Search results
Search for "palladium" in Full Text gives 630 result(s) in Beilstein Journal of Organic Chemistry. Showing first 200.
Amino- and polyaminophthalazin-1(2H)-ones: synthesis, coordination properties, and biological activity
Beilstein J. Org. Chem. 2021, 17, 558–568, doi:10.3762/bjoc.17.50

- Group, Faculty of Chemistry, University of Lodz, Pomorska 163/165, 90-236 Łódź, Poland 10.3762/bjoc.17.50 Abstract Amino- and polyaminophthalazinones were synthesized by the palladium‐catalyzed amination (alkyl- and arylamines, polyamines) of 4-bromophthalazinones in good yields. The coordinating
- quinazolinones [30][31] and taking into consideration the biological importance of aminophthalazine derivatives, we decided to apply the methodology based on the palladium-catalyzed C–N-bond formation (Buchwald–Hartwig-type reaction) as a convenient and effective approach for the synthesis of the new
- -bromobenzoate via palladium-catalyzed isocyanide insertion [32][33] (a method that is limited to tertiary-substituted isocyanides) or 2) the palladium or copper-catalyzed coupling of bromolactams with amines (a method that requires the usually lengthy synthesis of the bromoprecursors) [19][34]. Therefore, the
Helicene synthesis by Brønsted acid-catalyzed cycloaromatization in HFIP [(CF3)2CHOH]
Beilstein J. Org. Chem. 2021, 17, 396–403, doi:10.3762/bjoc.17.35
- -catalyzed cycloaromatization (Scheme 3, route a and Scheme 4a). Treatment of 1,2-dibromobenzene (2a) with 3 in the presence of a palladium catalyst with SPhos afforded o-terphenyl derivative 4a bearing two acetal groups in 96% yield (Scheme 4a). The obtained bisacetal 4a successfully underwent
- subsequent cycloaromatization (Scheme 6a). Again, phenylboronic acid ester 3, used above in the synthesis of [5]- and [6]helicenes, was adopted as the coupling partner for 10. In the presence of a palladium catalyst, treatment of 3-bromobenzofuran (10a), 3-bromobenzothiophene (10b), and 3-bromo(N-tosyl
The preparation and properties of 1,1-difluorocyclopropane derivatives
Beilstein J. Org. Chem. 2021, 17, 245–272, doi:10.3762/bjoc.17.25
- cleavage by the use of either palladium(II) oxide or Raney nickel as the catalyst (Scheme 66) [116]. Butylbenzene (152) and 2-fluoro-1-phenylbutane (153) were the main products, although the unsaturated intermediates 154 and 155 were also detected. The contribution of the fluorine substituents to the
- good yields and with high (Z)-selectivity. The proposed mechanism involved the oxidative addition of the distal C–C bond to palladium, followed by a nucleophilic attack at the less hindered carbon atom of a 2-fluorinated palladium–π-allyl complex. Other examples of Pd-catalyzed ring-oрening reactions
- reaction of 2-(2,2-difluorocyclopropyl)naphthalene (167) with sodium arylsulfinates 168 under palladium catalysis afforded the 2-fluoroallylic sulfones 166 in moderate to good yields with (Z)-selectivity. This method showed a good compatibility with a broad range of substrates and substituents. As
Selective synthesis of α-organylthio esters and α-organylthio ketones from β-keto esters and sodium S-organyl sulfurothioates under basic conditions
Beilstein J. Org. Chem. 2021, 17, 234–244, doi:10.3762/bjoc.17.24
- , and related compounds. Some of them are catalyzed by expensive transition metals, such as gold, iridium, palladium, and titanium. More recently, the selective formation of C–S bonds using 1,3-dicarbonyl compounds, followed by a C–C bond cleavage has emerged as a versatile and less expensive protocol
Total synthesis of decarboxyaltenusin
Beilstein J. Org. Chem. 2021, 17, 224–228, doi:10.3762/bjoc.17.22
- -3,5-dimethoxybenzene, where the longest linear sequence consists of five steps. The key reaction was a palladium-catalyzed Suzuki coupling of an aromatic boronate with a brominated resorcin derivative. Keywords: biaryls; boronates; mycotoxins; polyketides; Suzuki coupling; Introduction 5’-Methoxy-6
- a 73% yield. The Suzuki coupling of boronate 6a and aryl bromide 9a using palladium acetate and cesium carbonate in the presence of the ligand SPhos [25] yielded biaryl 10a with virtually quantitative yield (98%, Scheme 4). Unfortunately, the removal of non-identified byproducts and of 9a (which had
- protection to the bis(benzyl ether) 5b using standard conditions (Scheme 2) [27]. The preparation of boronate 6b applying the conditions used for the silylated substrate 6a (vide supra) led to a mediocre 44% yield, but the utilization of a palladium-catalyzed borylation with bis(pinacolato)diboron afforded
Novel library synthesis of 3,4-disubstituted pyridin-2(1H)-ones via cleavage of pyridine-2-oxy-7-azabenzotriazole ethers under ionic hydrogenation conditions at room temperature
Beilstein J. Org. Chem. 2021, 17, 156–165, doi:10.3762/bjoc.17.16
- goal of creating a rapid, 3-step route requiring a single preparative LC–MS purification at the end of the sequence (Figure 1). Results and Discussion Exploration of the C-3 amide vector: formation of the pyridine-2-(1H)-one motif by palladium catalysis We decided to validate the route by preparing
- . Finally, we turned out attention to transition metal-catalyzed formation of phenols from aryl halides [5]. After another round of screening, we successfully applied palladium-catalyzed conditions discovered by the Buchwald group [6], using KOH as the nucleophile and X-Phos as the ligand, to afford 7 in 83
Progress in the total synthesis of inthomycins
Beilstein J. Org. Chem. 2021, 17, 58–82, doi:10.3762/bjoc.17.7
- mixture of (Z,E)/(E,E)-36a, respectively). Aldehyde 35 was then converted into dibromide 37 using PPh3/CBr4 followed by stereoselective palladium-catalyzed monoreduction according to the literature available protocol [41] to give vinyl bromide 38 in 76% yield (Z/E as 99:1 mixture). Iodide 15a [42] was
- 19). For the synthesis of inthomycin B ((+)-2), a Suzuki coupling reaction between (E,E)-128 and (Z)-(+)-130a was performed in the presence of palladium(II) acetate, triphenylphosphine, and aqueous sodium carbonate to give (E,E,Z)-triene (+)-131 selectively in 64% yield. Treatment of triene (+)-131
Recent progress in the synthesis of homotropane alkaloids adaline, euphococcinine and N-methyleuphococcinine
Beilstein J. Org. Chem. 2021, 17, 28–41, doi:10.3762/bjoc.17.4
- diastereoisomeric mixture, converted to ketone (±)-37 via methylene derivative (±)-36, in 63% over two steps (Scheme 5). Ketone (±)-37 was converted to alkenyl triflate (±)-38 after treatment with LDA at −78 °C, followed by the Comins reagent [47]. (±)-38 was subjected to palladium-catalyzed hydrogenation
Pentannulation of N-heterocycles by a tandem gold-catalyzed [3,3]-rearrangement/Nazarov reaction of propargyl ester derivatives: a computational study on the crucial role of the nitrogen atom
Beilstein J. Org. Chem. 2020, 16, 3059–3068, doi:10.3762/bjoc.16.255
- was prepared via the palladium-catalyzed reduction of the corresponding phosphate 17 [54]. Iodination and Sonogashira coupling, followed by acetylation led to the formation of the desired enynyl acetate 20. This compound was treated with 5 mol % Ph3PAuCl/AgSbF6 in DCM, and after 6 h, this afforded the
All-carbon [3 + 2] cycloaddition in natural product synthesis
Beilstein J. Org. Chem. 2020, 16, 3015–3031, doi:10.3762/bjoc.16.251

- ] cycloaddition through diyl trapping with an olefin [14][15] and Trost’s palladium-catalyzed trimethylenemethane cycloaddition [16], which allows the preparation of five-membered carbocycles, have been emerged since the 1970s. Thereafter, many novel and important all-carbon [3 + 2] cycloaddition reactions, such
- ] cycloaddition to give fused tricycle 25 in 85% yield. The synthesis of the hydroxykempenone 3β-hydroxykemp-7(8)-en-6-one (7) features Trost’s palladium-catalyzed trimethylenemethane [3 + 2] cycloaddition [27] and was reported by Paquette and co-workers in 1992 [23] (Scheme 1B). Catalytic TMM [3 + 2
- -mediated conjugated addition of methyllithium to enone 59 in the presence of boron trifluoride ether [34][35] produced desired ketone 60 in 75% yield. The resultant ketone 60 was converted to waihoensene (16) in two steps. Palladium-catalyzed carboxylative trimethylenemethane cycloaddition In 1986, Trost
Bifurcated synthesis of methylene-lactone- and methylene-lactam-fused spirolactams via electrophilic amide allylation of γ-phenylthio-functionalized γ-lactams
Beilstein J. Org. Chem. 2020, 16, 2769–2775, doi:10.3762/bjoc.16.227
- electrophilic allylation. We initially chose N-phenyl-3-(acetoxy)isoindolinone (2a) as a reactant. However, 2a showed no reactivity under the reaction conditions determined for the palladium-catalyzed allylation in our previous work and was recovered in 98% yield [17] (Table 1, entry 1). The use of strong bases
- sodium hydride, affording the corresponding adduct in 50% and 81% yields, respectively (Table 1, entries 6 and 7). Surprisingly, the desired allylation underwent even in the absence of palladium catalyst, probably due to high nucleophilicity of the deprotonated intermediate, to give 3b in 87% yield
Palladium nanoparticles supported on chitin-based nanomaterials as heterogeneous catalysts for the Heck coupling reaction
Beilstein J. Org. Chem. 2020, 16, 2477–2483, doi:10.3762/bjoc.16.201

Recent developments in enantioselective photocatalysis
Beilstein J. Org. Chem. 2020, 16, 2363–2441, doi:10.3762/bjoc.16.197

Synthetic approaches to bowl-shaped π-conjugated sumanene and its congeners
Beilstein J. Org. Chem. 2020, 16, 2212–2259, doi:10.3762/bjoc.16.186

- context, their journey started from syn-tris(oxonorborneno)benzene 23, obtained via palladium-catalyzed cyclotrimerization of iodonorbornene. The C3-symmetric compound 23 was then converted into the methylene groups containing compound 103 using Wittig reaction which on further treatment with
- palladium(II) was carried out in the presence of PdCl2(MeCN)2 in a stepwise manner, confirmed by UV–vis spectroscopic technique. The cyclopentadienyl (Cp) ligand has its own identity in the field of organometallic chemistry as a plethora of transition metal complexes contain this moiety in their structures
Synergy between supported ionic liquid-like phases and immobilized palladium N-heterocyclic carbene–phosphine complexes for the Negishi reaction under flow conditions
Beilstein J. Org. Chem. 2020, 16, 1924–1935, doi:10.3762/bjoc.16.159
- cross-coupling; NHC complex; palladium; supported ionic liquid; Introduction N-heterocyclic carbenes (NHCs) are known as efficient coordination ligands for different types of metals. The main feature of NHC complexes is their structural tunability [1]. Thus, their catalytic efficiency can be easily
- homogeneous catalysts for Negishi reactions [4]. Although these systems are highly efficient, their homogeneous nature hamper the separation of the products and recovery of the excess of the palladium from the reaction solution. A possible solution to this issue is the preparation of the related immobilized
- pursuit of NHC immobilized metal complexes many different materials of organic and inorganic nature have been used as supports [5]. Several reports describe the synthesis of supported palladium–NHC complexes (Pd–NHC) and their application in cross-coupling reactions [8][9][10]. The main flaw of this type
When metal-catalyzed C–H functionalization meets visible-light photocatalysis
Beilstein J. Org. Chem. 2020, 16, 1754–1804, doi:10.3762/bjoc.16.147

- synthesis of 1,3-oxazin-6-ones by merging palladium and photoredox catalysis (Figure 13) [77]. The transformation was performed at 80 °C under air atmosphere. This efficient approach for the construction of heterocycles exhibited a good functional group tolerance thus providing a broad range of products
- bicoordinating DGs to facilitate C(sp3)–H metalation, Polyzos’s group reported the direct arylation of aliphatic bonds with aryldiazonium salts via a palladium/photoredox dual catalysis (Figure 26) [88]. The method utilized an 8-aminoquinoline-derived bidentate auxiliary, and, in contrast with a vast majority of
- combining palladium-catalyzed C–H activation and photoredox catalysis (Figure 27) [89]. Interestingly, the transformation occurred smoothly in the presence of eosin Y, an inexpensive (in comparison with Ir- or Ru-based photoredox catalysts), and easily accessible organic photocatalyst. The reaction, carried
Pauson–Khand reaction of fluorinated compounds
Beilstein J. Org. Chem. 2020, 16, 1662–1682, doi:10.3762/bjoc.16.138

- described, including the use of metals other than cobalt (such as rhodium, iridium, titanium, ruthenium, nickel, and palladium), or the use of CO surrogates such as aldehydes, alcohols and formates. Recently, its utility in flow chemistry has also been described [42]. Intramolecular Pauson–Khand reactions
- corresponding aldehyde in a 41% global yield. Bicyclic product 49a underwent diastereoselective (dr > 20:1) hydrogenation using palladium over activated charcoal under an atmosphere of hydrogen to afford saturated derivative 50 (Scheme 26). On the other hand, the tert-butanesulfonyl group could be removed
Facile synthesis of 7-alkyl-1,2,3,4-tetrahydro-1,8-naphthyridines as arginine mimetics using a Horner–Wadsworth–Emmons-based approach
Beilstein J. Org. Chem. 2020, 16, 1617–1626, doi:10.3762/bjoc.16.134
- . This represents a 64% overall yield which was increased to 68% when no column chromatography was undertaken between transformations, with no loss of purity (Scheme 5). Olefin reduction and Cbz deprotection could not be performed simultaneously by palladium-catalysed hydrogenation as this resulted in
Heterogeneous photocatalysis in flow chemical reactors
Beilstein J. Org. Chem. 2020, 16, 1495–1549, doi:10.3762/bjoc.16.125

In silico rationalisation of selectivity and reactivity in Pd-catalysed C–H activation reactions
Beilstein J. Org. Chem. 2020, 16, 1465–1475, doi:10.3762/bjoc.16.122
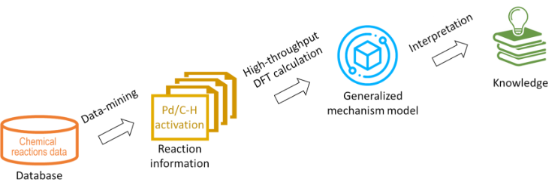
- intermediates of palladium-catalysed carbon–hydrogen (C–H) activation reactions as well as to predict the final products. Implemented as a high-performance computing cluster tool, it has been shown to correctly choose the mechanism and rationalise regioselectivity of chosen examples from open literature reports
- mild conditions [13]. In particular, reactions involving palladium-catalysed activation of sp2 or sp3 C–H bonds of arenes or alkanes have been extensively investigated due to their wide scope and functional group tolerance [14]. A number of different mechanisms are proposed in the literature
- was proposed for classifying whether the carbon atoms are active or inactive toward electrophilic aromatic substitution [17]. Also, a quantum mechanical approach was introduced to compute ortho-directing groups (DGs) in palladium-catalysed aromatic C–H activation reactions [18]. However, there is a
Highly selective Diels–Alder and Heck arylation reactions in a divergent synthesis of isoindolo- and pyrrolo-fused polycyclic indoles from 2-formylpyrrole
Beilstein J. Org. Chem. 2020, 16, 1320–1334, doi:10.3762/bjoc.16.113
- yields. Once having the optimal reaction conditions for the palladium(0)-catalyzed cross-coupling reaction leading to the cyclization of N-(2-bromobenzyl)pyrroles 8, the endo-octahydropyrrolo[3,4-e]indole-1,3-diones 9g–j and 9m were converted into pentacycles 11a–e through the same methodology (Table 3
- ). Optimized reaction conditions were established by using 9j as the model substrate and changing the catalyst, base and solvent. Palladium acetate was not efficient for the conversion, even though the base was modified (Table 3, entries 1 and 2). Pd(PPh3)4 was a better catalyst when employing potassium
Palladium-catalyzed regio- and stereoselective synthesis of aryl and 3-indolyl-substituted 4-methylene-3,4-dihydroisoquinolin-1(2H)-ones
Beilstein J. Org. Chem. 2020, 16, 1084–1091, doi:10.3762/bjoc.16.95
- aminopalladation/reductive elimination. Keywords: alkynylanilines; arylboronic acids; indoles; isoquinolinones; palladium; Introduction The isoquinolinone nucleus is a key constituent of many natural products [1][2][3] and pharmaceuticals [4][5][6]. Substituted isoquinolinones have been found in biologically
- stereoselective formation of carbon–carbon bonds [19]. Intramolecular palladium-catalyzed versions are particularly attractive, since they afford polycarbo- and heterocyclic systems via sequential reactions of the vinylpalladium intermediate [20][21][22][23][24][25]. In this field, a variety of regio- and
- stereoselective Pd-catalyzed cascade reactions, consisting of the addition of in situ-generated arylpalladium complexes over a proximate carbon–carbon triple bond, followed by cross-coupling reactions, have been reported [26][27][28][29][30][31]. Our continuing interest in the palladium-catalyzed reactions of
Synthesis of novel multifunctional carbazole-based molecules and their thermal, electrochemical and optical properties
Beilstein J. Org. Chem. 2020, 16, 1066–1074, doi:10.3762/bjoc.16.93
- potassium acetate (KOAc) and dichlorobis(triphenylphosphine)palladium(II) in 1,4-dioxane [36][37]. Upon chromatography (9-hexylcarbazole-3-yl)boronic acid pinacol ester (5) was obtained as a liquid in good yield. (9-Hexylcarbazole-3-yl)boronic acid pinacol ester (5) was subjected to Suzuki–Miyaura reaction
- either with 2-bromopyridine-4-carbaldehyde (6a) or 2-bromopyridine-5-carbaldehyde (6b) in the presence of potassium carbonate and bis(triphenylphosphine)palladium(II) dichloride in tetrahydrofuran [2]. Upon repeated purification by chromatography, compounds 7a and 7b were obtained as liquids in good
- ), 0.86 (t, J = 7.0 Hz, 4H). (9-Hexylcarbazole-3-yl)boronic acid pinacol ester (5): 5 was synthesised as reported previously [36][37]. Bis(pinacolato)diboron (423 mg, 1.7 mmol), potassium acetate (446 mg, 4.5 mmol) and dichlorobis(triphenylphosphine)palladium(II) (35 mg, 0.05 mmol) catalyst were added to
Fluorinated phenylalanines: synthesis and pharmaceutical applications
Beilstein J. Org. Chem. 2020, 16, 1022–1050, doi:10.3762/bjoc.16.91

- )-iodoalanine 2. The reaction was activated using Pd(0) as a catalyst. A palladium-catalyzed cross-coupling reaction between an organozinc iodide and aryl halides offers a convenient method for the direct preparation of protected fluorinated Phe analogues 3. Thus, cross coupling of the protected iodoalanine 2
- other hand, when the quinoline-based ligand 162 was used, it was shown to promote the palladium-catalyzed direct electrophilic fluorination of β-methylene C(sp3)–H bonds. Thus, fluorinations of ʟ-phenylalanine 4-trifluoromethylphenylamides 161a–l carrying a range of functional groups such as fluoro
Aryl-substituted acridanes as hosts for TADF-based OLEDs
Beilstein J. Org. Chem. 2020, 16, 989–1000, doi:10.3762/bjoc.16.88

- presence of a palladium catalyst, with yields ranging from 27 to 50%. The chemical structures of 3–6 were confirmed by 1H and 13C NMR spectroscopy, elemental analysis and mass spectrometry. Transparent thin films of these compounds could be prepared by vacuum evaporation or by spin coating from solutions



































































































































































































































































































































































































































































































































































































































































