Search results
Search for "electrolyte" in Full Text gives 296 result(s) in Beilstein Journal of Nanotechnology. Showing first 200.
Nanostructure-induced performance degradation of WO3·nH2O for energy conversion and storage devices
Beilstein J. Nanotechnol. 2018, 9, 2845–2854, doi:10.3762/bjnano.9.265

- electrochemical performance degradation of the three samples, CV tests were carried out at a scan rate of 50 mV·s−1 within the potential range from −0.8 V to +0.8 V (vs Ag/AgCl). The electrochemical energy conversion and storage of WO3·nH2O in H2SO4 electrolyte are based on the intercalation of protons and
- between the interlayer water molecules and the electrolyte. For the WO3·H2O sample synthesized at 120 °C (Figure 7b), the sheets were found to swell heavily due to the 2D intercalation/deintercalation processes in the CV cycles, turning almost twice as thick as their original thickness. Under the strong
Accurate control of the covalent functionalization of single-walled carbon nanotubes for the electro-enzymatically controlled oxidation of biomolecules
Beilstein J. Nanotechnol. 2018, 9, 2750–2762, doi:10.3762/bjnano.9.257

- electrolyte), and a platinum wire auxiliary electrode. A working electrode (WE) incorporating the f-SWCNTs deposited on the GCE surface. a) HRTEM micrographs of the raw HIPCO material. The arrows point out residual iron nanoparticles. b) HRSTEM BF image showing carbonaceous impurities at the surface of SWCNTs
Impact of the anodization time on the photocatalytic activity of TiO2 nanotubes
Beilstein J. Nanotechnol. 2018, 9, 2628–2643, doi:10.3762/bjnano.9.244

- Columbia, V6T 1Z4, Canada 10.3762/bjnano.9.244 Abstract Titanium oxide nanotubes (TNTs) were anodically grown in ethylene glycol electrolyte. The influence of the anodization time on their physicochemical and photoelectrochemical properties was evaluated. Concomitant with the anodization time, the NT
- importantly, it has been recognized that several parameters of the anodization, such as electric field strength, water content in the electrolyte, concentration of fluorine ions and pH value, have a direct influence on the electronic properties of the TNTs [20]. Nevertheless, the modification procedures for
- ] observed that a water-based electrolyte containing NH4F induced a co-doping with F and N in the TNTs. Their study suggested that a combination of applied potential and annealing temperature were responsible for the high photocatalytic activity (PCA) of their materials in the oxidation of methyl orange. In
Thickness-dependent photoelectrochemical properties of a semitransparent Co3O4 photocathode
Beilstein J. Nanotechnol. 2018, 9, 2432–2442, doi:10.3762/bjnano.9.228

- Co3O4 samples, which exhibit light-induced photocurrent in photoelectrochemical cells (PEC) containing the alkaline electrolyte. The thickness-dependent properties of Co3O4 related to its use as a working electrode in PEC cells are extensively studied and show potential for the application in water
- electrode was swept from 1.5 to −0.3 V vs RHE in a 0.1 M NaOH electrolyte (pH 12.5). The thickness-dependent linear sweep voltammogram (LSV) of the PEC cell under chopped light illumination is shown in Figure 2d. These results provide an overview of the photoinduced OER at 1.23 V vs RHE, the HER at 0 V vs
- splitting (Co3O4||Pt electrodes) in our previous report [20]. In order to verify the PEC performance, a PEC cell with dual Co3O4 electrodes was set up for volumetric measurements. The Co3O4 electrodes were loaded into two seperated vials (15 mL, Figure 7) and placed into the 1 M KOH electrolyte bath as
Hydrothermal-derived carbon as a stabilizing matrix for improved cycling performance of silicon-based anodes for lithium-ion full cells
Beilstein J. Nanotechnol. 2018, 9, 2381–2395, doi:10.3762/bjnano.9.223

- ; prelithiation; silicon/carbon composite; solid–electrolyte interphase (SEI); Introduction Since their market launch in 1991, the energy density of lithium-ion batteries (LIBs) has increased steadily. However, further improvements in terms of power density and energy density are essential to meet the rising
- drastic volume changes during cycling hinder the formation of a dimensionally stable solid electrolyte interphase (SEI), as it is known for carbonaceous anodes, formed on the negative electrode surface from electrolyte decomposition products in the first charge/discharge cycles [18][19][20]. In the case
- of Si anodes, the SEI formation is an ongoing process because of the recurring breakage of the already formed SEI and exposure of fresh Si to the electrolyte. Consequently, a very thick SEI may form after several cycles, affecting the reaction kinetics detrimentally. All these aforementioned factors
Lead-free hybrid perovskites for photovoltaics
Beilstein J. Nanotechnol. 2018, 9, 2209–2235, doi:10.3762/bjnano.9.207

- counter electron by a dissolved benzoquinone redox-couple BQ0/BQ− showed a PCE of 1.51% [137]. An FTO/TiO2/MASnCl3 photoanode (Eg = 2.1 eV) was combined with an FTO/Pt counter electrode and a solid/liquid electrolyte consisting of polyethylene oxide soaked with an acetonitrile solution of KI/I2 into a
Filling nanopipettes with apertures smaller than 50 nm: dynamic microdistillation
Beilstein J. Nanotechnol. 2018, 9, 2181–2187, doi:10.3762/bjnano.9.204

- completely filled using this new technique. The nanopipettes are first filled with pure water, which is later replaced with the desired electrolyte via electromigration. Electrical measurements are used to check that filling is complete. Keywords: current rectification; distillation; filling; nanopipette
- is recorded at the same time. The first measurement is made directly after the first electrolyte loading at a concentration of [KCl] = 10−4 mol·L−1. This first electrical measurement confirms that the nanopipette is filled: The current flows and ion exchange is ensured between the Ag/AgCl electrodes
- . However, the tip of the nanopipette is still filled with pure deionized water. The intensity level does not correspond to the stationary regime, instead it changes slowly with diffusion. To ensure that electrolyte concentration reaches its nominal value throughout the nanopipette, electromigration is
Phosphorus monolayer doping (MLD) of silicon on insulator (SOI) substrates
Beilstein J. Nanotechnol. 2018, 9, 2106–2113, doi:10.3762/bjnano.9.199

- to draw holes to the surface and enable the dissolution of the semiconductor into the electrolyte. Applying this voltage near the insulator layer becomes problematic and prevents etching and analysis in this region. Hall effect measurements were instead used, which required careful handling during
- profiling (CVP21 Profiler) was used to determine the active carrier concentrations in the samples after the doping process was completed. Ammonium hydrogen difluoride (0.1 M) was chosen as a suitable electrolyte/etchant as it can remove the native oxide layer without etching into the underlying substrate
Localized photodeposition of catalysts using nanophotonic resonances in silicon photocathodes
Beilstein J. Nanotechnol. 2018, 9, 2097–2105, doi:10.3762/bjnano.9.198

- light absorption and conversion to chemical energy take place. The photo-electrodes are in contact with an electrolyte that is the primary source of fuel together with the sunlight. In such a system, light absorption by the electrodes leads to the creation of electron–hole pairs, which after their
- separation participate in chemical reactions in the electrolyte to make fuels. One example is water splitting for H2 generation [5][6]. Carefully designed photo-electrodes are necessary for low cost and high efficiency, which are both needed to make solar fuels competitive with fossil fuels as an energy
- -precursor electrolyte (4 mM H2PtCl6, pH 11) and the current flow to the working electrode was recorded as a function of time at a constant electrochemical potential, i.e., in the chronoamperometry mode. The samples had an open-circuit voltage potential of around −0.1 V (vs Ag/AgCl) and were biased by 700 mV
Metal-free catalysis based on nitrogen-doped carbon nanomaterials: a photoelectron spectroscopy point of view
Beilstein J. Nanotechnol. 2018, 9, 2015–2031, doi:10.3762/bjnano.9.191

- with a better-defined surface area of the aligned tips at the interface with the electrolyte solution, which facilitates the electrolyte/reactant diffusion. A strong enhancement of the currents was observed when comparing N-CNTs to undoped nanotubes. Furthermore, they demonstrated that the glassy N
- introduction of nitrogen into various carbon-based cathode catalysts for the polymer electrolyte fuel cell (PEFC) [105]. Different preparation methods were used: nitrogen doping using ammonia resulted in high amounts of pyridinic N, while using pyrolysis of nitrogen-containing precursors the amount of
- rearrangement of the nitrogen species: a decrease of pyrrolic N and a general transformation into graphitic N, consistent with other reports [58]. The electrocatalytic performances were measured by cyclic voltammetry in oxygen-saturated 1 M KOH electrolyte. By correlating XPS and ORR, the samples with the
Synthesis of rare-earth metal and rare-earth metal-fluoride nanoparticles in ionic liquids and propylene carbonate
Beilstein J. Nanotechnol. 2018, 9, 1881–1894, doi:10.3762/bjnano.9.180
- the transformation of Er3+ to Er2+, which can be reversibly oxidized. During the oxidation process, oxidation of the electrolyte was observed, starting around 3.4 V. Hence, such standard electrolytes cannot be applied to this redox couple. In the range from 1.0 to 0.05 V, there is an overpotential
- + + 3e− → Er (−2.33 V vs SHE; 0.71 V vs Li+/Li) and Er2+ + 2e → Er (−2.0 V vs SHE; 1.04 V vs Li+/Li). In conclusion, ErF3 does not exhibit reversible redox behaviour using common electrolytes, and thus more elaborate experimental effort is needed, including changing the potential range or electrolyte, or
- lithium foil as a counter electrode and 1 M LiPF6 in ethylene carbonate–ethyl methyl carbonate (50:50) as the electrolyte. The cyclic voltammetry (CV) data of these half-cells were collected utilizing an electrochemical workstation (Autolab 302) with different cut-off potentials. TEM images and particle
A visible-light-controlled platform for prolonged drug release based on Ag-doped TiO2 nanotubes with a hydrophobic layer
Beilstein J. Nanotechnol. 2018, 9, 1793–1801, doi:10.3762/bjnano.9.170

- full XPS spectrum of Zn-Ag-TNTs, in which Zn, Ti, Ag and O elements are detected. These spectra suggest the successful decoration of Ag as well as loading of Zn. The presence of C may be attributed to the incorporation from the electrolyte and the organic contamination adsorbed from environment. Figure
- conducted using a programmable power supply (Maynuo DC source Meter, Shanghai, China) and a three-electrode configuration with a bicathode composed of two circular titanium plates as counter electrodes. The electrolyte was ethylene glycol containing 6 vol % distilled water and 0.3 wt % NH4F and the
Nitrogen-doped carbon nanotubes coated with zinc oxide nanoparticles as sulfur encapsulator for high-performance lithium/sulfur batteries
Beilstein J. Nanotechnol. 2018, 9, 1677–1685, doi:10.3762/bjnano.9.159

- energy density of 2600 Wh·kg−1, sulfur has been considered as a promising cathode material for lithium/sulfur (Li/S) batteries [1]. Additionally, sulfur is naturally abundant, has low cost and is environmentally friendly. But it is not conductive, and it dissolves into the electrolyte in the form of
- a strong bonding capacity for S. This will reduce the S losses to the electrolyte, and thus improve the cycling performance of the Li/S battery. In fact, the S–Zn and S–O bonds were confirmed by X-ray photoelectron spectroscopy (XPS) of the as-obtained S/ZnO@NCNT composite (Figure 5). In the S 2p
- a separator. The electrolyte was 1 M lithium bistrifluoromethanesulfonamide (LiTFSI) in tetraethylene glycol dimethyl ether as a solvent. The CR2025 coin cells assembly was carried out in an argon-filled glovebox (Mikrouna, Shanghai). The charge/discharge cycling performances was investigated using
Nanoscale electrochemical response of lithium-ion cathodes: a combined study using C-AFM and SIMS
Beilstein J. Nanotechnol. 2018, 9, 1623–1628, doi:10.3762/bjnano.9.154

- [1][2][3]. The 3D all-solid-state microbattery (ASB) is a promising new architecture built using processing techniques compatible with semiconductor processing, which provides more power and more capacity compared to conventional planar designs [4]. In this kind of battery, the electrolyte is
- generally a solid and dense material while crystalline conductive oxides are used for the anode and cathode. As a solid electrolyte is significantly safer compared to its flammable organic liquid counterparts, its use does represent a clear advantage [2]. Moreover, the presence of crystalline ordering in
- the electrodes and the electrolyte, which are complex to characterize, give rise to failure and reduced performance of cells. This puts the outcome of our work in context of a wide range of applications. These pending problems pose severe challenges for the physical characterization of battery
Interaction-tailored organization of large-area colloidal assemblies
Beilstein J. Nanotechnol. 2018, 9, 1582–1593, doi:10.3762/bjnano.9.150

- an electrolyte solution, the particles interact at a sufficiently large distance r (greater than the particle radius) through a screened Coulomb potential u(r) e−κr / r [25][26]. The range of particle electrostatic repulsion is determined by the Debye length where q is the elementary charge, NA is
- Avogadro’s number, kB is Boltzmann’s constant, T is the absolute temperature and is the ionic strength whereby the electrolyte solution contains ion of type i with valence zi and molar concentration ci. Therefore, the strength of electrostatic interaction between the colloidal particles on the surface can
- electrostatic repulsion between the particles is more screened by an increased electrolyte concentration, which allows them to adsorb more closely and achieves a higher surface coverage. At 5 mM salt concentration, the particles are so close that doublet and triplet aggregates begin to form on the surface
Correlative electrochemical strain and scanning electron microscopy for local characterization of the solid state electrolyte Li1.3Al0.3Ti1.7(PO4)3
Beilstein J. Nanotechnol. 2018, 9, 1564–1572, doi:10.3762/bjnano.9.148

- for replacing the flammable liquid electrolyte in LIBs, especially in safety-related environments like automotive applications [6][7]. Furthermore, the increased electrochemical window in the case of SSEs opens the path to use advanced electrode materials with improved volumetric and gravimetric
- energy density [1][8][9][10]. Lithium aluminum titanium phosphate Li1.3Al0.3Ti1.7(PO4)3 (LATP), a ceramic with NASICON-type structure, is especially considered as a beneficial solid state electrolyte due to its superior lithium-ion conductivity in the range of 2 mS cm−1 in the “bulk” and 2 µS cm−1 at
- ]. Information about the behavior of the material as a solid state electrolyte cannot be derived based on SEM and EDX mappings alone, hence we performed ESM. Figure 2 shows correlative images of SEM and AFM topography as well as ESM on identical regions of LATP sintered at 1050 °C. The SEM image (Figure 2a
Preparation and morphology-dependent wettability of porous alumina membranes
Beilstein J. Nanotechnol. 2018, 9, 1423–1436, doi:10.3762/bjnano.9.135

- work (type I–III) is shown. In the inset, a scheme describing non-isotropic etching of the pore walls [30] is shown. The authors of this work assume that the concentration of the electrolyte is initially higher on the surface of narrow channels (in the mouth of a nanopore, region 1). It then becomes
- –Baxter model is more suitable for the description of large thickness oxides with small pores (received, for example, in sulfuric electrolyte). The Wenzel model was found to be more suitable for the description of small thickness oxides (less than 30 µm) with larger diameter pores (received, for example
- , in oxalate electrolyte). The comparison of the wetting nature of the two surfaces of the PAM allows the contributions due to morphology and chemical properties to wetting of the nanostructure surface to be distinguished. It was shown that the etching method influences the surface morphology of the
Nanoporous silicon nitride-based membranes of controlled pore size, shape and areal density: Fabrication as well as electrophoretic and molecular filtering characterization
Beilstein J. Nanotechnol. 2018, 9, 1390–1398, doi:10.3762/bjnano.9.131

- (Supporting Information File 1, details in the text and Figure S1). Subsequently, the chambers were filled with a KCl electrolyte and the conductance was determined by applying dc voltages to the Ag/AgCl electrodes and automated current measurements. The results of the according experiments on the membranes A
- and assuming a homogenous resistivity of the electrolyte; for further details see Supporting Information File 1. Note the following in this context: The applied RIE processes involving CHF3/CF4 plasmas have a propensity for the formation of Teflon-like CF layers which, by influencing the wettability
- electrolyte concentrations in the range of 5∙10−2 to 12 mM. Additionally, the measured conductance of the setup without membrane and the modeled one (calculated by FEM) are shown. A seal test with a non-porous membrane reveals the contribution of leakage currents. Molecule transport through a porous membrane
Semi-automatic spray pyrolysis deposition of thin, transparent, titania films as blocking layers for dye-sensitized and perovskite solar cells
Beilstein J. Nanotechnol. 2018, 9, 1135–1145, doi:10.3762/bjnano.9.105

- acetylacetone), concentration (0.05 and 0.2 M) and subsequent post-calcination at 500 °C. The photo-electrochemical properties were evaluated in aqueous electrolyte solution under UV irradiation. The blocking properties were tested by cyclic voltammetry with a model redox probe with a simple one-electron
- sensitized with a dye. This is in contact with an electrolyte solution with a redox mediator which transports holes from the photo-oxidized dye towards the counter electrode. In SSDSSCs or PSCs, the photogenerated holes are transported by a solid conductive material (e.g., spiro-OMeTAD) [1][2]. This is
- be achieved with good blocking properties and low sensitivity to calcination at the same time. Such fabricated BL TiO2 films were characterized by photo-electrochemical measurements in aqueous electrolyte solution. The blocking ability was quantified by cyclic voltammetry with a K3[Fe(CN)6]/K4[Fe(CN
Cyclodextrin inhibits zinc corrosion by destabilizing point defect formation in the oxide layer
Beilstein J. Nanotechnol. 2018, 9, 936–944, doi:10.3762/bjnano.9.86
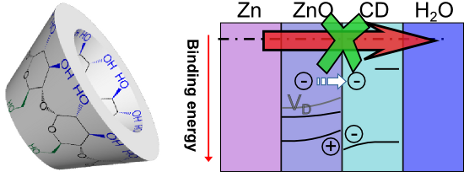
- containing electrolyte, samples were analysed by angle-dependent X-ray photoelectron spectroscopy (ADXPS) combined with ultraviolet photoelectron spectroscopy (UPS). Results and Discussion Electrochemical measurements of the corrosion potential Ecorr displayed in Figure 1a show a cathodic shift by several
- tens of millivolts of the initial Ecorr in the presence of β-CD in the electrolyte. Lower values of Ecorr are an indication of a suppression of the cathodic process of oxygen reduction [16], Ecorr stablized quickly in the presence of the inhibitor, while reference measurements showed a slower decrease
- (Supporting Information File 1, Figure S1) shows that this morphology is retained after exposure to the electrolyte. The inhibition efficiencies η (Figure 1c), based on EIS data (Supporting Information File 1, Figure S2, Figure S3 and Table S1), show that with only 19 μM of β-CD in KCl, an inhibition
Synthesis and characterization of two new TiO2-containing benzothiazole-based imine composites for organic device applications
Beilstein J. Nanotechnol. 2018, 9, 721–739, doi:10.3762/bjnano.9.67

- the internal standard. Cyclic voltammetry experiments were conducted in a standard one-compartment cell, in CH2Cl2 (Carlo Erba, HPLC grade), under argon. 0.2 M Bu4NPF6 (Aldrich, 99%) was used as the supporting electrolyte. The compound concentration was 1.0 × 10−6 mol/dm3. Deaeration of the solution
Facile synthesis of ZnFe2O4 photocatalysts for decolourization of organic dyes under solar irradiation
Beilstein J. Nanotechnol. 2018, 9, 436–446, doi:10.3762/bjnano.9.42

- working photo anode, Ag/AgCl as reference electrode and Pt as counter electrode. The electrolyte chosen for the study was 0.1 M Na2SO4 aqueous solution of pH 6.8. A 400 nm cut-off filter was used for the light irradiation during linear sweep voltammetry (LSV) analysis. Results and Discussion XRD analysis
- band-edge potential, a mechanism of the photocatalytic reaction has been proposed which is discussed later. Electrochemical impedance study Impedance measurements are commonly used to determine the charge transfer, resistance, and effective charge separation processes occurring at electrode–electrolyte
The nanofluidic confinement apparatus: studying confinement-dependent nanoparticle behavior and diffusion
Beilstein J. Nanotechnol. 2018, 9, 301–310, doi:10.3762/bjnano.9.30

- role in a nanofluidic system, in particular when a particle is close to a charged wall. Whereas diffusion measurements for uncharged particles [15] and for particles in electrolyte with higher ionic concentration [33] are in agreement with predictions that consider only a hydrodynamically hindered drag
- . There is considerable evidence of an increased drag of charged particles near charged walls in a weak electrolyte [18][38]. In a similar experimental configuration Eichmann et al. [18] measured a ≈30% (≈55%) lower lateral diffusion coefficient for 60 nm (100 nm) gold nanospheres with a relative radius
Synthesis and characterization of electrospun molybdenum dioxide–carbon nanofibers as sulfur matrix additives for rechargeable lithium–sulfur battery applications
Beilstein J. Nanotechnol. 2018, 9, 262–270, doi:10.3762/bjnano.9.28

- caused by dissolved polysulfide molecules [1]. All of these issues still pose a challenge to overcome for the production of reversible, stable, and efficient sulfur cathodes. The currently proposed approaches to solve these issues include sulfur-based cathode modification, electrolyte modification and
- , Shanghai Mapada Instrument Co. Ltd). Electrochemical measurements The electrochemical performance of the samples was measured in CR 2032-type coin cells. The electrolyte contained 1 M lithium bis(trifluoromethanesulfone)imide (LITFSI) and 0.1 M LiNO3 dissolved in 1,3-dioxolane (DOL) and 1.2-dimethoxyethane
- (DME) at a volume ratio of 1:1. The electrolyte solution volume used in the cells was 75 μL. The coin cells were galvanostatically charged–discharged at 0.25 mA/cm2 (1 C = 1675 mA g−1) and a voltage ranging from 1.7 and to 3.0 V (vs Li/Li+) using a CT2001A cell test instrument (LAND model, Wuhan RAMBO
Dielectric properties of a bisimidazolium salt with dodecyl sulfate anion doped with carbon nanotubes
Beilstein J. Nanotechnol. 2018, 9, 164–174, doi:10.3762/bjnano.9.19

- movements in the bulk of the electrolyte. At low frequencies (10−1–103 Hz), approximately region 1 in Figure 11, the behavior is controlled by “electrode polarization” effects. Thus, the electric conductivity decreases significantly when the frequency decreases. In the frequency range below 100 MHz, the
































































































































































































































