Search results
Search for "azide–alkyne" in Full Text gives 123 result(s) in Beilstein Journal of Organic Chemistry.
1,2,3-Triazolium macrocycles in supramolecular chemistry
Beilstein J. Org. Chem. 2019, 15, 2142–2155, doi:10.3762/bjoc.15.211
- (iodine, bromine) and chalcogens (selenium and tellurium) [18]. While there are several strategies for the synthesis of triazoles, the Cu(II)-catalyzed azide–alkyne cycloaddition reaction (CuAAC click reaction) is considered as one of the most efficient, simple and mild approaches towards the preparation
Archangelolide: A sesquiterpene lactone with immunobiological potential from Laserpitium archangelica
Beilstein J. Org. Chem. 2019, 15, 1933–1944, doi:10.3762/bjoc.15.189
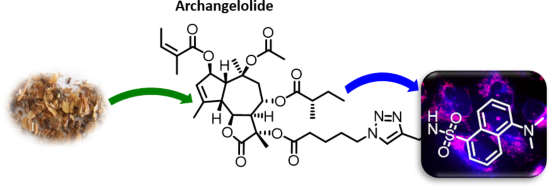
- studied by us and others, there are only scarce reports on the biological activity of archangelolide. Here we present the preparation of its fluorescent derivative based on a dansyl moiety using azide–alkyne Huisgen cycloaddition having obtained the two sesquiterpene lactones from the seeds of Laserpitium
- these compounds for further work including synthetic modifications using azide–alkyne Huisgen cycloaddition. We previously showed the preparation of fluorescent trilobolide conjugates [8] that retained the activity of the parental compounds and proved to be useful for live-cell imaging. In this article
Synthesis of a [6]rotaxane with singly threaded γ-cyclodextrins as a single stereoisomer
Beilstein J. Org. Chem. 2019, 15, 1829–1837, doi:10.3762/bjoc.15.177

- azide–alkyne cycloaddtion (CBAAC) with the anthracene-derived propagylamine stopper 2. In CBAAC, the strong ammonium–CB[6] binding places the alkyne and azide in a close proximity inside the CB[6] cavity and facilitates the cycloaddition [40][41][42]. Since CB[6] binding is required for the covalent
Solid-phase synthesis of biaryl bicyclic peptides containing a 3-aryltyrosine or a 4-arylphenylalanine moiety
Beilstein J. Org. Chem. 2019, 15, 761–768, doi:10.3762/bjoc.15.72
- -terminal groups of the peptide and their putative target. This method has been used for the macrocyclization of peptides through, for example, copper-catalyzed azide–alkyne cycloadditions [14], ring-closing olefin metathesis [13] or the formation of an aryl–aryl bond between the side chain of two aromatic
Cyclopropene derivatives of aminosugars for metabolic glycoengineering
Beilstein J. Org. Chem. 2019, 15, 584–601, doi:10.3762/bjoc.15.54
- , azides and alkynes can be visualized by the Staudinger ligation [8] or the azide–alkyne cycloaddition, that can be performed either copper-catalyzed [9][10] or strain-promoted [11][12]. Another type of reporter group that has been proven to be a valuable tool are electron-rich or strained alkenes, that
Synthesis and fluorescent properties of N(9)-alkylated 2-amino-6-triazolylpurines and 7-deazapurines
Beilstein J. Org. Chem. 2019, 15, 474–489, doi:10.3762/bjoc.15.41

- synthesis of a novel library of 9-alkyl-2-amino-6-triazolylpurine derivatives. Thus, copper-catalyzed azide–alkyne 1,3-dipolar cycloaddition reaction of compounds 6a–c with different para-substituted phenylacetylenes produced the expected compounds 7a–f, 8a–f and 9 (Scheme 1). The yields of 1,3-dipolar
- derivative 4 was synthesized using previously reported procedure of Cu(I)-catalyzed azide–alkyne cycloaddition reaction on 2,6-diazidopurine derivatives [25]. Synthesis of 7-deazapurine derivatives 3, 10a, 11a and their characterization are described in our preliminary communication [39]. Synthesis of 9
Copper(I)-catalyzed tandem reaction: synthesis of 1,4-disubstituted 1,2,3-triazoles from alkyl diacyl peroxides, azidotrimethylsilane, and alkynes
Beilstein J. Org. Chem. 2018, 14, 2916–2922, doi:10.3762/bjoc.14.270
- Academy of Sciences, 155 Yangqiao Road West, Fuzhou, Fujian 350002, P. R. China University of Chinese academy of Science (UCAS), Beijing 100190, P. R. China 10.3762/bjoc.14.270 Abstract A copper-catalyzed azide–alkyne cycloaddition (CuAAC) reaction for the synthesis of 1,4-disubstituted 1,2,3-triazoles
- research and synthesis of functionalized compounds that have applications in medicinal chemistry, drug discovery, materials chemistry, and as well as in bioconjugates [2][3][4][5][6][7][8][9][10][11][12]. The copper-catalyzed azide–alkyne cycloaddition (CuAAC) reaction [13][14][15][16][17][18][19][20][21
Nucleoside macrocycles formed by intramolecular click reaction: efficient cyclization of pyrimidine nucleosides decorated with 5'-azido residues and 5-octadiynyl side chains
Beilstein J. Org. Chem. 2018, 14, 2404–2410, doi:10.3762/bjoc.14.217
- and deprotection steps are necessary to control the cyclization process. Preorganization of the molecules can help to make cyclization more efficient. Azide–alkyne "click" chemistry has been executed to generate cyclic peptides [11][12][13], cyclic oligonucleotides [14][15][16][17] and other
- cyclic molecules when alkynyl side chains are functionalizing nucleobases in 5-position and azido substituents replace sugar 5'-hydroxy groups. Cyclic molecules (Figure 1) should be accessible when a copper(I)-azide–alkyne cycloaddition [29][30][31] is performed. The resulting "nucleoides" represent a
- intramolecular cyclization to a macrocycle was not observed. Next, the 5’-azido compound 2 was employed in the copper(I)-catalyzed azide–alkyne cycloaddition (CuAAC) "click" reaction [38][39] to build up macrocycle 3. In this regard, two reaction pathways have to be considered: (i) an intramolecular “click
Tetrathiafulvalene – a redox-switchable building block to control motion in mechanically interlocked molecules
Beilstein J. Org. Chem. 2018, 14, 2163–2185, doi:10.3762/bjoc.14.190

- (6) ring threaded onto a water-soluble axle [80]. The rotaxane was synthesized in 23% yield by a template/capping strategy where one stopper is attached using copper-catalyzed azide–alkyne click chemistry after the formation of the precursor pseudorotaxane. Due to the hydrophobic effect, the neutral
- = 6,300 M−1) which is embedded in an axle molecule with two azide residues. The second station, the dihydroxynaphthalene moiety (green), displays a lower association constant of Ka = 5,800 M−1. The pseudorotaxane precursor was end-capped by a double copper-catalyzed azide–alkyne click reaction in CH2Cl2
Revisiting ring-degenerate rearrangements of 1-substituted-4-imino-1,2,3-triazoles
Beilstein J. Org. Chem. 2018, 14, 2098–2105, doi:10.3762/bjoc.14.184
- recent years [1][2][3][4][5][6][7], enabled by efficient preparation from the Sharpless–Meldal copper-catalyzed azide–alkyne cycloaddition (CuAAC) reaction [8][9][10][11]. Click chelators with a variety of N-donor units connected at the 4-triazolyl position have been reported, including pyridine [12][13
A switchable [2]rotaxane with two active alkenyl groups
Beilstein J. Org. Chem. 2018, 14, 2074–2081, doi:10.3762/bjoc.14.181

- +) containing intermediate compound 5 were assembled in dichloromethane through host–guest interaction and capped with compound 8 under Cu(I)-catalyzed azide–alkyne cycloaddition to get the final [2]rotaxane with two distinguishable recognition sites. The target [2]rotaxane R1 was then characterized by 1H NMR
Synthesis of new p-tert-butylcalix[4]arene-based polyammonium triazolyl amphiphiles and their binding with nucleoside phosphates
Beilstein J. Org. Chem. 2018, 14, 1980–1993, doi:10.3762/bjoc.14.173

- /bjoc.14.173 Abstract The synthesis of new calix[4]arenes adopting a cone stereoisomeric form bearing two or four azide fragments on the upper rim and water-soluble triazolyl amphiphilic receptors with two or four polyammonium headgroups via copper-catalyzed azide–alkyne cycloaddition reaction has been
- solutions. Results and Discussion Synthesis of polyammonium calix[4]arene derivatives The functionalization of calix[4]arenes with azide groups paves the way to introduce a wide variety of functional groups [27] on the upper rim of the macrocycle by, e.g., the copper-catalyzed azide–alkyne cycloaddition
Efficient catenane synthesis by cucurbit[6]uril-mediated azide–alkyne cycloaddition
Beilstein J. Org. Chem. 2018, 14, 1846–1853, doi:10.3762/bjoc.14.158

- other recognition units render them promising building blocks for the construction of other high-order interlocked structures. Keywords: azide–alkyne cycloaddition; catenane; click chemistry; cucurbit[6]uril; mechanical bond; Introduction Catenanes are topologically non-trivial molecules possessing
- a preliminary study of a [6]catenane synthesis featuring the CB[6]-mediated azide–alkyne cycloaddition (CBAAC) using phenanthroline-based building blocks [24]. To further explore the applicability and generality of the CBAAC in the construction of mechanically interlocked molecules, we report here
- ] binding to the ammoniums on the building blocks to improve the efficiency of CBAAC [23]. In view of the low solubility of CB[6] and the possibility of thermal-assisted azide–alkyne cycloaddition of the unbound building blocks that may lead to the incomplete cycloaddition and other side products, we
Synthesis and photophysical studies of a multivalent photoreactive RuII-calix[4]arene complex bearing RGD-containing cyclopentapeptides
Beilstein J. Org. Chem. 2018, 14, 1758–1768, doi:10.3762/bjoc.14.150

- anchoring i) of the photoreactive [Ru(TAP)2phen]2+ complex on the calix[4]arene small rim through a peptide-type coupling and ii) of the four c-[RGDfK] moieties on the opposite rim through a copper-catalyzed azide–alkyne cycloaddition (CuAAC) [66][67][68] (Figure 1). It was thus necessary to block the calix
- + CF3COO−]+ by comparison between the experimental and theoretical isotope distributions (see Supporting Information File 1). With RuII-calix[4]arene complex 7 in hands, we next moved to the introduction of the cellular targeting units on the large rim through copper-catalyzed azide–alkyne cycloaddition
- ), as these nanomaterials are known to catalyze efficiently a wide range of organic reactions and notably the azide–alkyne cycloaddition [79]. Calixarene 7 was reacted with a slight excess (5 equiv) of cyclopeptide 8 in the presence of CuNPs and the mixture was heated by microwave (100 W) at 50 °C for 1
Hyper-reticulated calixarene polymers: a new example of entirely synthetic nanosponge materials
Beilstein J. Org. Chem. 2018, 14, 1498–1507, doi:10.3762/bjoc.14.127
- extend and possibly improve the supramolecular binding abilities of CyNSs, mixed cyclodextrin-calixarene co-polymers nanosponges (CyCaNSs) were recently synthesized by exploiting a classical “click-chemistry” approach, namely the CuAAC reaction (Cu-catalyzed azide–alkyne cycloaddition [28][29][30
[3 + 2]-Cycloaddition reaction of sydnones with alkynes
Beilstein J. Org. Chem. 2018, 14, 1317–1348, doi:10.3762/bjoc.14.113
- reaction conditions together with very high and reverse regioselectivity and efficiency (in most cases 85–99% yields) makes the CuSAC reaction a very good alternative to the well-established azide–alkyne click-reaction [117] useful not only in classical organic synthesis but also in bioconjugation
- ; SPSAC) which takes place without any catalyst and at ambient temperature. Such mild reaction conditions, (ultra) fast and unambiguous product formation make SPSAC useful in bio-orthogonal applications and competitive in comparison with analogous strain-promoted azide–alkyne cycloaddition (SPAAC). The
An overview of recent advances in duplex DNA recognition by small molecules
Beilstein J. Org. Chem. 2018, 14, 1051–1086, doi:10.3762/bjoc.14.93

- betulinic acid analogs were synthesized by using azide–alkyne click reaction and their anticancer activities against different cancer cell lines and normal human PBMC cell line were evaluated by MTT assay. Conjugate 42 was found to be extremely potent against HT-29 cell line with an IC50 value of 14.9 μM
Mechanochemistry of nucleosides, nucleotides and related materials
Beilstein J. Org. Chem. 2018, 14, 955–970, doi:10.3762/bjoc.14.81

- (Scheme 5) [42]. In the absence of light, azobenzene derivatives were isolated as the pure E-isomers. In their original report, Sharpless and co-workers described the use of copper turnings to promote a regioselective azide–alkyne [3 + 2]-cycloaddition ("click") reaction over 24 hours [43]. High-speed
- and carboxylic acid Boc protection using an improvised attritor-type mill. Nucleobase Boc protection via transient silylation using an improvised attritor-type mill. Chemoselective N-acylation of an aminonucleoside using LAG in a MBM. Azide–alkyne cycloaddition reactions performed in a copper vessel
Sequential Ugi reaction/base-induced ring closing/IAAC protocol toward triazolobenzodiazepine-fused diketopiperazines and hydantoins
Beilstein J. Org. Chem. 2018, 14, 626–633, doi:10.3762/bjoc.14.49
- together all necessary functionalities for further transformations. The Ugi adducts were then subjected to a base-induced ring closing and an intramolecular azide–alkyne cycloaddition reaction in succession to obtain highly fused benzodiazepine frameworks. Keywords: benzodiazepine; 2,5-diketopiperazine
- ; hydantoin; intramolecular azide–alkyne cycloaddition; Ugi reaction; Introduction The versatile bioactivities of multiring-fused heterocyclic scaffolds continue to attract significant attention in developing new methods for their synthesis. A plethora of functionalized fused heterocycles can be easily
- multicomponent reactions with intramolecular azide–alkyne cycloaddition (IAAC) for the generation of triazole-fused heterocycles [12][13][14][15][16][17][18][19][20][21][22][23]. In this report we disclose our results on the development of a sequential synthetic approach involving Ugi 4-component reaction (4-CR
Synthesis and biological evaluation of RGD and isoDGR peptidomimetic-α-amanitin conjugates for tumor-targeting
Beilstein J. Org. Chem. 2018, 14, 407–415, doi:10.3762/bjoc.14.29
- disulfide linker in the cytoplasm. In another example, Perrin and co-workers conjugated the N-propargylasparagine of an amanitin analog to a cycloRGD integrin ligand (cyclo[RGDfK]) using a copper-catalyzed azide–alkyne cycloaddition [8]. The conjugates were tested in the U87 glioblastoma cell line, but only
Recent applications of click chemistry for the functionalization of gold nanoparticles and their conversion to glyco-gold nanoparticles
Beilstein J. Org. Chem. 2018, 14, 11–24, doi:10.3762/bjoc.14.2
- glycoscience. In this review we present recent advances made in the use of one type of click chemistry, namely the azide–alkyne Huisgen cycloaddition, for the functionalization of gold nanoparticles and their conversion to glyco-gold nanoparticles. Keywords: azide–alkyne Huisgen cycloaddition; carbohydrates
- synthesis and application of GAuNPs can be found in the reviews by Penadés and co-workers [9][26] and also in a recent review by Compostella et al. [10]. In this regard, azide–alkyne click chemistry is an attractive approach that could be used to synthesize GAuNPs. The functionalization of AuNPs using the
- azide–alkyne Huisgen cycloaddition AuNP surface modification using NCAAC The azide–alkyne Huisgen cycloaddition (AAC) is a 1,3-dipolar cycloaddition between an organic azide and an alkyne that gives triazole products [37][38]. The non-catalysed azide–alkyne Huisgen cycloaddition (NCAAC) is very slow
Synthetic mRNA capping
Beilstein J. Org. Chem. 2017, 13, 2819–2832, doi:10.3762/bjoc.13.274

- ) followed by treatment with aqueous ammonia (rt, 3 h). Click chemistry for the preparation of capped RNA and cap analogues. (A) Preparation of capped RNA via a copper-catalyzed azide–alkyne cycloaddition (CuAAC) of an azido-modified cap analogue with a 5'-alkyne bearing RNA [120]. (B) An alkyne-modified
Asymmetric synthesis of propargylamines as amino acid surrogates in peptidomimetics
Beilstein J. Org. Chem. 2017, 13, 2428–2441, doi:10.3762/bjoc.13.240
- intriguing versatility as building blocks in organic and medicinal chemistry, as their reactivity is unique. Their chemistry involves several highly selective reactions, e.g., [3 + 2] cycloadditions with azides and isoelectronic functional groups (among them the copper or ruthenium-catalyzed azide–alkyne
Solvent-free copper-catalyzed click chemistry for the synthesis of N-heterocyclic hybrids based on quinoline and 1,2,3-triazole
Beilstein J. Org. Chem. 2017, 13, 2352–2363, doi:10.3762/bjoc.13.232
- spin resonance (ESR) spectroscopy; in situ Raman monitoring; mechanochemistry; quinoline; solid-state click chemistry; Introduction The copper-catalyzed azide–alkyne cycloaddition (CuAAC) represents a prime example of click chemistry. Click chemistry describes “a set of near-perfect” reactions [1] for
- bond bioisosteres made the click reaction a valuable synthetic methodology for conjugation of bioactive molecules [7][8][9] aiming to improve their biological activities [4][10][11]. Discovery of copper(I) ion catalysis in azide–alkyne cycloadditions was decisive for applications of this reaction, as
- it increases reaction rates and yields and directs the azide–alkyne cycloaddition exclusively towards 1,4-substituted regioisomers, whereas the non-catalyzed process results in a non-stoichiometric mixture of 1,4- and 1,5-regioisomers. Even though CuAAC reactions are efficiently performed in solution
The chemistry and biology of mycolactones
Beilstein J. Org. Chem. 2017, 13, 1596–1660, doi:10.3762/bjoc.13.159












































































































































































































































































































































