Search results
Search for "charge transfer" in Full Text gives 266 result(s) in Beilstein Journal of Organic Chemistry. Showing first 200.
Recent developments in photoredox-catalyzed remote ortho and para C–H bond functionalizations
Beilstein J. Org. Chem. 2020, 16, 248–280, doi:10.3762/bjoc.16.26

- seen in Figure 8, the mechanism of the reaction commences with the deprotonation of the biphenyl carboxylic acid 36, followed by the reaction of 38 with dimethyl dicarbonate (DMDC) to generate compound 39. On the other hand, the photocatalyst is excited by metal–ligand charge transfer, which produces
Synthesis, liquid crystalline behaviour and structure–property relationships of 1,3-bis(5-substituted-1,3,4-oxadiazol-2-yl)benzenes
Beilstein J. Org. Chem. 2020, 16, 149–158, doi:10.3762/bjoc.16.17

- electron D–A concept may be regarded as Lewis base–Lewis acid type or charge-transfer. Based upon the above considerations, we could attribute the close proximity of fluorinated chains in conformation B to a throw space electron D–A intramolecular interaction between the perfluoroalkyl chains (electron
Reversible photoswitching of the DNA-binding properties of styrylquinolizinium derivatives through photochromic [2 + 2] cycloaddition and cycloreversion
Beilstein J. Org. Chem. 2020, 16, 111–124, doi:10.3762/bjoc.16.13
- , the strong donor–acceptor system in 3a led to an intramolecular charge-transfer (ICT) state that did not lead to a subsequent photoreaction [77]. In contrast, the absorption bands of the substrates 3b–d decreased relatively fast upon irradiation, but the maxima did not disappear completely (Figure 5B
Synthesis and optoelectronic properties of benzoquinone-based donor–acceptor compounds
Beilstein J. Org. Chem. 2019, 15, 2914–2921, doi:10.3762/bjoc.15.285
- benzoquinone (BQ)-based charge-transfer (CT) derivatives in good yields. The optoelectronic properties of these compounds were explored both theoretically and experimentally and correlations to their structures were identified as a function of the nature and position of the donor group (meta and para) attached
- arylboronic acids are readily accessible, we envisaged that our Pd-catalyzed direct C–H functionalization method would be ideal for attempting a facile and direct route to a series of novel substituted benzoquinone-based charge-transfer derivatives, with the aim of exploring their electroluminescent (EL
- is difficult to realize highly efficient TADF emitters, which is especially the case for red emitters where nonradiative rates are high due to the well-known energy gap law [28]. While anthraquinone-based charge-transfer compounds have recently been shown to exhibit TADF that then translated to their
Synthesis of aryl-substituted thieno[3,2-b]thiophene derivatives and their use for N,S-heterotetracene construction
Beilstein J. Org. Chem. 2019, 15, 2678–2683, doi:10.3762/bjoc.15.261
- used to architect various ring-fused S-heteroacenes, which have been studied extensively [8][9][10] due to their better characteristics compared to heteroatom-free acenes. For instance, S-heteroacenes have a better oxidation stability due to lower-lying HOMO levels, as well as more efficient charge
- transfer because of their tendency to π-stack, with non-bonded sulfur–sulfur interactions in the solid state, which results in large intermolecular orbital coupling of HOMOs and, as a consequence, enhanced carrier transport properties [10]. However, there is a problem of poor solubility of a number of S
Plasma membrane imaging with a fluorescent benzothiadiazole derivative
Beilstein J. Org. Chem. 2019, 15, 2644–2654, doi:10.3762/bjoc.15.257

- agreement with the experimental data and helped to understand the stabilizing intramolecular charge-transfer process from the first excited state. The new fluorescent derivative could be applied as selective bioprobe in several cell lines and displayed plasma-membrane affinity during the imaging experiments
- noted in all tested solvents (134–173 nm), thus pointing to efficient stabilizations through intramolecular charge-transfer (ICT) processes from the excited states. The largest Stokes shift was noted in the aqueous solution, indicating the dye’s stability in this solvent. The solvatochromic analyses of
Arylisoquinoline-derived organoboron dyes with a triaryl skeleton show dual fluorescence
Beilstein J. Org. Chem. 2019, 15, 2612–2622, doi:10.3762/bjoc.15.254

- , boron with sp2 hybridization, such as in triarylboranes, offers the possibility to modulate fluorescence properties by the addition of Lewis bases (e.g., fluoride ions [27][28][29][30][31]) or by exploring the electron-accepting properties of the boron, including charge-transfer and photoinduced
- these dyes such as intramolecular charge-transfer processes and tunable red-shifted emission bands. Generally, the so far investigated borylated arylisoquinoline dyes show principally fluorescence quenching (on-off switching) on the formation of the corresponding fluoroboronate complexes [37]. Herein
- situated in between. These trends support that for the herein investigated dyes intramolecular charge-transfer (ICT) phenomena might play a role in the observation of the LW emission features. According to our previous observations the electron-acceptor moiety is likely constituted by the isoquinolinyl
Probing of local polarity in poly(methyl methacrylate) with the charge transfer transition in Nile red
Beilstein J. Org. Chem. 2019, 15, 2552–2562, doi:10.3762/bjoc.15.248

- films on a glass support was probed by the energy of the charge transfer transition in the oxazine dye Nile red (NR) at 25 °C. The absorption and fluorescence spectra of NR were observed to shift to the red with increasing solvent polarity, because of the intramolecular charge transfer character of the
- NR molecules in the ground state and slow sub-glass transition (Tg) relaxations in PMMA. Keywords: charge transfer; dipole moment; fluorescence; inhomogeneous broadening; oxazine dye; polarity probe; polymer permittivity; Introduction The chain and segment mobility as well as the permittivity of
- the phenomenon is still far from accurate. Fortunately, a contribution of solute to the large amplitude motion of the diethylamino group (twisting) in intramolecular charge transfer excited state of NR, postulated in references [21][24][25] was later associated with an artefact [26]. However, the
Experimental and computational electrochemistry of quinazolinespirohexadienone molecular switches – differential electrochromic vs photochromic behavior
Beilstein J. Org. Chem. 2019, 15, 2473–2485, doi:10.3762/bjoc.15.240
- focused on the preparation and investigation of electron-deficient analogs of the perimidinespirohexadienone (PSHD) family of photochromic molecular switches for potential application as "photochromic photooxidants" for gating sensitivity to photoinduced charge transfer. We previously reported the
- PSHD system was of interest to us as a potential photochromic photooxidant that would add an additional level of gating to photoinduced charge transfer (PICT) initiated processes (Figure 1). PSHDs were promising for this, as their photochromic reversion of LW back to SW proceeds purely thermally
- , and thus it makes sense that any thermal or solvatochromic LW is the eLW isomer. Conclusion While moving from PSHD (7) to QSHD (3) increased ΔEored between SW and pLW isomers, and therefore capacity to gate photoinduced charge transfer, by about 130 mV, the excited state reduction potential E*red of
Ultrafast processes triggered by one- and two-photon excitation of a photochromic and luminescent hydrazone
Beilstein J. Org. Chem. 2019, 15, 2438–2446, doi:10.3762/bjoc.15.236

- isomerization occurs on a fast <1 ps timescale in both toluene and acetonitrile, while the excited state lifetime of the Z-form depends on solvent polarity, suggesting a partial charge transfer nature of its low lying excited state. Time-resolved luminescence measurements evidence the presence of a main
- solvent polarity. On the contrary, the excited state lifetime of the Z-isomer depends on the solvent properties and is especially short in MeOH, suggesting that the excited state of the molecule could have a partial charge transfer nature. Finally, transient absorption spectroscopy was employed as an
- further decreases in methanol. As shown in Supporting Information File 1 (Figure S3) the bleaching recovery of the not isomerized population fraction follows the same kinetics. The observed solvent dependence of the ESA decay could indicate that the excited state of the Z-form has a partial charge
Photochromic diarylethene ligands featuring 2-(imidazol-2-yl)pyridine coordination site and their iron(II) complexes
Beilstein J. Org. Chem. 2019, 15, 2428–2437, doi:10.3762/bjoc.15.235
- complex 8 shows a single major absorption band in the low energy UV region at 346 nm (ε = 6.22 × 104 M−1·cm−1), which appears bathochromically shifted compared to the free ligand. No charge transfer (CT) bands are visible in the spectrum. The low intensity broad shoulder, which spans almost the entire
Small anion-assisted electrochemical potential splitting in a new series of bistriarylamine derivatives: organic mixed valency across a urea bridge and zwitterionization
Beilstein J. Org. Chem. 2019, 15, 2277–2286, doi:10.3762/bjoc.15.220

- intervalence charge transfer characteristics of the zwitterionic MV state. Keywords: anion binding; electrochemistry; hydrogen bonding; triarylamine; urea; zwitterionic mixed valency; Introduction Mixed-valence (MV) compounds have received increasing attention from the viewpoint of fundamental research on
- on evaluating the intervalence charge transfer (IVCT) transition near-infrared (NIR) absorption from NAr3 to NAr3•+ units [1][2][3][4][5]. The IVCT absorptions of MV compounds are generally more pronounced in organic species [19][20] than in their inorganic counterparts. The strong IVCT
1,2,3-Triazolium macrocycles in supramolecular chemistry
Beilstein J. Org. Chem. 2019, 15, 2142–2155, doi:10.3762/bjoc.15.211
- helped to increase the ability of complexation of 12 with iodide [56]. 2.7. Functional molecular crystal and materials Combining anion–arene interactions and controlling the electron-transfer or charge-transfer process concerning an anionic guest by using a cyclophane is uncommon [57] but can be realized
- between anions and the NDI motif [59]. NDI was chosen due to its excellent photochemical and electrochemical properties. In addition, the electron deficiency and aromatic nature of NDI is important for anion–π interactions [60] to control the charge-transfer properties. It also has been observed that in
- the solid state the movement of the molecules is restricted because of charge-transfer complexes between NDI and Cl−, Br− or I−. Because macrocycle 13 precipitated in less polar organic solvents upon addition of halides, the binding affinities were studied in DMSO in which the binding constants for Cl
Synthesis and properties of sulfur-functionalized triarylmethylium, acridinium and triangulenium dyes
Beilstein J. Org. Chem. 2019, 15, 2133–2141, doi:10.3762/bjoc.15.210
- rhodamines [43][44]. The weak tails on the red side of these bands are tentatively assigned to internal charge-transfer transitions from the perpendicularly [19][42] arranged ethylthio(dimethoxy)phenyl group to the xanthenium/acridinium systems polarized along the y-axes (Figure 4, inset). This
An overview of the cycloaddition chemistry of fulvenes and emerging applications
Beilstein J. Org. Chem. 2019, 15, 2113–2132, doi:10.3762/bjoc.15.209
- combinatorial chemistry. This highlight article discusses the unusual properties of fulvenes and their varied cycloaddition chemistry, focussing on applications in organic and natural synthesis, dynamic combinatorial chemistry and materials chemistry, including dynamers, hydrogels and charge transfer complexes
- the formation of several materials, including dynamic polymers (dynamers) [190], hydrogels [191], and precursors to charge-transfer complexes [181][234][235]. Dynamers, referred to as dynamers, are a class of adaptive polymers formed through reversible covalent bonds or noncovalent interactions
- fuelled advances in organic and natural product synthesis, dynamic combinatorial chemistry and materials science, including dynamers, hydrogels and charge transfer complexes. The recent advances show that potential applications for fulvene cycloaddition reactions are varied and wide in scope. We believe
1,2,3,4-Tetrahydro-1,4,5,8-tetraazaanthracene revisited: properties and structural evidence of aromaticity loss
Beilstein J. Org. Chem. 2019, 15, 2059–2068, doi:10.3762/bjoc.15.203

- –C8–N9 atom chains and the respective bond lengths of the second independent molecule of 3 reveals a contribution of the intramolecular charge transfer from the electron donating ethylenediamino moiety (D) toward the electron accepting ethylenediimino (A) moiety as shown by the narrow range of the C–N
- aromaticity of quinoxaline derivative 3 decreases compared to aromatic and planar 1 [23][24] because of the presence of the amino groups that give rise to intramolecular charge transfer. This effect is even stronger in 6a and 7a because of delocalization of the positive charge. The equilibrium geometries of 3
- , 6a and 7a obtained by calculations are in good agreement with the X-ray structural data (Table 1). The geometry optimized structures confirm that the bond lengths equalization and the aromaticity loss observed in the X-ray structures stem from the intramolecular charge transfer from the electron
Naphthalene diimides with improved solubility for visible light photoredox catalysis
Beilstein J. Org. Chem. 2019, 15, 2043–2051, doi:10.3762/bjoc.15.201
- solubilities. The electron-donating diaminoalkyl substituents together with the electron-deficient aromatic core of the naphthalene diimides increase the charge-transfer character of their photoexcited states and thus shift their absorption into the visible light (500–650 nm). The excited state reduction
- species – a photocatalyst. If the interacting mode between the sensitizer and the reactant is via charge transfer, it is named photoredox catalysis. This research field has been established over the past decade [1][2][3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20]. In principle, it is a
- catalysis is mainly [Ru(bpy)3]2+ [21], due to its strong MLCT (metal-to-ligand charge transfer) absorption, the excellent yield of its triplet state and the long lifetime, the versatile redox behavior (Ru3+ vs Ru+) in quenching processes and the chemical and photochemical robustness. Despite their positive
Functional panchromatic BODIPY dyes with near-infrared absorption: design, synthesis, characterization and use in dye-sensitized solar cells
Beilstein J. Org. Chem. 2019, 15, 1758–1768, doi:10.3762/bjoc.15.169

- thiophene ring. As we are dealing with charge-transfer electronic excitations, we have adopted a tuned range-separated CAM-B3LYP functional and the polarizable continuum model (PCM) to account for (implicit) solvent effects (in THF). The one-electron energy diagram reported in Figure 2 shows that: (i
Synthesis, photophysical and electrochemical properties of pyridine, pyrazine and triazine-based (D–π–)2A fluorescent dyes
Beilstein J. Org. Chem. 2019, 15, 1712–1721, doi:10.3762/bjoc.15.167

- properties of the three dyes were investigated by photoabsorption and fluorescence spectroscopy, Lippert–Mataga plots, cyclic voltammetry and density functional theory calculations. The photoabsorption maximum (λmax,abs) and the fluorescence maximum (λmax,fl) for the intramolecular charge-transfer
- . Thus, the D–π–A dyes exhibit intense photoabsorption and fluorescence emission properties based on the intramolecular charge transfer (ICT) excitation from the D moiety to the A moiety [1][2][3][4]. Moreover, the D–π–A structure possesses considerable structural characteristics: the increase in the
- pyridine, pyrazine or triazine ring as the electron-withdrawing group (electron-accepting group, A), and their photophysical and electrochemical properties were investigated. It was found that the intramolecular charge-transfer (ICT)-based photoabsorption and fluorescence bands of the three dyes appear at
Host–guest interactions in nor-seco-cucurbit[10]uril: novel guest-dependent molecular recognition and stereoisomerism
Beilstein J. Org. Chem. 2019, 15, 1705–1711, doi:10.3762/bjoc.15.166
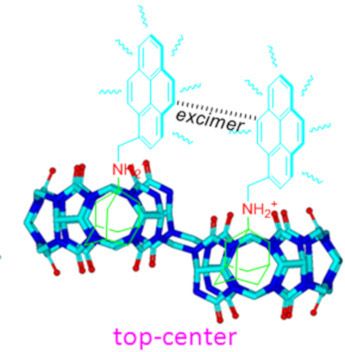
- guests in its cavity through host-stabilized charge-transfer or π–π interactions [14][15]. This novel property of Q[8] has been utilized as molecular container for biological substrates [16][17], as well as in the construction of various supramolecular assemblies with specific structures and properties
Complexation of a guanidinium-modified calixarene with diverse dyes and investigation of the corresponding photophysical response
Beilstein J. Org. Chem. 2019, 15, 1394–1406, doi:10.3762/bjoc.15.139

- intramolecular charge-transfer dyes. Phosphated tetraphenylethylene was involved as the classical aggregation-induced emission dye. Sulfonated acedan representing one example of two-photon fluorescent probes, was also investigated. A ruthenium(II) complex with carboxylated bipyridyl ligands was included as a
- charge-transfer dyes. P-TPE was included in the study as a classical aggregation-induced emission (AIE) dye and TPS as a representative of a two-photon fluorescent probe. Ru(dcbpy)3 was involved as a member of luminescent transition-metal complexes. Of our special interest in the present study is to
- excited-state photophysics, redox behavior and utilizable luminescence properties [60][61]. These Ru complexes exhibit transitions involving charge transfer from the metal-centered d orbital to the ligand p orbital, commonly known as metal-to-ligand charge transfer (MLCT). Upon excitation, the excited
Precious metal-free molecular machines for solar thermal energy storage
Beilstein J. Org. Chem. 2019, 15, 1096–1106, doi:10.3762/bjoc.15.106

- higher energy form has to be with quite small molar absorptivity in comparison to the lower one; (iv) the quantum yield of the photoisomerization has to be as high as possible, which requires the design of MOST systems with completely suppressed or minimal fluorescence, intramolecular charge transfer or
Photochemical generation of the 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) radical from caged nitroxides by near-infrared two-photon irradiation and its cytocidal effect on lung cancer cells
Beilstein J. Org. Chem. 2019, 15, 863–873, doi:10.3762/bjoc.15.84

- yields in DMSO of 5a and 5b were determined to be 16.1 and 8.6%, respectively, although no emission was observed from these compounds in non-polar benzene, indicating that the excited state has zwitterionic character. The charge transfer transition was supported by time-dependent density functional
- %) for 2a, and 390 (70%) and 890 ps (30%) for 2b (Table 1, entries 1 and 2). For 2a and 2b, intermolecular charge transfer processes induced by the TEMPO moiety may account for the double-exponential decay curves to some extent. OP photolysis of 2a (5 mM) was first conducted in benzene at ≈298 K using
Coordination chemistry and photoswitching of dinuclear macrocyclic cadmium-, nickel-, and zinc complexes containing azobenzene carboxylato co-ligands
Beilstein J. Org. Chem. 2019, 15, 840–851, doi:10.3762/bjoc.15.81
- -allowed 3A2g(F) → 3T2g(F) (ν3) transition is expected around 440 nm, but is obscured by the stronger RS → Ni2+ charge transfer and π→π* transitions in this region. We carried out orienting irradiation experiments with regard to a possible trans to cis photoisomerization of the bound azobenzene-carboxylate
- [Cd2L]2+ fragment. The switch from the trans to the cis form induces significant change of the π–π transitions of the supporting N6S2 macrocycle, which may be indicative of some increased π–π- (or charge transfer) interactions between the aromatic rings of the electron rich amino thiophenolato
Synthesis and fluorescent properties of N(9)-alkylated 2-amino-6-triazolylpurines and 7-deazapurines
Beilstein J. Org. Chem. 2019, 15, 474–489, doi:10.3762/bjoc.15.41

- heterocycles, which were additionally extended by triazole moieties, the compounds with electron-donating groups showed intramolecular charge transfer character (ICT/TICT) of the excited states which was proved by solvatochromic dynamics and supported by DFT calculations. In the 7-deazapurine series this led
- solvent properties such as viscosity or hydrogen bonding. Interestingly, purine class compound 8c showed a dual-fluorescence character in the solvents of higher polarity (DMSO, MeCN) arising from the locally excited and charge transfer excited states, however, dual-fluorescence was not evident for the 7
- with a possible character of TICT [55], which could explain a dual-fluorescence and fluorescence quenching in the high polarity media. The charge transfer nature of the transitions for the compounds 8c and 11c, compared to reference compounds 8a and 11a, was confirmed by quantum chemical calculations
























































































































































































































































































































