.

Scheme 7: MMA (26), DEABP (27), and Michler’s ketone (28).

Scheme 8: Radical intermediates of DEABP.

Scheme 9: Photoinitiated polymerization of monomeric MMA (26) using the quinoxalines 32 and benzaldehyde (8).

Scheme 10: Acetone (4) and formaldehyde (35) as photografting initiators.

Scheme 11: Photografting by employing acetaldehyde (36) as the photoinitiator.

Scheme 12: Proposed photolysis mechanism for aliphatic ketones 44 and formaldehyde (35).

Scheme 13: Initiator 50, reductant 51, and benzaldehyde derivatives 52–54 for the polymerization of the methac...

Scheme 14: Proposed mechanism of the photomediated atom transfer radical polymerization employing the benzalde...

Scheme 15: cis/trans isomerization employing triplet states of photosensitizers.

Scheme 16: Salicylaldehyde (68) forms an internal hydrogen bond.

Scheme 17: Olefin isomerization via energy transfer from a carbonyl compound.

Scheme 18: Mechanistic pathways for the Paterno–Büchi reaction.

Scheme 19: Isomeric oxetanes formed after photochemical addition of aryl aldehydes to 2-butenes.

Scheme 20: Rotation of the C3–C4 bond of the biradical intermediate may lead to all four conformations.

Scheme 21: Photolysis products of benzaldehyde (8) in different solvents. a) In benzene or ethanol. b) In hex-...

Scheme 22: N-tert-Butylbenzamide formation proceeds via a benzoyl radical.

Scheme 23: Photochemical pinacol coupling.

Scheme 24: Photochemical ATRA catalyzed by 4-anisaldehyde (52).

Scheme 25: Proposed triplet sensitization mechanism of the ATRA reaction in the presence of 4-anisaldehyde (52...

Scheme 26: Benzaldehyde-mediated photoredox CDC reaction: compatible amides and ethers.

Scheme 27: Photoredox cross-dehydrogenative coupling (CDC) conditions and proposed reaction mechanism.

Scheme 28: Optimized conditions for the photoredox merger reaction.

Scheme 29: Proposed mechanism for the C(sp3)–H alkylation/arylation of ethers.

Scheme 30: Substrate scope for the photochemical alkylation of ethers.

Scheme 31: C(sp3)–H Functionalization of N-containing molecules.

Scheme 32: Substrate scope for the photochemical alkylation of N-containing molecules.

Scheme 33: Additional products yielded by the photochemical alkylation reaction of N-containing molecules.

Scheme 34: C(sp3)–H functionalization of thioethers.

Scheme 35: Proposed mechanism for the C(sp3)–H alkylation/arylation of N-containing molecules and thioethers.

Scheme 36: Hydroacylation using 4-cyanobenzaldehyde (53) as the photoinitiator.

Scheme 37: Selectivity for the formation of the α,α-disubstituted aldehydes.

Scheme 38: Substrate scope for the photochemical addition of aldehydes to Michael acceptors.

Scheme 39: Proposed mechanism for the hydroacylation of Michael acceptors using 4-cyanobenzaldehyde (53) as th...

Scheme 40: Catalytic arylation of aromatic aldehydes by aryl bromides in which the reaction product acts as th...

Scheme 41: Proposed mechanism for the catalytic arylation of benzaldehydes by aryl bromides in which the react...

Scheme 42: Functionalization of the chiral cyclobutanes 180.

Scheme 43: Optimized reaction conditions and proposed mechanism for the sulfonylcyanation of cyclobutenes.
Preparation of 2-phospholene oxides by the isomerization of 3-phospholene oxides
- Péter Bagi,
- Réka Herbay,
- Nikolett Péczka,
- Zoltán Mucsi,
- István Timári and
- György Keglevich
Beilstein J. Org. Chem. 2020, 16, 818–832, doi:10.3762/bjoc.16.75
- more and more dominant role as the catalysis of the 3-phospholene oxide (1) 2-phospholene oxide (4) rearrangement, which explains the change in the reaction rate in the kinetic experiments. Mechanistic studies of the isomerization of 3-phospholene oxides 1 under acidic conditions The isomerization of 3

Graphical Abstract

Figure 1: Examples for catalytically or biologically active molecules containing five-membered P-heterocyclic...

Scheme 1: Comparison of the isomerization of 1-phenyl-3-phospholene oxide (5), 1-phenyl-3-methyl-3-phospholen...

Scheme 2: Three possible reaction mechanisms considered in the theoretical studies for the isomerization of 3...

Figure 2: The full time experimental kinetic curves (a); The initial part of the kinetic curves of 1c–f and 1h...

Scheme 3: Computed reaction mechanism of the 3-phospholene oxide (1) 2-phospholene oxide (4) isomerization un...

Scheme 4: Computed reaction mechanism of the 3-phospholene oxide (1) 2-phospholene oxide (4) isomerization un...
Recent advances in Cu-catalyzed C(sp3)–Si and C(sp3)–B bond formation
- Balaram S. Takale,
- Ruchita R. Thakore,
- Elham Etemadi-Davan and
- Bruce H. Lipshutz
Beilstein J. Org. Chem. 2020, 16, 691–737, doi:10.3762/bjoc.16.67

- featuring a 1,4-borylation of α,β-unsaturated compounds 405 was reported by Hosomi in the presence of Cu(OTf)2·C6H6/(t-Bu)3P in DMF. No reaction occurred using (CuOTf)2·C6H6 in the absence of (t-Bu)3P. Mechanistic studies showed that the coordination of phosphorus(III) to Cu(I) accelerated the reaction, as
- an appropriate electrophile led to C–C bond formation, ultimately delivering chiral tertiary alcohols. Mechanistic studies and DFT calculations showed that an in situ-formed borylcopper(I) species is responsible for the 1,2-addition (Scheme 73) [136]. C,O-Diboration of ketones 464 was explored using

Graphical Abstract

Scheme 1: Pharmaceuticals possessing a silicon or boron atom.

Scheme 2: The first Cu-catalyzed C(sp3)–Si bond formation.

Scheme 3: Conversion of benzylic phosphate 6 to the corresponding silane.

Scheme 4: Conversion of alkyl triflates to alkylsilanes.

Scheme 5: Conversion of secondary alkyl triflates to alkylsilanes.

Scheme 6: Conversion of alkyl iodides to alkylsilanes.

Scheme 7: Trapping of intermediate radical through cascade reaction.

Scheme 8: Radical pathway for conversion of alkyl iodides to alkylsilanes.

Scheme 9: Conversion of alkyl ester of N-hydroxyphthalimide to alkylsilanes.

Scheme 10: Conversion of gem-dibromides to bis-silylalkanes.

Scheme 11: Conversion of imines to α-silylated amines (A) and the reaction pathway (B).

Scheme 12: Conversion of N-tosylimines to α-silylated amines.

Scheme 13: Screening of diamine ligands.

Scheme 14: Conversion of N-tert-butylsulfonylimines to α-silylated amines.

Scheme 15: Conversion of aldimines to nonracemic α-silylated amines.

Scheme 16: Conversion of N-tosylimines to α-silylated amines.

Scheme 17: Reaction pathway [A] and conversion of aldehydes to α-silylated alcohols [B].

Scheme 18: Conversion of aldehydes to benzhydryl silyl ethers.

Scheme 19: Conversion of ketones to 1,2-diols (A) and conversion of imines to 1,2-amino alcohols (B).

Scheme 20: Ligand screening (A) and conversion of aldehydes to α-silylated alcohols (B).

Scheme 21: Conversion of aldehydes to α-silylated alcohols.

Scheme 22: 1,4-Additions to α,β-unsaturated ketones.

Scheme 23: 1,4-Additions to unsaturated ketones to give β-silylated derivatives.

Scheme 24: Additions onto α,β-unsaturated lactones to give β-silylated lactones.

Scheme 25: Conversion of α,β-unsaturated to β-silylated lactams.

Scheme 26: Conversion of N-arylacrylamides to silylated oxindoles.

Scheme 27: Conversion of α,β-unsaturated carbonyl compounds to silylated tert-butylperoxides.

Scheme 28: Catalytic cycle for Cu(I) catalyzed α,β-unsaturated compounds.

Scheme 29: Conversion of p-quinone methides to benzylic silanes.

Scheme 30: Conversion of α,β-unsaturated ketimines to regio- and stereocontrolled allylic silanes.

Scheme 31: Conversion of α,β-unsaturated ketimines to enantioenriched allylic silanes.

Scheme 32: Regioselective conversion of dienedioates to allylic silanes.

Scheme 33: Conversion of alkenyl-substituted azaarenes to β-silylated adducts.

Scheme 34: Conversion of conjugated benzoxazoles to enantioenriched β-silylated adducts.

Scheme 35: Conversion of α,β-unsaturated carbonyl indoles to α-silylated N-alkylated indoles.

Scheme 36: Conversion of β-amidoacrylates to α-aminosilanes.

Scheme 37: Conversion of α,β-unsaturated ketones to enantioenriched β-silylated ketones, nitriles, and nitro d...

Scheme 38: Regio-divergent silacarboxylation of allenes.

Scheme 39: Silylation of diazocarbonyl compounds, (A) asymmetric and (B) racemic.

Scheme 40: Enantioselective hydrosilylation of alkenes.

Scheme 41: Conversion of 3-acylindoles to indolino-silanes.

Scheme 42: Proposed mechanism for the silylation of 3-acylindoles.

Scheme 43: Silyation of N-chlorosulfonamides.

Scheme 44: Conversion of acyl silanes to α-silyl alcohols.

Scheme 45: Conversion of N-tosylaziridines to β-silylated N-tosylamines.

Scheme 46: Conversion of N-tosylaziridines to silylated N-tosylamines.

Scheme 47: Conversion of 3,3-disubstituted cyclopropenes to silylated cyclopropanes.

Scheme 48: Conversion of conjugated enynes to 1,3-bis(silyl)propenes.

Scheme 49: Proposed sequence for the Cu-catalyzed borylation of substituted alkenes.

Scheme 50: Cu-catalyzed synthesis of nonracemic allylic boronates.

Scheme 51: Cu–NHC catalyzed synthesis of α-substituted allylboronates.

Scheme 52: Synthesis of α-chiral (γ-alkoxyallyl)boronates.

Scheme 53: Cu-mediated formation of nonracemic cis- or trans- 2-substituted cyclopropylboronates.

Scheme 54: Cu-catalyzed synthesis of γ,γ-gem-difluoroallylboronates.

Scheme 55: Cu-catalyzed hydrofunctionalization of internal alkenes and vinylarenes.

Scheme 56: Cu-catalyzed Markovnikov and anti-Markovnikov borylation of alkenes.

Scheme 57: Cu-catalyzed borylation/ortho-cyanation/Cope rearrangement.

Scheme 58: Borylfluoromethylation of alkenes.

Scheme 59: Cu-catalyzed synthesis of tertiary nonracemic alcohols.

Scheme 60: Synthesis of densely functionalized and synthetically versatile 1,2- or 4,3-borocyanated 1,3-butadi...

Scheme 61: Cu-catalyzed trifunctionalization of allenes.

Scheme 62: Cu-catalyzed selective arylborylation of arenes.

Scheme 63: Asymmetric borylative coupling between styrenes and imines.

Scheme 64: Regio-divergent aminoboration of unactivated terminal alkenes.

Scheme 65: Cu-catalyzed 1,4-borylation of α,β-unsaturated ketones.

Scheme 66: Cu-catalyzed protodeboronation of α,β-unsaturated ketones.

Scheme 67: Cu-catalyzed β-borylation of α,β-unsaturated imines.

Scheme 68: Cu-catalyzed synthesis of β-trifluoroborato carbonyl compounds.

Scheme 69: Asymmetric 1,4-borylation of α,β-unsaturated carbonyl compounds.

Scheme 70: Cu-catalyzed ACB and ACA reactions of α,β-unsaturated 2-acyl-N-methylimidazoles.

Scheme 71: Cu-catalyzed diborylation of aldehydes.

Scheme 72: Umpolung pathway for chiral, nonracemic tertiary alcohol synthesis (top) and proposed mechanism for...

Scheme 73: Cu-catalyzed synthesis of α-hydroxyboronates.

Scheme 74: Cu-catalyzed borylation of ketones.

Scheme 75: Cu-catalyzed borylation of unactivated alkyl halides.

Scheme 76: Cu-catalyzed borylation of allylic difluorides.

Scheme 77: Cu-catalyzed borylation of cyclic and acyclic alkyl halides.

Scheme 78: Cu-catalyzed borylation of unactivated alkyl chlorides and bromides.

Scheme 79: Cu-catalyzed decarboxylative borylation of carboxylic acids.

Scheme 80: Cu-catalyzed borylation of benzylic, allylic, and propargylic alcohols.
Recent advances in photocatalyzed reactions using well-defined copper(I) complexes
- Mingbing Zhong,
- Xavier Pannecoucke,
- Philippe Jubault and
- Thomas Poisson
Beilstein J. Org. Chem. 2020, 16, 451–481, doi:10.3762/bjoc.16.42
- isolated in moderate to good yields. Using the more reactive allyl sulfone, a panel of symmetrical diaryliodonium salts was reacted, giving the products in good yields. The authors conducted some mechanistic studies and suggested a plausible catalytic cycle involving [Cu(I)]/[Cu(I)]*/[Cu(II)] species. The
- conducted mechanistic studies and demonstrated the role of [Cu(dap)2]Cl as a precatalyst since the catalytically active species was a [Cu(II)] complex. To explain the formation of the product, the authors suggested that after excitation, the [Cu(I)] catalyst is oxidized to a [Cu(II)] species along with the
- , probably due to their intrinsic reduction potential. Interestingly, the bromide substrate derived from menthol was readily reduced in an excellent 74% isolated yield. The authors conducted some mechanistic studies (including cyclic voltammetry and Stern–Volmer quenching experiments, for instance), and

Graphical Abstract

Scheme 1: [Cu(I)(dap)2]Cl-catalyzed ATRA reaction under green light irradiation.

Scheme 2: Photocatalytic allylation of α-haloketones.

Scheme 3: [Cu(I)(dap)2]Cl-photocatalyzed chlorosulfonylation and chlorotrifluoromethylation of alkenes.

Scheme 4: Photocatalytic perfluoroalkylchlorination of electron-deficient alkenes using the Sauvage catalyst.

Scheme 5: Photocatalytic synthesis of fluorinated sultones.

Scheme 6: Photocatalyzed haloperfluoroalkylation of alkenes and alkynes.

Scheme 7: Chlorosulfonylation of alkenes catalyzed by [Cu(I)(dap)2]Cl. aNo Na2CO3 was added. b1 equiv of Na2CO...

Scheme 8: Copper-photocatalyzed reductive allylation of diaryliodonium salts.

Scheme 9: Copper-photocatalyzed azidomethoxylation of olefins.

Scheme 10: Benzylic azidation initiated by [Cu(I)(dap)2]Cl.

Scheme 11: Trifluoromethyl methoxylation of styryl derivatives using [Cu(I)(dap)2]PF6. All redox potentials ar...

Scheme 12: Trifluoromethylation of silyl enol ethers.

Scheme 13: Synthesis of annulated heterocycles upon oxidation with the Sauvage catalyst.

Scheme 14: Oxoazidation of styrene derivatives using [Cu(dap)2]Cl as a precatalyst.

Scheme 15: [Cu(I)(dpp)(binc)]PF6-catalyzed ATRA reaction.

Scheme 16: Allylation reaction of α-bromomalonate catalyzed by [Cu(I)(dpp)(binc)]PF6 following an ATRA mechani...

Scheme 17: Bromo/tribromomethylation reaction using [Cu(I)(dmp)(BINAP)]PF6.

Scheme 18: Chlorotrifluoromethylation of alkenes catalyzed by [Cu(I)(N^N)(xantphos)]PF6.

Scheme 19: Chlorosulfonylation of styrene and alkyne derivatives by ATRA reactions.

Scheme 20: Reduction of aryl and alkyl halides with the complex [Cu(I)(bcp)(DPEPhos)]PF6. aIrradiation was car...

Scheme 21: Meerwein arylation of electron-rich aromatic derivatives and 5-exo-trig cyclization catalyzed by th...

Scheme 22: [Cu(I)(bcp)(DPEPhos)]PF6-photocatalyzed synthesis of alkaloids. aYield over two steps (cyclization ...

Scheme 23: Copper-photocatalyzed decarboxylative amination of NHP esters.

Scheme 24: Photocatalytic decarboxylative alkynylation using [Cu(I)(dq)(binap)]BF4.

Scheme 25: Copper-photocatalyzed alkylation of glycine esters.

Scheme 26: Copper-photocatalyzed borylation of organic halides. aUnder continuous flow conditions.

Scheme 27: Copper-photocatalyzed α-functionalization of alcohols with glycine ester derivatives.

Scheme 28: δ-Functionalization of alcohols using [Cu(I)(dmp)(xantphos)]BF4.

Scheme 29: Photocatalytic synthesis of [5]helicene and phenanthrene.

Scheme 30: Oxidative carbazole synthesis using in situ-formed [Cu(I)(dmp)(xantphos)]BF4.

Scheme 31: Copper-photocatalyzed functionalization of N-aryl tetrahydroisoquinolines.

Scheme 32: Bicyclic lactone synthesis using a copper-photocatalyzed PCET reaction.

Scheme 33: Photocatalytic Pinacol coupling reaction catalyzed by [Cu(I)(pypzs)(BINAP)]BF4. The ligands of the ...

Scheme 34: Azide photosensitization using a Cu-based photocatalyst.
Room-temperature Pd/Ag direct arylation enabled by a radical pathway
- Amy L. Mayhugh and
- Christine K. Luscombe
Beilstein J. Org. Chem. 2020, 16, 384–390, doi:10.3762/bjoc.16.36
- paper, we investigated the mechanism of room-temperature direct arylation and DArP by extending the method reported by Larrosa to synthesize a conjugated polymer, polyindole (PIn). Small molecule mechanistic studies were undertaken that will help future development of mild, efficient DArP conditions

Graphical Abstract

Scheme 1: A high yielding, highly selective room-temperature direct arylation reaction between indole and iod...

Figure 1: 1H NMR (500 MHz, CDCl3) of (a) 5-iodo-1-octylindole monomer (b) PIn prepared according to condition...

Figure 2: MALDI–TOF MS of PIn, indicating octylindole repeat units with three different types of end groups. ...

Scheme 2: Commonly discussed mechanisms for C2 selective direct arylation, none containing radical intermedia...

Scheme 3: Proposed mechanism for palladium radical involved reaction between indole and iodobenzene.

Scheme 4: Radical trap effects on literature methods for the direct arylation at room temperature. A) From re...
p-Pyridinyl oxime carbamates: synthesis, DNA binding, DNA photocleaving activity and theoretical photodegradation studies
- Panagiotis S. Gritzapis,
- Panayiotis C. Varras,
- Nikolaos-Panagiotis Andreou,
- Katerina R. Katsani,
- Konstantinos Dafnopoulos,
- George Psomas,
- Zisis V. Peitsinis,
- Alexandros E. Koumbis and
- Konstantina C. Fylaktakidou
Beilstein J. Org. Chem. 2020, 16, 337–350, doi:10.3762/bjoc.16.33

- sulfonylethanone oxime derivatives were less reactive than the PNP one [10]. Due to the similarity in DNA photocleavage by p-pyridine amidoxime, ethanome oxime and aldoxime carbamoyl derivatives, mechanistic studies were performed for two active compounds from the series of amidoximes and aldoximes, 12 and 26
- derivatives, of both amidoxime, ethanone oxime and aldoxime derivatives showed, similarly, significant DNA photocleavage. Mechanistic studies on DNA photocleavage showed that these “synthetic nucleases” act independently of oxygen and pH. As photobase generators, upon the homolysis of their N–O bond, oxime
- 32.3% of the initial EB–DNA fluorescence intensity for 11 and 31.5% for 12). DNA photocleavage of amidoxime carbamates at a concentration of 500 μM and mechanistic studies of aldoxime carbamate 26. Gel electrophoreses pictures. For (A) and (B) wells 1 and 2 represent DNA in dark and DNA UV irradiated

Graphical Abstract

Figure 1: General structures of oxime derivatives with possible DNA photocleavage ability. Left: Oxime carbox...

Scheme 1: Synthesis of O-carbamoyl amidoximes (8–13), ethanone oximes (15–20) and aldoximes (22–27). Oxime 1 ...

Figure 2: UV–vis spectra of CT DNA ([DNA] = 1.1 × 10−4 M) in buffer solution in the absence or presence of in...

Figure 3: Relative viscosity (η/η0)1/3 of CT DNA (0.1 mM) in buffer solution in the presence of compounds 11 ...

Figure 4: Plot of EB-DNA relative fluorescence emission intensity at λ = 592 nm (I/I0, %) vs r (= [compound]/...

Figure 5: DNA photocleavage of amidoxime carbamates at a concentration of 500 μM and mechanistic studies of a...

Figure 6: Potential energy curve for the dissociation of 12 in the first excited triplet state, T1. For compo...

Scheme 2: Photodissociation reaction of the derivative 12 in the T1 state and the formation of ground state r...

Scheme 3: Decarboxylation reaction of the p-chlorophenylcarbamoyloxyl radical.

Figure 7: Proposed scheme showing a possible energy transfer from acetophenone sensitizer to oxime carbamate ...

Figure 8: DNA photocleavage of compounds 8–10 and 12–13 at concentration of 500 μM, at 365 nm, in the absence...

Figure 9: DNA photocleavage of compound 12 at a concentration of 500 μM, at 312 nm, in the absence and presen...
Recent developments in photoredox-catalyzed remote ortho and para C–H bond functionalizations
- Rafia Siddiqui and
- Rashid Ali
Beilstein J. Org. Chem. 2020, 16, 248–280, doi:10.3762/bjoc.16.26

- ]. From mechanistic studies it can be inferred that the photoredox catalysis process is independent from the C–H activation process. They also successfully demonstrated the importance of a photoredox catalysts in the generation of superoxide radicals. On the other hand, recently, Xiong and co-workers
- competent organic photoredox catalyst 9,10-dihydroacridine (1) under mild conditions at room temperature [128]. As usual, no reaction was observed without photoredox catalyst 1 or LED light. Mechanistic studies showed that the reaction proceeded via the generation of an aryl radical. As such, a list of

Graphical Abstract

Figure 1: List of photoredox catalysts used for C–H bond functionalizations.

Figure 2: List of metal-based photoredox catalysts used in this review article.

Figure 3: Jablonski diagram.

Figure 4: Photoredox catalysis via reductive or oxidative pathways. D = donor, A = acceptor, S = substrate, P...

Figure 5: Schematic representation of the combination of photoredox catalysis and transition metal catalysis.

Scheme 1: Weinreb amide C–H olefination.

Figure 6: Mechanism for the formation of 21 from 19 using photoredox catalyst 11.

Scheme 2: C–H olefination of phenolic ethers.

Scheme 3: Decarboxylative acylation of acetanilides.

Figure 7: Mechanism for the formation of 30 from acetanilide derivatives.

Scheme 4: Synthesis of fluorenone derivatives by intramolecular deoxygenative acylation of biaryl carboxylic ...

Figure 8: Mechanism for the photoredox-catalyzed synthesis of fluorenone derivatives.

Scheme 5: Synthesis of benzothiazoles via aerobic C–H thiolation.

Figure 9: Plausible mechanism for the construction of benzothiazoles from benzothioamides.

Scheme 6: Synthesis of benzothiazoles via oxidant-free C–H thiolation.

Figure 10: Mechanism involved in the synthesis of benzothiazoles via oxidant-free C–H thiolation.

Scheme 7: Synthesis of indoles via C–H cyclization of anilides with alkynes.

Scheme 8: Preparation of 3-trifluoromethylcoumarins via C–H cyclization of arylpropiolate esters.

Figure 11: Mechanistic pathway for the synthesis of coumarin derivatives via C–H cyclization.

Scheme 9: Monobenzoyloxylation without chelation assistance.

Figure 12: Plausible mechanism for the formation of 71 from 70.

Scheme 10: Aryl-substituted arenes prepared by inorganic photoredox catalysis using 12a.

Figure 13: Proposed mechanism for C–H arylations in the presence of 12a and a Pd catalyst.

Scheme 11: Arylation of purines via dual photoredox catalysis.

Scheme 12: Arylation of substituted arenes with an organic photoredox catalyst.

Scheme 13: C–H trifluoromethylation.

Figure 14: Proposed mechanism for the trifluoromethylation of 88.

Scheme 14: Synthesis of benzo-3,4-coumarin derivatives.

Figure 15: Plausible mechanism for the synthesis of substituted coumarins.

Scheme 15: Oxidant-free oxidative phosphonylation.

Figure 16: Mechanism proposed for the phosphonylation reaction of 100.

Scheme 16: Nitration of anilines.

Figure 17: Plausible mechanism for the nitration of aniline derivatives via photoredox catalysis.

Scheme 17: Synthesis of carbazoles via intramolecular amination.

Figure 18: Proposed mechanism for the formation of carbazoles from biaryl derivatives.

Scheme 18: Synthesis of substituted phenols using QuCN.

Figure 19: Mechanism for the synthesis of phenol derivatives with photoredox catalyst 8.

Scheme 19: Synthesis of substituted phenols with DDQ (5).

Figure 20: Possible mechanism for the generation of phenols with the aid of photoredox catalyst 5.

Scheme 20: Aerobic bromination of arenes using an acridinium-based photocatalyst.

Scheme 21: Aerobic bromination of arenes with anthraquinone.

Figure 21: Proposed mechanism for the synthesis of monobrominated compounds.

Scheme 22: Chlorination of benzene derivatives with Mes-Acr-MeClO4 (2).

Figure 22: Mechanism for the synthesis of 131 from 132.

Scheme 23: Chlorination of arenes with 4CzIPN (5a).

Figure 23: Plausible mechanism for the oxidative photocatalytic monochlorination using 5a.

Scheme 24: Monofluorination using QuCN-ClO4 (8).

Scheme 25: Fluorination with fluorine-18.

Scheme 26: Aerobic amination with acridinium catalyst 3a.

Figure 24: Plausible mechanism for the aerobic amination using acridinium catalyst 3a.

Scheme 27: Aerobic aminations with semiconductor photoredox catalyst 18.

Scheme 28: Perfluoroalkylation of arenes.

Scheme 29: Synthesis of benzonitriles in the presence of 3a.

Figure 25: Plausible mechanism for the synthesis of substituted benzonitrile derivatives in the presence of 3a....
Synthesis of 3-alkenylindoles through regioselective C–H alkenylation of indoles by a ruthenium nanocatalyst
- Abhijit Paul,
- Debnath Chatterjee,
- Srirupa Banerjee and
- Somnath Yadav
Beilstein J. Org. Chem. 2020, 16, 140–148, doi:10.3762/bjoc.16.16
- . Mechanistic studies have unambiguously proven the heterogeneous nature of the catalysis. The ability of the nanocatalyst to activate the C–H bond is due to the presence of minimal stabilising groups on its surface. Studies of the surface morphology of the catalyst have revealed the presence of surface oxides

Graphical Abstract

Figure 1: Biologically and medicinally important 3-alkenylindoles.

Scheme 1: a) Previous and b) present work related to the synthesis of 3-alkenylindoles.

Scheme 2: Substrate scope for the C–H alkenylation of the indoles 1. Reaction conditions: 1 (1 mmol), 2 (2 mm...

Scheme 3: a) Three-phase test to determine a homogeneous or heterogeneous catalytic mechanism of action for t...

Scheme 4: Probable catalytic mechanism for the transformation of 1a by the RuNC.
Potent hemithioindigo-based antimitotics photocontrol the microtubule cytoskeleton in cellulo
- Alexander Sailer,
- Franziska Ermer,
- Yvonne Kraus,
- Rebekkah Bingham,
- Ferdinand H. Lutter,
- Julia Ahlfeld and
- Oliver Thorn-Seshold
Beilstein J. Org. Chem. 2020, 16, 125–134, doi:10.3762/bjoc.16.14

- azobenzenes or styrylbenzothiazoles) is desirable, in particular for cell-free mechanistic studies [33]. More broadly, this work also shows that the HTI scaffold robustly enables the photoswitchable use of resonance-capable substituents that can establish high-affinity interactions (such as para-hydroxy

Graphical Abstract

Figure 1: a) The potent tubulin inhibitor colchicine as a lead scaffold led to the development of the HOTub g...

Figure 2: Chemical structures of HITubs. Key variations with respect to HITub-4 are highlighted in dashed box...

Figure 3: Photocharacterisation of HITub-4. a) Photochemical and thermal isomerisation. b) UV–vis spectra aft...

Figure 4: a) Resazurin reduction assay for HITub-4 and nocodazole in HeLa cells (n = 3), demonstrating the di...

Figure 5: Confocal microscopy images of immunofluorescently labelled MT networks after treatment with HITubs ...

Figure 6: Cell cycle analysis of HITub-4-treated cells. a) and b) (Z)-HITub-4 caused significant G2/M arrest ...
[1,3]/[1,4]-Sulfur atom migration in β-hydroxyalkylphosphine sulfides
- Katarzyna Włodarczyk,
- Piotr Borowski and
- Marek Stankevič
Beilstein J. Org. Chem. 2020, 16, 88–105, doi:10.3762/bjoc.16.11
- control experiments and DFT calculations. Keywords: Brønsted and Lewis acids; DFT; mechanistic studies; rearrangement; stereochemistry; sulfur atom migration; Introduction Among all organic reactions, rearrangements are an exciting class of transformations where unusual or even unexpected products can

Graphical Abstract

Scheme 1: Arbusov, phospha-Fries, and phospha-Brook rearrangements.

Scheme 2: Cyclization of 1a and 1b under acidic conditions.

Scheme 3: The synthesis of P-stereogenic β-hydroxyalkylphosphine sulfides.

Scheme 4: Cyclization of 8 and 19 in the presence of H3PO4.

Scheme 5: Cyclization of (SP)-19 in the presence of H3PO4.

Figure 1: 1H NMR spectra of compounds 12 and 29.

Figure 2: 13C NMR spectra of compounds 12 and 29.

Scheme 6: Synthesis of the alkenylphosphine sulfides used in study.

Scheme 7: The reaction of mesylate compounds with Lewis-acidic AlCl3.

Scheme 8: The reaction of alkenylphosphine sulfides with AlCl3.

Scheme 9: Rearrangement of 20 in the presence of Brønsted acid. The calculated energies next to the arrows ar...

Scheme 10: Rearrangement of 20 in the presence of Lewis acid. The calculated energies next to the arrows are r...

Scheme 11: The synthesis of chiral substrates for rearrangement reactions.

Scheme 12: The reaction of (SP)-60 and (SP)-65 with AlCl3.

Scheme 13: Reaction of chiral β-hydroxyalkylphosphine sulfides with Brønsted acid.

Scheme 14: Attempted cyclization of enantiomerically enriched 53 and 46.






