Search results
Search for "disulfide" in Full Text gives 206 result(s) in Beilstein Journal of Organic Chemistry. Showing first 200.
Identification of volatiles from six marine Celeribacter strains
- Anuj Kumar Chhalodia,
- Jan Rinkel,
- Dorota Konvalinkova,
- Jörn Petersen and
- Jeroen S. Dickschat
Beilstein J. Org. Chem. 2021, 17, 420–430, doi:10.3762/bjoc.17.38
- ·OEt2-catalyzed reaction of bis(benzothiazol-2-yl)disulfane with dimethyl disulfide, giving access to 41 with a yield of 64% (Scheme 2). The synthetic compound 41 showed an identical mass spectrum and retention index compared to the volatile in the Celeribacter extracts. The Z and E stereoisomers of 42

Graphical Abstract

Scheme 1: Sulfur metabolism in bacteria from the roseobacter group. A) DMSP demethylation by DmdABCD, B) DMSP...

Figure 1: Total ion chromatograms of headspace extracts from A) C. marinus DSM 100036T, B) C. neptunius DSM 2...

Figure 2: Structures of the identified volatile compounds in the headspace extracts from six Celeribacter typ...

Figure 3: EI mass spectra of A) unlabeled 2-(methyldisulfanyl)benzothiazole (41) and of labeled 41 after feed...

Scheme 2: Synthesis of sulfur-containing compounds detected in the Celeribacter headspace extracts. A) Synthe...
Recent progress in the synthesis of homotropane alkaloids adaline, euphococcinine and N-methyleuphococcinine
- Dimas J. P. Lima,
- Antonio E. G. Santana,
- Michael A. Birkett and
- Ricardo S. Porto
Beilstein J. Org. Chem. 2021, 17, 28–41, doi:10.3762/bjoc.17.4
- -Ni (W-2), H2O, 30 °C, 95%; vii) PCC, CH2Cl2, rt, 30%. Synthesis (+)-euphococcinine (2). Reagents and conditions: i) 2,4-bis(4-phenoxyphenyl)-1,3-dithia-2,4-diphosphetane 2,4-disulfide (Belleau’s reagent), 100%; ii) 33, triethylamine, trimethyl phosphite, reflux, 69%; iii) H2 (60 psi), Pt/C, Na2CO3

Graphical Abstract

Figure 1: Homotropane (azabicyclononane) systems.

Figure 2: Alkaloids (−)-adaline (1), (+)-euphococcinine (2) and (+)-N-methyleuphococcinine (3).

Scheme 1: Synthetic strategies before 1995.

Scheme 2: Synthesis (±)-adaline (1) and (±)-euphococcinine (2). Reagents and conditions: i) 1. dihydropyran, ...

Scheme 3: Synthesis (+)-euphococcinine (2). Reagents and conditions: i) H2O2, SeO2 (cat), acetone, rt, 88%; i...

Scheme 4: Synthesis (+)-euphococcinine (2). Reagents and conditions: i) 2,4-bis(4-phenoxyphenyl)-1,3-dithia-2...

Scheme 5: Synthesis of (±)-euphococcinine precursor (±)-42. Reagents and conditions: i) Bu3SnH, AIBN, toluene...

Scheme 6: Synthesis of (−)-adaline (1). Reagents and conditions: i) LiH2NBH3, THF, 40 °C, 88%; ii) TPAP, NMO,...

Scheme 7: Synthesis of (−)-adaline (1) and (−)-euphococcinine (2). Reagents and conditions: i) 1. BuLi, t-BuO...

Scheme 8: Synthesis of (−)-adaline (1). Reagents and conditions: i) Ref. [52]; ii) Et3N, TBDMSOTf, CH2Cl2, 0 °C t...

Scheme 9: Synthesis of (+)-euphococcinine (2). Reagents and conditions: i) 1. Cp2ZrCl2,AlMe3, CH2Cl2; 2. p-me...

Scheme 10: Synthesis of (−)-adaline 1. Reagents and conditions: i) 1. CuBr.DMS, Et2O/DMS, -42 ºC; 2. 1-heptyne...

Scheme 11: Synthesis of (−)-euphococcinine (2) and (−)-adaline (1). Reagents and conditions: i) 102, KHMDS, Et2...

Scheme 12: Synthesis of N-methyleuphococcinine 3. Reagents and conditions: i) 108 (1.5 equiv), 3,5-di-F-C6H3B(...
3-Acetoxy-fatty acid isoprenyl esters from androconia of the ithomiine butterfly Ithomia salapia
- Florian Mann,
- Daiane Szczerbowski,
- Lisa de Silva,
- Melanie McClure,
- Marianne Elias and
- Stefan Schulz
Beilstein J. Org. Chem. 2020, 16, 2776–2787, doi:10.3762/bjoc.16.228
- acylium ion m/z 263 is formed. In contrast, isoprenyl esters lack the M − 69 ion, but additionally show acylium +1 and +2 ions (m/z 264 and 265 in A). The location of the double bonds in the unsaturated esters was determined by dimethyl disulfide (DMDS) addition [25][26]. Because the double bond in the

Graphical Abstract

Figure 1: Extended hairs (arrow) of the androconia of a male Ithomia salapia aquinia (Photo: Melanie McClure)....

Scheme 1: Pyrrolizidine alkaloid lycopsamine (1) and the putative pheromone compounds methyl hydroxydanaidoat...

Scheme 2: Biosynthetic formation of hedycaryol (7) and α-elemol (8).

Figure 2: Total ion current chromatogram of androconial extracts of male butterflies of the two subspecies I....

Figure 3: Proposed mass spectrometric formation of characteristic ions in prenyl and isoprenyl esters. Format...

Figure 4: Mass spectra and fragmentation of A: isoprenyl (3-methyl-3-butenyl) 9-octadenoate (9) and B: prenyl...

Figure 5: Mass spectra and fragmentation of A: isoprenyl 3-acetoxyoctadecanoate (11); B: isoprenyl (Z)-3-acet...

Scheme 3: Synthesis of isoprenyl 3-acetoxyoctadecanoate (11). a) IBX, EtOAc, 60 °C, 3.15 h, 99%; b) SnCl2, CH2...

Scheme 4: a) 48% HBraq, toluene, 24 h, 110 °C, 79%; b) IBX, EtOAc, 60 °C, 3.15 h, 90%; c) C5H11PPh3Br, LDA, T...

Figure 6: Separation of the enantiomers of methyl (Z)-3-hydroxy-13-octadecenoate (25) on a β-6-TBDMS hydrodex...

Scheme 5: Proposed biosynthetic pathway of fatty acids leading to the observed regioisomers of the isoprenyl ...
Optical detection of di- and triphosphate anions with mixed monolayer-protected gold nanoparticles containing zinc(II)–dipicolylamine complexes
- Lena Reinke,
- Julia Bartl,
- Marcus Koch and
- Stefan Kubik
Beilstein J. Org. Chem. 2020, 16, 2687–2700, doi:10.3762/bjoc.16.219

- and Discussion Ligand synthesis. The ligands used in this work are depicted in Figure 1. All of them derived from lipoic acid, which was chosen as the anchor group because the immobilization of a cyclic disulfide on gold involves the formation of two Au–S bonds, causing the ligands to have a smaller

Graphical Abstract

Figure 1: Schematic illustration of the analyte-induced crosslinking of gold nanoparticles containing a mixtu...

Scheme 1: Syntheses of the ligands rac-1 and (R)-1. Conditions: i) TsCl, NaOH, THF, 0 °C, 60 min → 25 °C, 80 ...

Scheme 2: Synthesis of ligand 2. Conditions: i) potassium phthalimide, DMF, 25 °C, 18 h, 67%; ii) 2,2'-dipico...

Figure 2: Photographs of solutions of NPrac-1 in water (0.25 mg/mL) containing different sodium salts at a co...

Figure 3: Sections of the 1H NMR spectra of solutions of NP25 in D2O/CD3OD 1:2 (v/v) between 8.9 and 3.9 ppm ...

Figure 4: Images of vials containing solutions of NP10-Zn (0.25 mg/mL) in water/methanol 1:2 (v/v) and additi...

Figure 5: Photograph of the solutions of the competition experiment. Vial (a) only contained NP10-Zn (and the...

Figure 6: UV–vis spectra of NP10-Zn (0.25 mg/mL in the initial measurement) in water/methanol 1:2 (v/v) conta...

Figure 7: TEM images of NP10-Zn (0.25 mg/mL) in water/methanol 1:2 (v/v) before (a) and after the addition of...
Selective and reversible 1,3-dipolar cycloaddition of 6-aryl-1,5-diazabicyclo[3.1.0]hexanes with 1,3-diphenylprop-2-en-1-ones under microwave irradiation
- Alexander P. Molchanov,
- Mariia M. Efremova,
- Mariya A. Kryukova and
- Mikhail A. Kuznetsov
Beilstein J. Org. Chem. 2020, 16, 2679–2686, doi:10.3762/bjoc.16.218
- with carbon disulfide [26], diphenylketene [27], β-nitrostyrenes [28][29], if ionic liquids (IL) were used as reaction media. The reaction of β-nitrostyrenes with azomethine imines proceeds giving mixtures of Michael and anti-Michael-type adducts, containing a nitro group at the C2- or C1-position of

Graphical Abstract

Scheme 1: The two types of azomethine imines (AMI).

Scheme 2: Reaction of 1,5-diazabicyclo[3.1.0]hexanes 1a–d with diarylpropenones 2a–l.

Figure 1: Single-crystal X-ray structure of compound 3e.

Figure 2: Single-crystal X-ray structure of compound 3g.

Scheme 3: Control experiments.

Scheme 4: Mechanistic hypothesis for cycloaddition and cycloreversion reactions of diazabicyclohexane 1a with...

Scheme 5: Experiments on the trapping of azomethine imine, generated from pyrazolopyrazole 3g.
Synthesis of 4-substituted azopyridine-functionalized Ni(II)-porphyrins as molecular spin switches
- Jannis Ludwig,
- Tobias Moje,
- Fynn Röhricht and
- Rainer Herges
Beilstein J. Org. Chem. 2020, 16, 2589–2597, doi:10.3762/bjoc.16.210

- shown that the spin switching efficiency is strongly dependent on the solvent and on the substituent at the 4-position of the pyridine unit. We now introduced thiol, disulfide, thioethers, nitrile and carboxylic acid groups and investigated their spin switching efficiency. Keywords: azopyridines; Ni(II
- porphyrin 22 and the disulfide 12, obviously because of catalyst poisoning by sulfur [32][33]. To circumvent these problems, protection groups were introduced. Protection with methyl 3-mercaptopropionate (15) [29] was successful, however, inhibition of the Suzuki reaction was observed. We assume an alkaline
- procedure of Nishimura et al. using mercury acetate in trifluoroacetic acid and anisole as radical scavenger (Scheme 5) [34]. Cleavage of the tert-butyl protection group yielded the disulfide 1g, which was reduced with catecholborane in tetrahydrofuran to give the free thiol 1h, which readily reoxidizes

Graphical Abstract

Figure 1: “Record player” approach for molecular spin switching. a) General principle b) Variation of the sub...

Scheme 1: Synthesis of the nitroso compounds 3 and 6 using the two different methods described by Wegner et a...

Scheme 2: Synthesis of azopyridines 11, 14, 16 and 18 by nucleophilic aromatic substitution.

Scheme 3: Synthesis of 3-(3-bromophenylazo)-4-cyanopyridine (20), which was hydrolyzed to yield 3-(3-bromophe...

Scheme 4: Modular approach for the C–C connection of the Ni(II)-porphyrin 22 and the different 4-substituted ...

Scheme 5: Cleavage of 1f to yield disulfide 1g [34].

Figure 2: Hammett plot of the investigated pyridine substituents [36].

Figure 3: UV–vis spectra of 1e (top), 1h (left) and 1j (right) in acetone water (1:9) (solid line) and after ...
A new method for the synthesis of diamantane by hydroisomerization of binor-S on treatment with sulfuric acid
- Rishat I. Aminov and
- Ravil I. Khusnutdinov
Beilstein J. Org. Chem. 2020, 16, 2534–2539, doi:10.3762/bjoc.16.205
- , experiment B), or in carbon disulfide (CS2, experiment C). In experiment А, the major isomer 1-D2, which is formed upon hydroisomerization of binor-S (2), contains two deuterium atoms. Two more hydrogen atoms are probably provided by cyclohexane. Unexpectedly, the reaction also gave undeuterated diamantane
- (2) acts as the hydrogen source for the isomer C14H17D3, 1-D3. Our attempt to carry out the deuteration of diamantane (1) with D2SO4 in carbon disulfide for 7 h at 20 °C was unsuccessful. Evidently, the deuterium exchange, resulting in the formation of diamantanes 1-D7 and 1-D8 containing 7 and 8
- acid (98%) in carbon disulfide or cyclohexane. It was found that both, sulfuric acid and cyclohexane can serve as the main hydrogen sources. In the presence of H2SO4 with a lower concentration (75–80%), the reaction stops at the step of formation of endo-endo-pentacyclo[7.3.1.12,5.18,10.03,7

Graphical Abstract

Scheme 1: Isomerization of 3а–с to diamantane (1). Reaction conditions: (a) CoBr2·2PPh3–BF3·OEt2, 110 °C, 12 ...

Scheme 2: Isomerization of binor-S (2) to diamantane (1).

Scheme 3: Selective synthesis of tetrahydrobinor-S (3c) from binor-S (2).

Scheme 4: Isomerization of binor-S (2) to hydrocarbons 4а and b.
Dawn of a new era in industrial photochemistry: the scale-up of micro- and mesostructured photoreactors
- Emine Kayahan,
- Mathias Jacobs,
- Leen Braeken,
- Leen C.J. Thomassen,
- Simon Kuhn,
- Tom van Gerven and
- M. Enis Leblebici
Beilstein J. Org. Chem. 2020, 16, 2484–2504, doi:10.3762/bjoc.16.202

- of the photocatalytic oxidation of thiophenol to phenyl disulfide using a Taylor flow in a microreactor that consists of a capillary tube. By varying the flow rate of the gas and liquid phase and comparing the oxygen concentration in the liquid phase with the equilibrium concentration without a

Graphical Abstract

Figure 1: The momentum transport affects the mass transfer and the light field. All transport phenomena need ...

Figure 2: Common photomicroreactor designs: (a) Straight channel, (b) serpentine channel, (c) square serpenti...

Figure 3: Benchmarked photoreactors: (a) Microcapillaries in parallel, (b) microcapillaries in series, (c) fl...

Figure 4: Photochemical reactions that are detailed in Table 1.

Figure 5: Structured reactors designed for enhancing the mass transfer: (a) Packed bed photoreactor, (b) mono...

Figure 6: Comparison of the LED board designs of photomicroreactors: (a) CC array design, (b) MC array design...

Figure 7: Illustration of the light scattering phenomenon inside a photocatalytic flow reactor.

Figure 8: Efficiency of the absorption process in scattering situations with respect to pure absorption situa...

Figure 9: Different types of distributors: (a) Traditional or consecutive manifold, (b) bifurcation unit dist...
Synthetic approaches to bowl-shaped π-conjugated sumanene and its congeners
- Shakeel Alvi and
- Rashid Ali
Beilstein J. Org. Chem. 2020, 16, 2212–2259, doi:10.3762/bjoc.16.186


Graphical Abstract

Figure 1: Representation of corannulene (1) and sumanene (2), the subunits of fullerene (C60).

Scheme 1: Mehta’s unsuccessful effort for the synthesis of sumanene scaffold 2.

Scheme 2: First synthesis of sumanene 2 by Sakurai et al. from norbornadiene 10.

Scheme 3: Synthesis of trimethylsumanene 28 from easily accessible norbornadiene (10).

Scheme 4: Generation of anions 29–31 and the preparation of tris(trimethylsilyl)sumanene 32.

Scheme 5: Synthesis of tri- and hexa-substituted sumanene derivatives.

Scheme 6: Synthesis of bowl-shaped π-extended sumanene derivatives 37a–f.

Scheme 7: Synthesis of monooxasumanene 38, trioxosumanene 40 along with imination of them.

Scheme 8: Synthesis of trimethylsumanenetrione 46 and exo-functionalized products 45a,b.

Scheme 9: Synthesis of bisumanenylidene 47 and sumanene dimer 48 from 2.

Scheme 10: The mono-substitution of 2 to generate diverse mono-sumanene derivatives 49a–d.

Scheme 11: Synthesis of sumanene building block 53 useful for further extension.

Scheme 12: Synthesis of hexafluorosumanene derivative 55 by Sakurai and co-workers.

Scheme 13: Preparation of sumanene-based carbene 60 and its reaction with cyclohexane.

Scheme 14: Barton–Kellogg reaction for the synthesis of sterically hindered alkenes.

Scheme 15: Synthesis of hydroxysumanene 68 by employing Baeyer–Villiger oxidation.

Scheme 16: Synthesis of sumanene derivatives having functionality at an internal carbon.

Scheme 17: Mechanism for nucleophilic substitution reaction at the internal carbon.

Scheme 18: Synthesis of diverse monosubstituted sumanene derivatives.

Scheme 19: Synthesis of di- and trisubstituted sumanene derivatives from sumanene (2).

Scheme 20: Preparation of monochlorosumanene 88 and hydrogenation of sumanene (2).

Scheme 21: The dimer 90 and bissumanenyl 92 achieved from halosumannes.

Scheme 22: Pyrenylsumanene 93 involving the Suzuki-coupling as a key transformation.

Scheme 23: Synthesis of various hexaarylsumanene derivatives using the Suzuki-coupling reaction.

Scheme 24: Synthesis of hexasubstituted sumanene derivatives 96 and 97.

Scheme 25: Synthesis of thioalkylsumanenes via an aromatic nucleophilic substitution reaction.

Scheme 26: Synthesis of tris(ethoxycarbonylethenyl)sumanene derivative 108.

Scheme 27: Synthesis of ferrocenyl-based sumanene derivatives.

Scheme 28: Synthesis of sumanenylferrocene architectures 118 and 119 via Negishi coupling.

Scheme 29: Diosmylation and the synthesis of phenylboronate ester 121 of sumanene.

Scheme 30: Synthesis of the iron-complex of sumanene.

Scheme 31: Synthesis of tri- and mononuclear sumanenyl zirconocene complexes.

Scheme 32: Synthesis of [CpRu(η6-sumanene)]PF6.

Scheme 33: Preparation of sumanene-based porous coordination networks 127 (spherical tetramer units) and 128 (...

Scheme 34: Synthesis of sumanenylhafnocene complexes 129 and 130.

Scheme 35: Synthesis of 134 and 135 along with PdII coordination complex 136.

Scheme 36: Synthesis of alkali metals sumanene complex K7(C21H102−)2(C21H93−)·8THF (137) containing di- and tr...

Scheme 37: The encapsulation of a Cs+ ion between two sumanenyl anions.

Scheme 38: Synthesis of monothiasumanene 140 and dithiasumanene 141 from 139.

Scheme 39: Synthesis of trithiasumanene 151 by Otsubo and his co-workers.

Scheme 40: Synthesis of trithiasumanene derivatives 155 and 156.

Scheme 41: Synthetic route towards hexathiolated trithiasumanenes 158.

Scheme 42: Synthesis of triselenasumanene 160 by Shao and teammates.

Scheme 43: Synthesis of tritellurasumanene derivatives from triphenylene skeletons.

Scheme 44: Synthesis of pyrazine-fused sumanene architectures through condensation reaction.

Scheme 45: Treatment of the trichalcogenasumanenes with diverse oxidative reagents.

Scheme 46: Ring-opening reaction with H2O2 and oxone of heterasumanenes 178 and 179.

Scheme 47: Synthesis of polycyclic compounds from sumanene derivatives.

Scheme 48: Synthesis of diimide-based heterocycles reported by Shao’s and co-workers.

Scheme 49: Synthesis of pristine trichalcogenasumanenes, 151, 205, and 206.

Scheme 50: Synthesis of trichalcogenasumanenes via hexaiodotriphenylene precursor 208.

Scheme 51: Synthesis of trisilasumanenes 214 and 215.

Scheme 52: Synthesis of trisilasumanene derivatives 218 and 219.

Scheme 53: Synthesis of novel trigermasumanene derivative 223.

Scheme 54: An attempt towards the synthesis of tristannasumanene derivative 228.

Scheme 55: Synthesis of triphosphasumanene trisulfide 232 from commercially available 229.

Scheme 56: The doping of sumanene derivatives with chalcogens (S, Se, Te) and phosphorus.

Scheme 57: Synthesis of heterasumanene containing three different heteroatoms.

Scheme 58: Synthesis of trichalcogenasumanene derivatives 240 and 179.

Scheme 59: Preparation of trichalcogenasumanenes 245 and 248.

Scheme 60: Design and synthesis of trichalcogenasumanene derivatives 252 and 178.

Scheme 61: Synthesis of spirosumanenes 264–269 and non-spiroheterasumanenes 258–263.

Scheme 62: Synthesis of sumanene-type hetero polycyclic compounds.

Scheme 63: Synthesis of triazasumanenes 288 and its sulfone congener 287.

Scheme 64: Synthesis of C3-symmetric chiral triaryltriazasumanenes via cross-coupling reaction.

Scheme 65: Synthesis of mononaphthosumanene 293 using Suzuki coupling as a key step.

Scheme 66: Synthesis of di- and trinaphthosumanene derivatives 302–304.

Scheme 67: Synthesis of hemifullerene skeletons by Hirao’s group.

Scheme 68: Design and construction of C70 fragment from a C60 sumanene fragment.
Automated high-content imaging for cellular uptake, from the Schmuck cation to the latest cyclic oligochalcogenides
- Rémi Martinent,
- Javier López-Andarias,
- Dimitri Moreau,
- Yangyang Cheng,
- Naomi Sakai and
- Stefan Matile
Beilstein J. Org. Chem. 2020, 16, 2007–2016, doi:10.3762/bjoc.16.167

- chemistry tools to deliver into living cells has seen a shift of attention from counterion-mediated uptake of cell-penetrating peptides (CPPs) and their mimics, particularly the Schmuck cation, toward thiol-mediated uptake with cell-penetrating poly(disulfide)s (CPDs) and cyclic oligochalcogenides (COCs
- increasing number of charges and substrate size [18]. To address both problems, the peptide backbone has been replaced by poly(disulfide)s [18][37]. The resulting cell-penetrating poly(disulfide)s (CPDs) 18 are at least as efficient as CPPs but less toxic because they are degraded by reductive
- depolymerization as soon as they reach the cytosol, and their endosomal capture is minimal because they enter cells by thiol-mediated uptake (Figure 4) [38][39][40][41][42][43][44][45][46][47][48]. This mechanism operates by dynamic covalent disulfide exchange on the cell surface and on the way into the cell

Graphical Abstract

Figure 1: Schematic representation of binding models between organic cations (simple ammonium, guanidinium, S...

Figure 2: From Schmuck cations to cell-penetrating dipeptides, with schematic representation of the binding m...

Figure 3: Peptide tweezers and cyclic peptides with Schmuck cations for gene transfection.

Figure 4: Evolution from CPPs to CPDs and COCs.

Figure 5: Structure of a) the trifunctional transporter 23 and c) the HaloTag reporter 26. b) Schematic mecha...

Figure 6: CAPA assay for the complex 25, composed of three transporters 23 bound to one streptavidin 24 (with...

Figure 7: Examples from the automated HC imaging of stable HGM cells with HaloTag–GFP on mitochondria, labele...

Figure 8: Evaluation of the automated HC imaging of stable HGM cells with HaloTag–GFP on mitochondria, labele...

Figure 9: a) Automated HC imaging of the cellular uptake of 25, covering the concentration dependence for the...

Figure 10: Examples of automated HC imaging of transiently transfected HeLa cells with HaloTag–GFP on Golgi, l...

Figure 11: Evaluation of the automated HC imaging of the transiently transfected HeLa cells with HaloTag–GFP o...
A dynamic combinatorial library for biomimetic recognition of dipeptides in water
- Florian Klepel and
- Bart Jan Ravoo
Beilstein J. Org. Chem. 2020, 16, 1588–1595, doi:10.3762/bjoc.16.131

- ), while AA binds significantly weaker (K ≈ 65–71 M−1). Keywords: artificial receptor; dynamic combinatorial chemistry; dynamic covalent chemistry; molecular recognition; thiolate–disulfide exchange; Introduction Peptides are one of the most abundant and structurally versatile motifs in nature. Thus
- library to be under thermodynamic control. Therefore, the equilibrium responds to stimuli such as addition of a template, which would stabilize a suitable receptor and thus amplifies its formation. This strategy has led to the discovery of many artificial receptors [7][8][9][10], with thiolate disulfide
- cysteines are converted quantitively to disulfides by oxidation with oxygen. Until fully oxidized library members can exchange building blocks in a thiol–disulfide exchange reaction. While a closed monomer can be observed in the beginning (Figure S1, Supporting Information File 1), exclusively cyclic

Graphical Abstract

Scheme 1: a) Building blocks included in this study. b) Antiparallel and parallel constitutional isomers of t...

Figure 1: HPLC–MS chromatograms of a reference library for all possible tripeptide dimers ([M + H]+ ions).

Figure 2: a) HPLC–MS chromatograms of the dimers (CFC)2 and templates YY and FF. b) Amplification of the peak...

Scheme 2: a) Synthesis of the parallel and antiparallel isomers p(CFC)2 and a(CFC)2. b) Templates FF. YY and ...

Figure 3: ITC of YY (30 mM) to a(CFC)2 (1.5 mM) in phosphate buffer (pH 7.4, 100 mM).

Figure 4: Continuously varied NMR measurements of a) p(CFC)2 to YY b) p(CFC)2 to FF c) a(CFC)2 to YY d) a(CFC)...

Figure 5: Job plots derived from the continuously varied NMR measurements of a) p(CFC)2 to YY b) p(CFC)2 to FF...
Heterogeneous photocatalysis in flow chemical reactors
- Christopher G. Thomson,
- Ai-Lan Lee and
- Filipe Vilela
Beilstein J. Org. Chem. 2020, 16, 1495–1549, doi:10.3762/bjoc.16.125


Graphical Abstract

Figure 1: A) Bar chart of the publications per year for the topics “Photocatalysis” (49,662 instances) and “P...

Figure 2: A) Professor Giacomo Ciamician and Dr. Paolo Silber on their roof laboratory at the University of B...

Scheme 1: PRC trifluoromethylation of N-methylpyrrole (1) using hazardous gaseous CF3I safely in a flow react...

Figure 3: A) Unit cells of the three most common crystal structures of TiO2: rutile, brookite, and anatase. R...

Figure 4: Illustration of the key semiconductor photocatalysis events: 1) A photon with a frequency exceeding...

Figure 5: Photocatalytic splitting of water by oxygen vacancies on a TiO2(110) surface. Reprinted with permis...

Figure 6: Proposed adsorption modes of A) benzene, B) chlorobenzene, C) toluene, D) phenol, E) anisole, and F...

Figure 7: Structures of the sulfonate-containing organic dyes RB5 (3) and MX-5B (4) and the adsorption isothe...

Figure 8: Idealised triclinic unit cell of a g-C3N4 type polymer, displaying possible hopping transport scena...

Figure 9: Idealised structure of a perfect g-C3N4 sheet. The central unit highlighted in red represents one t...

Figure 10: Timeline of the key processes of charge transport following the photoexcitation of g-C3N4, leading ...

Scheme 2: Photocatalytic bifunctionalisation of heteroarenes using mpg-C3N4, with the selected examples 5 and ...

Figure 11: A) Structure of four linear conjugated polymer photocatalysts for hydrogen evolution, displaying th...

Figure 12: Graphical representation of the common methods used to immobilise molecular photocatalysts (PC) ont...

Figure 13: Wireless light emitter-supported TiO2 (TiO2@WLE) HPCat spheres powered by resonant inductive coupli...

Figure 14: Graphical representation of zinc–perylene diimide (Zn-PDI) supramolecular assembly photocatalysis v...

Scheme 3: Upconversion of NIR photons to the UV frequency by NaYF4:Yb,Tm nanocrystals sequentially coated wit...

Figure 15: Types of reactors employed in heterogeneous photocatalysis in flow. A) Fixed bed reactors and the s...

Figure 16: Electrochemical potential of common semiconductor, transition metal, and organic dye-based photocat...

Scheme 4: Possible mechanisms of an immobilised molecular photoredox catalyst by oxidative or reductive quenc...

Scheme 5: Scheme of the CMB-C3N4 photocatalytic decarboxylative fluorination of aryloxyacetic acids, with the...

Scheme 6: Scheme of the g-C3N4 photocatalytic desilylative coupling reaction in flow and proposed mechanism [208].

Scheme 7: Proposed mechanism of the radical cyclisation of unsaturated alkyl 2-bromo-1,3-dicarbonyl compounds...

Scheme 8: N-alkylation of benzylamine and schematic of the TiO2-coated microfluidic device [213].

Scheme 9: Proposed mechanism of the Pt@TiO2 photocatalytic deaminitive cyclisation of ʟ-lysine (23) to ʟ-pipe...

Scheme 10: A) Proposed mechanism for the photocatalytic oxidation of phenylboronic acid (24). B) Photos and SE...

Scheme 11: Proposed mechanism for the DA-CMP3 photocatalytic aza-Henry reaction performed in a continuous flow...

Scheme 12: Proposed mechanism for the formation of the cyclic product 32 by TiO2-NC HPCats in a slurry flow re...

Scheme 13: Reaction scheme for the photocatalytic synthesis of homo and hetero disulfides in flow and scope of...

Scheme 14: Reaction scheme for the MoOx/TiO2 HPCat oxidation of cyclohexane (34) to benzene. The graph shows t...

Scheme 15: Proposed mechanism of the TiO2 HPC heteroarene C–H functionalisation via aryl radicals generated fr...

Scheme 16: Scheme of the oxidative coupling of benzylamines with the HOTT-HATN HPCat and selected examples of ...

Scheme 17: Photocatalysis oxidation of benzyl alcohol (40) to benzaldehyde (41) in a microflow reactor coated ...

Figure 17: Mechanisms of Dexter and Forster energy transfer.

Scheme 18: Continuous flow process for the isomerisation of alkenes with an ionic liquid-immobilised photocata...

Scheme 19: Singlet oxygen synthetic step in the total synthesis of canataxpropellane [265].

Scheme 20: Scheme and proposed mechanism of the singlet oxygen photosensitisation by CMP_X HPCats, with the st...

Scheme 21: Structures of CMP HPCat materials applied by Vilela and co-workers for the singlet oxygen photosens...

Scheme 22: Polyvinylchloride resin-supported TDCPP photosensitisers applied for singlet oxygen photosensitisat...

Scheme 23: Structure of the ionically immobilised TPP photosensitiser on amberlyst-15 ion exchange resins (TPP...

Scheme 24: Photosensitised singlet oxygen oxidation of citronellol (46) in scCO2, with automatic phase separat...

Scheme 25: Schematic of PS-Est-BDP-Cl2 being applied for singlet oxygen photosensitisation in flow. A) Pseudo-...

Scheme 26: Reaction scheme of the singlet oxygen oxidation of furoic acid (54) using a 3D-printed microfluidic...

Figure 18: A) Photocatalytic bactericidal mechanism by ROS oxidative cleavage of membrane lipids (R = H, amino...

Figure 19: A) Suggested mechanisms for the aqueous pollutant degradation by TiO2 in a slurry flow reactor [284-287]. B)...

Figure 20: Schematic of the flow system used for the degradation of aqueous oxytetracycline (56) solutions [215]. M...

Scheme 27: Degradation of a salicylic acid (57) solution by a coupled solar photoelectro-Fenton (SPEF) process...

Figure 21: A) Schematic flow diagram using the TiO2-coated NETmix microfluidic device for an efficient mass tr...
An overview on disulfide-catalyzed and -cocatalyzed photoreactions
- Yeersen Patehebieke
Beilstein J. Org. Chem. 2020, 16, 1418–1435, doi:10.3762/bjoc.16.118

- , mild, and chemoselective radical catalysts that deserve more attention. The present review highlights the recent progress in the field of disulfide-catalyzed and -cocatalyzed photocatalytic reactions for different reaction types. Keywords: cycloaddition; disulfide catalyst; isomerization; oxidation
- ; photocatalysis; thiyl radical; Introduction Organic disulfides are often used as the skeleton for drugs, pesticides, rubber auxiliaries, polymers, and electronic materials [1]. Over the past decade, organic disulfide-involving photoreactions have attracted increasing attention. Disulfides have versatile
- , oxidations, or isomerizations, disulfides have increasingly proven their power. Herein, we briefly describe the progress in the field of disulfide-involving photocatalysis in recent years for different reaction types. Review Cycloaddition reactions As early as 1988, Feldman and co-workers reported an example

Graphical Abstract

Scheme 1: [3 + 2] cyclization catalyzed by diaryl disulfide.

Scheme 2: [3 + 2] cycloaddition catalyzed by disulfide.

Scheme 3: Disulfide-bridged peptide-catalyzed enantioselective cycloaddition.

Scheme 4: Disulfide-catalyzed [3 + 2] methylenecyclopentane annulations.

Scheme 5: Disulfide as a HAT cocatalyst in the [4 + 2] cycloaddition reaction.

Scheme 6: Proposed mechanism of the [4 + 2] cycloaddition reaction using disulfide as a HAT cocatalyst.

Scheme 7: Disulfide-catalyzed ring expansion of vinyl spiro epoxides.

Scheme 8: Disulfide-catalyzed aerobic oxidation of diarylacetylene.

Scheme 9: Disulfide-catalyzed aerobic photooxidative cleavage of olefins.

Scheme 10: Disulfide-catalyzed aerobic oxidation of 1,3-dicarbonyl compounds.

Scheme 11: Proposed mechanism of the disulfide-catalyzed aerobic oxidation of 1,3-dicarbonyl compounds.

Scheme 12: Disulfide-catalyzed oxidation of allyl alcohols.

Scheme 13: Disulfide-catalyzed diboration of alkynes.

Scheme 14: Dehalogenative radical cyclization catalyzed by disulfide.

Scheme 15: Hydrodifluoroacetamidation of alkenes catalyzed by disulfide.

Scheme 16: Plausible mechanism of the hydrodifluoroacetamidation of alkenes catalyzed by disulfide.

Scheme 17: Disulfide-cocatalyzed anti-Markovnikov olefin hydration reactions.

Scheme 18: Disulfide-catalyzed decarboxylation of carboxylic acids.

Scheme 19: Proposed mechanism of the disulfide-catalyzed decarboxylation of carboxylic acids.

Scheme 20: Disulfide-catalyzed decarboxylation of carboxylic acids.

Scheme 21: Disulfide-catalyzed conversion of maleate esters to fumarates and 5H-furanones.

Scheme 22: Disulfide-catalyzed isomerization of difluorotriethylsilylethylene.

Scheme 23: Disulfide-catalyzed isomerization of allyl alcohols to carbonyl compounds.

Scheme 24: Proposed mechanism for the disulfide-catalyzed isomerization of allyl alcohols to carbonyl compound...

Scheme 25: Diphenyl disulfide-catalyzed enantioselective synthesis of ophirin B.

Scheme 26: Disulfide-catalyzed isomerization in the total synthesis of (+)-hitachimycin.

Scheme 27: Disulfide-catalyzed isomerization in the synthesis of (−)-gloeosporone.
Recent synthesis of thietanes
- Jiaxi Xu
Beilstein J. Org. Chem. 2020, 16, 1357–1410, doi:10.3762/bjoc.16.116
- transform 1,3-diols 62 into the precursors of γ-mercaptoalkanols with dibenzoxazol-2-yl disulfide (63) and phosphines. They reacted primary or secondary 1,3-diols 62 with disulfide 63 in the presence of Bu3P or Ph3P to selectively synthesize 2-(3-hydroxyalkylthio)benzoxazoles 64. These were treated with KH
- of quadricyclane 218 with thiocarbonyl derivatives 219. With carbon disulfide, mono- and biscycloadducts 221 and 222 were formed depending on concentration, temperature, and pressure conditions [69] (Scheme 43). In the same year, Kanaoka and co-workers reported the intermolecular photo [2 + 2

Graphical Abstract

Figure 1: Examples of biologically active thietane-containing molecules.

Figure 2: The diverse methods for the synthesis of thietanes.

Scheme 1: Synthesis of 1-(thietan-2-yl)ethan-1-ol (10) from 3,5-dichloropentan-2-ol (9).

Scheme 2: Synthesis of thietanose nucleosides 2,14 from 2,2-bis(bromomethyl)propane-1,3-diol (11).

Scheme 3: Synthesis of methyl 3-vinylthietane-3-carboxylate (19).

Scheme 4: Synthesis of 1,6-thiazaspiro[3.3]heptane (24).

Scheme 5: Synthesis of 6-amino-2-thiaspiro[3.3]heptane hydrochloride (28).

Scheme 6: Synthesis of optically active thietane 31 from vitamin C.

Scheme 7: Synthesis of an optically active thietane nucleoside from diethyl L-tartrate (32).

Scheme 8: Synthesis of thietane-containing spironucleoside 40 from 5-aldo-3-O-benzyl-1,2-O-isopropylidene-α-D...

Scheme 9: Synthesis of optically active 2-methylthietane-containing spironucleoside 43.

Scheme 10: Synthesis of a double-linked thietane-containing spironucleoside 48.

Scheme 11: Synthesis of two diastereomeric thietanose nucleosides via 2,4-di(benzyloxymethyl)thietane (49).

Scheme 12: Synthesis of the thietane-containing PI3k inhibitor candidate 54.

Scheme 13: Synthesis of the spirothietane 57 as the key intermediate to Nuphar sesquiterpene thioalkaloids.

Scheme 14: Synthesis of spirothietane 61 through a direct cyclic thioetherification of 3-mercaptopropan-1-ol.

Scheme 15: Synthesis of thietanes 66 from 1,3-diols 62.

Scheme 16: Synthesis of thietanylbenzimidazolone 75 from (iodomethyl)thiazolobenzimidazole 70.

Scheme 17: Synthesis of 2-oxa-6-thiaspiro[3.3]heptane (80) from bis(chloromethyl)oxetane 76 and thiourea.

Scheme 18: Synthesis of the thietane-containing glycoside, 2-O-p-toluenesulfonyl-4,6-thioanhydro-α-D-gulopyran...

Scheme 19: Synthesis of methyl 4,6-thioanhydro-α-D-glucopyranoside (89).

Scheme 20: Synthesis of thietane-fused α-D-galactopyranoside 93.

Scheme 21: Synthesis of thietane-fused α-D-gulopyranoside 100.

Scheme 22: Synthesis of 3,5-anhydro-3-thiopentofuranosides 104.

Scheme 23: Synthesis of anhydro-thiohexofuranosides 110, 112 and 113 from from 1,2:4,5-di-O-isopropylidene D-f...

Scheme 24: Synthesis of optically active thietanose nucleosides from D- and L-xyloses.

Scheme 25: Synthesis of thietane-fused nucleosides.

Scheme 26: Synthesis of 3,5-anhydro-3-thiopentofuranosides.

Scheme 27: Synthesis of 2-amino-3,5-anhydro-3-thiofuranoside 141.

Scheme 28: Synthesis of thietane-3-ols 145 from (1-chloromethyl)oxiranes 142 and hydrogen sulfide.

Scheme 29: Synthesis of thietane-3-ol 145a from chloromethyloxirane (142a).

Scheme 30: Synthesis of thietane-3-ols 145 from 2-(1-haloalkyl)oxiranes 142 and 147 with ammonium monothiocarb...

Scheme 31: Synthesis of 7-deoxy-5(20)thiapaclitaxel 154a, a thietane derivative of taxoids.

Scheme 32: Synthesis of 5(20)-thiadocetaxel 158 from 10-deacetylbaccatin III (155).

Scheme 33: Synthesis of thietane derivatives 162 as precursors for deoxythiataxoid synthesis through oxiraneme...

Scheme 34: Synthesis of 7-deoxy 5(20)-thiadocetaxel 154b.

Scheme 35: Mechanism for the formation of the thietane ring in 171 from oxiranes with vicinal leaving groups 1...

Scheme 36: Synthesis of cis-2,3-disubstituted thietane 175 from thiirane-2-methanol 172.

Scheme 37: Synthesis of a bridged thietane 183 from aziridine cyclohexyl tosylate 179 and ammonium tetrathiomo...

Scheme 38: Synthesis of thietanes via the photochemical [2 + 2] cycloaddition of thiobenzophenone 184a with va...

Scheme 39: Synthesis of spirothietanes through the photo [2 + 2] cycloaddition of cyclic thiocarbonyls with ol...

Scheme 40: Photochemical synthesis of spirothietane-thioxanthenes 210 from thioxanthenethione (208) and butatr...

Scheme 41: Synthesis of thietanes 213 from 2,4,6-tri(tert-butyl)thiobenzaldehyde (211) with substituted allene...

Scheme 42: Photochemical synthesis of spirothietanes 216 and 217 from N-methylthiophthalimide (214) with olefi...

Scheme 43: Synthesis of fused thietanes from quadricyclane with thiocarbonyl derivatives 219.

Scheme 44: Synthesis of tricyclic thietanes via the photo [2 + 2] cycloaddition of N-methyldithiosuccinimides ...

Scheme 45: Synthesis of tricyclic thietanes via the photo [2 + 2] cycloaddition of N-methylthiosuccinimide/thi...

Scheme 46: Synthesis of tricyclic thietanes via the photo [2 + 2] cycloaddition of N-alkylmonothiophthalimides...

Scheme 47: Synthesis of spirothietanes from dithiosuccinimides 223 with 2,3-dimethyl-2-butene (215a).

Scheme 48: Synthesis of thietanes 248a,b from diaryl thione 184b and ketene acetals 247a,b.

Scheme 49: Photocycloadditions of acridine-9-thiones 249 and pyridine-4(1H)-thione (250) with 2-methylacrynitr...

Scheme 50: Synthesis of thietanes via the photo [2 + 2] cycloaddition of mono-, di-, and trithiobarbiturates 2...

Scheme 51: Synthesis of spirothietanes via the photo [2 + 2] cycloaddition of 1,1,3-trimethyl-2-thioxo-1,2-dih...

Scheme 52: Synthesis of spirothietanes via the photo [2 + 2] cycloaddition of thiocoumarin 286 with olefins.

Scheme 53: Photochemical synthesis of thietanes 296–299 from semicyclic and acyclic thioimides 292–295 and 2,3...

Scheme 54: Photochemical synthesis of spirothietane 301 from 1,3,3-trimethylindoline-2-thione (300) and isobut...

Scheme 55: Synthesis of spirobenzoxazolethietanes 303 via the photo [2 + 2] cycloaddition of alkyl and aryl 2-...

Scheme 56: Synthesis of spirothietanes from tetrahydrothioxoisoquinolines 306 and 307 with olefins.

Scheme 57: Synthesis of spirothietanes from 1,3-dihydroisobenzofuran-1-thiones 311 and benzothiophene-1-thione...

Scheme 58: Synthesis of 2-triphenylsilylthietanes from phenyl triphenylsilyl thioketone (316) with electron-po...

Scheme 59: Diastereoselective synthesis of spiropyrrolidinonethietanes 320 via the photo [2 + 2] cycloaddition...

Scheme 60: Synthesis of bicyclic thietane 323 via the photo [2 + 2] cycloaddition of 2,4-dioxo-3,4-dihydropyri...

Scheme 61: Photo-induced synthesis of fused thietane-2-thiones 325 and 326 from silacyclopentadiene 324 and ca...

Scheme 62: Synthesis of highly strained tricyclic thietanes 328 via the intramolecular photo [2 + 2] cycloaddi...

Scheme 63: Synthesis of tri- and pentacyclic thietanes 330 and 332, respectively, through the intramolecular p...

Scheme 64: Synthesis of tricyclic thietanes 334 via the intramolecular photo [2 + 2] cycloaddition of N-vinylt...

Scheme 65: Synthesis of tricyclic thietanes 336 via the intramolecular photo [2 + 2] cycloaddition of N-but-3-...

Scheme 66: Synthesis of tricyclic thietanes via the intramolecular photo [2 + 2] cycloaddition of N-but-3-enyl...

Scheme 67: Synthesis of tetracyclic thietane 344 through the intramolecular photo [2 + 2] cycloaddition of N-[...

Scheme 68: Synthesis of tri- and tetracyclic thietanes 348, 350, and 351, through the intramolecular photo [2 ...

Scheme 69: Synthesis of tetracyclic fused thietane 354 via the photo [2 + 2] cycloaddition of vinyl 2-thioxo-3H...

Scheme 70: Synthesis of highly rigid thietane-fused β-lactams via the intramolecular photo [2 + 2] cycloadditi...

Scheme 71: Asymmetric synthesis of a highly rigid thietane-fused β-lactam 356a via the intramolecular photo [2...

Scheme 72: Diastereoselective synthesis of the thietane-fused β-lactams via the intramolecular photo [2 + 2] c...

Scheme 73: Asymmetric synthesis of thietane-fused β-lactams 356 via the intramolecular photo [2 + 2] cycloaddi...

Scheme 74: Synthesis of the bridged bis(trifluoromethyl)thietane from 2,2,4,4-tetrakis(trifluoromethyl)-1,3-di...

Scheme 75: Synthesis of the bridged-difluorothietane 368 from 2,2,4,4-tetrafluoro-1,3-dithietane (367) and qua...

Scheme 76: Synthesis of bis(trifluoromethyl)thietanes from 2,2,4,4-tetrakis(trifluoromethyl)-1,3-dithietane (3...

Scheme 77: Synthesis of 2,2-dimethylthio-4,4-di(trifluoromethyl)thietane (378) from 2,2,4,4-tetrakis(trifluoro...

Scheme 78: Formation of bis(trifluoromethyl)thioacetone (381) through nucleophilic attack of dithietane 363 by...

Scheme 79: Synthesis of 2,2-bis(trifluoromethyl)thietanes from 2,2,4,4-tetrakis(trifluoromethyl)-1,3-dithietan...

Scheme 80: Synthesis of the bridged bis(trifluoromethyl)thietane 364 from of 2,2,4,4-tetrakis(trifluoromethyl)...

Scheme 81: Synthesis of 2,4-diiminothietanes 390 from alkenimines and 4-methylbenzenesulfonyl isothiocyanate (...

Scheme 82: Synthesis of arylidene 2,4-diiminothietanes 393 starting from phosphonium ylides 391 and isothiocya...

Scheme 83: Synthesis of thietane-2-ylideneacetates 397 through a DABCO-catalyzed formal [2 + 2] cycloaddition ...

Scheme 84: Synthesis of 3-substituted thietanes 400 from (1-chloroalkyl)thiiranes 398.

Scheme 85: Synthesis of N-(thietane-3-yl)azaheterocycles 403 and 404 through reaction of chloromethylthiirane (...

Scheme 86: Synthesis of 3-sulfonamidothietanes 406 from sulfonamides and chloromethylthiirane (398a).

Scheme 87: Synthesis of N-(thietane-3-yl)isatins 408 from chloromethylthiirane (398a) and isatins 407.

Scheme 88: Synthesis of 3-(nitrophenyloxy)thietanes 410 from nitrophenols 409 and chloromethylthiirane (398a).

Scheme 89: Synthesis of N-aryl-N-(thietane-3-yl)cyanamides 412 from N-arylcyanamides 411 and chloromethylthiir...

Scheme 90: Synthesis of 1-(thietane-3-yl)pyrimidin-2,4(1H,3H)-diones 414 from chloromethylthiirane (398a) and ...

Scheme 91: Synthesis of 2,4-diiminothietanes 418 from 2-iminothiiranes 416 and isocyanoalkanes 415.

Scheme 92: Synthesis of 2-vinylthietanes 421 from thiiranes 419 and 3-chloroallyl lithium (420).

Scheme 93: Synthesis of thietanes from thiiranes 419 and trimethyloxosulfonium iodide 424.

Scheme 94: Mechanism for synthesis of thietanes 425 from thiiranes 419 and trimethyloxosulfonium iodide 424.

Scheme 95: Synthesis of functionalized thietanes from thiiranes and dimethylsulfonium acylmethylides.

Scheme 96: Mechanism for the rhodium-catalyzed synthesis of functionalized thietanes 429 from thiiranes 419 an...

Scheme 97: Synthesis of 3-iminothietanes 440 through thermal isomerization from 4,5-dihydro-1,3-oxazole-4-spir...

Scheme 98: Synthesis of thietanes 443 from 3-chloro-2-methylthiolane (441) through ring contraction.

Scheme 99: Synthesis of an optically active thietanose 447 from D-xylose involving a ring contraction.

Scheme 100: Synthesis of optically thietane 447 via the DAST-mediated ring contraction of 448.

Scheme 101: Synthesis of the optically thietane nucleoside 451 via the ring contraction of thiopentose in 450.

Scheme 102: Synthesis of spirothietane 456 from 3,3,5,5-tetramethylthiolane-2,4-dithione (452) and benzyne (453...

Scheme 103: Synthesis of thietanes 461 via photoisomerization of 2H,6H-thiin-3-ones 459.

Scheme 104: Phosphorodithioate-mediated synthesis of 1,4-diarylthietanes 465.

Scheme 105: Mechanism of the phosphorodithioate-mediated synthesis of 1,4-diarylthietanes 465.

Scheme 106: Phosphorodithioate-mediated synthesis of trisubstituted thietanes (±)-470.

Scheme 107: Mechanism on the phosphorodithioate-mediated synthesis of trisubstituted thietanes.

Scheme 108: Phosphorodithioate-mediated synthesis of thietanes (±)-475.

Scheme 109: Phosphorodithioate-mediated synthesis of 1,2-disubstituted thietanes from aldehydes 476 and acrylon...

Scheme 110: Phosphorodithioate-mediated synthesis of 1,2-disubstituted thietanes via a one-pot three-component ...

Scheme 111: Mechanism for the phosphorodithioate-mediated synthesis of 1,2-disubstituted thietanes via three-co...

Scheme 112: Phosphorodithioate-mediated synthesis of substituted 3-nitrothietanes.

Scheme 113: Mechanism on the phosphorodithioate-mediated synthesis of 1,2-disubstituted thietanes (±)-486.

Scheme 114: Asymmetric synthesis of (S)-2-phenylthietane (497).

Scheme 115: Asymmetric synthesis of optically active 2,4-diarylthietanes.

Scheme 116: Synthesis of 3-acetamidothietan-2-one 503 via the intramolecular thioesterification of 3-mercaptoal...

Scheme 117: Synthesis of 4-substituted thietan-2-one via the intramolecular thioesterification of 3-mercaptoalk...

Scheme 118: Synthesis of 4,4-disubstituted thietan-2-one 511 via the intramolecular thioesterification of the 3...

Scheme 119: Synthesis of a spirothietan-2-one 514 via the intramolecular thioesterification of 3-mercaptoalkano...

Scheme 120: Synthesis of thiatetrahydrolipstatin starting from (S)-(−)-epichlorohydrin ((S)-142a).

Scheme 121: Synthesis of 2-phenethyl-4-(propan-2-ylidene)thietane (520) from 5-bromo-6-methyl-1-phenylhept-5-en...

Scheme 122: Synthesis of 2-phenethyl-4-(propan-2-ylidene)thietane (520) directly from S-(5-bromo-6-methyl-1-phe...

Scheme 123: Synthesis of 2-alkylidenethietanes from S-(2-bromoalk-1-en-4-yl)thioacetates.

Scheme 124: Synthesis of 2-alkylidenethietanes from S-(2-bromo/chloroalk-1-en-4-yl)thiols.

Scheme 125: Synthesis of spirothietan-3-ol 548 from enone 545 and ammonium hydrosulfide.

Scheme 126: Asymmetric synthesis of the optically active thietanoside from cis-but-2-ene-1,4-diol (47).

Scheme 127: Synthesis of 2-alkylidenethietan-3-ols 557 via the fluoride-mediated cyclization of thioacylsilanes ...

Scheme 128: Synthesis of 2-iminothietanes via the reaction of propargylbenzene (558) and isothiocyanates 560 in...

Scheme 129: Synthesis of 2-benzylidenethietane 567 via the nickel complex-catalyzed electroreductive cyclizatio...

Scheme 130: Synthesis of 2-iminothietanes 569 via the photo-assisted electrocyclic reaction of N-monosubstitute...

Scheme 131: Synthesis of ethyl 3,4-diiminothietane-2-carboxylates from ethyl thioglycolate (570) and bis(imidoy...

Scheme 132: Synthesis of N-(thietan-3-yl)-α-oxoazaheterocycles from azaheterocyclethiones and chloromethyloxira...

Scheme 133: Synthesis of thietan-3-yl benzoate (590) via the nickel-catalyzed intramolecular reductive thiolati...

Scheme 134: Synthesis of 2,2-bis(trifluoromethyl)thietane from 3,3-bis(trifluoromethyl)-1,2-dithiolane.

Scheme 135: Synthesis of thietanes from enamines and sulfonyl chlorides.

Scheme 136: Synthesis of spirothietane 603 via the [2 + 3] cycloaddition of 2,2,4,4-tetramethylcyclobutane-1,3-...

Scheme 137: Synthesis of thietane (605) from 1-bromo-3-chloropropane and sulfur.
Oxime radicals: generation, properties and application in organic synthesis
- Igor B. Krylov,
- Stanislav A. Paveliev,
- Alexander S. Budnikov and
- Alexander O. Terent’ev
Beilstein J. Org. Chem. 2020, 16, 1234–1276, doi:10.3762/bjoc.16.107

Graphical Abstract

Figure 1: Imine-N-oxyl radicals (IV) discussed in the present review and other classes of N-oxyl radicals (I–...

Figure 2: The products of decomposition of iminoxyl radicals generated from oximes by oxidation with Ag2O.

Scheme 1: Generation of oxime radicals and study of the kinetics of their decay by photolysis of the solution...

Scheme 2: Synthesis of di-tert-butyliminoxyl radical and its decomposition products.

Scheme 3: The proposed reaction pathway of the decomposition of di-tert-butyliminoxyl radical (experimentally...

Scheme 4: Monomolecular decomposition of the tert-butyl(triethylmethyl)oxime radical.

Scheme 5: The synthesis and stability of the most stable dialkyl oxime radicals – di-tert-butyliminoxyl and d...

Scheme 6: The formation of iminoxyl radicals from β-diketones under the action of NO2.

Scheme 7: Synthesis of the diacetyliminoxyl radical.

Scheme 8: Examples of long-living oxime radicals with electron-withdrawing groups and the conditions for thei...

Figure 3: The electronic structure iminoxyl radicals and their geometry compared to the corresponding oximes.

Figure 4: Bond dissociation enthalpies (kcal/mol) of oximes and N,N-disubstituted hydroxylamines calculated o...

Scheme 9: Examples demonstrating the low reactivity of the di-tert-butyliminoxyl radical towards the substrat...

Scheme 10: The reactions of di-tert-butyliminoxyl radical with unsaturated hydrocarbons involving hydrogen ato...

Scheme 11: Possible mechanisms of reaction of di-tert-butyliminoxyl radical with alkenes.

Scheme 12: Products of the reaction between di-tert-butyliminoxyl radical and phenol derivatives.

Scheme 13: The reaction of di-tert-butyliminoxyl radical with amines.

Scheme 14: Reaction of di-tert-butyliminoxyl radicals with organolithium reagents.

Scheme 15: Cross-dehydrogenative C–O coupling of 1,3-dicarbonyl compounds with oximes under the action of mang...

Scheme 16: Cross-dehydrogenative C–O coupling of 1,3-dicarbonyl compounds with oximes under the action of Cu(BF...

Scheme 17: Oxidative C–O coupling of benzylmalononitrile (47) with 3-(hydroxyimino)pentane-2,4-dione (19).

Scheme 18: The proposed mechanism of the oxidative coupling of benzylmalononitrile (47) with diacetyl oxime (19...

Scheme 19: Oxidative C–O coupling of pyrazolones with oximes under the action of Fe(ClO4)3.

Scheme 20: The reaction of diacetyliminoxyl radical with pyrazolones.

Scheme 21: Oxidative C–O coupling of oximes with acetonitrile, ketones, and esters.

Scheme 22: Intramolecular cyclizations of oxime radicals to form substituted isoxazolines or cyclic nitrones.

Scheme 23: TEMPO-mediated oxidative cyclization of oximes with C–H bond cleavage.

Scheme 24: Proposed reaction mechanism of oxidative cyclization of oximes with C–H bond cleavage.

Scheme 25: Selectfluor/Bu4NI-mediated C–H oxidative cyclization of oximes.

Scheme 26: Oxidative cyclization of N-benzyl amidoximes to 1,2,4-oxadiazoles.

Scheme 27: The formation of quinazolinone 73a from 5-phenyl-4,5-dihydro-1,2,4-oxadiazole 74 under air.

Scheme 28: DDQ-mediated oxidative cyclization of thiohydroximic acids.

Scheme 29: Plausible mechanism of the oxidative cyclization of thiohydroximic acids.

Scheme 30: Silver-mediated oxidative cyclization of α-halogenated ketoximes and 1,3-dicarbonyl compounds.

Scheme 31: Possible pathway of one-pot oxidative cyclization of α-halogenated ketoximes and 1,3-dicarbonyl com...

Scheme 32: T(p-F)PPT-catalyzed oxidative cyclization of oximes with the formation of 1,2,4-oxadiazolines.

Scheme 33: Intramolecular cyclization of iminoxyl radicals involving multiple C=C and N=N bonds.

Scheme 34: Oxidative cyclization of β,γ- and γ,δ-unsaturated oximes employing the DEAD or TEMPO/DEAD system wi...

Scheme 35: Cobalt-catalyzed aerobic oxidative cyclization of β,γ-unsaturated oximes.

Scheme 36: Manganese-catalyzed aerobic oxidative cyclization of β,γ-unsaturated oximes.

Scheme 37: Visible light photocatalytic oxidative cyclization of β,γ-unsaturated oximes.

Scheme 38: TBAI/TBHP-mediated radical cascade cyclization of the β,γ-unsaturated oximes.

Scheme 39: TBAI/TBHP-mediated radical cascade cyclization of vinyl isocyanides with β,γ-unsaturated oximes.

Scheme 40: tert-Butylnitrite-mediated oxidative cyclization of unsaturated oximes with the introduction of an ...

Scheme 41: Transformation of unsaturated oxime to oxyiminomethylisoxazoline via the confirmed dimeric nitroso ...

Scheme 42: tert-Butylnitrite-mediated oxidative cyclization of unsaturated oximes with the introduction of a n...

Scheme 43: Synthesis of cyano-substituted oxazolines from unsaturated oximes using the TBN/[RuCl2(p-cymene)]2 ...

Scheme 44: Synthesis of trifluoromethylthiolated isoxazolines from unsaturated oximes.

Scheme 45: Copper-сatalyzed oxidative cyclization of β,γ-unsaturated oximes with the introduction of an azido ...

Scheme 46: TBHP-mediated oxidative cascade cyclization of β,γ-unsaturated oximes and unsaturated N-arylamides.

Scheme 47: Copper-сatalyzed oxidative cyclization of unsaturated oximes with the introduction of an amino grou...

Scheme 48: TEMPO-mediated oxidative cyclization of unsaturated oximes followed by elimination.

Scheme 49: Oxidative cyclization of β,γ-unsaturated oximes with the introduction of a trifluoromethyl group.

Scheme 50: Oxidative cyclization of unsaturated oximes with the introduction of a nitrile group.

Scheme 51: Oxidative cyclization of β,γ-unsaturated oximes to isoxazolines with the introduction of a nitrile ...

Scheme 52: Oxidative cyclization of β,γ-unsaturated oximes to isoxazolines with the introduction of a sulfonyl...

Scheme 53: Oxidative cyclization of β,γ- and γ,δ-unsaturated oximes to isoxazolines with the introduction of a...

Scheme 54: Oxidative cyclization of β,γ-unsaturated oximes to isoxazolines with the introduction of a thiocyan...

Scheme 55: PhI(OAc)2-mediated oxidative cyclization of oximes with C–S and C–Se bond formation.

Scheme 56: PhI(OAc)2-mediated oxidative cyclization of unsaturated oximes accompanied by alkoxylation.

Scheme 57: PhI(OAc)2-mediated cyclization of unsaturated oximes to methylisoxazolines.

Scheme 58: Oxidative cyclization-alkynylation of unsaturated oximes.

Scheme 59: TEMPO-mediated oxidative cyclization of C-glycoside ketoximes to C-glycosylmethylisoxazoles.

Scheme 60: Silver-сatalyzed oxidative cyclization of β,γ-unsaturated oximes with formation of fluoroalkyl isox...

Scheme 61: Oxidative cyclization of β,γ-unsaturated oximes with the formation of haloalkyl isoxazolines.

Scheme 62: Cyclization of β,γ-unsaturated oximes into haloalkyl isoxazolines under the action of the halogenat...

Scheme 63: Synthesis of haloalkyl isoxazoles and cyclic nitrones via oxidative cyclization and 1,2-halogen shi...

Scheme 64: Electrochemical oxidative cyclization of diaryl oximes.

Scheme 65: Copper-сatalyzed cyclization and dioxygenation oximes containing a triple C≡C bond.

Scheme 66: Photoredox-catalyzed sulfonylation of β,γ-unsaturated oximes by sulfonyl hydrazides.

Scheme 67: Oxidative cyclization of β,γ-unsaturated oximes with introduction of sulfonate group.

Scheme 68: Ultrasound-promoted oxidative cyclization of β,γ-unsaturated oximes.
Photocatalysis with organic dyes: facile access to reactive intermediates for synthesis
- Stephanie G. E. Amos,
- Marion Garreau,
- Luca Buzzetti and
- Jerome Waser
Beilstein J. Org. Chem. 2020, 16, 1163–1187, doi:10.3762/bjoc.16.103
- use of the strong oxidant Mes-Acr-Ph+ (OD3, E(PC+*/PC) ≈ 2 V) as organic photocatalyst leads to the oxidation/decarboxylation of the in situ-generated carboxylates (Eox ≈ 1.3 V). An organic disulfide cocatalyst, (PhS)2, activated by the reduced photocatalyst, was found to act as a co-base (PhS−) and a

Graphical Abstract

Figure 1: Selected examples of organic dyes. Mes-Acr+: 9-mesityl-10-methylacridinium, DCA: 9,10-dicyanoanthra...

Scheme 1: Activation modes in photocatalysis.

Scheme 2: Main strategies for the formation of C(sp3) radicals used in organophotocatalysis.

Scheme 3: Illustrative example for the photocatalytic oxidative generation of radicals from carboxylic acids:...

Scheme 4: Illustrative example for the photocatalytic reductive generation of C(sp3) radicals from redoxactiv...

Figure 2: Common substrates for the photocatalytic oxidative generation of C(sp3) radicals.

Scheme 5: Illustrative example for the photocatalytic oxidative generation of radicals from dihydropyridines ...

Scheme 6: Illustrative example for the photocatalytic oxidative generation of C(sp3) radicals from trifluorob...

Scheme 7: Illustrative example for the photocatalytic reductive generation of C(sp3) radicals from benzylic h...

Scheme 8: Illustrative example for the photocatalytic generation of C(sp3) radicals via direct HAT: the cross...

Scheme 9: Illustrative example for the photocatalytic generation of C(sp3) radicals via indirect HAT: the deu...

Scheme 10: Selected precursors for the generation of aryl radicals using organophotocatalysis.

Scheme 11: Illustrative example for the photocatalytic reductive generation of aryl radicals from aryl diazoni...

Scheme 12: Illustrative examples for the photocatalytic reductive generation of aryl radicals from haloarenes:...

Scheme 13: Illustrative example for the photocatalytic reductive generation of aryl radicals from aryl halides...

Scheme 14: Illustrative example for the photocatalytic reductive generation of aryl radicals from arylsulfonyl...

Scheme 15: Illustrative example for the reductive photocatalytic generation of aryl radicals from triaryl sulf...

Scheme 16: Main strategies towards acyl radicals used in organophotocatalysis.

Scheme 17: Illustrative example for the decarboxylative photocatalytic generation of acyl radicals from α-keto...

Scheme 18: Illustrative example for the oxidative photocatalytic generation of acyl radicals from acyl silanes...

Scheme 19: Illustrative example for the oxidative photocatalytic generation of carbamoyl radicals from 4-carba...

Scheme 20: Illustrative example of the photocatalytic HAT approach for the generation of acyl radicals from al...

Scheme 21: General reactivity of a) radical cations; b) radical anions; c) the main strategies towards aryl an...

Scheme 22: Illustrative example for the oxidative photocatalytic generation of alkene radical cations from alk...

Scheme 23: Illustrative example for the reductive photocatalytic generation of an alkene radical anion from al...

Figure 3: Structure of C–X radical anions and their neutral derivatives.

Scheme 24: Illustrative example for the photocatalytic reduction of imines and the generation of an α-amino C(...

Scheme 25: Illustrative example for the oxidative photocatalytic generation of aryl radical cations from arene...

Scheme 26: NCR classifications and generation.

Scheme 27: Illustrative example for the photocatalytic reductive generation of iminyl radicals from O-aryl oxi...

Scheme 28: Illustrative example for the photocatalytic oxidative generation of iminyl radicals from α-N-oxy ac...

Scheme 29: Illustrative example for the photocatalytic oxidative generation of iminyl radicals via an N–H bond...

Scheme 30: Illustrative example for the photocatalytic oxidative generation of amidyl radicals from Weinreb am...

Scheme 31: Illustrative example for the photocatalytic reductive generation of amidyl radicals from hydroxylam...

Scheme 32: Illustrative example for the photocatalytic reductive generation of amidyl radicals from N-aminopyr...

Scheme 33: Illustrative example for the photocatalytic oxidative generation of amidyl radicals from α-amido-ox...

Scheme 34: Illustrative example for the photocatalytic oxidative generation of aminium radicals: the N-aryltet...

Scheme 35: Illustrative example for the photocatalytic oxidative generation of nitrogen-centered radical catio...

Scheme 36: Illustrative example for the photocatalytic oxidative generation of nitrogen-centered radical catio...

Scheme 37: Illustrative example for the photocatalytic oxidative generation of hydrazonyl radical from hydrazo...

Scheme 38: Generation of O-radicals.

Scheme 39: Illustrative examples for the photocatalytic generation of O-radicals from N-alkoxypyridinium salts...

Scheme 40: Illustrative examples for the photocatalytic generation of O-radicals from alkyl hydroperoxides: th...

Scheme 41: Illustrative example for the oxidative photocatalytic generation of thiyl radicals from thiols: the...

Scheme 42: Main strategies and reagents for the generation of sulfonyl radicals used in organophotocatalysis.

Scheme 43: Illustrative example for the reductive photocatalytic generation of sulfonyl radicals from arylsulf...

Scheme 44: Illustrative example of a Cl atom abstraction strategy for the photocatalytic generation of sulfamo...

Scheme 45: Illustrative example for the oxidative photocatalytic generation of sulfonyl radicals from sulfinic...

Scheme 46: Illustrative example for the photocatalytic generation of electronically excited triplet states: th...

Scheme 47: Illustrative example for the photocatalytic generation of electronically excited triplet states: th...
Recent applications of porphyrins as photocatalysts in organic synthesis: batch and continuous flow approaches
- Rodrigo Costa e Silva,
- Luely Oliveira da Silva,
- Aloisio de Andrade Bartolomeu,
- Timothy John Brocksom and
- Kleber Thiago de Oliveira
Beilstein J. Org. Chem. 2020, 16, 917–955, doi:10.3762/bjoc.16.83
- HEH were used as H donors for both the benzyl and thiyl radicals, respectively. The HEH was needed to avoid the dimerization of the thiophenol to the disulfide (Scheme 11). Fu and co-workers have demonstrated the huge versatility of rhodium porphyrins for photocatalysis. In 2015, they showed that the

Graphical Abstract

Figure 1: Chemical structures of the porphyrinoids and their absorption spectra: in bold are highlighted the ...

Figure 2: Photophysical and photochemical processes (Por = porphyrin). Adapted from [12,18].

Figure 3: Main dual photocatalysts and their oxidative/reductive excited state potentials, including porphyri...

Scheme 1: Photoredox alkylation of aldehydes with diazo acetates using porphyrins and a Ru complex. aUsing a ...

Scheme 2: Proposed mechanism for the alkylation of aldehydes with diazo acetates in the presence of TPP.

Scheme 3: Arylation of heteroarenes with aryldiazonium salts using TPFPP as photocatalyst, and corresponding ...

Scheme 4: A) Scope with different aryldiazonium salts and enol acetates. B) Photocatalytic cycles and compari...

Scheme 5: Photoarylation of isopropenyl acetate A) Comparison between batch and continuous-flow approaches an...

Scheme 6: Dehalogenation induced by red light using thiaporphyrin (STPP).

Scheme 7: Applications of NiTPP as both photoreductant and photooxidant.

Scheme 8: Proposed mechanism for obtaining tetrahydroquinolines by reductive quenching.

Scheme 9: Selenylation and thiolation of anilines.

Scheme 10: NiTPP as photoredox catalyst in oxidative and reductive quenching, in comparison with other photoca...

Scheme 11: C–O bond cleavage of 1-phenylethanol using a cobalt porphyrin (CoTMPP) under visible light.

Scheme 12: Hydration of terminal alkynes by RhIII(TSPP) under visible light irradiation.

Scheme 13: Regioselective photocatalytic hydro-defluorination of perfluoroarenes by RhIII(TSPP).

Scheme 14: Formation of 2-methyl-2,3-dihydrobenzofuran by intramolecular hydro-functionalization of allylpheno...

Scheme 15: Photocatalytic oxidative hydroxylation of arylboronic acids using UNLPF-12 as heterogeneous photoca...

Scheme 16: Photocatalytic oxidative hydroxylation of arylboronic acids using MOF-525 as heterogeneous photocat...

Scheme 17: Preparation of the heterogeneous photocatalyst CNH.

Scheme 18: Photoinduced sulfonation of alkenes with sulfinic acid using CNH as photocatalyst.

Scheme 19: Sulfonic acid scope of the sulfonation reactions.

Scheme 20: Regioselective sulfonation reaction of arimistane.

Scheme 21: Synthesis of quinazolin-4-(3H)-ones.

Scheme 22: Selective photooxidation of aromatic benzyl alcohols to benzaldehydes using Pt/PCN-224(Zn).

Scheme 23: Photooxidation of benzaldehydes to benzoic acids using Pt or Pd porphyrins.

Scheme 24: Photocatalytic reduction of various nitroaromatics using a Ni-MOF.

Scheme 25: Photoinduced cycloadditions of CO2 with epoxides by MOF1.

Figure 4: Electronic configurations of the species of oxygen. Adapted from [66].

Scheme 26: TPP-photocatalyzed generation of 1O2 and its application in organic synthesis. Adapted from [67-69].

Scheme 27: Pericyclic reactions involving singlet oxygen and their mechanisms. Adapted from [67].

Scheme 28: First scaled up ascaridole preparation from α-terpinene.

Scheme 29: Antimalarial drug synthesis using an endoperoxidation approach.

Scheme 30: Photooxygenation of colchicine.

Scheme 31: Synthesis of (−)-pinocarvone from abundant (+)-α-pinene.

Scheme 32: Seeberger’s semi-synthesis of artemisinin.

Scheme 33: Synthesis of artemisinin using TPP and supercritical CO2.

Scheme 34: Synthesis of artemisinin using chlorophyll a.

Scheme 35: Quercitol stereoisomer preparation.

Scheme 36: Photocatalyzed preparation of naphthoquinones.

Scheme 37: Continuous endoperoxidation of conjugated dienes and subsequent rearrangements leading to oxidized ...

Scheme 38: The Opatz group total synthesis of (–)-oxycodone.

Scheme 39: Biomimetic syntheses of rhodonoids A, B, E, and F.

Scheme 40: α-Photooxygenation of chiral aldehydes.

Scheme 41: Asymmetric photooxidation of indanone β-keto esters by singlet oxygen using PTC as a chiral inducer...

Scheme 42: Asymmetric photooxidation of both β-keto esters and β-keto amides by singlet oxygen using PTC-2 as ...

Scheme 43: Bifunctional photo-organocatalyst used for the asymmetric oxidation of β-keto esters and β-keto ami...

Scheme 44: Mechanism of singlet oxygen oxidation of sulfides to sulfoxides.

Scheme 45: Controlled oxidation of sulfides to sulfoxides using protonated porphyrins as photocatalysts. aIsol...

Scheme 46: Photochemical oxidation of sulfides to sulfoxides using PdTPFPP as photocatalyst.

Scheme 47: Controlled oxidation of sulfides to sulfoxides using SnPor@PAF as a photosensitizer.

Scheme 48: Syntheses of 2D-PdPor-COF and 3D-Pd-COF.

Scheme 49: Photocatalytic oxidation of A) thioanisole to methyl phenyl sulfoxide and B) various aryl sulfides,...

Scheme 50: General mechanism for oxidation of amines to imines.

Scheme 51: Oxidation of secondary amines to imines.

Scheme 52: Oxidation of secondary amines using Pd-TPFPP as photocatalyst.

Scheme 53: Oxidative amine coupling using UNLPF-12 as heterogeneous photocatalyst.

Scheme 54: Synthesis of Por-COF-1 and Por-COF-2.

Scheme 55: Photocatalytic oxidation of amines to imines by Por-COF-2.

Scheme 56: Photocyanation of primary amines.

Scheme 57: Synthesis of ᴅ,ʟ-tert-leucine hydrochloride.

Scheme 58: Photocyanation of catharanthine and 16-O-acetylvindoline using TPP.

Scheme 59: Photochemical α-functionalization of N-aryltetrahydroisoquinolines using Pd-TPFPP as photocatalyst.

Scheme 60: Ugi-type reaction with 1,2,3,4-tetrahydroisoquinoline using molecular oxygen and TPP.

Scheme 61: Ugi-type reaction with dibenzylamines using molecular oxygen and TPP.

Scheme 62: Mannich-type reaction of tertiary amines using PdTPFPP as photocatalyst.

Scheme 63: Oxidative Mannich reaction using UNLPF-12 as heterogeneous photocatalyst.

Scheme 64: Transformation of amines to α-cyanoepoxides and the proposed mechanism.
One-pot synthesis of dicyclopenta-fused peropyrene via a fourfold alkyne annulation
- Ji Ma,
- Yubin Fu,
- Junzhi Liu and
- Xinliang Feng
Beilstein J. Org. Chem. 2020, 16, 791–797, doi:10.3762/bjoc.16.72
- 1 were obtained by slow evaporation from a carbon disulfide solution, allowing us to disclose the molecular structure by X-ray crystallography (Figure 2a). As shown in Figure 2a, the crystal structure of 1 clearly displayed a nonplanar conformation, resulting from steric repulsion between the phenyl

Graphical Abstract

Scheme 1: Chemical structures of dicyclopenta-fused pyrene derivatives i–iii, peropyrene and the dicyclopenta...

Scheme 2: Synthetic route towards compound 1. a) B2pin2, dtbpy, [Ir(OMe)cod]2, cyclohexane, 70 °C, 20 h, 67%;...

Figure 1: High-resolution MALDI-TOF mass spectrum of 1. Inset: isotopic distribution compared to mass spectru...

Figure 2: Single-crystal X-ray structure of 1. (a) Top view and (b) side view of the (P,P) isomer. c) Crystal...

Figure 3: (a) UV–vis absorption spectra of precursor 5 and 1 in CH2Cl2 solution (10−5 M). Inset: photograph o...

Figure 4: Molecular orbitals of peropyrene derivative 6 and the dicyclopenta-fused peropyrene 1.
The use of isoxazoline and isoxazole scaffolding in the design of novel thiourea and amide liquid-crystalline compounds
- Itamar L. Gonçalves,
- Rafaela R. da Rosa,
- Vera L. Eifler-Lima and
- Aloir A. Merlo
Beilstein J. Org. Chem. 2020, 16, 175–184, doi:10.3762/bjoc.16.20
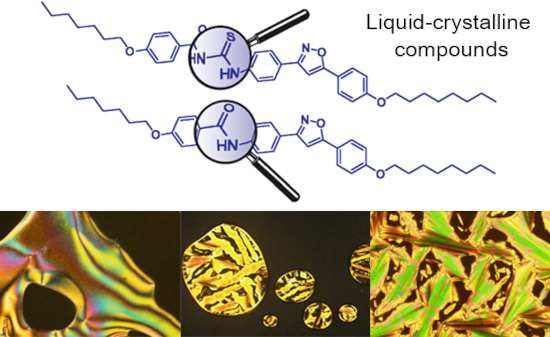
- thioureas is the reaction of carbon disulfide with either one or two different amines [9]. Due to their self-assembly and self-organization through intermolecular hydrogen bonding, thioureas display interesting technological applications to this group of molecules, one of which explores its application in

Graphical Abstract

Scheme 1: Amines 3, 4, 8, 9, 12 and 13 installed on 5-membered isoxazoline and isoxazole rings.

Scheme 2: Synthesis of acylisoxazolinylthioureas 17a–c and acylisoxazolylthioureas 18a–c. (i) SOCl2, reflux, ...

Scheme 3: Synthesis of amides. Part A: (i) SOCl2, reflux; (ii) KOCN, acetone, reflux; (iii) amines 3, 4, 8 an...

Figure 1: Optical textures observed on POM for thioureas 17a (a), 17b (b), 18a (c), 18b (d) and 18c (e,f). Al...

Figure 2: Optical textures observed on POM of amide 19. (a) Fan-shaped focal conic texture of the SmA mesopha...

Figure 3: DSC curves for the thiourea 17a (A), amide 19 (B) and 20 (C) upon the first heating and cooling cur...

Figure 4: TGA analysis for thiourea 18c; and amides 20 and 22.
Synthesis, liquid crystalline behaviour and structure–property relationships of 1,3-bis(5-substituted-1,3,4-oxadiazol-2-yl)benzenes
- Afef Mabrouki,
- Malek Fouzai,
- Armand Soldera,
- Abdelkader Kriaa and
- Ahmed Hedhli
Beilstein J. Org. Chem. 2020, 16, 149–158, doi:10.3762/bjoc.16.17

- acids in the presence of phosphorus oxychloride according to standard methods [29], oxadiazole derivatives 2 were obtained (Scheme 1). On the other hand, we converted compound 1 into sulfanyloxadiazole derivatives 4 by treatment with carbon disulfide and subsequent alkylation of the obtained

Graphical Abstract

Scheme 1: Synthesis of oxadiazole derivatives 2 and 4.

Scheme 2: Tautomeric equilibrium of compound 3.

Figure 1: DSC thermograms of fluorinated compounds 2b, 4a and 4b recorded at 5 °C/mn at heating (down traces)...

Figure 2: Optical texture (×10) of liquid crystal phase for fluorinated compounds, (a): SmA phase observed in...

Figure 3: Typical diffractogram observed for compound 2b at 398 K.

Figure 4: Typical diffractogram observed for compound 4a at 411 K.

Figure 5: Conformer of lowest energy of compounds: 4c, conformation A, (a) front view, (a’) top view, (a”) si...

Figure 6: Vector of dipole moment of compounds 4c, 4b and 2b.

Figure 7: Plot of molecular dipole moments. Orange, fluorocarbon compounds; blue, hydrocarbon compounds; gree...
Potent hemithioindigo-based antimitotics photocontrol the microtubule cytoskeleton in cellulo
- Alexander Sailer,
- Franziska Ermer,
- Yvonne Kraus,
- Rebekkah Bingham,
- Ferdinand H. Lutter,
- Julia Ahlfeld and
- Oliver Thorn-Seshold
Beilstein J. Org. Chem. 2020, 16, 125–134, doi:10.3762/bjoc.16.14

- , lithium diisopropylamide (LDA)-mediated cyclisation of 2-(methylthio)benzamides, which were obtained by directed ortho-metalation of the respective benzamides followed by quenching with dimethyl disulfide [27] (Supporting Information File 1, Scheme S1). In general, we found the LDA-mediated cyclisation

Graphical Abstract

Figure 1: a) The potent tubulin inhibitor colchicine as a lead scaffold led to the development of the HOTub g...

Figure 2: Chemical structures of HITubs. Key variations with respect to HITub-4 are highlighted in dashed box...

Figure 3: Photocharacterisation of HITub-4. a) Photochemical and thermal isomerisation. b) UV–vis spectra aft...

Figure 4: a) Resazurin reduction assay for HITub-4 and nocodazole in HeLa cells (n = 3), demonstrating the di...

Figure 5: Confocal microscopy images of immunofluorescently labelled MT networks after treatment with HITubs ...

Figure 6: Cell cycle analysis of HITub-4-treated cells. a) and b) (Z)-HITub-4 caused significant G2/M arrest ...
Synthesis of novel sulfide-based cyclic peptidomimetic analogues to solonamides
- José Brango-Vanegas,
- Luan A. Martinho,
- Lucinda J. Bessa,
- Andreanne G. Vasconcelos,
- Alexandra Plácido,
- Alex L. Pereira,
- José R. S. A. Leite and
- Angelo H. L. Machado
Beilstein J. Org. Chem. 2019, 15, 2544–2551, doi:10.3762/bjoc.15.247
- cocktail afforded the resin-free linear peptidomimetics 8. The SN2’ macrocyclization step was performed immediately after the cleavage procedure to avoid the oxidative disulfide dimerization observed when preparative HPLC purification of the resin-free linear peptidomimetics 8 was tried. Thus, a 1 mM

Graphical Abstract

Figure 1: Chemical structure of solonamides and autoinducing peptides (AIP).

Scheme 1: Macrocyclization strategy based on SN2’.

Scheme 2: Chemical synthesis of the MBH adducts 2 and their carboxylic acids 3.

Scheme 3: Chemical synthesis of the linear peptidomimetics 8.

Scheme 4: Macrocyclization strategy based on SN2’ reaction to affords the solonamide analogues 9 and their ov...

Figure 2: Effect of compounds 9e and 9g at three concentrations on the hemolysis production by S. aureus ATCC...

Figure 3: Inhibition of hemolysis (%) on human red blood cells caused by S. aureus ATCC 25923 after being in ...

Figure 4: Cell viability of human fibroblast exposed to compounds 9e (A) and 9g (B) for 24 and 48 hours. The ...
Targeted photoswitchable imaging of intracellular glutathione by a photochromic glycosheet sensor
- Xianzhi Chai,
- Hai-Hao Han,
- Yi Zang,
- Jia Li,
- Xiao-Peng He,
- Junji Zhang and
- He Tian
Beilstein J. Org. Chem. 2019, 15, 2380–2389, doi:10.3762/bjoc.15.230

- high intracellular concentration of GSH is another feature for HepG2 cells. Therefore, amounts of work on HepG2 cell imaging have targeted GSH as the characteristic biomarker [40][41]. Strategies like reduction of disulfide [42][43][44] and Michael addition [45][46][47] have been utilized to design

Graphical Abstract

Scheme 1: The structure (A) of reporter Glyco-DTE and working principle (B) of photochromic glycosheet Glyco-...

Scheme 2: Synthetic route to dithienylethene fluorescence reporters Glyco-DTE and 8o. VcNa: sodium ascorbate.

Figure 1: Absorption spectral changes (A), absorption fatigue resistance (B), emission spectral changes (C) a...

Figure 2: (A) The absorbance spectrum and (B) high resolution TEM image of 2D MnO2 nanosheets (1 × 10−5 g/mL)...

Figure 3: (A) Emission spectral changes of reporter Glyco-DTE (1 × 10−5 mol/L in PBS buffer, 0.25‰ Triton X-1...

Figure 4: (A) Fluorescence imaging of HepG2 cells and HeLa cells after incubated with reporters Glyco-DTE (20...

Figure 5: (A) Fluorescence imaging of HepG2 cells and HeLa cells after incubated with Glyco-DTE@MnO2 photochr...
Recent advances in transition-metal-catalyzed incorporation of fluorine-containing groups
- Xiaowei Li,
- Xiaolin Shi,
- Xiangqian Li and
- Dayong Shi
Beilstein J. Org. Chem. 2019, 15, 2213–2270, doi:10.3762/bjoc.15.218

Graphical Abstract

Scheme 1: The main three strategies of fluorination: nucleophilic, electrophilic and radical fluorination.

Scheme 2: Doyle’s Pd-catalyzed fluorination of allylic chlorides.

Scheme 3: Allylic fluorination of 2- and 3-substituted propenyl esters.

Scheme 4: Regioselective allylic fluorination of cinnamyl phosphorothioate esters.

Scheme 5: Palladium-catalyzed aliphatic C–H fluorination reported by Doyle.

Scheme 6: Pd-catalyzed enantioselective fluorination of α-ketoesters followed by stereoselective reduction to...

Scheme 7: Pd-catalyzed C(sp3)–H fluorination of oxindoles.

Scheme 8: C–H fluorination of 8-methylquinoline derivatives with F− reagents.

Scheme 9: Fluorination of α-cyano acetates reported by van Leeuwen.

Scheme 10: The catalytic enantioselective electrophilic C–H fluorination of α-chloro-β-keto phosphonates.

Scheme 11: Fluorination of unactivated C(sp3)–H bonds directed by the bidentate PIP auxiliary.

Scheme 12: Fluorination of C(sp3)–H bonds at the β-position of carboxylic acids.

Scheme 13: Enantioselective benzylic C–H fluorination with a chiral transient directing group.

Scheme 14: Microwave-heated Pd-catalyzed fluorination of aryl alcohols.

Scheme 15: Fluorination of aryl potassium trifluoroborates.

Scheme 16: C(sp2)–F bond formation using precatalyst [L·Pd]2(cod).

Scheme 17: Pd-catalyzed fluorination of (hetero)aryl triflates and bromides.

Scheme 18: The Pd-catalyzed C–H fluorination of arenes with Selectfluor/NFSI.

Scheme 19: Pd(II)-catalyzed ortho-monofluorination protocol for benzoic acids.

Scheme 20: Pd-catalyzed C(sp2)–H bond fluorination of 2-arylbenzothiazoles.

Scheme 21: Nitrate-promoted fluorination of aromatic and olefinic C(sp2)–H bonds and proposed mechanism.

Scheme 22: Fluorination of oxalyl amide-protected benzylamine derivatives.

Scheme 23: C–H fluorination of benzaldehydes with orthanilic acids as transient directing group.

Scheme 24: Pd(II)-catalyzed aryl C–H fluorination with various directing groups.

Scheme 25: Cu-catalyzed aliphatic, allylic, and benzylic fluorination.

Scheme 26: Cu-catalyzed SN2 fluorination of primary and secondary alkyl bromides.

Scheme 27: Copper-catalyzed fluorination of alkyl triflates.

Scheme 28: Cu-catalyzed fluorination of allylic bromides and chlorides.

Scheme 29: Synthetic strategy for the fluorination of active methylene compounds.

Scheme 30: Fluorination of β-ketoesters using a tartrate-derived bidentate bisoxazoline-Cu(II) complex.

Scheme 31: Highly enantioselective fluorination of β-ketoesters and N-Boc-oxindoles.

Scheme 32: Amide group-assisted site-selective fluorination of α-bromocarbonyl compounds.

Scheme 33: Cu-mediated aryl fluorination reported by Sanford [77].

Scheme 34: Mono- or difluorination reactions of benzoic acid derivatives.

Scheme 35: Cu-catalyzed fluorination of diaryliodonium salts with KF.

Scheme 36: Copper(I)-catalyzed cross-coupling of 2-pyridylaryl bromides.

Scheme 37: AgNO3-catalyzed decarboxylative fluorination of aliphatic carboxylic acids.

Scheme 38: The Mn-catalyzed aliphatic and benzylic C–H fluorination.

Scheme 39: Iron(II)-promoted C–H fluorination of benzylic substrates.

Scheme 40: Ag-catalyzed fluorodecarboxylation of carboxylic acids.

Scheme 41: Vanadium-catalyzed C(sp3)–H fluorination.

Scheme 42: AgNO3-catalyzed radical deboronofluorination of alkylboronates and boronic acids.

Scheme 43: Selective heterobenzylic C–H fluorination with Selectfluor reported by Van Humbeck.

Scheme 44: Fe(II)-catalyzed site-selective fluorination guided by an alkoxyl radical.

Scheme 45: Fluorination of allylic trichloroacetimidates reported by Nguyen et al.

Scheme 46: Iridium-catalyzed fluorination of allylic carbonates with TBAF(t-BuOH)4.

Scheme 47: Iridium-catalyzed asymmetric fluorination of allylic trichloroacetimidates.

Scheme 48: Cobalt-catalyzed α-fluorination of β-ketoesters.

Scheme 49: Nickel-catalyzed α-fluorination of various α-chloro-β-ketoesters.

Scheme 50: Ni(II)-catalyzed enantioselective fluorination of oxindoles and β-ketoesters.

Scheme 51: Scandium(III)-catalyzed asymmetric C–H fluorination of unprotected 3-substituted oxindoles.

Scheme 52: Iron-catalyzed directed C–H fluorination.

Scheme 53: Electrophilic silver-catalyzed Ar–F bond-forming reaction from arylstannanes.

Figure 1: Nucleophilic, electrophilic and radical CF3 sources.

Scheme 54: Cu(I)-catalyzed allylic trifluoromethylation of unactivated terminal olefins.

Scheme 55: Direct copper-catalyzed trifluoromethylation of allylsilanes.

Scheme 56: Cupper-catalyzed enantioselective trifluoromethylation of five and six-membered ring β-ketoesters.

Scheme 57: Cu-catalyzed highly stereoselective trifluoromethylation of secondary propargyl sulfonates.

Scheme 58: Remote C(sp3)–H trifluoromethylation of carboxamides and sulfonamides.

Scheme 59: Trifluoromethylation of allylsilanes with photoredox catalysis.

Scheme 60: Ag-catalyzed decarboxylative trifluoromethylation of aliphatic carboxylic acids in aqueous CH3CN.

Scheme 61: Decarboxylative trifluoromethylation of aliphatic carboxylic acids via combined photoredox and copp...

Scheme 62: Palladium-catalyzed Ar–CF3 bond-forming reaction.

Scheme 63: Palladium-catalyzed trifluoromethylation of arenes with diverse heterocyclic directing groups.

Scheme 64: Pd-catalyzed trifluoromethylation of indoles as reported by Liu.

Scheme 65: Pd-catalyzed trifluoromethylation of vinyl triflates and vinyl nonaflates.

Scheme 66: Pd(II)-catalyzed ortho-trifluoromethylation of aromatic C–H bonds.

Scheme 67: Visible-light-induced Pd(OAc)2-catalyzed ortho-trifluoromethylation of acetanilides with CF3SO2Na.

Scheme 68: CuI-catalyzed trifluoromethylation of aryl- and alkenylboronic acids.

Scheme 69: Cu-catalyzed trifluoromethylation of aryl- and vinylboronic acids.

Scheme 70: Copper-catalyzed trifluoromethylation of α,β-unsaturated carboxylic acids.

Scheme 71: Formation of C(sp2)–CF3 bond catalyzed by copper(I) complex.

Scheme 72: Loh’s Cu(I)-catalyzed trifluoromethylation of enamides and electron-deficient alkenes.

Scheme 73: Copper and iron-catalyzed decarboxylative tri- and difluoromethylation.

Scheme 74: Cu-catalyzed trifluoromethylation of hydrazones developed by Bouyssi.

Scheme 75: Cu(I)-catalyzed trifluoromethylation of terminal alkenes.

Scheme 76: Cu/Ag-catalyzed decarboxylative trifluoromethylation of cinnamic acids.

Scheme 77: Copper-catalyzed direct alkenyl C–H trifluoromethylation.

Scheme 78: Copper(I/II)-catalyzed direct trifluoromethylation of styrene derivatives.

Scheme 79: Regioselective trifluoromethylation of pivalamido arenes and heteroarenes.

Scheme 80: Synthesis of trifluoromethylquinones in the presence of copper(I).

Scheme 81: Oxidative trifluoromethylation of imidazoheterocycles in ionic liquid/water.

Scheme 82: A mild and fast continuous-flow trifluoromethylation of coumarins using a CuI/CF3SO2Na/TBHP system.

Scheme 83: Copper-catalyzed oxidative trifluoromethylation of various 8-aminoquinolines.

Scheme 84: PA-directed copper-catalyzed trifluoromethylation of anilines.

Scheme 85: Trifluoromethylation of potassium vinyltrifluoroborates catalyzed by Fe(II).

Scheme 86: Alkenyl trifluoromethylation catalyzed by Ru(phen)3Cl2 as photocatalyst.

Scheme 87: Ru-catalyzed trifluoromethylation of alkenes by Akita’s group.

Scheme 88: Ir-catalyzed Cvinyl–CF3 bond formation of α,β-unsaturated carboxylic acids.

Scheme 89: Ag(I)-catalyzed denitrative trifluoromethylation of β-nitrostyrenes.

Scheme 90: Photocatalyzed direct trifluoromethylation of aryl and heteroaryl C–H bonds.

Scheme 91: Rhenium (MTO)-catalyzed direct trifluoromethylation of aromatic substrates.

Scheme 92: Trifluoromethylation of unprotected anilines under [Ir(ppy)3] catalyst.

Scheme 93: Oxidative trifluoromethylation of imidazopyridines and imidazoheterocycles.

Scheme 94: Ruthenium-catalyzed trifluoromethylation of (hetero)arenes with trifluoroacetic anhydride.

Scheme 95: Phosphovanadomolybdic acid-catalyzed direct C–H trifluoromethylation.

Scheme 96: Picolinamide-assisted ortho-trifluoromethylation of arylamines.

Scheme 97: A nickel-catalyzed C–H trifluoromethylation of free anilines.

Scheme 98: Cu-mediated trifluoromethylation of terminal alkynes reported by Qing.

Scheme 99: Huang’s C(sp)–H trifluoromethylation using Togni’s reagent.

Scheme 100: Cu-catalyzed methods for trifluoromethylation with Umemoto’s reagent.

Scheme 101: The synthesis of alkynyl-CF3 compounds in the presence of fac-[Ir(ppy)3] under visible-light irradi...

Scheme 102: Pd-catalyzed Heck reaction reported by Reutrakul.

Scheme 103: Difluoromethylation of enamides and ene-carbamates.

Scheme 104: Difluoromethylation of α,β-unsaturated carboxylic acids.

Scheme 105: Copper-catalyzed direct C(sp2)–H difluoroacetylation reported by Pannecoucke and co-workers.

Scheme 106: Difluoroalkylation of aldehyde-derived hydrazones with functionalized difluoromethyl bromides.

Scheme 107: Photoredox-catalyzed C–H difluoroalkylation of aldehyde-derived hydrazones.

Scheme 108: Synergistic ruthenium(II)-catalyzed C–H difluoromethylation reported by Ackermann.

Scheme 109: Visible-light photocatalytic decarboxylation of α,β-unsaturated carboxylic acids.

Scheme 110: Synthesis of difluorinated ketones via S-alkyl dithiocarbamates obtained from acyl chlorides and po...

Scheme 111: Synthesis of aryl and heteroaryl difluoromethylated phosphonates.

Scheme 112: Difluoroalkylation of secondary propargyl sulfonates using Cu as the catalyst.

Scheme 113: Ru(II)-mediated para-selective difluoromethylation of anilides and their derivatives.

Scheme 114: Bulky diamine ligand promoted cross-coupling of difluoroalkyl bromides.

Scheme 115: Copper-catalyzed C3–H difluoroacetylation of quinoxalinones.

Scheme 116: Copper(I) chloride-catalyzed trifluoromethylthiolation of enamines, indoles and β-ketoesters.

Scheme 117: Copper-boxmi-catalyzed asymmetric trifluoromethylthiolation of β-ketoesters.

Scheme 118: Direct Cu-catalyzed trifluoromethylthiolation of boronic acids and alkynes.

Scheme 119: Cu-catalyzed synthesis of α-trifluoromethylthio-substituted ketones.

Scheme 120: Trifluoromethylthiolation reactions promoted by diazotriflone and copper.

Scheme 121: Halide activation of N-(trifluoromethylthio)phthalimide.

Scheme 122: The visible light-promoted trifluoromethylthiolation reported by Glorius.

Scheme 123: Synthesis of α-trifluoromethylthioesters via Goossen’s approach.

Scheme 124: Photoinduced trifluoromethylthiolation of diazonium salts.

Scheme 125: Ag-mediated trifluoromethoxylation of aryl stannanes and arylboronic acids.

Scheme 126: Catalytic (hetero)aryl C–H trifluoromethoxylation under visible light.

Scheme 127: Photoinduced C–H-bond trifluromethoxylation of (hetero)arenes.
A review of the total syntheses of triptolide
- Xiang Zhang,
- Zaozao Xiao and
- Hongtao Xu
Beilstein J. Org. Chem. 2019, 15, 1984–1995, doi:10.3762/bjoc.15.194
- new methodologies of butenolide formation. The first butenolide formation started with the reaction of ketone 68 with carbon disulfide (CS2) and iodomethane (MeI) to give the ketene dithioacetal intermediate 69, which was subjected to a Corey–Chaykovsky epoxidation, followed by acid hydrolysis to give

Graphical Abstract

Figure 1: Structures of triptolide (1), triptonide (2), tripdiolide (3), 16-hydroxytriptolide (4), triptrioli...

Figure 2: Syntheses of triptolide.

Scheme 1: Berchtold’s synthesis of triptolide.

Scheme 2: Li’s formal synthesis of triptolide.

Scheme 3: van Tamelen’s asymmetric synthesis of triptonide and triptolide.

Scheme 4: Van Tamelen’s (method II) formal synthesis of triptolide.

Scheme 5: Sherburn’s formal synthesis of triptolide.

Scheme 6: van Tamelen’s biogenetic type total synthesis of triptolide.

Scheme 7: Yang’s total synthesis of triptolide.

Scheme 8: Key intermediates or transformations of routes J–N.






