Search results
Search for "photochemistry" in Full Text gives 139 result(s) in Beilstein Journal of Organic Chemistry.
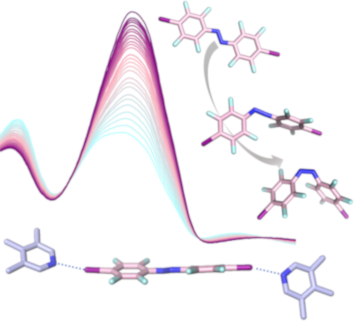
1,2,3,4-Tetrahydro-1,4,5,8-tetraazaanthracene revisited: properties and structural evidence of aromaticity loss
Beilstein J. Org. Chem. 2019, 15, 2059–2068, doi:10.3762/bjoc.15.203

- of moisture. The photochemistry of both compounds is currently under investigation in our labs. Crystal structures Numerous short intermolecular contacts are revealed upon inspection the crystal lattice of 3, 6a and 7a. There are two independent molecules of 3 in the unit cell with slightly different
Naphthalene diimides with improved solubility for visible light photoredox catalysis
Beilstein J. Org. Chem. 2019, 15, 2043–2051, doi:10.3762/bjoc.15.201
- light range between 520 nm and 640 nm. The irradiation by visible light together with the use of an organic dye instead of a transition metal complex as photoredox catalyst improve the sustainability and make photoredox catalysis “greener”. Keywords: chromophore; dyes; electrochemistry; photochemistry
Fluorinated azobenzenes as supramolecular halogen-bonding building blocks
Beilstein J. Org. Chem. 2019, 15, 2013–2019, doi:10.3762/bjoc.15.197
- building blocks both in solution and in the solid state in combination with neutral halogen bonding acceptors, such as lutidines. Therefore, we investigate the photochemistry of a series of azobenzene photoswitches. Upon introduction of iodoethynyl groups, the halogen bonding donor properties are
- azobenzenes with different halogen bonding donor properties are discussed in relation to their changing photophysical properties, rationalized by DFT calculations. Keywords: azobenzene; DFT calculations; fluorine chemistry; halogen bonding; photochemistry; Introduction The halogen bond is an attractive
- boxes [35]. Therefore, we herein present a comprehensive investigation of the photochemistry of highly fluorinated azobenzenes. Our efforts are supported by theoretical calculations, showing that these azobenzenes are suitable for the use as building blocks in supramolecular architectures. Results and
Tandem copper and photoredox catalysis in photocatalytic alkene difunctionalization reactions
Beilstein J. Org. Chem. 2019, 15, 351–356, doi:10.3762/bjoc.15.30
- renewed interest in synthetic photochemistry has resulted in the discovery of a broad range of powerful new bond-forming transformations [1][2][3][4]. Much of this work has been premised on the ability of visible light-activated photocatalysts to generate highly reactive odd-electron intermediates via
N-Arylphenothiazines as strong donors for photoredox catalysis – pushing the frontiers of nucleophilic addition of alcohols to alkenes
Beilstein J. Org. Chem. 2019, 15, 52–59, doi:10.3762/bjoc.15.5
- after 20 h of irradiation. Keywords: addition; phenothiazine; photochemistry; photoredox catalysis; redox potential; Introduction Visible-light photoredox catalysis has become a precious tool in modern synthetic organic chemistry and experiences a continuously growing interest in industrial
- chemical redox dynamics between −2.1 V up to +2.1 V. One of the key problems in photochemistry, which was recently addressed by the development of the consecutive photoelectron transfer process (conPET) [5], is the need to push the frontiers by accessing high reduction potentials. While the classical
Organometallic vs organic photoredox catalysts for photocuring reactions in the visible region
Beilstein J. Org. Chem. 2018, 14, 3025–3046, doi:10.3762/bjoc.14.282

- absorbing compound and suitable additives under light to induce a catalytical cycle. As light is an inexhaustible and renewable energy, photochemistry has dealt a great interest into organic chemistry and green chemistry since the early 20th century [15]. By absorbing light, the compound reaches an
- photoredox catalysts The first photoredox catalysts to emerge were the metal-based complexes. Indeed, metal complexes, also named coordination compounds, have been recognized in the photochemistry field since the second half of the last century [33][34][35]. However, these compounds are still the subject of
Oxidative and reductive cyclization in stiff dithienylethenes
Beilstein J. Org. Chem. 2018, 14, 2812–2821, doi:10.3762/bjoc.14.259
- components for optical memories [3][4]. In addition to photochemistry, the isomerization of DAEs can also be triggered by electrochemical means, therefore providing a stimulus orthogonal to light [5]. Electrochemically induced isomerization of DAEs is almost exclusively based on oxidation. Either cyclization
- achieve cyclization, via both the oxidative and reductive pathway. Experimental A detailed description of the experimental conditions and applied analytical methods, including synthesis, photochemistry, and electrochemistry, can be found in Supporting Information File 1. Cyclic voltammograms of sDTE66-Me
Photocatalyic Appel reaction enabled by copper-based complexes in continuous flow
Beilstein J. Org. Chem. 2018, 14, 2730–2736, doi:10.3762/bjoc.14.251
- anhydrides. The protocol was also amendable and optimized under continuous flow conditions. Keywords: Appel; continuous flow; copper; halides; photocatalysis; Introduction Synthetic photochemistry and photocatalysis continues to influence molecular synthesis [1][2][3][4]. In exploring photochemical
Synthesis of aryl sulfides via radical–radical cross coupling of electron-rich arenes using visible light photoredox catalysis
Beilstein J. Org. Chem. 2018, 14, 2520–2528, doi:10.3762/bjoc.14.228
- ps/div, 10 ns/div, 10µs/div; tmax = 3.5 µs to 10 µs) provided information on photophysics and photochemistry of the single reactants. Figure 4 shows the strong luminescence of the catalyst (red line) that overlaps with the transient absorption of the postulated 1,3,5-TMB•+ in the 2-component mixture
Microfluidic light-driven synthesis of tetracyclic molecular architectures
Beilstein J. Org. Chem. 2018, 14, 2418–2424, doi:10.3762/bjoc.14.219
- molecules. Keywords: [4 + 2] photoenol; cycloaddition; flow chemistry; microfluidic photoreactor; photoredox catalysis; synthetic photochemistry; Introduction In recent years synthetic photochemistry has become highly sophisticated [1]. The opportunity of using renewable energy sources to transform and
Novel photochemical reactions of carbocyclic diazodiketones without elimination of nitrogen – a suitable way to N-hydrazonation of C–H-bonds
Beilstein J. Org. Chem. 2018, 14, 2250–2258, doi:10.3762/bjoc.14.200
- with up to 90–97% yield. Keywords: C–H insertion; diazo compounds; excited state; photochemistry; Wolff rearrangement; Introduction Photochemical reactions of diazocarbonyl compounds are well-known transformations in the synthesis of the diversified acyclic, carbo- and heterocyclic structures [1][2
Applications of organocatalysed visible-light photoredox reactions for medicinal chemistry
Beilstein J. Org. Chem. 2018, 14, 2035–2064, doi:10.3762/bjoc.14.179
- damaging and toxic to various life forms. Photochemistry also offers benefits compared to conventional thermally driven processes. Several transformation processes that utilise sunlight as the energy source to drive a particular reaction have been reported and some reactions presented in this review
- Figure 2). It is ideal if a photocatalyst has a local absorbance maximum (λmax) at a relatively long wavelength. Lower energy photons avoid exciting other reactants and prevent competing photochemistry from occurring, cf. ultraviolet light. However, the energy of the absorbed photon also determines the
Diazirine-functionalized mannosides for photoaffinity labeling: trouble with FimH
Beilstein J. Org. Chem. 2018, 14, 1890–1900, doi:10.3762/bjoc.14.163
- . They differ with respect to their steric properties, photochemistry, and reactivity. In order to be applied in a biological environment, the wavelength of the light required for activation of the photolabile ligand has to be biocompatible. Furthermore, small photophores are desirable. Diazirines meet
Synthesis and photophysical studies of a multivalent photoreactive RuII-calix[4]arene complex bearing RGD-containing cyclopentapeptides
Beilstein J. Org. Chem. 2018, 14, 1758–1768, doi:10.3762/bjoc.14.150

- a photoreactive [Ru(TAP)2phen]2+ complex tethered to a calix[4]arene platform bearing four c-[RGDfK] moieties [65] (Figure 1). Before studying this conjugate in vitro, it was first mandatory to check that the photochemistry of the RuII complex was not altered by the presence of the targeting
- the Ru complex and the cyclic peptides by the calixarene should be an advantage by preventing any negative effect of the RGD peptidic units on the photochemistry of the complex and, alternatively, prevents any influence of the complex on the affinity of the RGD patterns to interact with the targeted
An unusual thionyl chloride-promoted C−C bond formation to obtain 4,4'-bipyrazolones
Beilstein J. Org. Chem. 2018, 14, 1287–1292, doi:10.3762/bjoc.14.110
- nitrosation and subsequent heating [28], by heating with an aqueous NaHSO3 solution [29], and by photochemistry [30][31]. In nearly all cases these reactions have been carried out with 2-pyrazolin-5-ones unsubstituted at pyrazole C4 position or with derivatives carrying an alkyl or aryl substituent at the
One hundred years of benzotropone chemistry
Beilstein J. Org. Chem. 2018, 14, 1120–1180, doi:10.3762/bjoc.14.98
Mechanochemistry of nucleosides, nucleotides and related materials
Beilstein J. Org. Chem. 2018, 14, 955–970, doi:10.3762/bjoc.14.81

- four taxa along with thermochemistry, electrochemistry and photochemistry [1]. A general definition commonly cited is that developed by The International Union of Pure and Applied Chemistry (IUPAC) to encompass both the chemical and physical effects of shearing, stretching or grinding polymeric
The photodecarboxylative addition of carboxylates to phthalimides as a key-step in the synthesis of biologically active 3-arylmethylene-2,3-dihydro-1H-isoindolin-1-ones
Beilstein J. Org. Chem. 2017, 13, 2833–2841, doi:10.3762/bjoc.13.275
- ; arylmethylenedihydroisoindolinones; photochemistry; photodecarboxylation; phthalimide; Introduction Phthalimides and their related 3-alkyl- and 3-arylmethylene-2,3-dihydro-1H-isoindolin-1-ones play an important role in medicinal chemistry due to their biological activities for a wide range of therapeutic applications [1][2][3][4
Mechanochemical synthesis of small organic molecules
Beilstein J. Org. Chem. 2017, 13, 1907–1931, doi:10.3762/bjoc.13.186
- received the Nobel Prize in 1909, mentioned the term “Mechanochemistry” as, like a branch of physical chemistry, i.e., thermochemistry, photochemistry and electrochemistry [13][14]. He defined the subject as “Mechanochemistry is a branch of chemistry which is concerned with chemical and physio-chemical
Chemoselective synthesis of diaryl disulfides via a visible light-mediated coupling of arenediazonium tetrafluoroborates and CS2
Beilstein J. Org. Chem. 2017, 13, 903–909, doi:10.3762/bjoc.13.91
- has also been studied [14][15] by taking advantage of visible light as abundant and environmentally friendly energy source for organic syntheses. The photochemistry of diazonium salts has been widely studied since the early 19th century, at which time, it was noticed that benzenediazonium nitrate
How and why kinetics, thermodynamics, and chemistry induce the logic of biological evolution
Beilstein J. Org. Chem. 2017, 13, 665–674, doi:10.3762/bjoc.13.66

- origin of life that more or less correspond to the conditions for the development of life on the primitive Earth (organic chemistry, liquid water and visible or UV light). Here again, some recent experimental work has shown how photochemistry could lead to biochemical building blocks compatible with
Exploring endoperoxides as a new entry for the synthesis of branched azasugars
Beilstein J. Org. Chem. 2017, 13, 644–647, doi:10.3762/bjoc.13.63

- these building blocks provided access to a variety of derivatives including tetrahydrofurans, epoxides and protected amino-tetraols. Keywords: azasugar; carbohydrate; cycloaddition; endoperoxide; photochemistry; Introduction Azasugars are small organic compounds that can mimic carbohydrates or their
Novel β-cyclodextrin–eosin conjugates
Beilstein J. Org. Chem. 2017, 13, 543–551, doi:10.3762/bjoc.13.52

- 43, Prague 2, Czech Republic Laboratory of Photochemistry, Department of Drug Sciences, University of Catania, I-95125 Viale A. Doria 6, Italy Department of Pharmacognosy, Semmelweis University, H-1085 Üllői út 26, Hungary STMicroelectronics, Stradale Primosole 50, I-95121, Catania, Italy 10.3762
Contribution of microreactor technology and flow chemistry to the development of green and sustainable synthesis
Beilstein J. Org. Chem. 2017, 13, 520–542, doi:10.3762/bjoc.13.51

- photochemistry got a sort of renaissance, emerging as useful approach in modern sustainable and green synthesis. Concerning the heterogeneous catalysis with palladium, practical procedures for recovering and reusing of the catalysts have been recently reported [51][52][53]. A versatile Pd-catalysed synthesis of
- prepared with this greener flow approach (Scheme 15) starting from mesitylene 13, and haloarenes using short irradiation times (<6 h), and a 5:1 MeCN/H2O mixture. The reported results show how photochemistry hold the potential to become a green tool for the development of sustainable photochemical flow
NMR reaction monitoring in flow synthesis
Beilstein J. Org. Chem. 2017, 13, 285–300, doi:10.3762/bjoc.13.31

- (photochemistry, electrochemistry, microwave, ultrasound, etc.) [6][7][8][9]. In this regard, in a recent paper a compact reconfigurable flow system was described for the continuous flow production of pharmaceuticals. The system comprised different types of preparation, reaction and elaboration modules that could





























































































































































































































































































































































































































































































