Search results
Search for "Cleavage" in Full Text gives 871 result(s) in Beilstein Journal of Organic Chemistry. Showing first 200.
Design, synthesis and application of carbazole macrocycles in anion sensors
- Alo Rüütel,
- Ville Yrjänä,
- Sandip A. Kadam,
- Indrek Saar,
- Mihkel Ilisson,
- Astrid Darnell,
- Kristjan Haav,
- Tõiv Haljasorg,
- Lauri Toom,
- Johan Bobacka and
- Ivo Leito
Beilstein J. Org. Chem. 2020, 16, 1901–1914, doi:10.3762/bjoc.16.157

- reference [6]. It is worth noting that the described cleavage problem during nitration was specific to tert-butyl groups. We also studied the possibility of hexyl substituents instead of tert-butyl groups [25]. In this case, the nitration process yielded only the desired product. However, since this

Graphical Abstract

Figure 1: The biscarbazolylurea moiety.

Figure 2: The structure of the solid-contact ion-selective electrode (sensor): a) glassy carbon as the electr...

Figure 3: Studied receptor molecules.

Figure 4: MC001 and MC003 lowest energy conformers (COSMO-RS) showing intramolecular bonds. Color coding: whi...

Figure 5: a) Complex of MC008 with acetate; b) complex of MC006 with formate; c) complex of MC007 with lactat...

Scheme 1: The synthetic pathway to receptors CZ016 and MC001–MC014. The reaction yield for 2–3a/3b is given a...

Figure 6: Binding affinities of the studied receptors towards different carboxylates in DMSO-d6/H2O (99.5%:0....

Figure 7: Impedance spectra of sensors with each of the membranes. The spectra were recorded in 0.1 M sodium ...

Figure 8: Calibration curves for each of the membranes. The calibrations were performed by diluting 0.1 M sod...

Figure 9: The influence of solution pH on the potential responses of the sensor prototypes (three sensors for...

Figure 10: Potentiometric selectivity coefficients of interfering anions (relative to acetate) determined usin...
On the hydrolysis of diethyl 2-(perfluorophenyl)malonate
- Ilya V. Taydakov and
- Mikhail A. Kiskin
Beilstein J. Org. Chem. 2020, 16, 1863–1868, doi:10.3762/bjoc.16.153
- of diethyl 2-(perfluorophenyl)malonate, acid-catalyzed reactions were also tested. Trifluoroacetic acid (TFA) is a common reagent for the catalytic cleavage of tert-butyl esters, but in the stoichiometric quantity it is also suitable for the cleavage of nonvolatile methyl or ethyl esters due to

Graphical Abstract

Figure 1: Phenylmalonic acids.

Scheme 1: Synthesis of diethyl 2-phenylmalonate (4).

Scheme 2: Synthesis of diethyl 2-(perfluorophenyl)malonate (3).

Figure 2: Esters of fluorine-substituted 2-phenylmalonic acids.

Scheme 3: Hydrolysis of diethyl 2-(perfluorophenyl)malonate (3).

Figure 3: Molecular structure of 2-(perfluorophenyl)acetic acid (12).

Scheme 4: Formation of 2-(perfluorophenyl)acetic acid (12).
Synthesis, docking study and biological evaluation of ᴅ-fructofuranosyl and ᴅ-tagatofuranosyl sulfones as potential inhibitors of the mycobacterial galactan synthesis targeting the galactofuranosyltransferase GlfT2
- Marek Baráth,
- Jana Jakubčinová,
- Zuzana Konyariková,
- Stanislav Kozmon,
- Katarína Mikušová and
- Maroš Bella
Beilstein J. Org. Chem. 2020, 16, 1853–1862, doi:10.3762/bjoc.16.152
- % and 54% yield, respectively. Moderate yields of 2-thio-ᴅ-tagatofuranosides 17 can be explained by cleavage of the benzyl protecting group [21] as well as further side reactions (Scheme 4). In order to increase the yield of 2-thio-ᴅ-tagatofuranosides 17, compound 12 was converted to the more reactive
- diacetate 18 under acetolysis conditions (Ac2O/BF3∙OEt2) [22]. However, besides the expected diacetate 18, 1,2,6-tri-O-acetate 19 was observed and isolated as a byproduct (Scheme 4). The cleavage of the O-benzyl ethers followed by acetylation of the liberated hydroxy groups under acidic conditions was

Graphical Abstract

Figure 1: Target ᴅ-fructofuranosyl and ᴅ-tagatofuranosyl sulfones 1‒3.

Figure 2: Molecular representation of the best binding poses of the four compounds with the predicted highest...

Scheme 1: Reagents and conditions: a) 1. (BnO)2P-NMe2, 1H-tetrazole, 0 °C→rt, 1 h, 2. m-CPBA, 0 °C→rt, 1.5‒2 ...

Scheme 2: Reagents and conditions: a) PivCl, pyridine, CH2Cl2, rt, overnight; b) BnBr, NaOH, TBAB, THF, reflu...

Scheme 3: Reagents and conditions: a) EtSH, BF3∙OEt2, CH2Cl2, 0 °C, 2 h; b) 1. (BnO)2P-NMe2, 1H-tetrazole, 0 ...

Scheme 4: Reagents and conditions: a) PhSH, or iPrSH, BF3·OEt2, CH2Cl2, −5 °C, 1 h; b) BF3·OEt2, Ac2O, 0 °C, ...

Scheme 5: Reagents and conditions: a) 1. (BnO)2P-NMe2, 1H-tetrazole, 0 °C→rt, 1 h, 2. m-CPBA, 0 °C→rt, 1.5‒2 ...

Figure 3: TLC analysis of the effects of the target compounds at 500 μM on the production of lower lipid-link...

Figure 4: TLC analysis of the dose effects of the compounds 1bα and 3a on production of lower lipid-linked ga...
One-pot synthesis of oxazolidinones and five-membered cyclic carbonates from epoxides and chlorosulfonyl isocyanate: theoretical evidence for an asynchronous concerted pathway
- Esra Demir,
- Ozlem Sari,
- Yasin Çetinkaya,
- Ufuk Atmaca,
- Safiye Sağ Erdem and
- Murat Çelik
Beilstein J. Org. Chem. 2020, 16, 1805–1819, doi:10.3762/bjoc.16.148
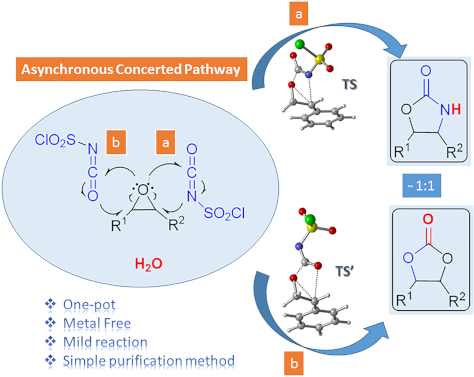
- reaction of CSI with epoxides in different solvents under mild conditions and compared the reaction mechanism with previously proposed mechanisms using theoretical calculations. Keshava Murthy and Dhar [41] postulated a mechanism involving a zwitterionic intermediate. C–O bond cleavage in this unstable and

Graphical Abstract

Scheme 1: Oxazolidinone (1), five-membered cyclic carbonate (2) and some important compounds containing an ox...

Scheme 2: Proposed mechanisms by Keshava Murthy and Dhar [41] and De Meijere and co-workers [42].

Figure 1: Possible pathways for the formation of oxazolidinone intermediates 10 and 11. Optimized transition ...

Figure 2: Potential energy profile related to the formation of oxazolidinone intermediates 10 and 11 at the P...

Figure 3: IRC calculated for the formation of (a) 10 and (b) 11 at M06-2X/6-31+G(d,p) level. I-1, I-15, I-35, ...

Figure 4: Optimized geometries for the stationary points for the formation of 10 at PCM(DCM)/M06-2X/6-31+G(d,...

Scheme 3: Proposed mechanisms for the formation of oxazolidinone 9f.

Figure 5: Potential energy profiles for paths 1a (blue), 1b (red), 2 (green) and relative Gibbs free energies...

Figure 6: Optimized geometries for the stationary points of path 1b at PCM(DCM)/M06-2X/6-31+G(d,p)//M06-2X/6-...

Scheme 4: Proposed mechanism for the formation of five-membered cyclic carbonate 8f.

Figure 7: Potential energy profile and relative Gibbs free energies (kcal/mol) in DCM related to the formatio...

Figure 8: Optimized geometries for the stationary points of step 1 for the formation of 16 at PCM(DCM)/M06-2X...

Figure 9: Optimized geometries for the stationary points of step 2 for the formation of 17 at PCM(DCM)/M06-2X...

Figure 10: Optimized geometries for the stationary points of step 3 for the formation of PC8 at PCM(DCM)/M06-2...
When metal-catalyzed C–H functionalization meets visible-light photocatalysis
- Lucas Guillemard and
- Joanna Wencel-Delord
Beilstein J. Org. Chem. 2020, 16, 1754–1804, doi:10.3762/bjoc.16.147

- the C–H cleavage step slightly, yet significantly absorbed light in the relevant blue region. This absorption was believed to trigger the overall catalytic cycle at room temperature as no product formation was observed in the dark. The mechanistic scenario thus foresees an initial pyridine-directed

Graphical Abstract

Figure 1: Concept of dual synergistic catalysis.

Figure 2: Classification of catalytic systems involving two catalysts.

Figure 3: General mechanism for the dual nickel/photoredox catalytic system.

Figure 4: General mechanisms for C–H activation catalysis involving different reoxidation strategies.

Figure 5: Indole synthesis via dual C–H activation/photoredox catalysis.

Figure 6: Proposed mechanism for the indole synthesis via dual catalysis.

Figure 7: Oxidative Heck reaction on arenes via the dual catalysis.

Figure 8: Proposed mechanism for the Heck reaction on arenes via dual catalysis.

Figure 9: Oxidative Heck reaction on phenols via the dual catalysis.

Figure 10: Proposed mechanism for the Heck reaction on phenols via dual catalysis.

Figure 11: Carbazole synthesis via dual C–H activation/photoredox catalysis.

Figure 12: Proposed mechanism for the carbazole synthesis via dual catalysis.

Figure 13: Carbonylation of enamides via the dual C–H activation/photoredox catalysis.

Figure 14: Proposed mechanism for carbonylation of enamides via dual catalysis.

Figure 15: Annulation of benzamides via the dual C–H activation/photoredox catalysis.

Figure 16: Proposed mechanism for the annulation of benzamides via dual catalysis.

Figure 17: Synthesis of indoles via the dual C–H activation/photoredox catalysis.

Figure 18: Proposed mechanism for the indole synthesis via dual catalysis.

Figure 19: General concept of dual catalysis merging C–H activation and photoredox catalysis.

Figure 20: The first example of dual catalysis merging C–H activation and photoredox catalysis.

Figure 21: Proposed mechanism for the C–H arylation with diazonium salts via dual catalysis.

Figure 22: Dual catalysis merging C–H activation/photoredox using diaryliodonium salts.

Figure 23: Direct arylation via the dual catalytic system reported by Xu.

Figure 24: Direct arylation via dual catalytic system reported by Balaraman.

Figure 25: Direct arylation via dual catalytic system reported by Guo.

Figure 26: C(sp3)–H bond arylation via the dual Pd/photoredox catalytic system.

Figure 27: Acetanilide derivatives acylation via the dual C–H activation/photoredox catalysis.

Figure 28: Proposed mechanism for the C–H acylation with α-ketoacids via dual catalysis.

Figure 29: Acylation of azobenzenes via the dual catalysis C–H activation/photoredox.

Figure 30: C2-acylation of indoles via the dual C–H activation/photoredox catalysis.

Figure 31: Proposed mechanism for the C2-acylation of indoles with aldehydes via dual catalysis.

Figure 32: C2-acylation of indoles via the dual C–H activation/photoredox catalysis.

Figure 33: Perfluoroalkylation of arenes via the dual C–H activation/photoredox catalysis.

Figure 34: Proposed mechanism for perfluoroalkylation of arenes via dual catalysis.

Figure 35: Sulfonylation of 1-naphthylamides via the dual C–H activation/photoredox catalysis.

Figure 36: Proposed mechanism for sulfonylation of 1-naphthylamides via dual catalysis.

Figure 37: meta-C–H Alkylation of arenes via visible-light metallaphotocatalysis.

Figure 38: Alternative procedure for meta-C–H alkylation of arenes via metallaphotocatalysis.

Figure 39: Proposed mechanism for meta-C–H alkylation of arenes via metallaphotocatalysis.

Figure 40: C–H borylation of arenes via visible-light metallaphotocatalysis.

Figure 41: Proposed mechanism for C–H borylation of arenes via visible-light metallaphotocatalysis.

Figure 42: Undirected C–H aryl–aryl cross coupling via dual gold/photoredox catalysis.

Figure 43: Proposed mechanism for the undirected C–H aryl–aryl cross-coupling via dual catalysis.

Figure 44: Undirected C–H arylation of (hetero)arenes via dual manganese/photoredox catalysis.

Figure 45: Proposed mechanism for the undirected arylation of (hetero)arenes via dual catalysis.

Figure 46: Photoinduced C–H arylation of azoles via copper catalysis.

Figure 47: Photo-induced C–H chalcogenation of azoles via copper catalysis.

Figure 48: Decarboxylative C–H adamantylation of azoles via dual cobalt/photoredox catalysis.

Figure 49: Proposed mechanism for the C–H adamantylation of azoles via dual catalysis.

Figure 50: General mechanisms for the “classical” (left) and Cu-free variant (right) Sonogoshira reaction.

Figure 51: First example of a dual palladium/photoredox catalysis for Sonogashira-type couplings.

Figure 52: Arylation of terminal alkynes with diazonium salts via dual gold/photoredox catalysis.

Figure 53: Proposed mechanism for the arylation of terminal alkynes via dual catalysis.

Figure 54: C–H Alkylation of alcohols promoted by H-atom transfer (HAT).

Figure 55: Proposed mechanism for the C–H alkylation of alcohols promoted by HAT.

Figure 56: C(sp3)–H arylation of latent nucleophiles promoted by H-atom transfer.

Figure 57: Proposed mechanism for the C(sp3)–H arylation of latent nucleophiles promoted by HAT.

Figure 58: Direct α-arylation of alcohols promoted by H-atom transfer.

Figure 59: Proposed mechanism for the direct α-arylation of alcohols promoted by HAT.

Figure 60: C–H arylation of amines via dual Ni/photoredox catalysis.

Figure 61: Proposed mechanism for the C–H arylation of amines via dual Ni/photoredox catalysis.

Figure 62: C–H functionalization of nucleophiles via excited ketone/nickel dual catalysis.

Figure 63: Proposed mechanism for the C–H functionalization enabled by excited ketones.

Figure 64: Selective sp3–sp3 cross-coupling promoted by H-atom transfer.

Figure 65: Proposed mechanism for the selective sp3–sp3 cross-coupling promoted by HAT.

Figure 66: Direct C(sp3)–H acylation of amines via dual Ni/photoredox catalysis.

Figure 67: Proposed mechanism for the C–H acylation of amines via dual Ni/photoredox catalysis.

Figure 68: C–H hydroalkylation of internal alkynes via dual Ni/photoredox catalysis.

Figure 69: Proposed mechanism for the C–H hydroalkylation of internal alkynes.

Figure 70: Alternative procedure for the C–H hydroalkylation of ynones, ynoates, and ynamides.

Figure 71: Allylic C(sp3)–H activation via dual Ni/photoredox catalysis.

Figure 72: Proposed mechanism for the allylic C(sp3)–H activation via dual Ni/photoredox catalysis.

Figure 73: Asymmetric allylation of aldehydes via dual Cr/photoredox catalysis.

Figure 74: Proposed mechanism for the asymmetric allylation of aldehydes via dual catalysis.

Figure 75: Aldehyde C–H functionalization promoted by H-atom transfer.

Figure 76: Proposed mechanism for the C–H functionalization of aldehydes promoted by HAT.

Figure 77: Direct C–H arylation of strong aliphatic bonds promoted by HAT.

Figure 78: Proposed mechanism for the C–H arylation of strong aliphatic bonds promoted by HAT.

Figure 79: Direct C–H trifluoromethylation of strong aliphatic bonds promoted by HAT.

Figure 80: Proposed mechanism for the C–H trifluoromethylation of strong aliphatic bonds.
Et3N/DMSO-supported one-pot synthesis of highly fluorescent β-carboline-linked benzothiophenones via sulfur insertion and estimation of the photophysical properties
- Dharmender Singh,
- Vipin Kumar and
- Virender Singh
Beilstein J. Org. Chem. 2020, 16, 1740–1753, doi:10.3762/bjoc.16.146
- addition of the trisulfur radical anion to the double bond in 2-nitrochalcone (1bA) may yield the intermediate 7. The further abstraction of hydrogen in intermediate 7 may result in formation of intermediate 8. The cleavage of the S–S bond in 8 under basic conditions may generate sulfur anion 9. The

Graphical Abstract

Figure 1: Representative examples of some commercial drugs and biologically active alkaloids.

Scheme 1: Synthesis of β-carboline-linked 2-nitrochalcones.

Scheme 2: Synthesis of β-carboline-linked benzothiophenone frameworks.

Scheme 3: Comparison of outcome of one-pot vs two-pot approach.

Scheme 4: One-pot synthesis of β-carboline C-1-tethered benzothiophenone derivatives.

Scheme 5: One-pot synthesis of β-carboline C-3-linked benzothiophenone derivatives.

Scheme 6: One-pot synthesis of β-carboline-linked benzothiophene derivative 6C.

Scheme 7: Control experiment in the presence of a radical scavenger.

Figure 2: Proposed reaction mechanism.

Figure 3: Fluorescence spectra of 2aA–nA, 2bB, 2hB, and 6C.

Figure 4: Fluorescence spectra of 4aA–gA, and 4eB.
Synthesis of Streptococcus pneumoniae serotype 9V oligosaccharide antigens
- Sharavathi G. Parameswarappa,
- Claney L. Pereira and
- Peter H. Seeberger
Beilstein J. Org. Chem. 2020, 16, 1693–1699, doi:10.3762/bjoc.16.140
- mannosamine was obtained from 33, that in turn was a product of the benzylation of 32. Cleavage of the benzylidene group in 33 yielded 34 that was selectively acetylated at the primary alcohol at low temperature to obtain 35 [30]. The subsequent removal of the benzyl groups using hydrogenation with Pd/C in an

Graphical Abstract

Figure 1: Streptococcus pneumoniae 9V repeating unit. The numbers refer to the version concerning the structu...

Scheme 1: Retrosynthesis of Streptococcus pneumoniae 9V deacetylated (4) and acetylated (5) repeating units.

Scheme 2: Synthesis of trisaccharide acceptor 25.

Scheme 3: Synthesis of disaccharide 29.

Scheme 4: Synthesis of the pentasaccharide repeating unit oligosaccharide antigens without C-6 O-acetate (4) ...
A dynamic combinatorial library for biomimetic recognition of dipeptides in water
- Florian Klepel and
- Bart Jan Ravoo
Beilstein J. Org. Chem. 2020, 16, 1588–1595, doi:10.3762/bjoc.16.131

- SPPS. Thus one cysteine moiety is left deprotected and can be addressed selectively either by dimerization with another CFC(Acm) or with inversely substituted tripeptide (C(Acm)FC). These linear peptide dimers were subsequently cyclized by oxidative cleavage of the Acm groups to give a(CFC)2 and p(CFC

Graphical Abstract

Scheme 1: a) Building blocks included in this study. b) Antiparallel and parallel constitutional isomers of t...

Figure 1: HPLC–MS chromatograms of a reference library for all possible tripeptide dimers ([M + H]+ ions).

Figure 2: a) HPLC–MS chromatograms of the dimers (CFC)2 and templates YY and FF. b) Amplification of the peak...

Scheme 2: a) Synthesis of the parallel and antiparallel isomers p(CFC)2 and a(CFC)2. b) Templates FF. YY and ...

Figure 3: ITC of YY (30 mM) to a(CFC)2 (1.5 mM) in phosphate buffer (pH 7.4, 100 mM).

Figure 4: Continuously varied NMR measurements of a) p(CFC)2 to YY b) p(CFC)2 to FF c) a(CFC)2 to YY d) a(CFC)...

Figure 5: Job plots derived from the continuously varied NMR measurements of a) p(CFC)2 to YY b) p(CFC)2 to FF...
Photoredox-catalyzed silyldifluoromethylation of silyl enol ethers
- Vyacheslav I. Supranovich,
- Vitalij V. Levin and
- Alexander D. Dilman
Beilstein J. Org. Chem. 2020, 16, 1550–1553, doi:10.3762/bjoc.16.126
- )trimethylsilane followed by a reduction of the primary products with sodium borohydride is described. An 18 W, 375 nm LED was used as the light source. The reaction is performed in the presence of a gold photocatalyst, which effects the generation of a (trimethylsilyl)difluoromethyl radical via cleavage of the
- carbon-based radical via cleavage of the carbon–bromine bond by a gold photocatalyst. Reaction of silyl enol ethers. Yields refer to isolated yields. aReaction time 24 h; b1.0 equiv of silane 1 was used; cketone was isolated. Reactions of (bromodifluoromethyl)trimethylsilane (1). Optimization studies

Graphical Abstract

Scheme 1: Reactions of (bromodifluoromethyl)trimethylsilane (1).

Scheme 2: Optimization studies. Yield determined by 19F NMR spectroscopy using an internal standard.

Figure 1: Reaction of silyl enol ethers. Yields refer to isolated yields. aReaction time 24 h; b1.0 equiv of ...

Scheme 3: Proposed mechanism of the fluoroalkylation reaction.
Heterogeneous photocatalysis in flow chemical reactors
- Christopher G. Thomson,
- Ai-Lan Lee and
- Filipe Vilela
Beilstein J. Org. Chem. 2020, 16, 1495–1549, doi:10.3762/bjoc.16.125


Graphical Abstract

Figure 1: A) Bar chart of the publications per year for the topics “Photocatalysis” (49,662 instances) and “P...

Figure 2: A) Professor Giacomo Ciamician and Dr. Paolo Silber on their roof laboratory at the University of B...

Scheme 1: PRC trifluoromethylation of N-methylpyrrole (1) using hazardous gaseous CF3I safely in a flow react...

Figure 3: A) Unit cells of the three most common crystal structures of TiO2: rutile, brookite, and anatase. R...

Figure 4: Illustration of the key semiconductor photocatalysis events: 1) A photon with a frequency exceeding...

Figure 5: Photocatalytic splitting of water by oxygen vacancies on a TiO2(110) surface. Reprinted with permis...

Figure 6: Proposed adsorption modes of A) benzene, B) chlorobenzene, C) toluene, D) phenol, E) anisole, and F...

Figure 7: Structures of the sulfonate-containing organic dyes RB5 (3) and MX-5B (4) and the adsorption isothe...

Figure 8: Idealised triclinic unit cell of a g-C3N4 type polymer, displaying possible hopping transport scena...

Figure 9: Idealised structure of a perfect g-C3N4 sheet. The central unit highlighted in red represents one t...

Figure 10: Timeline of the key processes of charge transport following the photoexcitation of g-C3N4, leading ...

Scheme 2: Photocatalytic bifunctionalisation of heteroarenes using mpg-C3N4, with the selected examples 5 and ...

Figure 11: A) Structure of four linear conjugated polymer photocatalysts for hydrogen evolution, displaying th...

Figure 12: Graphical representation of the common methods used to immobilise molecular photocatalysts (PC) ont...

Figure 13: Wireless light emitter-supported TiO2 (TiO2@WLE) HPCat spheres powered by resonant inductive coupli...

Figure 14: Graphical representation of zinc–perylene diimide (Zn-PDI) supramolecular assembly photocatalysis v...

Scheme 3: Upconversion of NIR photons to the UV frequency by NaYF4:Yb,Tm nanocrystals sequentially coated wit...

Figure 15: Types of reactors employed in heterogeneous photocatalysis in flow. A) Fixed bed reactors and the s...

Figure 16: Electrochemical potential of common semiconductor, transition metal, and organic dye-based photocat...

Scheme 4: Possible mechanisms of an immobilised molecular photoredox catalyst by oxidative or reductive quenc...

Scheme 5: Scheme of the CMB-C3N4 photocatalytic decarboxylative fluorination of aryloxyacetic acids, with the...

Scheme 6: Scheme of the g-C3N4 photocatalytic desilylative coupling reaction in flow and proposed mechanism [208].

Scheme 7: Proposed mechanism of the radical cyclisation of unsaturated alkyl 2-bromo-1,3-dicarbonyl compounds...

Scheme 8: N-alkylation of benzylamine and schematic of the TiO2-coated microfluidic device [213].

Scheme 9: Proposed mechanism of the Pt@TiO2 photocatalytic deaminitive cyclisation of ʟ-lysine (23) to ʟ-pipe...

Scheme 10: A) Proposed mechanism for the photocatalytic oxidation of phenylboronic acid (24). B) Photos and SE...

Scheme 11: Proposed mechanism for the DA-CMP3 photocatalytic aza-Henry reaction performed in a continuous flow...

Scheme 12: Proposed mechanism for the formation of the cyclic product 32 by TiO2-NC HPCats in a slurry flow re...

Scheme 13: Reaction scheme for the photocatalytic synthesis of homo and hetero disulfides in flow and scope of...

Scheme 14: Reaction scheme for the MoOx/TiO2 HPCat oxidation of cyclohexane (34) to benzene. The graph shows t...

Scheme 15: Proposed mechanism of the TiO2 HPC heteroarene C–H functionalisation via aryl radicals generated fr...

Scheme 16: Scheme of the oxidative coupling of benzylamines with the HOTT-HATN HPCat and selected examples of ...

Scheme 17: Photocatalysis oxidation of benzyl alcohol (40) to benzaldehyde (41) in a microflow reactor coated ...

Figure 17: Mechanisms of Dexter and Forster energy transfer.

Scheme 18: Continuous flow process for the isomerisation of alkenes with an ionic liquid-immobilised photocata...

Scheme 19: Singlet oxygen synthetic step in the total synthesis of canataxpropellane [265].

Scheme 20: Scheme and proposed mechanism of the singlet oxygen photosensitisation by CMP_X HPCats, with the st...

Scheme 21: Structures of CMP HPCat materials applied by Vilela and co-workers for the singlet oxygen photosens...

Scheme 22: Polyvinylchloride resin-supported TDCPP photosensitisers applied for singlet oxygen photosensitisat...

Scheme 23: Structure of the ionically immobilised TPP photosensitiser on amberlyst-15 ion exchange resins (TPP...

Scheme 24: Photosensitised singlet oxygen oxidation of citronellol (46) in scCO2, with automatic phase separat...

Scheme 25: Schematic of PS-Est-BDP-Cl2 being applied for singlet oxygen photosensitisation in flow. A) Pseudo-...

Scheme 26: Reaction scheme of the singlet oxygen oxidation of furoic acid (54) using a 3D-printed microfluidic...

Figure 18: A) Photocatalytic bactericidal mechanism by ROS oxidative cleavage of membrane lipids (R = H, amino...

Figure 19: A) Suggested mechanisms for the aqueous pollutant degradation by TiO2 in a slurry flow reactor [284-287]. B)...

Figure 20: Schematic of the flow system used for the degradation of aqueous oxytetracycline (56) solutions [215]. M...

Scheme 27: Degradation of a salicylic acid (57) solution by a coupled solar photoelectro-Fenton (SPEF) process...

Figure 21: A) Schematic flow diagram using the TiO2-coated NETmix microfluidic device for an efficient mass tr...
Photocatalyzed syntheses of phenanthrenes and their aza-analogues. A review
- Alessandra Del Tito,
- Havall Othman Abdulla,
- Davide Ravelli,
- Stefano Protti and
- Maurizio Fagnoni
Beilstein J. Org. Chem. 2020, 16, 1476–1488, doi:10.3762/bjoc.16.123
- conditions finally afforded the desired phenanthridine 8.7 in 90% yield [69]. Carbon-based radicals could be likewise generated via a C–H hydrogen-atom transfer path. As an example, ethers were used as hydrogen donors and underwent a C–H cleavage step promoted by a photogenerated tert-butoxyl radical. The so
- preparation of phenanthridines with the intermediacy of imidoyl radicals. As an example, the process depicted in Scheme 10 involved a visible-light homolytic radical aromatic substitution (HAS) starting from trifluoroacetimidoyl chlorides 10.1a–e. Thus, the photocatalyzed cleavage of the C(sp2)–Cl bond in
- photocatalyzed reduction of acyloximes 14.1a,b offered a smooth entry to iminyl radicals (Scheme 14) [76]. The process took place at room temperature and involved the cleavage of a C–O bond, followed by a cyclization to give access to the benzo[c]phenanthridine alkaloids noravicine (14.2a) and nornitidine (14.2b

Graphical Abstract

Figure 1: Bioactive phenanthridine and phenanthridinium derivatives.

Scheme 1: Synthesis of phenanthrenes by a photo-Pschorr reaction.

Scheme 2: Synthesis of phenanthrenes by a benzannulation reaction.

Scheme 3: Photocatalytic cyclization of α-bromochalcones for the synthesis of phenanthrenes.

Figure 2: Carbon-centered and nitrogen-centered radicals used for the synthesis of phenanthridines.

Scheme 4: General scheme describing the synthesis of phenanthridines from isocyanides via imidoyl radicals.

Scheme 5: Synthesis of substituted phenanthridines involving the intermediacy of electrophilic radicals.

Scheme 6: Photocatalyzed synthesis of 6-β-ketoalkyl phenanthridines.

Scheme 7: Synthesis of 6-substituted phenanthridines through the addition of trifluoromethyl (path a), phenyl...

Scheme 8: Synthesis of 6-(trifluoromethyl)-7,8-dihydrobenzo[k]phenanthridine.

Scheme 9: Phenanthridine syntheses by using photogenerated radicals formed through a C–H bond homolytic cleav...

Scheme 10: Trifluoroacetimidoyl chlorides as starting substrates for the synthesis of 6-(trifluoromethyl)phena...

Scheme 11: Synthesis of phenanthridines via aryl–aryl-bond formation.

Scheme 12: Oxidative conversion of N-biarylglycine esters to phenanthridine-6-carboxylates.

Scheme 13: Photocatalytic synthesis of benzo[f]quinolines from 2-heteroaryl-substituted anilines and heteroary...

Scheme 14: Synthesis of noravicine (14.2a) and nornitidine (14.2b) alkaloids.

Scheme 15: Gram-scale synthesis of the alkaloid trisphaeridine (15.3).

Scheme 16: Synthesis of phenanthridines starting from vinyl azides.

Scheme 17: Synthesis of pyrido[4,3,2-gh]phenanthridines 17.5a–d through the radical trifluoromethylthiolation ...

Scheme 18: The direct oxidative C–H amidation involving amidyl radicals for the synthesis of phenanthridones.
In silico rationalisation of selectivity and reactivity in Pd-catalysed C–H activation reactions
- Liwei Cao,
- Mikhail Kabeshov,
- Steven V. Ley and
- Alexei A. Lapkin
Beilstein J. Org. Chem. 2020, 16, 1465–1475, doi:10.3762/bjoc.16.122
- -addition, respectively [15]. Even though the mechanisms are inherently different, three most important aspects should be primarily taken into account when classifying and rationalising C–H activation reactions: the proximity of C–H bond to the transition metal; the energy of C–H bond cleavage within the
- feature of the proton abstraction (PA) mechanism [25] is that the formation of the metal–carbon bond (M–C) occurs simultaneously with the cleavage of the carbon–hydrogen (C–H) bond, while the hydrogen is being transferred to a basic centre, Scheme 1. Assuming the reaction proceeds through the formation of

Graphical Abstract

Figure 1: An approximate energy map for the electrophilic aromatic substitution mechanism.

Scheme 1: Schematic representation of the two mechanisms of Pd-catalysed C–H activation reaction considered i...
The McKenna reaction – avoiding side reactions in phosphonate deprotection
- Katarzyna Justyna,
- Joanna Małolepsza,
- Damian Kusy,
- Waldemar Maniukiewicz and
- Katarzyna M. Błażewska
Beilstein J. Org. Chem. 2020, 16, 1436–1446, doi:10.3762/bjoc.16.119
- reagent of choice for phosphonate ester cleavage, compared with its more and less reactive analogs, iodotrimethylsilane (ITMS) [11][12] and chlorotrimethylsilane (CTMS) [13], respectively. While being one of the most popular methods for the deprotection of organophosphorus esters, the McKenna reaction may
- be accompanied by side reactions such as the cleavage of tert-butyl carboxyester, [14][15] or other ester groups [16], as well as the formation of decomposition products [17][18][19][20]. Instead of focusing only on the experiments that “did work”, we decided to follow an alternative strategy
- reaction with BTMS, and underwent exchange of the chlorine substituent for bromine. This reaction can be partially circumvented by using nonpolar solvents, amines and lower temperatures. Section 5: Cleavage of the tert-butyl carboxyester group BTMS is known for its chemoselectivity and cleaves phosphonate

Graphical Abstract

Scheme 1: Schematic overview of the McKenna reaction including the decomposition of BTMS in protic solvents. ...

Figure 1: The model compounds used for this study (in red: the functionality of the molecules vulnerable to s...

Scheme 2: Formation of the side products derived from 10. Conditions: An equimolar mixture of propargylamide ...

Scheme 3: Addition of HBr to compound 11.

Scheme 4: N-Alkylation of 9.

Scheme 5: N-Alkylation of 12.

Scheme 6: Exchange of the chlorine substituent with bromine in 2-chloro-N-phenethylacetamide (13) under McKen...
An overview on disulfide-catalyzed and -cocatalyzed photoreactions
- Yeersen Patehebieke
Beilstein J. Org. Chem. 2020, 16, 1418–1435, doi:10.3762/bjoc.16.118

- , cyclopentanone, and three carbon ring-expanded 1,3-diones from vinyl spiro epoxides [14]. The reaction is initiated by the addition of a thiyl radical to the vinyl epoxide 24, followed by the epoxide fragmentation to the alkoxy radical 25. Then, the β-cleavage to form the carbon-centered radical 26, the final
- by the excitation with visible light, the homolytic cleavage of disulfide generates an arylthiyl radical (ArS•), which adds to diphenylacetylene to form a free-radical intermediate 28. Subsequently, this intermediate traps a molecule of singlet oxygen (1O2), and the thiyl radical is regenerated to
- give the four-membered ring intermediate 29. Finally, the rearrangement of the four-membered intermediate provides the diketone 30 as the product (Scheme 8). In 2017, Wang and co-workers reported an oxidative cleavage of aromatic alkenes at ambient temperature with visible-light irradiation, using

Graphical Abstract

Scheme 1: [3 + 2] cyclization catalyzed by diaryl disulfide.

Scheme 2: [3 + 2] cycloaddition catalyzed by disulfide.

Scheme 3: Disulfide-bridged peptide-catalyzed enantioselective cycloaddition.

Scheme 4: Disulfide-catalyzed [3 + 2] methylenecyclopentane annulations.

Scheme 5: Disulfide as a HAT cocatalyst in the [4 + 2] cycloaddition reaction.

Scheme 6: Proposed mechanism of the [4 + 2] cycloaddition reaction using disulfide as a HAT cocatalyst.

Scheme 7: Disulfide-catalyzed ring expansion of vinyl spiro epoxides.

Scheme 8: Disulfide-catalyzed aerobic oxidation of diarylacetylene.

Scheme 9: Disulfide-catalyzed aerobic photooxidative cleavage of olefins.

Scheme 10: Disulfide-catalyzed aerobic oxidation of 1,3-dicarbonyl compounds.

Scheme 11: Proposed mechanism of the disulfide-catalyzed aerobic oxidation of 1,3-dicarbonyl compounds.

Scheme 12: Disulfide-catalyzed oxidation of allyl alcohols.

Scheme 13: Disulfide-catalyzed diboration of alkynes.

Scheme 14: Dehalogenative radical cyclization catalyzed by disulfide.

Scheme 15: Hydrodifluoroacetamidation of alkenes catalyzed by disulfide.

Scheme 16: Plausible mechanism of the hydrodifluoroacetamidation of alkenes catalyzed by disulfide.

Scheme 17: Disulfide-cocatalyzed anti-Markovnikov olefin hydration reactions.

Scheme 18: Disulfide-catalyzed decarboxylation of carboxylic acids.

Scheme 19: Proposed mechanism of the disulfide-catalyzed decarboxylation of carboxylic acids.

Scheme 20: Disulfide-catalyzed decarboxylation of carboxylic acids.

Scheme 21: Disulfide-catalyzed conversion of maleate esters to fumarates and 5H-furanones.

Scheme 22: Disulfide-catalyzed isomerization of difluorotriethylsilylethylene.

Scheme 23: Disulfide-catalyzed isomerization of allyl alcohols to carbonyl compounds.

Scheme 24: Proposed mechanism for the disulfide-catalyzed isomerization of allyl alcohols to carbonyl compound...

Scheme 25: Diphenyl disulfide-catalyzed enantioselective synthesis of ophirin B.

Scheme 26: Disulfide-catalyzed isomerization in the total synthesis of (+)-hitachimycin.

Scheme 27: Disulfide-catalyzed isomerization in the synthesis of (−)-gloeosporone.
Synthesis of new fluorescent molecules having an aggregation-induced emission property derived from 4-fluoroisoxazoles
- Kazuyuki Sato,
- Akira Kawasaki,
- Yukiko Karuo,
- Atsushi Tarui,
- Kentaro Kawai and
- Masaaki Omote
Beilstein J. Org. Chem. 2020, 16, 1411–1417, doi:10.3762/bjoc.16.117
- diketone (Scheme 5). As an alternative method to synthesize F-BKIs 9, we turned our attention to the ring-opening reaction of isoxazoles. The reductive cleavage of the N–O bond in isoxazoles can be achieved by transition metals or their complexes to give the corresponding enaminoketones [35][37
- in the aggregated state and exhibited a redshift in the emission intensity. We also achieved the first synthesis of α-fluorinated boron ketoiminates (F-BKIs) by the reductive cleavage of the N–O bond in 4-fluorinated isoxazoles and demonstrated that F-BKIs exhibited AIE property similarly to their

Graphical Abstract

Scheme 1: Selective fluorination of isoxazoles and one-pot synthesis of 4-fluoroisoxazoles.

Scheme 2: One-pot reaction for the synthesis of 3,5-disubstituted 4-fluoroisoxazoles 3. aIsolated yield. bIso...

Figure 1: UV–vis and fluorescence (FL) spectra of compounds 3b and 3c.

Scheme 3: Synthesis of BKIs 6 either from 1,3-diketones 1 or from isoxazoles 2.

Scheme 4: Synthesis of enaminoketones 5 and 8 and their conversion to BKIs (yields refer to isolated yields; a...

Scheme 5: Attempted selective fluorination of BKI 6b.

Scheme 6: Ring-opening reaction of 4-fluoroisoxazoles 3 and their conversion into F-BKIs 9 (yields refer to i...

Figure 2: Photochemical properties comparisons of BKIs and F-BKIs. (a–c) BKI 6b: photograph (a), UV–vis (b), ...
Recent synthesis of thietanes
- Jiaxi Xu
Beilstein J. Org. Chem. 2020, 16, 1357–1410, doi:10.3762/bjoc.16.116
- second displacement was an intramolecular SN2 process performed under mild basic conditions, affording the desired thietane 116 in 92% yield. After deprotection, oxidative cleavage, and reduction, a thietanose 117 was obtained in 63% overall yield. The thietanose 117 was further applied to synthesize a
- cleavage and aromatization to give substituted pyridines 255 [75] (Scheme 49). In 1989, Kanaoka and co-workers further studied the photo [2 + 2] cycloadditions of thiobarbiturates 256–258, whose skeletons consisted of a combination of a thioamide and an amide or a thioamide (two-imides system), and olefins
- were obtained via a ring cleavage of the thietanes, that had formed in the [2 + 2] photocycloaddition of the thiocarbonyl moiety and the olefin. However, the reaction of 1,3,3-trimethylindoline-2-thione (300) and isobutene (215c) afforded the corresponding spiroindoline-thietane derivative 301 [80

Graphical Abstract

Figure 1: Examples of biologically active thietane-containing molecules.

Figure 2: The diverse methods for the synthesis of thietanes.

Scheme 1: Synthesis of 1-(thietan-2-yl)ethan-1-ol (10) from 3,5-dichloropentan-2-ol (9).

Scheme 2: Synthesis of thietanose nucleosides 2,14 from 2,2-bis(bromomethyl)propane-1,3-diol (11).

Scheme 3: Synthesis of methyl 3-vinylthietane-3-carboxylate (19).

Scheme 4: Synthesis of 1,6-thiazaspiro[3.3]heptane (24).

Scheme 5: Synthesis of 6-amino-2-thiaspiro[3.3]heptane hydrochloride (28).

Scheme 6: Synthesis of optically active thietane 31 from vitamin C.

Scheme 7: Synthesis of an optically active thietane nucleoside from diethyl L-tartrate (32).

Scheme 8: Synthesis of thietane-containing spironucleoside 40 from 5-aldo-3-O-benzyl-1,2-O-isopropylidene-α-D...

Scheme 9: Synthesis of optically active 2-methylthietane-containing spironucleoside 43.

Scheme 10: Synthesis of a double-linked thietane-containing spironucleoside 48.

Scheme 11: Synthesis of two diastereomeric thietanose nucleosides via 2,4-di(benzyloxymethyl)thietane (49).

Scheme 12: Synthesis of the thietane-containing PI3k inhibitor candidate 54.

Scheme 13: Synthesis of the spirothietane 57 as the key intermediate to Nuphar sesquiterpene thioalkaloids.

Scheme 14: Synthesis of spirothietane 61 through a direct cyclic thioetherification of 3-mercaptopropan-1-ol.

Scheme 15: Synthesis of thietanes 66 from 1,3-diols 62.

Scheme 16: Synthesis of thietanylbenzimidazolone 75 from (iodomethyl)thiazolobenzimidazole 70.

Scheme 17: Synthesis of 2-oxa-6-thiaspiro[3.3]heptane (80) from bis(chloromethyl)oxetane 76 and thiourea.

Scheme 18: Synthesis of the thietane-containing glycoside, 2-O-p-toluenesulfonyl-4,6-thioanhydro-α-D-gulopyran...

Scheme 19: Synthesis of methyl 4,6-thioanhydro-α-D-glucopyranoside (89).

Scheme 20: Synthesis of thietane-fused α-D-galactopyranoside 93.

Scheme 21: Synthesis of thietane-fused α-D-gulopyranoside 100.

Scheme 22: Synthesis of 3,5-anhydro-3-thiopentofuranosides 104.

Scheme 23: Synthesis of anhydro-thiohexofuranosides 110, 112 and 113 from from 1,2:4,5-di-O-isopropylidene D-f...

Scheme 24: Synthesis of optically active thietanose nucleosides from D- and L-xyloses.

Scheme 25: Synthesis of thietane-fused nucleosides.

Scheme 26: Synthesis of 3,5-anhydro-3-thiopentofuranosides.

Scheme 27: Synthesis of 2-amino-3,5-anhydro-3-thiofuranoside 141.

Scheme 28: Synthesis of thietane-3-ols 145 from (1-chloromethyl)oxiranes 142 and hydrogen sulfide.

Scheme 29: Synthesis of thietane-3-ol 145a from chloromethyloxirane (142a).

Scheme 30: Synthesis of thietane-3-ols 145 from 2-(1-haloalkyl)oxiranes 142 and 147 with ammonium monothiocarb...

Scheme 31: Synthesis of 7-deoxy-5(20)thiapaclitaxel 154a, a thietane derivative of taxoids.

Scheme 32: Synthesis of 5(20)-thiadocetaxel 158 from 10-deacetylbaccatin III (155).

Scheme 33: Synthesis of thietane derivatives 162 as precursors for deoxythiataxoid synthesis through oxiraneme...

Scheme 34: Synthesis of 7-deoxy 5(20)-thiadocetaxel 154b.

Scheme 35: Mechanism for the formation of the thietane ring in 171 from oxiranes with vicinal leaving groups 1...

Scheme 36: Synthesis of cis-2,3-disubstituted thietane 175 from thiirane-2-methanol 172.

Scheme 37: Synthesis of a bridged thietane 183 from aziridine cyclohexyl tosylate 179 and ammonium tetrathiomo...

Scheme 38: Synthesis of thietanes via the photochemical [2 + 2] cycloaddition of thiobenzophenone 184a with va...

Scheme 39: Synthesis of spirothietanes through the photo [2 + 2] cycloaddition of cyclic thiocarbonyls with ol...

Scheme 40: Photochemical synthesis of spirothietane-thioxanthenes 210 from thioxanthenethione (208) and butatr...

Scheme 41: Synthesis of thietanes 213 from 2,4,6-tri(tert-butyl)thiobenzaldehyde (211) with substituted allene...

Scheme 42: Photochemical synthesis of spirothietanes 216 and 217 from N-methylthiophthalimide (214) with olefi...

Scheme 43: Synthesis of fused thietanes from quadricyclane with thiocarbonyl derivatives 219.

Scheme 44: Synthesis of tricyclic thietanes via the photo [2 + 2] cycloaddition of N-methyldithiosuccinimides ...

Scheme 45: Synthesis of tricyclic thietanes via the photo [2 + 2] cycloaddition of N-methylthiosuccinimide/thi...

Scheme 46: Synthesis of tricyclic thietanes via the photo [2 + 2] cycloaddition of N-alkylmonothiophthalimides...

Scheme 47: Synthesis of spirothietanes from dithiosuccinimides 223 with 2,3-dimethyl-2-butene (215a).

Scheme 48: Synthesis of thietanes 248a,b from diaryl thione 184b and ketene acetals 247a,b.

Scheme 49: Photocycloadditions of acridine-9-thiones 249 and pyridine-4(1H)-thione (250) with 2-methylacrynitr...

Scheme 50: Synthesis of thietanes via the photo [2 + 2] cycloaddition of mono-, di-, and trithiobarbiturates 2...

Scheme 51: Synthesis of spirothietanes via the photo [2 + 2] cycloaddition of 1,1,3-trimethyl-2-thioxo-1,2-dih...

Scheme 52: Synthesis of spirothietanes via the photo [2 + 2] cycloaddition of thiocoumarin 286 with olefins.

Scheme 53: Photochemical synthesis of thietanes 296–299 from semicyclic and acyclic thioimides 292–295 and 2,3...

Scheme 54: Photochemical synthesis of spirothietane 301 from 1,3,3-trimethylindoline-2-thione (300) and isobut...

Scheme 55: Synthesis of spirobenzoxazolethietanes 303 via the photo [2 + 2] cycloaddition of alkyl and aryl 2-...

Scheme 56: Synthesis of spirothietanes from tetrahydrothioxoisoquinolines 306 and 307 with olefins.

Scheme 57: Synthesis of spirothietanes from 1,3-dihydroisobenzofuran-1-thiones 311 and benzothiophene-1-thione...

Scheme 58: Synthesis of 2-triphenylsilylthietanes from phenyl triphenylsilyl thioketone (316) with electron-po...

Scheme 59: Diastereoselective synthesis of spiropyrrolidinonethietanes 320 via the photo [2 + 2] cycloaddition...

Scheme 60: Synthesis of bicyclic thietane 323 via the photo [2 + 2] cycloaddition of 2,4-dioxo-3,4-dihydropyri...

Scheme 61: Photo-induced synthesis of fused thietane-2-thiones 325 and 326 from silacyclopentadiene 324 and ca...

Scheme 62: Synthesis of highly strained tricyclic thietanes 328 via the intramolecular photo [2 + 2] cycloaddi...

Scheme 63: Synthesis of tri- and pentacyclic thietanes 330 and 332, respectively, through the intramolecular p...

Scheme 64: Synthesis of tricyclic thietanes 334 via the intramolecular photo [2 + 2] cycloaddition of N-vinylt...

Scheme 65: Synthesis of tricyclic thietanes 336 via the intramolecular photo [2 + 2] cycloaddition of N-but-3-...

Scheme 66: Synthesis of tricyclic thietanes via the intramolecular photo [2 + 2] cycloaddition of N-but-3-enyl...

Scheme 67: Synthesis of tetracyclic thietane 344 through the intramolecular photo [2 + 2] cycloaddition of N-[...

Scheme 68: Synthesis of tri- and tetracyclic thietanes 348, 350, and 351, through the intramolecular photo [2 ...

Scheme 69: Synthesis of tetracyclic fused thietane 354 via the photo [2 + 2] cycloaddition of vinyl 2-thioxo-3H...

Scheme 70: Synthesis of highly rigid thietane-fused β-lactams via the intramolecular photo [2 + 2] cycloadditi...

Scheme 71: Asymmetric synthesis of a highly rigid thietane-fused β-lactam 356a via the intramolecular photo [2...

Scheme 72: Diastereoselective synthesis of the thietane-fused β-lactams via the intramolecular photo [2 + 2] c...

Scheme 73: Asymmetric synthesis of thietane-fused β-lactams 356 via the intramolecular photo [2 + 2] cycloaddi...

Scheme 74: Synthesis of the bridged bis(trifluoromethyl)thietane from 2,2,4,4-tetrakis(trifluoromethyl)-1,3-di...

Scheme 75: Synthesis of the bridged-difluorothietane 368 from 2,2,4,4-tetrafluoro-1,3-dithietane (367) and qua...

Scheme 76: Synthesis of bis(trifluoromethyl)thietanes from 2,2,4,4-tetrakis(trifluoromethyl)-1,3-dithietane (3...

Scheme 77: Synthesis of 2,2-dimethylthio-4,4-di(trifluoromethyl)thietane (378) from 2,2,4,4-tetrakis(trifluoro...

Scheme 78: Formation of bis(trifluoromethyl)thioacetone (381) through nucleophilic attack of dithietane 363 by...

Scheme 79: Synthesis of 2,2-bis(trifluoromethyl)thietanes from 2,2,4,4-tetrakis(trifluoromethyl)-1,3-dithietan...

Scheme 80: Synthesis of the bridged bis(trifluoromethyl)thietane 364 from of 2,2,4,4-tetrakis(trifluoromethyl)...

Scheme 81: Synthesis of 2,4-diiminothietanes 390 from alkenimines and 4-methylbenzenesulfonyl isothiocyanate (...

Scheme 82: Synthesis of arylidene 2,4-diiminothietanes 393 starting from phosphonium ylides 391 and isothiocya...

Scheme 83: Synthesis of thietane-2-ylideneacetates 397 through a DABCO-catalyzed formal [2 + 2] cycloaddition ...

Scheme 84: Synthesis of 3-substituted thietanes 400 from (1-chloroalkyl)thiiranes 398.

Scheme 85: Synthesis of N-(thietane-3-yl)azaheterocycles 403 and 404 through reaction of chloromethylthiirane (...

Scheme 86: Synthesis of 3-sulfonamidothietanes 406 from sulfonamides and chloromethylthiirane (398a).

Scheme 87: Synthesis of N-(thietane-3-yl)isatins 408 from chloromethylthiirane (398a) and isatins 407.

Scheme 88: Synthesis of 3-(nitrophenyloxy)thietanes 410 from nitrophenols 409 and chloromethylthiirane (398a).

Scheme 89: Synthesis of N-aryl-N-(thietane-3-yl)cyanamides 412 from N-arylcyanamides 411 and chloromethylthiir...

Scheme 90: Synthesis of 1-(thietane-3-yl)pyrimidin-2,4(1H,3H)-diones 414 from chloromethylthiirane (398a) and ...

Scheme 91: Synthesis of 2,4-diiminothietanes 418 from 2-iminothiiranes 416 and isocyanoalkanes 415.

Scheme 92: Synthesis of 2-vinylthietanes 421 from thiiranes 419 and 3-chloroallyl lithium (420).

Scheme 93: Synthesis of thietanes from thiiranes 419 and trimethyloxosulfonium iodide 424.

Scheme 94: Mechanism for synthesis of thietanes 425 from thiiranes 419 and trimethyloxosulfonium iodide 424.

Scheme 95: Synthesis of functionalized thietanes from thiiranes and dimethylsulfonium acylmethylides.

Scheme 96: Mechanism for the rhodium-catalyzed synthesis of functionalized thietanes 429 from thiiranes 419 an...

Scheme 97: Synthesis of 3-iminothietanes 440 through thermal isomerization from 4,5-dihydro-1,3-oxazole-4-spir...

Scheme 98: Synthesis of thietanes 443 from 3-chloro-2-methylthiolane (441) through ring contraction.

Scheme 99: Synthesis of an optically active thietanose 447 from D-xylose involving a ring contraction.

Scheme 100: Synthesis of optically thietane 447 via the DAST-mediated ring contraction of 448.

Scheme 101: Synthesis of the optically thietane nucleoside 451 via the ring contraction of thiopentose in 450.

Scheme 102: Synthesis of spirothietane 456 from 3,3,5,5-tetramethylthiolane-2,4-dithione (452) and benzyne (453...

Scheme 103: Synthesis of thietanes 461 via photoisomerization of 2H,6H-thiin-3-ones 459.

Scheme 104: Phosphorodithioate-mediated synthesis of 1,4-diarylthietanes 465.

Scheme 105: Mechanism of the phosphorodithioate-mediated synthesis of 1,4-diarylthietanes 465.

Scheme 106: Phosphorodithioate-mediated synthesis of trisubstituted thietanes (±)-470.

Scheme 107: Mechanism on the phosphorodithioate-mediated synthesis of trisubstituted thietanes.

Scheme 108: Phosphorodithioate-mediated synthesis of thietanes (±)-475.

Scheme 109: Phosphorodithioate-mediated synthesis of 1,2-disubstituted thietanes from aldehydes 476 and acrylon...

Scheme 110: Phosphorodithioate-mediated synthesis of 1,2-disubstituted thietanes via a one-pot three-component ...

Scheme 111: Mechanism for the phosphorodithioate-mediated synthesis of 1,2-disubstituted thietanes via three-co...

Scheme 112: Phosphorodithioate-mediated synthesis of substituted 3-nitrothietanes.

Scheme 113: Mechanism on the phosphorodithioate-mediated synthesis of 1,2-disubstituted thietanes (±)-486.

Scheme 114: Asymmetric synthesis of (S)-2-phenylthietane (497).

Scheme 115: Asymmetric synthesis of optically active 2,4-diarylthietanes.

Scheme 116: Synthesis of 3-acetamidothietan-2-one 503 via the intramolecular thioesterification of 3-mercaptoal...

Scheme 117: Synthesis of 4-substituted thietan-2-one via the intramolecular thioesterification of 3-mercaptoalk...

Scheme 118: Synthesis of 4,4-disubstituted thietan-2-one 511 via the intramolecular thioesterification of the 3...

Scheme 119: Synthesis of a spirothietan-2-one 514 via the intramolecular thioesterification of 3-mercaptoalkano...

Scheme 120: Synthesis of thiatetrahydrolipstatin starting from (S)-(−)-epichlorohydrin ((S)-142a).

Scheme 121: Synthesis of 2-phenethyl-4-(propan-2-ylidene)thietane (520) from 5-bromo-6-methyl-1-phenylhept-5-en...

Scheme 122: Synthesis of 2-phenethyl-4-(propan-2-ylidene)thietane (520) directly from S-(5-bromo-6-methyl-1-phe...

Scheme 123: Synthesis of 2-alkylidenethietanes from S-(2-bromoalk-1-en-4-yl)thioacetates.

Scheme 124: Synthesis of 2-alkylidenethietanes from S-(2-bromo/chloroalk-1-en-4-yl)thiols.

Scheme 125: Synthesis of spirothietan-3-ol 548 from enone 545 and ammonium hydrosulfide.

Scheme 126: Asymmetric synthesis of the optically active thietanoside from cis-but-2-ene-1,4-diol (47).

Scheme 127: Synthesis of 2-alkylidenethietan-3-ols 557 via the fluoride-mediated cyclization of thioacylsilanes ...

Scheme 128: Synthesis of 2-iminothietanes via the reaction of propargylbenzene (558) and isothiocyanates 560 in...

Scheme 129: Synthesis of 2-benzylidenethietane 567 via the nickel complex-catalyzed electroreductive cyclizatio...

Scheme 130: Synthesis of 2-iminothietanes 569 via the photo-assisted electrocyclic reaction of N-monosubstitute...

Scheme 131: Synthesis of ethyl 3,4-diiminothietane-2-carboxylates from ethyl thioglycolate (570) and bis(imidoy...

Scheme 132: Synthesis of N-(thietan-3-yl)-α-oxoazaheterocycles from azaheterocyclethiones and chloromethyloxira...

Scheme 133: Synthesis of thietan-3-yl benzoate (590) via the nickel-catalyzed intramolecular reductive thiolati...

Scheme 134: Synthesis of 2,2-bis(trifluoromethyl)thietane from 3,3-bis(trifluoromethyl)-1,2-dithiolane.

Scheme 135: Synthesis of thietanes from enamines and sulfonyl chlorides.

Scheme 136: Synthesis of spirothietane 603 via the [2 + 3] cycloaddition of 2,2,4,4-tetramethylcyclobutane-1,3-...

Scheme 137: Synthesis of thietane (605) from 1-bromo-3-chloropropane and sulfur.
Synthesis of 3-substituted isoxazolidin-4-ols using hydroboration–oxidation reactions of 4,5-unsubstituted 2,3-dihydroisoxazoles
- Lívia Dikošová,
- Júlia Laceková,
- Ondrej Záborský and
- Róbert Fischer
Beilstein J. Org. Chem. 2020, 16, 1313–1319, doi:10.3762/bjoc.16.112
- readily obtained through the reductive cleavage of benzoylated isoxazolidines, employing the Lewis acid-catalyzed SN reaction with triethylsilane as the hydride source (Scheme 1). For this reason, the benzoates 6a and 6b were readily prepared from the corresponding 2,3-dihydroisoxazoles 5a and 5b
- showed the applicability of the reductive cleavage of anomeric isoxazolidinyl carboxylates in the synthesis of the respective 5-unsubstituted 4-hydroxyisoxazolidines, this pioneering approach mainly suffered from a large number of synthetic steps starting from 2,3-dihydroisoxazoles, leading to the target
- 8b resulted only in a reductive cleavage of the N–O bond. A successful debenzylation was achieved in a two-step procedure using 2,2,2-trichloroethyl chloroformate (TrocCl). However, the protection of the hydroxy group was required first (for the reaction of 8b with benzoyl chloride, see Supporting

Graphical Abstract

Figure 1: 3-Substituted isoxazolidin-4-ols resembling 3-hydroxypyrrolidines.

Scheme 1: Synthetic approach towards isoxazolidin-4-ols via the regioselective reductive cleavage of the C5–O...

Scheme 2: Hydroboration-oxidation of 4,5-unsubstituted 2,3-dihydroisoxazoles.

Figure 2: Selected NOE enhancements observed in the isoxazolidin-4-ol trans-8a. The arrows show the NOESY cor...

Scheme 3: Dess-Martin oxidation of isoxazolidin-4-ols to ketones.

Scheme 4: Inversion of the relative configuration of the isoxazolidine ring.

Figure 3: Selected NOE enhancements observed in the isoxazolidin-4-ol cis-10a. The arrows show the NOESY corr...

Scheme 5: N-debenzylation via N-Troc-protected isoxazolidines.
Oxime radicals: generation, properties and application in organic synthesis
- Igor B. Krylov,
- Stanislav A. Paveliev,
- Alexander S. Budnikov and
- Alexander O. Terent’ev
Beilstein J. Org. Chem. 2020, 16, 1234–1276, doi:10.3762/bjoc.16.107
- reactions of oxime radicals. These reactions are classified into cyclizations involving C–H bond cleavage and cyclizations involving a double C=C bond cleavage. Keywords: iminoxyl radicals; isoxazolines; oxidative cyclization; oxime radicals; oximes; Introduction Free radicals in which an unpaired
- abstraction with cleavage of the C–H bond [12][13][14][15][16][17][18] and in the processes of functionalization of С=С double bonds [19][20]. Amidoxyl radicals (Figure 1, III) are applied in the functionalization of the double bonds [21][22][23][24][25][26] and in mild oxidations [27]. In contrast to the
- hardly achievable or not achievable by non-radical methods. In this review only works in which the participation of iminoxyl radicals was confirmed or assumed are discussed. Oxidative cyclization with the cleavage of the C–H bond In one of the first works in this area oximes with activated C–H bond in

Graphical Abstract

Figure 1: Imine-N-oxyl radicals (IV) discussed in the present review and other classes of N-oxyl radicals (I–...

Figure 2: The products of decomposition of iminoxyl radicals generated from oximes by oxidation with Ag2O.

Scheme 1: Generation of oxime radicals and study of the kinetics of their decay by photolysis of the solution...

Scheme 2: Synthesis of di-tert-butyliminoxyl radical and its decomposition products.

Scheme 3: The proposed reaction pathway of the decomposition of di-tert-butyliminoxyl radical (experimentally...

Scheme 4: Monomolecular decomposition of the tert-butyl(triethylmethyl)oxime radical.

Scheme 5: The synthesis and stability of the most stable dialkyl oxime radicals – di-tert-butyliminoxyl and d...

Scheme 6: The formation of iminoxyl radicals from β-diketones under the action of NO2.

Scheme 7: Synthesis of the diacetyliminoxyl radical.

Scheme 8: Examples of long-living oxime radicals with electron-withdrawing groups and the conditions for thei...

Figure 3: The electronic structure iminoxyl radicals and their geometry compared to the corresponding oximes.

Figure 4: Bond dissociation enthalpies (kcal/mol) of oximes and N,N-disubstituted hydroxylamines calculated o...

Scheme 9: Examples demonstrating the low reactivity of the di-tert-butyliminoxyl radical towards the substrat...

Scheme 10: The reactions of di-tert-butyliminoxyl radical with unsaturated hydrocarbons involving hydrogen ato...

Scheme 11: Possible mechanisms of reaction of di-tert-butyliminoxyl radical with alkenes.

Scheme 12: Products of the reaction between di-tert-butyliminoxyl radical and phenol derivatives.

Scheme 13: The reaction of di-tert-butyliminoxyl radical with amines.

Scheme 14: Reaction of di-tert-butyliminoxyl radicals with organolithium reagents.

Scheme 15: Cross-dehydrogenative C–O coupling of 1,3-dicarbonyl compounds with oximes under the action of mang...

Scheme 16: Cross-dehydrogenative C–O coupling of 1,3-dicarbonyl compounds with oximes under the action of Cu(BF...

Scheme 17: Oxidative C–O coupling of benzylmalononitrile (47) with 3-(hydroxyimino)pentane-2,4-dione (19).

Scheme 18: The proposed mechanism of the oxidative coupling of benzylmalononitrile (47) with diacetyl oxime (19...

Scheme 19: Oxidative C–O coupling of pyrazolones with oximes under the action of Fe(ClO4)3.

Scheme 20: The reaction of diacetyliminoxyl radical with pyrazolones.

Scheme 21: Oxidative C–O coupling of oximes with acetonitrile, ketones, and esters.

Scheme 22: Intramolecular cyclizations of oxime radicals to form substituted isoxazolines or cyclic nitrones.

Scheme 23: TEMPO-mediated oxidative cyclization of oximes with C–H bond cleavage.

Scheme 24: Proposed reaction mechanism of oxidative cyclization of oximes with C–H bond cleavage.

Scheme 25: Selectfluor/Bu4NI-mediated C–H oxidative cyclization of oximes.

Scheme 26: Oxidative cyclization of N-benzyl amidoximes to 1,2,4-oxadiazoles.

Scheme 27: The formation of quinazolinone 73a from 5-phenyl-4,5-dihydro-1,2,4-oxadiazole 74 under air.

Scheme 28: DDQ-mediated oxidative cyclization of thiohydroximic acids.

Scheme 29: Plausible mechanism of the oxidative cyclization of thiohydroximic acids.

Scheme 30: Silver-mediated oxidative cyclization of α-halogenated ketoximes and 1,3-dicarbonyl compounds.

Scheme 31: Possible pathway of one-pot oxidative cyclization of α-halogenated ketoximes and 1,3-dicarbonyl com...

Scheme 32: T(p-F)PPT-catalyzed oxidative cyclization of oximes with the formation of 1,2,4-oxadiazolines.

Scheme 33: Intramolecular cyclization of iminoxyl radicals involving multiple C=C and N=N bonds.

Scheme 34: Oxidative cyclization of β,γ- and γ,δ-unsaturated oximes employing the DEAD or TEMPO/DEAD system wi...

Scheme 35: Cobalt-catalyzed aerobic oxidative cyclization of β,γ-unsaturated oximes.

Scheme 36: Manganese-catalyzed aerobic oxidative cyclization of β,γ-unsaturated oximes.

Scheme 37: Visible light photocatalytic oxidative cyclization of β,γ-unsaturated oximes.

Scheme 38: TBAI/TBHP-mediated radical cascade cyclization of the β,γ-unsaturated oximes.

Scheme 39: TBAI/TBHP-mediated radical cascade cyclization of vinyl isocyanides with β,γ-unsaturated oximes.

Scheme 40: tert-Butylnitrite-mediated oxidative cyclization of unsaturated oximes with the introduction of an ...

Scheme 41: Transformation of unsaturated oxime to oxyiminomethylisoxazoline via the confirmed dimeric nitroso ...

Scheme 42: tert-Butylnitrite-mediated oxidative cyclization of unsaturated oximes with the introduction of a n...

Scheme 43: Synthesis of cyano-substituted oxazolines from unsaturated oximes using the TBN/[RuCl2(p-cymene)]2 ...

Scheme 44: Synthesis of trifluoromethylthiolated isoxazolines from unsaturated oximes.

Scheme 45: Copper-сatalyzed oxidative cyclization of β,γ-unsaturated oximes with the introduction of an azido ...

Scheme 46: TBHP-mediated oxidative cascade cyclization of β,γ-unsaturated oximes and unsaturated N-arylamides.

Scheme 47: Copper-сatalyzed oxidative cyclization of unsaturated oximes with the introduction of an amino grou...

Scheme 48: TEMPO-mediated oxidative cyclization of unsaturated oximes followed by elimination.

Scheme 49: Oxidative cyclization of β,γ-unsaturated oximes with the introduction of a trifluoromethyl group.

Scheme 50: Oxidative cyclization of unsaturated oximes with the introduction of a nitrile group.

Scheme 51: Oxidative cyclization of β,γ-unsaturated oximes to isoxazolines with the introduction of a nitrile ...

Scheme 52: Oxidative cyclization of β,γ-unsaturated oximes to isoxazolines with the introduction of a sulfonyl...

Scheme 53: Oxidative cyclization of β,γ- and γ,δ-unsaturated oximes to isoxazolines with the introduction of a...

Scheme 54: Oxidative cyclization of β,γ-unsaturated oximes to isoxazolines with the introduction of a thiocyan...

Scheme 55: PhI(OAc)2-mediated oxidative cyclization of oximes with C–S and C–Se bond formation.

Scheme 56: PhI(OAc)2-mediated oxidative cyclization of unsaturated oximes accompanied by alkoxylation.

Scheme 57: PhI(OAc)2-mediated cyclization of unsaturated oximes to methylisoxazolines.

Scheme 58: Oxidative cyclization-alkynylation of unsaturated oximes.

Scheme 59: TEMPO-mediated oxidative cyclization of C-glycoside ketoximes to C-glycosylmethylisoxazoles.

Scheme 60: Silver-сatalyzed oxidative cyclization of β,γ-unsaturated oximes with formation of fluoroalkyl isox...

Scheme 61: Oxidative cyclization of β,γ-unsaturated oximes with the formation of haloalkyl isoxazolines.

Scheme 62: Cyclization of β,γ-unsaturated oximes into haloalkyl isoxazolines under the action of the halogenat...

Scheme 63: Synthesis of haloalkyl isoxazoles and cyclic nitrones via oxidative cyclization and 1,2-halogen shi...

Scheme 64: Electrochemical oxidative cyclization of diaryl oximes.

Scheme 65: Copper-сatalyzed cyclization and dioxygenation oximes containing a triple C≡C bond.

Scheme 66: Photoredox-catalyzed sulfonylation of β,γ-unsaturated oximes by sulfonyl hydrazides.

Scheme 67: Oxidative cyclization of β,γ-unsaturated oximes with introduction of sulfonate group.

Scheme 68: Ultrasound-promoted oxidative cyclization of β,γ-unsaturated oximes.
Photocatalysis with organic dyes: facile access to reactive intermediates for synthesis
- Stephanie G. E. Amos,
- Marion Garreau,
- Luca Buzzetti and
- Jerome Waser
Beilstein J. Org. Chem. 2020, 16, 1163–1187, doi:10.3762/bjoc.16.103
- heteroarene 6.3. Other reductive fragmentations Another organophotocatalytic strategy for accessing C(sp3) radicals relies on the reductive homolytic cleavage of easily reducible functional groups. In this case, the substrates can act as acceptors in SET reductions, and the alkyl radical is obtained after the
- cleavage of various benzyl halides 7.1 (I, Br, Cl). The radical is then intercepted by an H atom donor (Hantzsch ester), which delivers the corresponding toluene derivative 7.2. Other organic dyes can promote the reductive fragmentation of alkyl halides. For instance, König and Zeitler demonstrated that a
- Photocatalytic hydrogen atom transfer (HAT) represents a valuable strategy for accessing C(sp3) radicals. This method allows the direct cleavage of a C–H bond and the consequent generation of alkyl radicals without relying on the presence of redox-active functional groups. This results in a superior atom economy

Graphical Abstract

Figure 1: Selected examples of organic dyes. Mes-Acr+: 9-mesityl-10-methylacridinium, DCA: 9,10-dicyanoanthra...

Scheme 1: Activation modes in photocatalysis.

Scheme 2: Main strategies for the formation of C(sp3) radicals used in organophotocatalysis.

Scheme 3: Illustrative example for the photocatalytic oxidative generation of radicals from carboxylic acids:...

Scheme 4: Illustrative example for the photocatalytic reductive generation of C(sp3) radicals from redoxactiv...

Figure 2: Common substrates for the photocatalytic oxidative generation of C(sp3) radicals.

Scheme 5: Illustrative example for the photocatalytic oxidative generation of radicals from dihydropyridines ...

Scheme 6: Illustrative example for the photocatalytic oxidative generation of C(sp3) radicals from trifluorob...

Scheme 7: Illustrative example for the photocatalytic reductive generation of C(sp3) radicals from benzylic h...

Scheme 8: Illustrative example for the photocatalytic generation of C(sp3) radicals via direct HAT: the cross...

Scheme 9: Illustrative example for the photocatalytic generation of C(sp3) radicals via indirect HAT: the deu...

Scheme 10: Selected precursors for the generation of aryl radicals using organophotocatalysis.

Scheme 11: Illustrative example for the photocatalytic reductive generation of aryl radicals from aryl diazoni...

Scheme 12: Illustrative examples for the photocatalytic reductive generation of aryl radicals from haloarenes:...

Scheme 13: Illustrative example for the photocatalytic reductive generation of aryl radicals from aryl halides...

Scheme 14: Illustrative example for the photocatalytic reductive generation of aryl radicals from arylsulfonyl...

Scheme 15: Illustrative example for the reductive photocatalytic generation of aryl radicals from triaryl sulf...

Scheme 16: Main strategies towards acyl radicals used in organophotocatalysis.

Scheme 17: Illustrative example for the decarboxylative photocatalytic generation of acyl radicals from α-keto...

Scheme 18: Illustrative example for the oxidative photocatalytic generation of acyl radicals from acyl silanes...

Scheme 19: Illustrative example for the oxidative photocatalytic generation of carbamoyl radicals from 4-carba...

Scheme 20: Illustrative example of the photocatalytic HAT approach for the generation of acyl radicals from al...

Scheme 21: General reactivity of a) radical cations; b) radical anions; c) the main strategies towards aryl an...

Scheme 22: Illustrative example for the oxidative photocatalytic generation of alkene radical cations from alk...

Scheme 23: Illustrative example for the reductive photocatalytic generation of an alkene radical anion from al...

Figure 3: Structure of C–X radical anions and their neutral derivatives.

Scheme 24: Illustrative example for the photocatalytic reduction of imines and the generation of an α-amino C(...

Scheme 25: Illustrative example for the oxidative photocatalytic generation of aryl radical cations from arene...

Scheme 26: NCR classifications and generation.

Scheme 27: Illustrative example for the photocatalytic reductive generation of iminyl radicals from O-aryl oxi...

Scheme 28: Illustrative example for the photocatalytic oxidative generation of iminyl radicals from α-N-oxy ac...

Scheme 29: Illustrative example for the photocatalytic oxidative generation of iminyl radicals via an N–H bond...

Scheme 30: Illustrative example for the photocatalytic oxidative generation of amidyl radicals from Weinreb am...

Scheme 31: Illustrative example for the photocatalytic reductive generation of amidyl radicals from hydroxylam...

Scheme 32: Illustrative example for the photocatalytic reductive generation of amidyl radicals from N-aminopyr...

Scheme 33: Illustrative example for the photocatalytic oxidative generation of amidyl radicals from α-amido-ox...

Scheme 34: Illustrative example for the photocatalytic oxidative generation of aminium radicals: the N-aryltet...

Scheme 35: Illustrative example for the photocatalytic oxidative generation of nitrogen-centered radical catio...

Scheme 36: Illustrative example for the photocatalytic oxidative generation of nitrogen-centered radical catio...

Scheme 37: Illustrative example for the photocatalytic oxidative generation of hydrazonyl radical from hydrazo...

Scheme 38: Generation of O-radicals.

Scheme 39: Illustrative examples for the photocatalytic generation of O-radicals from N-alkoxypyridinium salts...

Scheme 40: Illustrative examples for the photocatalytic generation of O-radicals from alkyl hydroperoxides: th...

Scheme 41: Illustrative example for the oxidative photocatalytic generation of thiyl radicals from thiols: the...

Scheme 42: Main strategies and reagents for the generation of sulfonyl radicals used in organophotocatalysis.

Scheme 43: Illustrative example for the reductive photocatalytic generation of sulfonyl radicals from arylsulf...

Scheme 44: Illustrative example of a Cl atom abstraction strategy for the photocatalytic generation of sulfamo...

Scheme 45: Illustrative example for the oxidative photocatalytic generation of sulfonyl radicals from sulfinic...

Scheme 46: Illustrative example for the photocatalytic generation of electronically excited triplet states: th...

Scheme 47: Illustrative example for the photocatalytic generation of electronically excited triplet states: th...
A smart deoxyribozyme-based fluorescent sensor for in vitro detection of androgen receptor mRNA
- Ekaterina A. Bryushkova,
- Erik R. Gandalipov and
- Julia V. Nuzhina
Beilstein J. Org. Chem. 2020, 16, 1135–1141, doi:10.3762/bjoc.16.100

- diagnosis. Keywords: androgen receptor; 10–23 deoxyribozyme; nucleic acid sensor; malachite green aptamer; RNA cleavage; Introduction The fast and precise diagnostics of diseases are one of the key factors that allow choosing the most effective method of treatment. Disease markers can be found at a few
- assembling was evaluated using agarose gel electrophoresis (Figure 1E). The single band proved that in the chosen conditions there was a complete hybridization of all 5 strands into an integrated SDFS complex. After hybridization between SDFS and 60-AR_RNA we expected to see a specific cleavage of target
- -nt fragment has steric and thermodynamic benefits to interact with MGA compared to 60-AR_RNA, or especially full-length AR mRNA. Due to the close proximity of “GU” cleavage sites in the selected 60-AR_RNA fragment, we decided to use asymmetric Dz1 design with an extremely short left RNA-binding part

Graphical Abstract

Figure 1: SDFS main components and its work model. A) Schematic representation of the SDFS structure. Dotted ...

Figure 2: The SDFS functional activity. A–C) Emission spectra of the assembled SDFS (green line), T1–T5 chain...

Figure 3: Emission spectra of SDFS activity on total cellular RNA. The green line represents a spectrum of SD...
Fluorinated phenylalanines: synthesis and pharmaceutical applications
- Laila F. Awad and
- Mohammed Salah Ayoup
Beilstein J. Org. Chem. 2020, 16, 1022–1050, doi:10.3762/bjoc.16.91

- . Thus, a three-component condensation of a series of fluorinated benzaldehydes 50a–h, N-acetyl- or N-benzoylglycine 51a or 51b, respectively, and an excess of acetic anhydride in the presence of sodium acetate afforded the oxazolones 52a–h. The subsequent reductive ring cleavage of 52a–h without

Graphical Abstract

Figure 1: Categories I–V of fluorinated phenylalanines.

Scheme 1: Synthesis of fluorinated phenylalanines via Jackson’s method.

Scheme 2: Synthesis of all-cis-tetrafluorocyclohexylphenylalanines.

Scheme 3: Synthesis of ʟ-4-[sulfono(difluoromethyl)]phenylalanine (nPt: neopentyl, TCE: trichloroethyl).

Scheme 4: Synthesis of ʟ-4-[sulfono(difluoromethyl)]phenylalanine derivatives 17.

Scheme 5: Synthesis of fluorinated Phe analogues from Cbz-protected aminomalonates.

Scheme 6: Synthesis of tetrafluorophenylalanine analogues via the 3-methyl-4-imidazolidinone auxiliary 25.

Scheme 7: Synthesis of tetrafluoro-Phe derivatives via chiral auxiliary 31.

Scheme 8: Synthesis of 2,5-difluoro-Phe and 2,4,5-trifluoro-Phe via Schöllkopf reagent 34.

Scheme 9: Synthesis of 2-fluoro- and 2,6-difluoro Fmoc-Phe derivatives starting from chiral auxiliary 39.

Scheme 10: Synthesis of 2-[18F]FPhe via chiral auxiliary 43.

Scheme 11: Synthesis of FPhe 49a via photooxidative cyanation.

Scheme 12: Synthesis of FPhe derivatives via Erlenmeyer azalactone synthesis.

Scheme 13: Synthesis of (R)- and (S)-2,5-difluoro Phe via the azalactone method.

Scheme 14: Synthesis of 3-bromo-4-fluoro-(S)-Phe (65).

Scheme 15: Synthesis of [18F]FPhe via radiofluorination of phenylalanine with [18F]F2 or [18F]AcOF.

Scheme 16: Synthesis of 4-borono-2-[18F]FPhe.

Scheme 17: Synthesis of protected 4-[18F]FPhe via arylstannane derivatives.

Scheme 18: Synthesis of FPhe derivatives via intermediate imine formation.

Scheme 19: Synthesis of FPhe derivatives via Knoevenagel condensation.

Scheme 20: Synthesis of FPhe derivatives 88a,b from aspartic acid derivatives.

Scheme 21: Synthesis of 2-(2-fluoroethyl)phenylalanine derivatives 93 and 95.

Scheme 22: Synthesis of FPhe derivatives via Zn2+ complexes.

Scheme 23: Synthesis of FPhe derivatives via Ni2+ complexes.

Scheme 24: Synthesis of 3,4,5-trifluorophenylalanine hydrochloride (109).

Scheme 25: Synthesis of FPhe derivatives via phenylalanine aminomutase (PAM).

Scheme 26: Synthesis of (R)-2,5-difluorophenylalanine 115.

Scheme 27: Synthesis of β-fluorophenylalanine via 2-amino-1,3-diol derivatives.

Scheme 28: Synthesis of β-fluorophenylalanine derivatives via the oxazolidinone chiral auxiliary 122.

Scheme 29: Synthesis of β-fluorophenylalanine from pyruvate hemiketal 130.

Scheme 30: Synthesis of β-fluorophenylalanine (136) via fluorination of β-hydroxyphenylalanine (137).

Scheme 31: Synthesis of β-fluorophenylalanine from aziridine derivatives.

Scheme 32: Synthesis of β-fluorophenylalanine 136 via direct fluorination of pyruvate esters.

Scheme 33: Synthesis of β-fluorophenylalanine via fluorination of ethyl 3-phenylpyruvate enol using DAST.

Scheme 34: Synthesis of β-fluorophenylalanine derivatives using photosensitizer TCB.

Scheme 35: Synthesis of β-fluorophenylalanine derivatives using Selectflour and dibenzosuberenone.

Scheme 36: Synthesis of protected β-fluorophenylalanine via aziridinium intermediate 150.

Scheme 37: Synthesis of β-fluorophenylalanine derivatives via fluorination of α-hydroxy-β-aminophenylalanine d...

Scheme 38: Synthesis of β-fluorophenylalanine derivatives from α- or β-hydroxy esters 152a and 155.

Scheme 39: Synthesis of a series of β-fluoro-Phe derivatives via Pd-catalyzed direct fluorination of β-methyle...

Scheme 40: Synthesis of series of β-fluorinated Phe derivatives using quinoline-based ligand 162 in the Pd-cat...

Scheme 41: Synthesis of β,β-difluorophenylalanine derivatives from 2,2-difluoroacetaldehyde derivatives 164a,b....

Scheme 42: Synthesis of β,β-difluorophenylalanine derivatives via an imine chiral auxiliary.

Scheme 43: Synthesis of α-fluorophenylalanine derivatives via direct fluorination of protected Phe 174.

Figure 2: Structures of PET radiotracers of 18FPhe derivatives.

Figure 3: Structures of melfufen (179) and melphalan (180) anticancer drugs.

Figure 4: Structure of gastrazole (JB95008, 181), a CCK2 receptor antagonist.

Figure 5: Dual CCK1/CCK2 antagonist 182.

Figure 6: Structure of sitagliptin (183), an antidiabetic drug.

Figure 7: Structure of retaglpitin (184) and antidiabetic drug.

Figure 8: Structure of evogliptin (185), an antidiabetic drug.

Figure 9: Structure of LY2497282 (186) a DPP-4 inhibitor for the treatment of type II diabetes.

Figure 10: Structure of ulimorelin (187).

Figure 11: Structure of GLP1R (188).

Figure 12: Structures of Nav1.7 blockers 189 and 190.
Recent applications of porphyrins as photocatalysts in organic synthesis: batch and continuous flow approaches
- Rodrigo Costa e Silva,
- Luely Oliveira da Silva,
- Aloisio de Andrade Bartolomeu,
- Timothy John Brocksom and
- Kleber Thiago de Oliveira
Beilstein J. Org. Chem. 2020, 16, 917–955, doi:10.3762/bjoc.16.83
- energies, as for example the thiaporphyrins that absorb beyond 650 nm [8][28]. Derksen and co-workers studied bond-cleavage reactions that can occur in biological microenvironments, using a light source with wavelengths frequently employed in photomedicine (650–850 nm) and thiophene-containing porphyrins
- platform for dual catalysis due to their ability to promote both metallocatalysis and photocatalysis in a one-pot system [36][37][38][39]. Martin and co-workers carried out the C–O bond cleavage of alcohols using a cobalt porphyrin under visible light irradiation and a carbon monoxide atmosphere (Scheme 11
- ) [36]. The authors hypothesized that the C–O bond cleavage could be achieved via cobalt-mediated alcohol carbonylation followed by radical decarboxylation of the alkoxycarbonyl intermediate. In a proof-of-concept study, they proceeded with the carbonylation of 1-phenylethanol using Co(II) tetrakis(4

Graphical Abstract

Figure 1: Chemical structures of the porphyrinoids and their absorption spectra: in bold are highlighted the ...

Figure 2: Photophysical and photochemical processes (Por = porphyrin). Adapted from [12,18].

Figure 3: Main dual photocatalysts and their oxidative/reductive excited state potentials, including porphyri...

Scheme 1: Photoredox alkylation of aldehydes with diazo acetates using porphyrins and a Ru complex. aUsing a ...

Scheme 2: Proposed mechanism for the alkylation of aldehydes with diazo acetates in the presence of TPP.

Scheme 3: Arylation of heteroarenes with aryldiazonium salts using TPFPP as photocatalyst, and corresponding ...

Scheme 4: A) Scope with different aryldiazonium salts and enol acetates. B) Photocatalytic cycles and compari...

Scheme 5: Photoarylation of isopropenyl acetate A) Comparison between batch and continuous-flow approaches an...

Scheme 6: Dehalogenation induced by red light using thiaporphyrin (STPP).

Scheme 7: Applications of NiTPP as both photoreductant and photooxidant.

Scheme 8: Proposed mechanism for obtaining tetrahydroquinolines by reductive quenching.

Scheme 9: Selenylation and thiolation of anilines.

Scheme 10: NiTPP as photoredox catalyst in oxidative and reductive quenching, in comparison with other photoca...

Scheme 11: C–O bond cleavage of 1-phenylethanol using a cobalt porphyrin (CoTMPP) under visible light.

Scheme 12: Hydration of terminal alkynes by RhIII(TSPP) under visible light irradiation.

Scheme 13: Regioselective photocatalytic hydro-defluorination of perfluoroarenes by RhIII(TSPP).

Scheme 14: Formation of 2-methyl-2,3-dihydrobenzofuran by intramolecular hydro-functionalization of allylpheno...

Scheme 15: Photocatalytic oxidative hydroxylation of arylboronic acids using UNLPF-12 as heterogeneous photoca...

Scheme 16: Photocatalytic oxidative hydroxylation of arylboronic acids using MOF-525 as heterogeneous photocat...

Scheme 17: Preparation of the heterogeneous photocatalyst CNH.

Scheme 18: Photoinduced sulfonation of alkenes with sulfinic acid using CNH as photocatalyst.

Scheme 19: Sulfonic acid scope of the sulfonation reactions.

Scheme 20: Regioselective sulfonation reaction of arimistane.

Scheme 21: Synthesis of quinazolin-4-(3H)-ones.

Scheme 22: Selective photooxidation of aromatic benzyl alcohols to benzaldehydes using Pt/PCN-224(Zn).

Scheme 23: Photooxidation of benzaldehydes to benzoic acids using Pt or Pd porphyrins.

Scheme 24: Photocatalytic reduction of various nitroaromatics using a Ni-MOF.

Scheme 25: Photoinduced cycloadditions of CO2 with epoxides by MOF1.

Figure 4: Electronic configurations of the species of oxygen. Adapted from [66].

Scheme 26: TPP-photocatalyzed generation of 1O2 and its application in organic synthesis. Adapted from [67-69].

Scheme 27: Pericyclic reactions involving singlet oxygen and their mechanisms. Adapted from [67].

Scheme 28: First scaled up ascaridole preparation from α-terpinene.

Scheme 29: Antimalarial drug synthesis using an endoperoxidation approach.

Scheme 30: Photooxygenation of colchicine.

Scheme 31: Synthesis of (−)-pinocarvone from abundant (+)-α-pinene.

Scheme 32: Seeberger’s semi-synthesis of artemisinin.

Scheme 33: Synthesis of artemisinin using TPP and supercritical CO2.

Scheme 34: Synthesis of artemisinin using chlorophyll a.

Scheme 35: Quercitol stereoisomer preparation.

Scheme 36: Photocatalyzed preparation of naphthoquinones.

Scheme 37: Continuous endoperoxidation of conjugated dienes and subsequent rearrangements leading to oxidized ...

Scheme 38: The Opatz group total synthesis of (–)-oxycodone.

Scheme 39: Biomimetic syntheses of rhodonoids A, B, E, and F.

Scheme 40: α-Photooxygenation of chiral aldehydes.

Scheme 41: Asymmetric photooxidation of indanone β-keto esters by singlet oxygen using PTC as a chiral inducer...

Scheme 42: Asymmetric photooxidation of both β-keto esters and β-keto amides by singlet oxygen using PTC-2 as ...

Scheme 43: Bifunctional photo-organocatalyst used for the asymmetric oxidation of β-keto esters and β-keto ami...

Scheme 44: Mechanism of singlet oxygen oxidation of sulfides to sulfoxides.

Scheme 45: Controlled oxidation of sulfides to sulfoxides using protonated porphyrins as photocatalysts. aIsol...

Scheme 46: Photochemical oxidation of sulfides to sulfoxides using PdTPFPP as photocatalyst.

Scheme 47: Controlled oxidation of sulfides to sulfoxides using SnPor@PAF as a photosensitizer.

Scheme 48: Syntheses of 2D-PdPor-COF and 3D-Pd-COF.

Scheme 49: Photocatalytic oxidation of A) thioanisole to methyl phenyl sulfoxide and B) various aryl sulfides,...

Scheme 50: General mechanism for oxidation of amines to imines.

Scheme 51: Oxidation of secondary amines to imines.

Scheme 52: Oxidation of secondary amines using Pd-TPFPP as photocatalyst.

Scheme 53: Oxidative amine coupling using UNLPF-12 as heterogeneous photocatalyst.

Scheme 54: Synthesis of Por-COF-1 and Por-COF-2.

Scheme 55: Photocatalytic oxidation of amines to imines by Por-COF-2.

Scheme 56: Photocyanation of primary amines.

Scheme 57: Synthesis of ᴅ,ʟ-tert-leucine hydrochloride.

Scheme 58: Photocyanation of catharanthine and 16-O-acetylvindoline using TPP.

Scheme 59: Photochemical α-functionalization of N-aryltetrahydroisoquinolines using Pd-TPFPP as photocatalyst.

Scheme 60: Ugi-type reaction with 1,2,3,4-tetrahydroisoquinoline using molecular oxygen and TPP.

Scheme 61: Ugi-type reaction with dibenzylamines using molecular oxygen and TPP.

Scheme 62: Mannich-type reaction of tertiary amines using PdTPFPP as photocatalyst.

Scheme 63: Oxidative Mannich reaction using UNLPF-12 as heterogeneous photocatalyst.

Scheme 64: Transformation of amines to α-cyanoepoxides and the proposed mechanism.
Cation-induced ring-opening and oxidation reaction of photoreluctant spirooxazine–quinolizinium conjugates
- Phil M. Pithan,
- Sören Steup and
- Heiko Ihmels
Beilstein J. Org. Chem. 2020, 16, 904–916, doi:10.3762/bjoc.16.82

- photoreaction [12][15][16]. The photochromism is based on a reversible electrocyclic reaction that proceeds through the UV light-induced cleavage of the C2’–O bond of the closed, colorless form to give the colored, metastable merocyanine form, whereas the back reaction can be initiated thermally or by the

Graphical Abstract

Scheme 1: Photo- or cation-induced ring-opening reaction of spirooxazine 1aSO; Mn+ = Pb2+, La3+, Eu3+, Tb3+ [17].

Scheme 2: Synthesis of the spirooxazine–quinolizinium conjugates 3a and 3b.

Figure 1: Colors of the solutions resulting from the addition of metal ions (c = 50 µM) to derivative 3a (c =...

Figure 2: Spectrophotometric titration of 3a (A) and 3b (B) (c = 20 µM) with Cu(BF4)2 (c = 2.44 mM) in MeCN. ...

Figure 3: Absorption (A) and fluorescence spectrum (B) of 3a in MeCN (c = 5 µM) in the absence (black) and in...

Figure 4: Emission colors of solutions resulting from the addition of metal ions (c = 50 µM) to derivative 3a...

Scheme 3: Cu2+-induced formation of the oxazole derivatives 4a and 4b.

Figure 5: 1H NMR spectra (600 MHz, 6.4–9.4 ppm) of 3a (c = 2.0 mM) in the absence (A) and in the presence (B–...

Figure 6: Spectral changes of 3a (c = 20 µM) upon the addition of Cu2+ (A) and Fe3+ (B) (cM+ = 60 µM) in MeCN...

Scheme 4: Proposed mechanism for the formation of the oxazole derivatives 4a and 4b (cf. Scheme 3); Mn+ = Cu2+, Fe3+,...
Synthesis of new asparagine-based glycopeptides for future scanning tunneling microscopy investigations
- Laura Sršan and
- Thomas Ziegler
Beilstein J. Org. Chem. 2020, 16, 888–894, doi:10.3762/bjoc.16.80

- significantly higher yield than HBTU. The Fmoc cleavage of the compounds 3–5 was performed with piperidine in DMF (20 vol %) [36]. The reaction products were not isolated and purified but instead immediately used for the succeeding peptide coupling step to give the dipeptides 4 and 5 from the glycosylated
- showed that the use of HATU reduced the reaction times but did not always result in higher yields, as anticipated from the literature. A simple cleavage protocol that did not necessitate any further purification step allowed for the preparation of the unprotected glycopeptides in solution. The
- investigation of the self assembly of such glycopeptides on metal surfaces will be performed via pMS and STM in further studies. Description of the starting materials 1a–f and 2a–f. Peptide coupling reactions, including the previous Fmoc cleavage. Cleavage of the fully protected peptides 6 and 7. Yields of the

Graphical Abstract

Scheme 1: Description of the starting materials 1a–f and 2a–f.

Scheme 2: Peptide coupling reactions, including the previous Fmoc cleavage.

Scheme 3: Cleavage of the fully protected peptides 6 and 7.
Aldehydes as powerful initiators for photochemical transformations
- Maria A. Theodoropoulou,
- Nikolaos F. Nikitas and
- Christoforos G. Kokotos
Beilstein J. Org. Chem. 2020, 16, 833–857, doi:10.3762/bjoc.16.76
- household 23 W compact fluorescent light (CFL) bulb at ambient temperature [55]. A base, 2,6-lutidine, was used to neutralize traces of HX (X = halogen), which formed by the homolytic cleavage of the C–X bond of the haloalkanes 93 (Scheme 24). Among the aldehydes tested, benzaldehyde (8) and 4
- -anisaldehyde (98), which possessed a relatively long lifetime and an energetic value sufficient for C–X bond cleavage. The participation of the triplet state 4-anisaldehyde (98) in the reaction mechanism was also confirmed by the complete inhibition of the ATRA reaction in the presence of oxygen, a triplet
- homolytic cleavage of the C–S bond, providing the sulfonyl radical 186. The sulfonyl radical 186 can then approach anti to the oxazolidinone moiety in 180, generating a β-sulfonyl radical 187, which, once trapped by another sulfonyl cyanide molecule 181, affords the desired product 182, and the sulfonyl

Graphical Abstract

Scheme 1: Norrish type I and II dissociations.

Scheme 2: Proposed radical pair formation after the photolysis of benzaldehyde (8).

Scheme 3: Aldehydes in the Paterno–Büchi reaction.

Scheme 4: 2,3-Diazabicyclo[2.2.1]hept-2-ene (DBH).

Scheme 5: Dissociation pathways of benzaldehyde.

Scheme 6: Reactions that lead to polarized products detectable by CIDNP.

Scheme 7: MMA (26), DEABP (27), and Michler’s ketone (28).

Scheme 8: Radical intermediates of DEABP.

Scheme 9: Photoinitiated polymerization of monomeric MMA (26) using the quinoxalines 32 and benzaldehyde (8).

Scheme 10: Acetone (4) and formaldehyde (35) as photografting initiators.

Scheme 11: Photografting by employing acetaldehyde (36) as the photoinitiator.

Scheme 12: Proposed photolysis mechanism for aliphatic ketones 44 and formaldehyde (35).

Scheme 13: Initiator 50, reductant 51, and benzaldehyde derivatives 52–54 for the polymerization of the methac...

Scheme 14: Proposed mechanism of the photomediated atom transfer radical polymerization employing the benzalde...

Scheme 15: cis/trans isomerization employing triplet states of photosensitizers.

Scheme 16: Salicylaldehyde (68) forms an internal hydrogen bond.

Scheme 17: Olefin isomerization via energy transfer from a carbonyl compound.

Scheme 18: Mechanistic pathways for the Paterno–Büchi reaction.

Scheme 19: Isomeric oxetanes formed after photochemical addition of aryl aldehydes to 2-butenes.

Scheme 20: Rotation of the C3–C4 bond of the biradical intermediate may lead to all four conformations.

Scheme 21: Photolysis products of benzaldehyde (8) in different solvents. a) In benzene or ethanol. b) In hex-...

Scheme 22: N-tert-Butylbenzamide formation proceeds via a benzoyl radical.

Scheme 23: Photochemical pinacol coupling.

Scheme 24: Photochemical ATRA catalyzed by 4-anisaldehyde (52).

Scheme 25: Proposed triplet sensitization mechanism of the ATRA reaction in the presence of 4-anisaldehyde (52...

Scheme 26: Benzaldehyde-mediated photoredox CDC reaction: compatible amides and ethers.

Scheme 27: Photoredox cross-dehydrogenative coupling (CDC) conditions and proposed reaction mechanism.

Scheme 28: Optimized conditions for the photoredox merger reaction.

Scheme 29: Proposed mechanism for the C(sp3)–H alkylation/arylation of ethers.

Scheme 30: Substrate scope for the photochemical alkylation of ethers.

Scheme 31: C(sp3)–H Functionalization of N-containing molecules.

Scheme 32: Substrate scope for the photochemical alkylation of N-containing molecules.

Scheme 33: Additional products yielded by the photochemical alkylation reaction of N-containing molecules.

Scheme 34: C(sp3)–H functionalization of thioethers.

Scheme 35: Proposed mechanism for the C(sp3)–H alkylation/arylation of N-containing molecules and thioethers.

Scheme 36: Hydroacylation using 4-cyanobenzaldehyde (53) as the photoinitiator.

Scheme 37: Selectivity for the formation of the α,α-disubstituted aldehydes.

Scheme 38: Substrate scope for the photochemical addition of aldehydes to Michael acceptors.

Scheme 39: Proposed mechanism for the hydroacylation of Michael acceptors using 4-cyanobenzaldehyde (53) as th...

Scheme 40: Catalytic arylation of aromatic aldehydes by aryl bromides in which the reaction product acts as th...

Scheme 41: Proposed mechanism for the catalytic arylation of benzaldehydes by aryl bromides in which the react...

Scheme 42: Functionalization of the chiral cyclobutanes 180.

Scheme 43: Optimized reaction conditions and proposed mechanism for the sulfonylcyanation of cyclobutenes.






