Search results
Search for "solvolysis" in Full Text gives 46 result(s) in Beilstein Journal of Organic Chemistry.
Synthesis of β-ketophosphonates through aerobic copper(II)-mediated phosphorylation of enol acetates
- Alexander S. Budnikov,
- Igor B. Krylov,
- Fedor K. Monin,
- Valentina M. Merkulova,
- Alexey I. Ilovaisky,
- Liu Yan,
- Bing Yu and
- Alexander O. Terent’ev
Beilstein J. Org. Chem. 2025, 21, 1192–1200, doi:10.3762/bjoc.21.96
- synthetic step distinguishes them from the less accessible substituted vinylarenes, alkynes, cinnamic and α,β-alkynyl carboxylic acids, as well as vinyl azides. In addition, compared to similar silyl and alkyl enol ethers, enol acetates are more resistant to solvolysis, which prevents unwanted side

Graphical Abstract

Scheme 1: Recent approaches for the synthesis of β-ketophosphonates by the oxyphosphorylation of unsaturated ...

Scheme 2: The scope of the discovered copper(II)-mediated phosphorylation of enol acetates.

Scheme 3: Gram-scale synthesis of 3a.

Scheme 4: Control experiments.

Scheme 5: Proposed mechanism for copper(II) mediated phosphorylation of enol acetates.
Photomechanochemistry: harnessing mechanical forces to enhance photochemical reactions
- Francesco Mele,
- Ana M. Constantin,
- Andrea Porcheddu,
- Raimondo Maggi,
- Giovanni Maestri,
- Nicola Della Ca’ and
- Luca Capaldo
Beilstein J. Org. Chem. 2025, 21, 458–472, doi:10.3762/bjoc.21.33

- hydrogen-atom transfer or solvolysis are often observed. A technological solution to cope with the Beer–Lambert law was offered by flow chemistry [19][20][21] by employing microreactors with reduced optical paths to enhance irradiation efficiency [22][23][24]. Photon-limited reactions, whose efficiency is

Graphical Abstract

Figure 1: The Grotthuss–Draper, Einstein–Stark, and Beer–Lambert laws. T: transmittance; ε: molar attenuation...

Figure 2: The benefits of merging photochemistry with mechanochemical setups (top). Most common setups for ph...

Scheme 1: Mechanochemically triggered pedal-like motion in solid-state [2 + 2] photochemical cycloaddition fo...

Scheme 2: Mechanically promoted [2 + 2] photodimerization of trans-1,2-bis(4-pyridyl)ethylene (2.1) via supra...

Scheme 3: Photo-thermo-mechanosynthesis of quinolines [65].

Scheme 4: Study of the mechanically assisted [2 + 2] photodimerization of chalcone [66].

Scheme 5: Liquid-assisted vortex grinding (LAVG) for the synthesis of [2.2]paracyclophane [68].

Scheme 6: Photomechanochemical approach for the riboflavin tetraacetate-catalyzed photocatalytic oxidation of...

Scheme 7: Photomechanochemical oxidation of 1,2-diphenylethyne to benzil. The photo in Scheme 7 was republished with ...

Scheme 8: Photomechanochemical borylation of aryldiazonium salts. The photo in Scheme 8 was reproduced from [72] (© 2017 ...

Scheme 9: Photomechanochemical control over stereoselectivity in the [2 + 2] dimerization of acenaphthylene. ...

Scheme 10: Photomechanochemical synthesis of polyaromatic compounds using UV light. The photo in Scheme 10 was reproduc...

Scheme 11: Mechanically assisted photocatalytic reactions: A) atom-transfer-radical addition, B) pinacol coupl...

Scheme 12: Use of mechanoluminescent materials as photon sources for photomechanochemistry. SAOED: SrAl2O4:Eu2+...

Figure 3: SWOT (strengths, weaknesses, opportunities, threats) analysis of photomechanochemistry.
Multicomponent reactions driving the discovery and optimization of agents targeting central nervous system pathologies
- Lucía Campos-Prieto,
- Aitor García-Rey,
- Eddy Sotelo and
- Ana Mallo-Abreu
Beilstein J. Org. Chem. 2024, 20, 3151–3173, doi:10.3762/bjoc.20.261

- solvolysis of the polyacetates. Also, the Ugi reaction gave the best results when imine preformation was implemented. In this preliminary paper, it was observed that the ferulic derived part was highly significant and for this reason, further experiments were conducted by Galante et al. [37] maintaining the

Graphical Abstract

Figure 1: Classical MCRs.

Figure 2: Different scaffolds that can be formed with the Ugi adduct.

Scheme 1: Oxoindole-β-lactam core produced in a U4C-3CR.

Figure 3: Most active oxoindole-β-lactam compounds developed by Brãndao et al. [33].

Scheme 2: Ugi-azide synthesis of benzofuran, pyrazole and tetrazole hybrids.

Figure 4: The most promising hybrids synthesized via the Ugi-azide multicomponent reaction reported by Kushwa...

Scheme 3: Four-component Ugi reaction for the synthesis of novel antioxidant compounds.

Figure 5: Most potent antioxidant compounds obtained through the Ugi four-component reaction developed by Pac...

Scheme 4: Four-component Ugi reaction to synthesize β-amiloyd aggregation inhibitors.

Figure 6: The most potential β-amiloyd aggregation inhibitors generated by Galante et al. [37].

Scheme 5: Four-component Ugi reaction to obtain FATH hybrids and the best candidate synthesized.

Scheme 6: Four-component Ugi reaction for the synthesis of FATMH hybrids and the best candidate synthesized.

Scheme 7: Petasis multicomponent reaction to produce pyrazine-based MTDLs.

Figure 7: Best pyrazine-based MTDLs synthesized by Madhav et al. [40].

Scheme 8: Synthesis of BCPOs employing a Knoevenagel-based multicomponent reaction and the best candidate syn...

Scheme 9: Hantzsch multicomponent reaction for the synthesis of DHPs as novel MTDLs.

Figure 8: Most active 1,4-dihydropyridines developed by Malek et al. [43].

Scheme 10: Chromone–donepezil hybrid MTDLs obtained via the Passerini reaction.

Figure 9: Best CDH-based MTDLs as AChE inhibitors synthesized by Malek et al. [46].

Scheme 11: Replacement of the nitrogen in lactams 11 with an oxygen in 12 to influence hydrogen-bond donating ...

Scheme 12: MCR 3 + 2 reaction to develop spirooxindole, spiroacenaphthylene, and bisbenzo[b]pyran compounds.

Figure 10: SIRT2 activity of best derivatives obtained by Hasaninejad et al. [49].

Scheme 13: Synthesis of ML192 analogs using the Gewald multicomponent reaction and the best candidate synthesi...

Scheme 14: Development of 1,5-benzodiazepines via Ugi/deprotection/cyclization (UDC) approach by Xu et al. [59].

Scheme 15: Synthesis of polysubstituted 1,4-benzodiazepin-3-ones using UDC strategy.

Scheme 16: Synthetic procedure to obtain 3-carboxamide-1,4-benzodiazepin-5-ones employing Ugi–reduction–cycliz...

Scheme 17: Ugi cross-coupling (U-4CRs) to synthesize triazolobenzodiazepines.

Scheme 18: Azido-Ugi four component reaction cyclization to obtain imidazotetrazolodiazepinones.

Scheme 19: Synthesis of oxazolo- and thiazolo[1,4]benzodiazepine-2,5-diones via Ugi/deprotection/cyclization a...

Scheme 20: General synthesis of 2,3-dichlorophenylpiperazine-derived compounds by the Ugi reaction and Ugi/dep...

Figure 11: Best DRD2 compounds synthesized using a multicomponent strategy.

Scheme 21: Bucherer–Bergs multicomponent reaction to obtain a key intermediate in the synthesis of pomaglumeta...

Scheme 22: Ugi reaction to synthesize racetam derivatives and example of two racetams synthesized by Cioc et a...
Evaluating the halogen bonding strength of a iodoloisoxazolium(III) salt
- Dominik L. Reinhard,
- Anna Schmidt,
- Marc Sons,
- Julian Wolf,
- Elric Engelage and
- Stefan M. Huber
Beilstein J. Org. Chem. 2024, 20, 2401–2407, doi:10.3762/bjoc.20.204
- (Scheme 1). The triflate salt 7OTf was transformed into the corresponding bromide salt by XB-activated solvolysis of α-methylbenzyl bromide in wet acetonitrile [13]. The DAI salt 7Br crystallized from this solution in the monoclinic space group P21/n with a cell volume of 1447.91(3) Å3 (a = 5.4707(1) Å, b
- halide abstraction is the crucial step towards the formation of a catalytically active gold species [18][19]. Furthermore, iodonium species 1BArF–4BArF have been shown to be halide abstracting agents in the Ritter-type solvolysis of α-methylbenzyl bromide and via the crystal structures of 1Cl, 2Cl, and
- 3Cl which resulted from crystallization of the respective cation with the abstracted chloride from the Ritter-type solvolysis of benzhydryl chloride [13]. The crystal structure of 5Br was also obtained directly from the halide-abstraction reaction (see Supporting Information File 1). These three facts

Graphical Abstract

Figure 1: Set of literature-known monocationic cyclic diaryliodonium(III) salts that were applied as XB donor...

Scheme 1: Synthesis of the iodoloisoxazolium salts 7Z: (a) 1.5 equiv 9, 0.2 equiv CuI, 2.0 equiv K2CO3, (THF)...

Figure 2: Halogen bonding dimer found in the crystal structure of 7Br. Ellipsoids are shown at 50% probabilit...

Scheme 2: Gold(I)-catalyzed cyclization of propargylic amide 11 as benchmark reaction for Au–Cl activation.

Figure 3: 1H NMR kinetics of the gold-catalyzed cyclization shown in Scheme 2. An equimolar amount of the gold comple...
Hydrogen-bond activation enables aziridination of unactivated olefins with simple iminoiodinanes
- Phong Thai,
- Lauv Patel,
- Diyasha Manna and
- David C. Powers
Beilstein J. Org. Chem. 2024, 20, 2305–2312, doi:10.3762/bjoc.20.197
- alcohol solvent to afford ArI(OR)2 and TsNH2 (Supporting Information File 1, Figure S3); similar solvolysis of PhIO in HFIP has been reported [10]. Reaction between cyclohexene and PhIO (2 equiv) in HFIP delivered <10% of cyclohexene oxide; meanwhile, both cyclohexene and cyclohexene oxide were shown to

Graphical Abstract

Scheme 1: a) Lewis acid activation of hypervalent iodine reagents can enhance the reactivity of these reagent...

Scheme 2: Scope and limitations of HFIP-promoted direct aziridination with iminoiodinane reagents. Conditions...

Scheme 3: Scope of nitrogen group transfer in the aziridination of aliphatic olefins. Conditions using synthe...

Scheme 4: a) The broadening of the hydroxide proton (denoted by asterisk *) of HFIP in the presence of iminoi...
Photoredox catalysis harvesting multiple photon or electrochemical energies
- Mattia Lepori,
- Simon Schmid and
- Joshua P. Barham
Beilstein J. Org. Chem. 2023, 19, 1055–1145, doi:10.3762/bjoc.19.81

Graphical Abstract

Figure 1: Oxidative and reductive activations of organic compounds harvesting photoredox catalysis.

Figure 2: General catalytic cycles of radical ion conPET (left) and radical ion e-PRC (right).

Figure 3: “Beginner’s guide”: comparison between advantages, capacities, and prospectives of conPET and PEC.

Figure 4: A) conPET reductive dehalogenation of aryl halides with PDI. B) Reductive C–H arylation with pyrrol...

Figure 5: A) Chromoselective mono- and disubstitution or polybrominated pyrimidines with pyrroles. B) Sequent...

Figure 6: A) Synthesis of pyrrolo[1,2-a]quinolines. B) Synthesis of ullazines.

Figure 7: A) Reductive phosphorylation of aryl halides via conPET. B) Selected examples from the substrate sc...

Figure 8: A) Reductive dehalogenation of aryl halides via conPET and selected examples from the substrate sco...

Figure 9: A) Reductive C–H arylation of aryl halides via conPET (top) and selected examples from the substrat...

Figure 10: A) Reductive hydrodehalogenation of aryl halides with Mes-Acr-BF4. B) Selected examples from the su...

Figure 11: A) Reductive hydrodechlorination of aryl chlorides with 4-DPAIPN. B) Proposed formation of CO2•−. C...

Figure 12: A) Reductive conPET borylation with 3CzEPAIPN (top) and selected examples from the substrate scope ...

Figure 13: Scale-up of conPET phosphorylation with 3CzEPAIPN.

Figure 14: A) Borylation of 1d. B) Characteristics and structure of PC1 with green and red parts showing the l...

Figure 15: A) Reductive C–H arylation scope with polysulfide conPET (top) and selected examples from the subst...

Figure 16: Scale-up of A) C–H arylation and B) dehaloborylation with polysulfide photocatalysis in continuous-...

Figure 17: A) Formation of [Ir1]0 and [Ir2]0 upon PET between [Ir1]+ and Et3N. B) Mechanism of multi-photon ta...

Figure 18: A) Reductive hydrodehalogenation of aryl halides via multi-photon tandem photocatalysis. B) Selecte...

Figure 19: A) Carbonylative amidation of aryl halides in continuous flow. B) Selected examples from the substr...

Figure 20: A) General scheme for reductive (RQ) and oxidative quenching (OQ) protocols using [FeIII(btz)3](PF6)...

Figure 21: A) Carbonylative amidation of alkyl iodides with [IrIII(ppy)2(dtbbpy)]PF6. B) Selected examples fro...

Figure 22: A) Carboxylative C–N bond cleavage in cyclic amines. B) Selected examples from the substrate scope....

Figure 23: A) Formal reduction of alkenes to alkanes via transfer hydrogenation. B) Selected examples from the...

Figure 24: A) Birch-type reduction of benzenes with PMP-BPI. B) Selected examples from the substrate scope (sc...

Figure 25: Proposed mechanism of the OH− mediated conPET Birch-type reduction of benzene via generation of sol...

Figure 26: Reductive detosylation of N-tosylated amides with Mes-Acr-BF4. B) Selected examples from the substr...

Figure 27: A) Reductive detosylation of N-tosyl amides by dual PRC. B) Selected examples from the substrate sc...

Figure 28: A) Mechanism of the dual PRC based on PET between [Cu(dap)2]+ and DCA. B) Mechanism of the dual PRC...

Figure 29: A) N–O bond cleavage in Weinreb amides with anthracene. B) N–O bond cleavage in Weinreb amides rely...

Figure 30: A) Pentafluorosulfanylation and fluoride elimination. B) Mechanism of the pentafluorosulfanylation ...

Figure 31: A) α-Alkoxypentafluorosulfanylation (top) and selected examples from the substrate scope (bottom). ...

Figure 32: A) Oxidative amination of arenes with azoles catalyzed by N-Ph PTZ. B) Selected examples from the s...

Figure 33: A) C(sp3)–H bond activation by HAT via chloride oxidation by *N-Ph PTZ•+. B) Proposed mechanism for...

Figure 34: A) Recycling e-PRC C–H azolation of electron-rich arenes with pyrazoles using Mes-Acr+ as a photoca...

Figure 35: A) Radical ion e-PRC direct oxidation of unactivated arenes using TAC+ as an electro-activated phot...

Figure 36: A) Radical ion e-PRC direct oxidation of unactivated arenes using TPA as an electro-activated photo...

Figure 37: Proposed mechanism (top) and mode of preassembly (bottom).

Figure 38: A) Possible preassemblies of reactive (left) vs unreactive (right) arenes. B) Calculated spin densi...

Figure 39: A) Recycling e-PRC C(sp2 )–H acetoxylation of arenes using DDQ as a photocatalyst. B) Proposed cata...

Figure 40: Gram scale hydroxylation of benzene in a recirculated flow setup.

Figure 41: A) Radical ion e-PRC vicinal diamination of alkylarenes using TAC+ as an electro-activated photocat...

Figure 42: A) Sequential oxygenation of multiple adjacent C–H bonds under radical ion e-PRC using TAC+ as an e...

Figure 43: A) Enantioselective recycling e-PRC cyanation of benzylic C–H bonds using ADQS as photocatalyst. B)...

Figure 44: Proposed tandem mechanism by Xu and co-workers.

Figure 45: A) Enantioselective recycling e-PRC decarboxylative cyanation using Cu(acac)2, Ce(OTf)3 and a box l...

Figure 46: A) Enantioselective recycling e-PRC benzylic cyanation using Cu(MeCN)4BF4, box ligand and anthraqui...

Figure 47: A) Radical ion e-PRC acetoxyhydroxylation of aryl olefins using TAC+ as an electro-activated photoc...

Figure 48: Selected examples from the substrate scope.

Figure 49: Photoelectrochemical acetoxyhydroxylation in a recirculated flow setup.

Figure 50: A) Radical ion e-PRC aminooxygenation of aryl olefins using TAC+ as an electro-activated photocatal...

Figure 51: A) Recycling e-PRC C–H alkylation of heteroarenes with organic trifluoroborates using Mes-Acr+ as p...

Figure 52: A) Recycling e-PRC decarboxylative C–H alkylation of heteroarenes using CeCl3·7H2O as catalyst. B) ...

Figure 53: A) Recycling e-PRC decarboxylative C–H alkylation of heteroarenes using Fe(NH4)2(SO4)2·6H2O as cata...

Figure 54: A) Recycling e-PRC C–H alkylation of heteroarenes with alkyl oxalates and 4CzIPN as photocatalyst. ...

Figure 55: A) Recycling e-PRC decarboxylative C–H carbamoylation of heteroarenes using 4CzIPN as photocatalyst...

Figure 56: A) Photoelectrochemical HAT-mediated hydrocarbon activation via the chlorine radical. B) Proposed m...

Figure 57: A) Selected examples from the substrate scope. B) Gram and decagram scale semi-continuous flow PEC ...

Figure 58: A) Photoelectrochemical HAT-mediated dehydrogenative coupling of benzothiazoles with aliphatic C–H ...

Figure 59: A) Photoelectrochemical HAT activation of ethers using electro-activated TAC+ as photocatalyst. B) ...

Figure 60: Selected examples from the substrate scope.

Figure 61: A) Photoelectrochemical HAT-mediated synthesis of alkylated benzimidazo-fused isoquinolinones using...

Figure 62: A) Decoupled photoelectrochemical cerium-catalyzed oxydichlorination of alkynes using CeCl3 as cata...

Figure 63: Proposed decoupled photoelectrochemical mechanism.

Figure 64: A) Decoupled photoelectrochemical ring-opening bromination of tertiary cycloalkanols using MgBr2 as...

Figure 65: A) Recycling e-PRC ring-opening functionalization of cycloalkanols using CeCl3 as catalyst. B) Prop...

Figure 66: Selected examples from the substrate scope of the PEC ring-opening functionalization.

Figure 67: A) Radical ion e-PRC reduction of chloro- and bromoarenes using DCA as catalyst and various accepto...

Figure 68: A) Screening of different phthalimide derivatives as catalyst for the e-PRC reduction of aryl halid...

Figure 69: Screening of different organic catalysts for the e-PRC reduction of trialkylanilium salts.

Figure 70: A) e-PRC reduction of phosphonated phenols and anilinium salts. B) Selected examples from the subst...

Figure 71: A) ConPET and e-PRC reduction of 4-bromobenzonitrile using a naphthalene diimide (NDI) precatalyst ...

Figure 72: A) Radical ion e-PRC reduction of phosphinated aliphatic alcohols with n-BuO-NpMI as catalyst. B) C...

Figure 73: Selected examples from the substrate scope.

Figure 74: A) Recycling e-PRC reductive dimerization of benzylic chlorides using a [Cu2] catalyst. B) Proposed...

Figure 75: A) Decoupled photoelectrochemical C–H alkylation of heteroarenes through deamination of Katritzky s...

Figure 76: Proposed mechanism by Chen and co-workers.
Dipeptide analogues of fluorinated aminophosphonic acid sodium salts as moderate competitive inhibitors of cathepsin C
- Karolina Wątroba,
- Małgorzata Pawełczak and
- Marcin Kaźmierczak
Beilstein J. Org. Chem. 2023, 19, 434–439, doi:10.3762/bjoc.19.33
- Poznań, Uniwersytetu Poznańskiego 10, 61-614 Poznań, Poland 10.3762/bjoc.19.33 Abstract In this paper, we present the solvolysis reaction of dipeptide analogues of fluorinated aminophosphonates with simultaneous quantitative deprotection of the amino group. To the best of our knowledge, this work is the
- development of fluorinated aminophosphonate-based inhibitors. Keywords: aminophosphonates; cathepsin C; dipeptide; fluorine; solvolysis; Introduction Cathepsin C, also known as dipeptidyl peptidase I (DPPI) belongs to the family of lysosomal cysteine proteases encompassing 11 human enzymes (cathepsins B, C
- with nucleophilic deoxyfluorinating reagents often does not lead to the expected products with a fluorine atom in place of the –OH group. They usually undergo rearrangement, and intramolecular cyclization leading to products that are constitutional isomers [15]. The solvolysis reaction of phosphonates

Graphical Abstract

Scheme 1: Synthetic strategy towards 5 and 7.

Scheme 2: Synthesis of 9 and 11. (a) R = -CH3; (b) R = -CH(CH3)2; (c) R = -CH2CH(CH3)2; (d) R = -CH(CH3)CH2CH3...

Figure 1: Dixon plot for the hydrolysis of Gly-Phe-pNA substrate catalyzed by bovine cathepsin C in the prese...
Synthesis of (−)-halichonic acid and (−)-halichonic acid B
- Keith P. Reber and
- Emma L. Niner
Beilstein J. Org. Chem. 2022, 18, 1629–1635, doi:10.3762/bjoc.18.174
- oxocarbenium ion 16. Subsequent loss of the ethyl group (either as ethyl formate upon solvolysis with the formic acid co-solvent or as ethanol upon aqueous workup) gives lactone 9, which features a strained trans-fused 6/5 ring system. Although this lactone survives aqueous workup at neutral pH, it is rapidly

Graphical Abstract

Figure 1: Structures of halichonic acid ((+)-1) and halichonic acid B ((+)-2).

Scheme 1: Synthesis of (−)-7-amino-7,8-dihydrobisabolene (4) and its conversion to cyclization precursor 7.

Scheme 2: Synthesis of the halichonic acids via a key intramolecular aza-Prins cyclization.

Scheme 3: Proposed intermediates for the intramolecular aza-Prins reaction leading to the formation of ethyl ...
Iridium-catalyzed hydroacylation reactions of C1-substituted oxabenzonorbornadienes with salicylaldehyde: an experimental and computational study
- Angel Ho,
- Austin Pounder,
- Krish Valluru,
- Leanne D. Chen and
- William Tam
Beilstein J. Org. Chem. 2022, 18, 251–261, doi:10.3762/bjoc.18.30
- its derivatives were used in all calculations rather than the experimentally used precatalyst [Ir(COD)Cl]2, as ligand exchange with the hydroxide ions present likely generates the [Ir(COD)OH]2 species in solution. Upon monomerization of [Ir(COD)OH]2, either through solvolysis or coordination of the

Graphical Abstract

Scheme 1: Previously reported metal-catalyzed reactions of heterobicyclic alkenes and applications towards th...

Scheme 2: Iridium-catalyzed hydroacylation of C1-substituted OBDs 13a–k with salicylaldehyde 14.

Scheme 3: Competition reaction of different C1-substituted OBDs.

Figure 1: Potential energy profile of the PCM solvation model for the hydrometalation/reductive elimination p...

Figure 2: Potential energy profile of the PCM solvation model for the carbometalation/reductive elimination p...

Figure 3: Potential energy profile of the PCM solvation model for the endo hydrometalation/reductive eliminat...

Figure 4: Potential energy profile of the PCM solvation model for the Ir/diene-catalyzed hydroacylation of Me...
Mechanistic studies of the solvolysis of alkanesulfonyl and arenesulfonyl halides
- Malcolm J. D’Souza and
- Dennis N. Kevill
Beilstein J. Org. Chem. 2022, 18, 120–132, doi:10.3762/bjoc.18.13
- several studies on the solvolysis mechanisms for alkanesulfonyl chlorides (RSO2Cl) and arenesulfonyl chlorides (ArSO2Cl). The earlier of these studies were reviewed a little over thirty years ago by Gordon, Maskill and Ruasse (Chem. Soc. Rev. 1989, 18, 123–151) in a contribution entitled “Sulfonyl
- Transfer Reactions”. The present review will emphasize more recent contributions and, in particular, the application of the extended Grunwald–Winstein equation and kinetic solvent isotope effects to the solvolysis reactions. There is also an appreciable number of reports concerning the corresponding
- anhydrides with the chloride leaving group replaced by the appropriate sulfonate leaving group, concerning sulfamoyl chlorides (ZZ'NSO2Cl) with Z and Z' being alkyl or aryl and concerning the solvolysis of chlorosulfate esters (alkoxy- or aryloxysulfonyl chlorides), with the structures ROSO2Cl or ArOSO2Cl

Graphical Abstract

Scheme 1: Organic reactions where the breaking of a C–X bond involves the formation of a high energy ion-pair...

Scheme 2: The chemical structures for the 1-adamantyl substrate, 2-adamantyl substrate, and the S-methyldiben...

Figure 1: The SN2 reaction plot of log (k/ko) vs (1.26 NT + 0.65 YCl) for the solvolyses of benzesulfonyl chl...

Figure 2: The SN2 reaction plot of log (k/ko) vs (1.35 NT + 0.70 YCl) for the solvolyses of 2-thiophenesulfon...
Efficient and regioselective synthesis of dihydroxy-substituted 2-aminocyclooctane-1-carboxylic acid and its bicyclic derivatives
- İlknur Polat,
- Selçuk Eşsiz,
- Uğur Bozkaya and
- Emine Salamci
Beilstein J. Org. Chem. 2022, 18, 77–85, doi:10.3762/bjoc.18.7
- expected product 9 failed. The product 8 was obtained as the major product in 80% yield, and the expected product 9 was formed as the minor product in 4% yield. We propose that diol isomer mixture 9 can be formed by solvolysis. The presence of the lactone ring in 8 was determined by 2D NMR spectroscopic
- . However, all attempts to isolate isomer mixture 11 failed. Again, we suggest that diol isomer mixture 11 can be formed by solvolysis. The presence of the lactone ring in 10 was determined by 2D NMR spectroscopic data (COSY and HMQC). The diagonal peak at 4.19 ppm has cross peaks with the protons

Graphical Abstract

Figure 1: Examples of natural products containing β-amino acids.

Scheme 1: Synthesis of cyclic β-amino acid 6.

Scheme 2: Epoxidation of Boc-protected amino ester 4 and hydrolysis of epoxide 7 with HCl(g)–MeOH.

Scheme 3: Reaction of epoxide 7 with NaHSO4 in methylene chloride/MeOH.

Figure 2: The X-ray crystal structure of 10.

Scheme 4: Synthesis of cyclic β-amino acid derivative 8.

Scheme 5: Suggested mechanism for the reaction of epoxide 7 with NaHSO4.

Figure 3: Solvent-corrected relative free energy profile at 298.15 K for the reaction mechanism of 14 shown i...

Figure 4: Solvent-corrected relative free energy profile at 298.15 K for the reaction mechanism of 17 shown i...

Figure 5: The optimized geometries of the conformers 7a and 7b with selected interatomic distances at the B3L...
Prins cyclization-mediated stereoselective synthesis of tetrahydropyrans and dihydropyrans: an inspection of twenty years
- Asha Budakoti,
- Pradip Kumar Mondal,
- Prachi Verma and
- Jagadish Khamrai
Beilstein J. Org. Chem. 2021, 17, 932–963, doi:10.3762/bjoc.17.77
- for the formation of 48a by exclusively using TMSBr as a Lewis acid, as shown in Scheme 11. The mechanistic rationale for an axially selective Prins cyclization is explained in Scheme 12 [38]. It is proposed that the reaction of 49 with TMSBr forms an intermediate 50, which, after solvolysis, affords

Graphical Abstract

Scheme 1: General strategy for the synthesis of THPs.

Scheme 2: Developments towards the Prins cyclization.

Scheme 3: General stereochemical outcome of the Prins cyclization.

Scheme 4: Regioselectivity in the Prins cyclization.

Scheme 5: Mechanism of the oxonia-Cope reaction in the Prins cyclization.

Scheme 6: Cyclization of electron-deficient enantioenriched alcohol 27.

Scheme 7: Partial racemization through 2-oxonia-Cope allyl transfer.

Scheme 8: Partial racemization by reversible 2-oxonia-Cope rearrangement.

Scheme 9: Rychnovsky modification of the Prins cyclization.

Scheme 10: Synthesis of (−)-centrolobine and the C22–C26 unit of phorboxazole A.

Scheme 11: Axially selective Prins cyclization by Rychnovsky et al.

Scheme 12: Mechanism for the axially selectivity Prins cyclization.

Scheme 13: Mukaiyama aldol–Prins cyclization reaction.

Scheme 14: Application of the aldol–Prins reaction.

Scheme 15: Hart and Bennet's acid-promoted Prins cyclization.

Scheme 16: Tetrahydropyran core of polycarvernoside A as well as (−)-clavoslide A and D.

Scheme 17: Scheidt and co-workers’ route to tetrahydropyran-4-one.

Scheme 18: Mechanism for the Lewis acid-catalyzed synthesis of tetrahydropyran-4-one.

Scheme 19: Hoveyda and co-workers’ strategy for 2,6-disubstituted 4-methylenetetrahydropyran.

Scheme 20: Funk and Cossey’s ene-carbamates strategy.

Scheme 21: Yadav and Kumar’s cyclopropane strategy for THP synthesis.

Scheme 22: 2-Arylcylopropylmethanolin in centrolobine synthesis.

Scheme 23: Yadav and co-workers’ strategy for the synthesis of THP.

Scheme 24: Yadav and co-workers’ Prins–Ritter reaction sequence for 4-amidotetrahydropyran.

Scheme 25: Yadav and co-workers’ strategy to prelactones B, C, and V.

Scheme 26: Yadav and co-workers’ strategy for the synthesis of (±)-centrolobine.

Scheme 27: Loh and co-workers’ strategy for the synthesis of zampanolide and dactylolide.

Scheme 28: Loh and Chan’s strategy for THP synthesis.

Scheme 29: Prins cyclization of cyclohexanecarboxaldehyde.

Scheme 30: Prins cyclization of methyl ricinoleate (127) and benzaldehyde (88).

Scheme 31: AlCl3-catalyzed cyclization of homoallylic alcohol 129 and aldehyde 130.

Scheme 32: Martín and co-workers’ stereoselective approach for the synthesis of highly substituted tetrahydrop...

Scheme 33: Ene-IMSC strategy by Marko and Leroy for the synthesis of tetrahydropyran.

Scheme 34: Marko and Leroy’s strategy for the synthesis of tetrahydropyrans 146.

Scheme 35: Sakurai dimerization/macrolactonization reaction for the synthesis of cyanolide A.

Scheme 36: Hoye and Hu’s synthesis of (−)-dactyloide by intramolecular Sakurai cyclization.

Scheme 37: Minehan and co-workers’ strategy for the synthesis of THPs 157.

Scheme 38: Yu and co-workers’ allylic transfer strategy for the construction of tetrahydropyran 161.

Scheme 39: Reactivity enhancement in intramolecular Prins cyclization.

Scheme 40: Floreancig and co-workers’ Prins cyclization strategy to (+)-dactyloide.

Scheme 41: Panek and Huang’s DHP synthesis from crotylsilanes: a general strategy.

Scheme 42: Panek and Huang’s DHP synthesis from syn-crotylsilanes.

Scheme 43: Panek and Huang’s DHP synthesis from anti-crotylsilanes.

Scheme 44: Roush and co-workers’ [4 + 2]-annulation strategy for DHP synthesis [82].

Scheme 45: TMSOTf-promoted annulation reaction.

Scheme 46: Dobb and co-workers’ synthesis of DHP.

Scheme 47: BiBr3-promoted tandem silyl-Prins reaction by Hinkle et al.

Scheme 48: Substrate scope of Hinkle and co-workers’ strategy.

Scheme 49: Cho and co-workers’ strategy for 2,6 disubstituted 3,4-dimethylene-THP.

Scheme 50: Furman and co-workers’ THP synthesis from propargylsilane.

Scheme 51: THP synthesis from silyl enol ethers.

Scheme 52: Rychnovsky and co-workers’ strategy for THP synthesis from hydroxy-substituted silyl enol ethers.

Scheme 53: Li and co-workers’ germinal bissilyl Prins cyclization strategy to (−)-exiguolide.

Scheme 54: Xu and co-workers’ hydroiodination strategy for THP.

Scheme 55: Wang and co-workers’ strategy for tetrahydropyran synthesis.

Scheme 56: FeCl3-catalyzed synthesis of DHP from alkynylsilane alcohol.

Scheme 57: Martín, Padrón, and co-workers’ proposed mechanism of alkynylsilane Prins cyclization for the synth...

Scheme 58: Marko and co-workers’ synthesis of 2,6-anti-configured tetrahydropyran.

Scheme 59: Loh and co-workers’ strategy for 2,6-syn-tetrahydropyrans.

Scheme 60: Loh and co-workers’ strategy for anti-THP synthesis.

Scheme 61: Cha and co-workers’ strategy for trans-2,6-tetrahydropyran.

Scheme 62: Mechanism proposed by Cha et al.

Scheme 63: TiCl4-mediated cyclization to trans-THP.

Scheme 64: Feng and co-workers’ FeCl3-catalyzed Prins cyclization strategy to 4-hydroxy-substituted THP.

Scheme 65: Selectivity profile of the Prins cyclization under participation of an iron ligand.

Scheme 66: Sequential reactions involving Prins cyclization.

Scheme 67: Banerjee and co-workers’ strategy of Prins cyclization from cyclopropane carbaldehydes and propargy...

Scheme 68: Mullen and Gagné's (R)-[(tolBINAP)Pt(NC6F5)2][SbF6]2-catalyzed asymmetric Prins cyclization strateg...

Scheme 69: Yu and co-workers’ DDQ-catalyzed asymmetric Prins cyclization strategy to trisubstituted THPs.

Scheme 70: Lalli and Weghe’s chiral-Brønsted-acid- and achiral-Lewis-acid-promoted asymmetric Prins cyclizatio...

Scheme 71: List and co-workers’ iIDP Brønsted acid-promoted asymmetric Prins cyclization strategy.

Scheme 72: Zhou and co-workers’ strategy for chiral phosphoric acid (CPA)-catalyzed cascade Prins cyclization.

Scheme 73: List and co-workers’ approach for asymmetric Prins cyclization using chiral imidodiphosphoric acid ...
Valorisation of plastic waste via metal-catalysed depolymerisation
- Francesca Liguori,
- Carmen Moreno-Marrodán and
- Pierluigi Barbaro
Beilstein J. Org. Chem. 2021, 17, 589–621, doi:10.3762/bjoc.17.53

- ], catalytic cracking [56] and gasification [57]. These are usually unselective, high-temperature treatments (300–1000 °C) that may efficiently provide light hydrocarbons or small molecules [58][59]. Chemolytic processes, wherein a chemical reagent is used to achieve depolymerisation, mainly involve solvolysis

Graphical Abstract

Figure 1: Potential classification of plastic recycling processes. The area covered by the present review is ...

Figure 2: EG produced during glycolytic depolymerisation of PET using DEG + DPG as solvent and titanium(IV) n...

Scheme 1: Simplified representation of the conversion of 1,4-PBD to C16–C44 macrocycles using Ru metathesis c...

Figure 3: Main added-value monomers obtainable by catalytic depolymerisation of PET via chemolytic methods.

Scheme 2: Hydrogenolytic depolymerisation of PET by ruthenium complexes.

Scheme 3: Depolymerisation of PET via catalytic hydrosilylation by Ir(III) pincer complex.

Scheme 4: Catalytic hydrolysis (top) and methanolysis (bottom) reactions of PET.

Scheme 5: Depolymerisation of PET by glycolysis with ethylene glycol.

Figure 4: Glycolysis of PET: evolution of BHET yield over time, with and without zinc acetate catalyst (196 °...

Scheme 6: Potential activated complex for the glycolysis reaction of PET catalysed by metallated ILs and evol...

Scheme 7: One-pot, two-step process for PET repurposing via chemical recycling.

Scheme 8: Synthetic routes to PLA.

Scheme 9: Structures of the zinc molecular catalysts used for PLA-methanolysis in various works. a) See [265], b) ...

Scheme 10: Depolymerisation of PLLA by Zn–N-heterocyclic carbene complex.

Scheme 11: Salalen ligands.

Scheme 12: Catalytic hydrogenolysis of PLA.

Scheme 13: Catalytic hydrosilylation of PLA.

Scheme 14: Hydrogenative depolymerisation of PBT and PCL by molecular Ru catalysts.

Scheme 15: Glycolysis reaction of PCT by diethylene glycol.

Scheme 16: Polymerisation–depolymerisation cycle of 3,4-T6GBL.

Scheme 17: Polymerisation–depolymerisation cycle of 2,3-HDB.

Scheme 18: Hydrogenative depolymerisation of PBPAC by molecular Ru catalysts.

Scheme 19: Catalytic hydrolysis (top), alcoholysis (middle) and aminolysis (bottom) reactions of PBPAC.

Scheme 20: Hydrogenative depolymerisation of PPC (top) and PEC (bottom) by molecular Ru catalysts.

Scheme 21: Polymerisation-depolymerisation cycle of BEP.

Scheme 22: Hydrogenolysis of polyamides using soluble Ru catalysts.

Scheme 23: Catalytic depolymerisation of epoxy resin/carbon fibres composite.

Scheme 24: Depolymerisation of polyethers with metal salt catalysts and acyl chlorides.

Scheme 25: Proposed mechanism for the iron-catalysed depolymerisation reaction of polyethers. Adapted with per...
CF3-substituted carbocations: underexploited intermediates with great potential in modern synthetic chemistry
- Anthony J. Fernandes,
- Armen Panossian,
- Bastien Michelet,
- Agnès Martin-Mingot,
- Frédéric R. Leroux and
- Sébastien Thibaudeau
Beilstein J. Org. Chem. 2021, 17, 343–378, doi:10.3762/bjoc.17.32

- 5) [38]. Tidwell et al. explored the influence of a CF3 group on the solvolysis reaction of various benzylic sulfonate derivatives [39][40]. They found a linear free-energy relationship between the solvolysis rate of sulfonate 13f in different solvents compared to the one of 2-adamantyl tosylate
- , the latter being known to undergo solvolysis via the formation of a carbenium ion. Hence, the formation of a highly destabilized α-(trifluoromethyl)carbenium ion 14fOTs was established as the rate-limiting step in the solvolysis reactions of 13f (Scheme 6). Furthermore, the authors determined a kCH3
- /kCD3 ratio of 1.54, highlighting an isotopic effect consistent with a solvolysis mechanism involving a carbenium ion (kCH3/kCD3 = 1.48 for 2-methyl-2-adamantyl tosylate). Also, kH/kCF3 = 2⋅105 was established, illustrating the retarding α-CF3 effect in the production of a carbenium ion [41]. In the

Graphical Abstract

Figure 1: Stabilizing interaction in the CF3CH2+ carbenium ion (top) and structure of the first observable fl...

Scheme 1: Isodesmic equations accounting for the destabilizing effect of the CF3 group. ΔE in kcal⋅mol−1, cal...

Scheme 2: Stabilizing effect of fluorine atoms by resonance electron donation in carbenium ions (δ in ppm).

Scheme 3: Direct in situ NMR observation of α-(trifluoromethyl)carbenium ion or protonated alcohols. Δδ = δ19...

Scheme 4: Reported 13C NMR chemical shifts for the α-(trifluoromethyl)carbenium ion 10c (δ in ppm).

Scheme 5: Direct NMR observation of α-(trifluoromethyl)carbenium ions in situ (δ in ppm).

Scheme 6: Illustration of the ion pair solvolysis mechanism for sulfonate 13f. YOH = solvent.

Figure 2: Solvolysis rate for 13a–i and 17.

Figure 3: Structures of allyl triflates 18 and 19 and allyl brosylate 20. Bs = p-BrC6H4SO2.

Figure 4: Structure of tosylate derivatives 21.

Figure 5: a) Structure of triflate derivatives 22. b) Stereochemistry outcomes of the reaction starting from (...

Scheme 7: Solvolysis reaction of naphthalene and anthracenyl derivatives 26 and 29.

Figure 6: Structure of bisarylated derivatives 34.

Figure 7: Structure of bisarylated derivatives 36.

Scheme 8: Reactivity of 9c in the presence of a Brønsted acid.

Scheme 9: Cationic electrocyclization of 38a–c under strongly acidic conditions.

Scheme 10: Brønsted acid-catalyzed synthesis of indenes 42 and indanes 43.

Scheme 11: Reactivity of sulfurane 44 in triflic acid.

Scheme 12: Solvolysis of triflate 45f in alcoholic solvents.

Scheme 13: Synthesis of labeled 18O-52.

Scheme 14: Reactivity of sulfurane 53 in triflic acid.

Figure 8: Structure of tosylates 56 and 21f.

Scheme 15: Resonance forms in benzylic carbenium ions.

Figure 9: Structure of pyrrole derivatives 58 and 59.

Scheme 16: Resonance structure 60↔60’.

Scheme 17: Ga(OTf)3-catalyzed synthesis of 3,3’- and 3,6’-bis(indolyl)methane from trifluoromethylated 3-indol...

Scheme 18: Proposed reaction mechanism.

Scheme 19: Metal-free 1,2-phosphorylation of 3-indolylmethanols.

Scheme 20: Superacid-mediated arylation of thiophene derivatives.

Scheme 21: In situ mechanistic NMR investigations.

Scheme 22: Proposed mechanisms for the prenyltransferase-catalyzed condensation.

Scheme 23: Influence of a CF3 group on the allylic SN1- and SN2-mechanism-based reactions.

Scheme 24: Influence of the CF3 group on the condensation reaction.

Scheme 25: Solvolysis of 90 in TFE.

Scheme 26: Solvolysis of allyl triflates 94 and 97 and isomerization attempt of 96.

Scheme 27: Proposed mechanism for the formation of 95.

Scheme 28: Formation of α-(trifluoromethyl)allylcarbenium ion 100 in a superacid.

Scheme 29: Lewis acid activation of CF3-substituted allylic alcohols.

Scheme 30: Bimetallic-cluster-stabilized α-(trifluoromethyl)carbenium ions.

Scheme 31: Reactivity of cluster-stabilized α-(trifluoromethyl)carbenium ions.

Scheme 32: α-(Trifluoromethyl)propargylium ion 122↔122’ generated from silyl ether 120 in a superacid.

Scheme 33: Formation of α-(trifluoromethyl)propargylium ions from CF3-substituted propargyl alcohols.

Scheme 34: Direct NMR observation of the protonation of some trifluoromethyl ketones in situ and the correspon...

Scheme 35: Selected resonance forms in protonated fluoroketone derivatives.

Scheme 36: Acid-catalyzed Friedel–Crafts reactions of trifluoromethyl ketones 143a,b and 147a–c.

Scheme 37: Enantioselective hydroarylation of CF3-substituted ketones.

Scheme 38: Acid-catalyzed arylation of ketones 152a–c.

Scheme 39: Reactivity of 156 in a superacid.

Scheme 40: Reactivity of α-CF3-substituted heteroaromatic ketones and alcohols as well as 1,3-diketones.

Scheme 41: Reactivity of 168 with benzene in the presence of a Lewis or Brønsted acid.

Scheme 42: Acid-catalyzed three-component asymmetric reaction.

Scheme 43: Anodic oxidation of amines 178a–c and proposed mechanism.

Scheme 44: Reactivity of 179b in the presence of a strong Lewis acid.

Scheme 45: Trifluoromethylated derivatives as precursors of trifluoromethylated iminium ions.

Scheme 46: Mannich reaction with trifluoromethylated hemiaminal 189.

Scheme 47: Suitable nucleophiles reacting with 192 after Lewis acid activation.

Scheme 48: Strecker reaction involving the trifluoromethylated iminium ion 187.

Scheme 49: Reactivity of 199 toward nucleophiles.

Scheme 50: Reactivity of 204a with benzene in the presence of a Lewis acid.

Scheme 51: Reactivity of α-(trifluoromethyl)-α-chloro sulfides in the presence of strong Lewis acids.

Scheme 52: Anodic oxidation of sulfides 213a–h and Pummerer rearrangement.

Scheme 53: Mechanism for the electrochemical oxidation of the sulfide 213a.

Scheme 54: Reactivity of (trifluoromethyl)diazomethane (217a) in HSO3F.

Figure 10: a) Structure of diazoalkanes 217a–c and b) rate-limiting steps of their decomposition.

Scheme 55: Deamination reaction of racemic 221 and enantioenriched (S)-221.

Scheme 56: Deamination reaction of labeled 221-d2. Elimination products were formed in this reaction, the yiel...

Scheme 57: Deamination reaction of 225-d2. Elimination products were also formed in this reaction in undetermi...

Scheme 58: Formation of 229 from 228 via 1,2-H-shift.

Scheme 59: Deamination reaction of 230. Elimination products were formed in this reaction, the yield of which ...

Scheme 60: Deamination of several diazonium ions. Elimination products were formed in these reactions, the yie...

Scheme 61: Solvolysis reaction mechanism of alkyl tosylates.

Scheme 62: Solvolysis outcome for the tosylates 248 and 249 in HSO3FSbF5.

Figure 11: Solvolysis rate of 248, 249, 252, and 253 in 91% H2SO4.

Scheme 63: Illustration of the reaction pathways. TsCl, pyridine, −5 °C (A); 98% H2SO4, 30 °C (B); 98% H2SO4, ...

Scheme 64: Proposed solvolysis mechanism for the aliphatic tosylate 248.

Scheme 65: Solvolysis of the derivatives 259 and 260.

Scheme 66: Solvolysis of triflate 261. SOH = solvent.

Scheme 67: Intramolecular Friedel–Crafts alkylations upon the solvolysis of triflates 264 and 267.

Scheme 68: α-CF3-enhanced γ-silyl elimination of cyclobutyltosylates 270a,b.

Scheme 69: γ-Silyl elimination in the synthesis of a large variety of CF3-substituted cyclopropanes. Pf = pent...

Scheme 70: Synthetic pathways to 281. aNMR yields.

Scheme 71: The cyclopropyl-substituted homoallylcyclobutylcarbenium ion manifold.

Scheme 72: Reactivity of CF3-substituted cyclopropylcarbinyl derivatives 287a–c. LG = leaving group.

Scheme 73: Reactivity of CF3-substituted cyclopropylcarbinyl derivatives 291a–c.

Scheme 74: Superacid-promoted dimerization or TFP.

Scheme 75: Reactivity of TFP in a superacid.

Scheme 76: gem-Difluorination of α-fluoroalkyl styrenes via the formation of a “hidden” α-RF-substituted carbe...

Scheme 77: Solvolysis of CF3-substituted pentyne 307.

Scheme 78: Photochemical rearrangement of 313.

Figure 12: Structure of 2-norbornylcarbenium ion 318 and argued model for the stabilization of this cation.

Figure 13: Structures and solvolysis rate (TFE, 25 °C) of the sulfonates 319–321. Mos = p-MeOC6H4SO2.

Scheme 79: Mechanism for the solvolysis of 323. SOH = solvent.

Scheme 80: Products formed by the hydrolysis of 328.

Scheme 81: Proposed carbenium ion intermediates in an equilibrium during the solvolysis of tosylates 328, 333,...
Metal-free synthesis of biarenes via photoextrusion in di(tri)aryl phosphates
- Hisham Qrareya,
- Lorenzo Meazza,
- Stefano Protti and
- Maurizio Fagnoni
Beilstein J. Org. Chem. 2020, 16, 3008–3014, doi:10.3762/bjoc.16.250
- investigations in the last decades [28][29][64][68]. Simple (electron-rich) monoaryl phosphates are known to undergo the photoheterolysis of the Ar–O bond to form aryl cations [28][36]. The presence of an electron-withdrawing group (e.g., NO2) may, however, divert the reactivity since a photoinduced solvolysis

Graphical Abstract

Scheme 1: Synthesis of biarenes via a) photogenerated triplet aryl cations and aryl radicals (PC = photocatal...

Scheme 2: Metal-free photochemical synthesis of biaryls 2 and 4.

Figure 1: Emission spectrum of compound 1e (red) and of diethyl p-tert-butylphenyl phosphate (black) in metha...

Figure 2: Emission spectrum of compound 1h (red) and of diethyl p-cyanophenyl phosphate (black) in methanol.

Figure 3: Emission spectrum of compound 3a in methanol (black) and in a methanol/TFE 4:1 mixture (red).

Figure 4: Emission spectrum of 3c in MeOH (dotted line) and in the presence of increasing amounts of TFE (up ...

Scheme 3: Photoreactivity of aryl phosphates 1 and 3 in protic media.
Synthetic approaches to bowl-shaped π-conjugated sumanene and its congeners
- Shakeel Alvi and
- Rashid Ali
Beilstein J. Org. Chem. 2020, 16, 2212–2259, doi:10.3762/bjoc.16.186

- reported hydroxysumanene 68 by means of a Baeyer–Villiger oxidation reaction of acyl- or formylsumanene derivatives in the presence of m-chloroperbenzoic acid (m-CPBA) followed by acid-catalyzed solvolysis in 10% HCl/MeOH (Scheme 15) [46]. Interestingly, it was found from both theoretically as well as

Graphical Abstract

Figure 1: Representation of corannulene (1) and sumanene (2), the subunits of fullerene (C60).

Scheme 1: Mehta’s unsuccessful effort for the synthesis of sumanene scaffold 2.

Scheme 2: First synthesis of sumanene 2 by Sakurai et al. from norbornadiene 10.

Scheme 3: Synthesis of trimethylsumanene 28 from easily accessible norbornadiene (10).

Scheme 4: Generation of anions 29–31 and the preparation of tris(trimethylsilyl)sumanene 32.

Scheme 5: Synthesis of tri- and hexa-substituted sumanene derivatives.

Scheme 6: Synthesis of bowl-shaped π-extended sumanene derivatives 37a–f.

Scheme 7: Synthesis of monooxasumanene 38, trioxosumanene 40 along with imination of them.

Scheme 8: Synthesis of trimethylsumanenetrione 46 and exo-functionalized products 45a,b.

Scheme 9: Synthesis of bisumanenylidene 47 and sumanene dimer 48 from 2.

Scheme 10: The mono-substitution of 2 to generate diverse mono-sumanene derivatives 49a–d.

Scheme 11: Synthesis of sumanene building block 53 useful for further extension.

Scheme 12: Synthesis of hexafluorosumanene derivative 55 by Sakurai and co-workers.

Scheme 13: Preparation of sumanene-based carbene 60 and its reaction with cyclohexane.

Scheme 14: Barton–Kellogg reaction for the synthesis of sterically hindered alkenes.

Scheme 15: Synthesis of hydroxysumanene 68 by employing Baeyer–Villiger oxidation.

Scheme 16: Synthesis of sumanene derivatives having functionality at an internal carbon.

Scheme 17: Mechanism for nucleophilic substitution reaction at the internal carbon.

Scheme 18: Synthesis of diverse monosubstituted sumanene derivatives.

Scheme 19: Synthesis of di- and trisubstituted sumanene derivatives from sumanene (2).

Scheme 20: Preparation of monochlorosumanene 88 and hydrogenation of sumanene (2).

Scheme 21: The dimer 90 and bissumanenyl 92 achieved from halosumannes.

Scheme 22: Pyrenylsumanene 93 involving the Suzuki-coupling as a key transformation.

Scheme 23: Synthesis of various hexaarylsumanene derivatives using the Suzuki-coupling reaction.

Scheme 24: Synthesis of hexasubstituted sumanene derivatives 96 and 97.

Scheme 25: Synthesis of thioalkylsumanenes via an aromatic nucleophilic substitution reaction.

Scheme 26: Synthesis of tris(ethoxycarbonylethenyl)sumanene derivative 108.

Scheme 27: Synthesis of ferrocenyl-based sumanene derivatives.

Scheme 28: Synthesis of sumanenylferrocene architectures 118 and 119 via Negishi coupling.

Scheme 29: Diosmylation and the synthesis of phenylboronate ester 121 of sumanene.

Scheme 30: Synthesis of the iron-complex of sumanene.

Scheme 31: Synthesis of tri- and mononuclear sumanenyl zirconocene complexes.

Scheme 32: Synthesis of [CpRu(η6-sumanene)]PF6.

Scheme 33: Preparation of sumanene-based porous coordination networks 127 (spherical tetramer units) and 128 (...

Scheme 34: Synthesis of sumanenylhafnocene complexes 129 and 130.

Scheme 35: Synthesis of 134 and 135 along with PdII coordination complex 136.

Scheme 36: Synthesis of alkali metals sumanene complex K7(C21H102−)2(C21H93−)·8THF (137) containing di- and tr...

Scheme 37: The encapsulation of a Cs+ ion between two sumanenyl anions.

Scheme 38: Synthesis of monothiasumanene 140 and dithiasumanene 141 from 139.

Scheme 39: Synthesis of trithiasumanene 151 by Otsubo and his co-workers.

Scheme 40: Synthesis of trithiasumanene derivatives 155 and 156.

Scheme 41: Synthetic route towards hexathiolated trithiasumanenes 158.

Scheme 42: Synthesis of triselenasumanene 160 by Shao and teammates.

Scheme 43: Synthesis of tritellurasumanene derivatives from triphenylene skeletons.

Scheme 44: Synthesis of pyrazine-fused sumanene architectures through condensation reaction.

Scheme 45: Treatment of the trichalcogenasumanenes with diverse oxidative reagents.

Scheme 46: Ring-opening reaction with H2O2 and oxone of heterasumanenes 178 and 179.

Scheme 47: Synthesis of polycyclic compounds from sumanene derivatives.

Scheme 48: Synthesis of diimide-based heterocycles reported by Shao’s and co-workers.

Scheme 49: Synthesis of pristine trichalcogenasumanenes, 151, 205, and 206.

Scheme 50: Synthesis of trichalcogenasumanenes via hexaiodotriphenylene precursor 208.

Scheme 51: Synthesis of trisilasumanenes 214 and 215.

Scheme 52: Synthesis of trisilasumanene derivatives 218 and 219.

Scheme 53: Synthesis of novel trigermasumanene derivative 223.

Scheme 54: An attempt towards the synthesis of tristannasumanene derivative 228.

Scheme 55: Synthesis of triphosphasumanene trisulfide 232 from commercially available 229.

Scheme 56: The doping of sumanene derivatives with chalcogens (S, Se, Te) and phosphorus.

Scheme 57: Synthesis of heterasumanene containing three different heteroatoms.

Scheme 58: Synthesis of trichalcogenasumanene derivatives 240 and 179.

Scheme 59: Preparation of trichalcogenasumanenes 245 and 248.

Scheme 60: Design and synthesis of trichalcogenasumanene derivatives 252 and 178.

Scheme 61: Synthesis of spirosumanenes 264–269 and non-spiroheterasumanenes 258–263.

Scheme 62: Synthesis of sumanene-type hetero polycyclic compounds.

Scheme 63: Synthesis of triazasumanenes 288 and its sulfone congener 287.

Scheme 64: Synthesis of C3-symmetric chiral triaryltriazasumanenes via cross-coupling reaction.

Scheme 65: Synthesis of mononaphthosumanene 293 using Suzuki coupling as a key step.

Scheme 66: Synthesis of di- and trinaphthosumanene derivatives 302–304.

Scheme 67: Synthesis of hemifullerene skeletons by Hirao’s group.

Scheme 68: Design and construction of C70 fragment from a C60 sumanene fragment.
Selective preparation of tetrasubstituted fluoroalkenes by fluorine-directed oxetane ring-opening reactions
- Clément Q. Fontenelle,
- Thibault Thierry,
- Romain Laporte,
- Emmanuel Pfund and
- Thierry Lequeux
Beilstein J. Org. Chem. 2020, 16, 1936–1946, doi:10.3762/bjoc.16.160
- resulted in slow solvolysis of alcohol 1d with acetic acid giving mainly the corresponding acetate (not shown). Product 1d was also obtained in a similar E/Z ratio (88:12) when the ring-opening reaction was performed in dichloromethane with tetrabutylammonium bromide (TBAB) as the bromide source and boron

Graphical Abstract

Figure 1: Representative fluorinated nucleos(t)ides and acyclonucleotides.

Figure 2: Acyclonucleotides as nucleotide surrogates.

Figure 3: Olefination approaches and ring-opening of oxetane derivatives.

Scheme 1: Preparation of fluoroakylidene-oxetanes and their ring-opening reactions.

Scheme 2: Synthesis of benzyloxy-substituted fluoroethylidene-oxetane derivative 8.

Scheme 3: Effect of the medium on the selective formation of derivative 10.

Scheme 4: Mechanism for the formation of dihydrofuran 10.

Scheme 5: Mechanism for the formation of unsaturated lactones 14 and 15.

Scheme 6: Opening reaction of ethyl 2-(oxetanyl-3-idene)acetate (16).

Scheme 7: Functionalization of bromomethyllactone 15 and its analogues.

Scheme 8: Functionalization by substitution reaction of the bromide E-1d vs ring-opening reaction of the oxet...

Scheme 9: Preparation of tetrasubstituted fluoroalkenes.
The McKenna reaction – avoiding side reactions in phosphonate deprotection
- Katarzyna Justyna,
- Joanna Małolepsza,
- Damian Kusy,
- Waldemar Maniukiewicz and
- Katarzyna M. Błażewska
Beilstein J. Org. Chem. 2020, 16, 1436–1446, doi:10.3762/bjoc.16.119
- ester [9], which is cleaved in the second step, upon solvolysis, forming the final product (Scheme 1). Bromotrimethylsilane (BTMS) is the main reagent in this reaction and is also known for its ability to cleave lactones, epoxides, acetals, and ethers [10]. BTMS also acted as a brominating agent and
- the side reactions, which originate from BTMS itself, and those resulting from not having taken appropriate precautions during the subsequent solvolysis step. By being alerted to the water-sensitive character of BTMS and the formation of the alkylating agent alkyl bromide (Scheme 1), side reactions
- occurring during the silylation step could be usually prevented. On the other hand, the side reactions during the solvolysis step are usually obviated by the use of a buffer or weak base, which neutralize the final organophosphorus acids. Herein we mainly addressed the problems related to the silylation

Graphical Abstract

Scheme 1: Schematic overview of the McKenna reaction including the decomposition of BTMS in protic solvents. ...

Figure 1: The model compounds used for this study (in red: the functionality of the molecules vulnerable to s...

Scheme 2: Formation of the side products derived from 10. Conditions: An equimolar mixture of propargylamide ...

Scheme 3: Addition of HBr to compound 11.

Scheme 4: N-Alkylation of 9.

Scheme 5: N-Alkylation of 12.

Scheme 6: Exchange of the chlorine substituent with bromine in 2-chloro-N-phenethylacetamide (13) under McKen...
Recent applications of porphyrins as photocatalysts in organic synthesis: batch and continuous flow approaches
- Rodrigo Costa e Silva,
- Luely Oliveira da Silva,
- Aloisio de Andrade Bartolomeu,
- Timothy John Brocksom and
- Kleber Thiago de Oliveira
Beilstein J. Org. Chem. 2020, 16, 917–955, doi:10.3762/bjoc.16.83
- the aryldiazonium salt leads to the other aryl radical species (path b, Scheme 9). The thio- and selenoethers were obtained after solvolysis. Excellent yields (up to 94%) were reported for both selenylation and thiolation of anilines with electron-withdrawing and electron-donating groups in the ortho

Graphical Abstract

Figure 1: Chemical structures of the porphyrinoids and their absorption spectra: in bold are highlighted the ...

Figure 2: Photophysical and photochemical processes (Por = porphyrin). Adapted from [12,18].

Figure 3: Main dual photocatalysts and their oxidative/reductive excited state potentials, including porphyri...

Scheme 1: Photoredox alkylation of aldehydes with diazo acetates using porphyrins and a Ru complex. aUsing a ...

Scheme 2: Proposed mechanism for the alkylation of aldehydes with diazo acetates in the presence of TPP.

Scheme 3: Arylation of heteroarenes with aryldiazonium salts using TPFPP as photocatalyst, and corresponding ...

Scheme 4: A) Scope with different aryldiazonium salts and enol acetates. B) Photocatalytic cycles and compari...

Scheme 5: Photoarylation of isopropenyl acetate A) Comparison between batch and continuous-flow approaches an...

Scheme 6: Dehalogenation induced by red light using thiaporphyrin (STPP).

Scheme 7: Applications of NiTPP as both photoreductant and photooxidant.

Scheme 8: Proposed mechanism for obtaining tetrahydroquinolines by reductive quenching.

Scheme 9: Selenylation and thiolation of anilines.

Scheme 10: NiTPP as photoredox catalyst in oxidative and reductive quenching, in comparison with other photoca...

Scheme 11: C–O bond cleavage of 1-phenylethanol using a cobalt porphyrin (CoTMPP) under visible light.

Scheme 12: Hydration of terminal alkynes by RhIII(TSPP) under visible light irradiation.

Scheme 13: Regioselective photocatalytic hydro-defluorination of perfluoroarenes by RhIII(TSPP).

Scheme 14: Formation of 2-methyl-2,3-dihydrobenzofuran by intramolecular hydro-functionalization of allylpheno...

Scheme 15: Photocatalytic oxidative hydroxylation of arylboronic acids using UNLPF-12 as heterogeneous photoca...

Scheme 16: Photocatalytic oxidative hydroxylation of arylboronic acids using MOF-525 as heterogeneous photocat...

Scheme 17: Preparation of the heterogeneous photocatalyst CNH.

Scheme 18: Photoinduced sulfonation of alkenes with sulfinic acid using CNH as photocatalyst.

Scheme 19: Sulfonic acid scope of the sulfonation reactions.

Scheme 20: Regioselective sulfonation reaction of arimistane.

Scheme 21: Synthesis of quinazolin-4-(3H)-ones.

Scheme 22: Selective photooxidation of aromatic benzyl alcohols to benzaldehydes using Pt/PCN-224(Zn).

Scheme 23: Photooxidation of benzaldehydes to benzoic acids using Pt or Pd porphyrins.

Scheme 24: Photocatalytic reduction of various nitroaromatics using a Ni-MOF.

Scheme 25: Photoinduced cycloadditions of CO2 with epoxides by MOF1.

Figure 4: Electronic configurations of the species of oxygen. Adapted from [66].

Scheme 26: TPP-photocatalyzed generation of 1O2 and its application in organic synthesis. Adapted from [67-69].

Scheme 27: Pericyclic reactions involving singlet oxygen and their mechanisms. Adapted from [67].

Scheme 28: First scaled up ascaridole preparation from α-terpinene.

Scheme 29: Antimalarial drug synthesis using an endoperoxidation approach.

Scheme 30: Photooxygenation of colchicine.

Scheme 31: Synthesis of (−)-pinocarvone from abundant (+)-α-pinene.

Scheme 32: Seeberger’s semi-synthesis of artemisinin.

Scheme 33: Synthesis of artemisinin using TPP and supercritical CO2.

Scheme 34: Synthesis of artemisinin using chlorophyll a.

Scheme 35: Quercitol stereoisomer preparation.

Scheme 36: Photocatalyzed preparation of naphthoquinones.

Scheme 37: Continuous endoperoxidation of conjugated dienes and subsequent rearrangements leading to oxidized ...

Scheme 38: The Opatz group total synthesis of (–)-oxycodone.

Scheme 39: Biomimetic syntheses of rhodonoids A, B, E, and F.

Scheme 40: α-Photooxygenation of chiral aldehydes.

Scheme 41: Asymmetric photooxidation of indanone β-keto esters by singlet oxygen using PTC as a chiral inducer...

Scheme 42: Asymmetric photooxidation of both β-keto esters and β-keto amides by singlet oxygen using PTC-2 as ...

Scheme 43: Bifunctional photo-organocatalyst used for the asymmetric oxidation of β-keto esters and β-keto ami...

Scheme 44: Mechanism of singlet oxygen oxidation of sulfides to sulfoxides.

Scheme 45: Controlled oxidation of sulfides to sulfoxides using protonated porphyrins as photocatalysts. aIsol...

Scheme 46: Photochemical oxidation of sulfides to sulfoxides using PdTPFPP as photocatalyst.

Scheme 47: Controlled oxidation of sulfides to sulfoxides using SnPor@PAF as a photosensitizer.

Scheme 48: Syntheses of 2D-PdPor-COF and 3D-Pd-COF.

Scheme 49: Photocatalytic oxidation of A) thioanisole to methyl phenyl sulfoxide and B) various aryl sulfides,...

Scheme 50: General mechanism for oxidation of amines to imines.

Scheme 51: Oxidation of secondary amines to imines.

Scheme 52: Oxidation of secondary amines using Pd-TPFPP as photocatalyst.

Scheme 53: Oxidative amine coupling using UNLPF-12 as heterogeneous photocatalyst.

Scheme 54: Synthesis of Por-COF-1 and Por-COF-2.

Scheme 55: Photocatalytic oxidation of amines to imines by Por-COF-2.

Scheme 56: Photocyanation of primary amines.

Scheme 57: Synthesis of ᴅ,ʟ-tert-leucine hydrochloride.

Scheme 58: Photocyanation of catharanthine and 16-O-acetylvindoline using TPP.

Scheme 59: Photochemical α-functionalization of N-aryltetrahydroisoquinolines using Pd-TPFPP as photocatalyst.

Scheme 60: Ugi-type reaction with 1,2,3,4-tetrahydroisoquinoline using molecular oxygen and TPP.

Scheme 61: Ugi-type reaction with dibenzylamines using molecular oxygen and TPP.

Scheme 62: Mannich-type reaction of tertiary amines using PdTPFPP as photocatalyst.

Scheme 63: Oxidative Mannich reaction using UNLPF-12 as heterogeneous photocatalyst.

Scheme 64: Transformation of amines to α-cyanoepoxides and the proposed mechanism.
Archangelolide: A sesquiterpene lactone with immunobiological potential from Laserpitium archangelica
- Silvie Rimpelová,
- Michal Jurášek,
- Lucie Peterková,
- Jiří Bejček,
- Vojtěch Spiwok,
- Miloš Majdl,
- Michal Jirásko,
- Miloš Buděšínský,
- Juraj Harmatha,
- Eva Kmoníčková,
- Pavel Drašar and
- Tomáš Ruml
Beilstein J. Org. Chem. 2019, 15, 1933–1944, doi:10.3762/bjoc.15.189
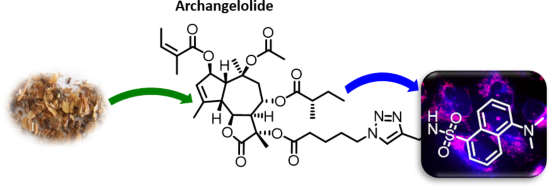
- ) of the dansyl-labeled archangelolide 5 started with a mild solvolysis of compound 1 by triethylamine in methanol. Surprisingly, the only product that we obtained after 48 h of treatment was 11-deacetylarchangelolide (3), in only 32% yield. The position in the structure of compound 1 where deacylation

Graphical Abstract

Figure 1: The structure of the sesquiterpene lactones archangelolide (1) and trilobolide (2).

Scheme 1: Reagents and conditions: a) MeOH, TEA, 48 h, yield 32%; b) (i) 5-azidopentanoic acid, DCC, DCM, 90 ...

Figure 2: Intracellular localization of archangelolide-dansyl (5) in human cells from osteosarcoma (U-2 OS). ...

Figure 3: Co-localization of dansylarchangelolide 5 with a marker of endoplasmic reticulum (top row) and with...

Figure 4: Cartoon representation of sarco/endoplasmic reticulum Ca2+ ATPase binding pocket with A, C) archang...

Figure 5: Molecular surface representation of sarco/endoplasmic reticulum Ca2+ ATPase binding pocket with A) ...

Figure 6: Structural formulae of (i) thapsigargin, (ii) trilobolide (2), and (iii) archangelolide (1). Red pa...

Figure 7: Viability of rat peritoneal cells treated with archangelolide (1), dansylarchangelolide 5 and dansy...

Figure 8: NO production in primary rat macrophages. The cells were treated with archangelolide (1) and dansyl...

Figure 9: Evaluation of cytokine TNF-α secretion in rat peritoneal cells. Stimulation of primary cells was in...

Figure 10: Structure of laserolide.
The cyclopropylcarbinyl route to γ-silyl carbocations
- Xavier Creary
Beilstein J. Org. Chem. 2019, 15, 1769–1780, doi:10.3762/bjoc.15.170
- solvolysis chemistry of mesylate and triflate derivatives of trans-1-hydroxymethyl-2-trimethylsilylcyclopropane and 1-substituted analogs can be quite different since these substrates do not generally lead to 3-trimethylsilylcyclobutyl cations. Keywords: bicyclobutane; carbocation; cyclopropylcarbinyl
- [12] leaving groups, reacted in aqueous solvents to give an identical mixture of products 3, 4, and 5 (Scheme 1). Additionally, solvolysis rates were far greater than expected for primary and strained secondary systems. To account for these facts, it has been suggested that there are common cationic
- intermediates in these solvolysis reactions of 1 and 2. Labelling [13][14][15], stable ion [16][17][18][19], and computational studies [19] implicate the involvement of three degenerate cyclopropylcarbinyl cations, 6a, 6b, and 6c, in equilibrium with cyclobutyl cation 7, as well as the homoallylic cation 8

Graphical Abstract

Scheme 1: Solvolyses of cyclopropylcarbinyl and cyclobutyl substrates.

Scheme 2: The cyclopropylcarbinyl–cyclobutyl–homoallyl cation manifold.

Figure 1: Electron-deficient carbocations.

Scheme 3: Solvolyses of γ-trimethylsilylcyclobutyl substrates.

Figure 2: Substrates of interest.

Scheme 4: Synthesis of mesylates 19 and 20.

Scheme 5: Reaction of mesylate 19 in CD3CO2D.

Scheme 6: Reaction of mesylate 20 in CD3CO2D.

Figure 3: M062X/6-311+G** calculated structures and relative energies of cations 24, 27, and transition state ...

Scheme 7: Synthesis of mesylates 31 and 32.

Scheme 8: Reaction of mesylate 31 in CD3CO2D.

Scheme 9: Reaction of mesylate 32 in CD3CO2D.

Scheme 10: Reaction of trifluoroacetate 48 in CD3CO2D.

Scheme 11: Bicyclobutane formation from a γ-trimethylsilyl cation.

Scheme 12: Formation of triflates 60 and 61.

Scheme 13: Formation of triflates 67, 68, and 69.

Scheme 14: Reactions of substrates with electron-withdrawing groups in CD3CO2D.

Figure 4: γ-Trimethylsilyl cations.

Scheme 15: Bicyclobutane formation from mesylate 76 in CH3CO2H.

Scheme 16: Reactions of triflates 60 and 67 in CD3CO2D.
Polyaminoazide mixtures for the synthesis of pH-responsive calixarene nanosponges
- Antonella Di Vincenzo,
- Antonio Palumbo Piccionello,
- Alberto Spinella,
- Delia Chillura Martino,
- Marco Russo and
- Paolo Lo Meo
Beilstein J. Org. Chem. 2019, 15, 633–641, doi:10.3762/bjoc.15.59
- , corresponding to the molecular formula C15H31N11O·H+, can be explained with the reaction of 1 with 1-bromo-3-methoxypropane, formed in situ by partial solvolysis of 4 (Scheme 5). A comprehensive reaction chart for mixture I is provided in Scheme 6, whereas the analogous chart for mixture II is reported in

Graphical Abstract

Scheme 1: Synthesis of the propargyloxy calixarene Ca.

Scheme 2: Synthesis of polyaminoazides from polyamines.

Scheme 3: Reaction of 1,3-dibromopropane (4) with sodium azide.

Scheme 4: Formation of the 1,3-oxazinan-2-one ring.

Scheme 5: Formation of the product at m/z 382.2765 u.

Scheme 6: Formation of the components of mixture I.

Figure 1: FTIR spectra (liquid) of mixture I (red) and mixture II (blue).

Figure 2: FTIR spectra (nujol) of Ca (red), CaNS-I (blue) and CaNS-II (green).

Figure 3: CP-MAS NMR spectra of CaNSs.

Figure 4: Structures of guests 6–15.
Quinolines from the cyclocondensation of isatoic anhydride with ethyl acetoacetate: preparation of ethyl 4-hydroxy-2-methylquinoline-3-carboxylate and derivatives
- Nicholas G. Jentsch,
- Jared D. Hume,
- Emily B. Crull,
- Samer M. Beauti,
- Amy H. Pham,
- Julie A. Pigza,
- Jacques J. Kessl and
- Matthew G. Donahue
Beilstein J. Org. Chem. 2018, 14, 2529–2536, doi:10.3762/bjoc.14.229
- chloride in THF [30]. Initially, the trimethylsilyl cyanohydrin 16 was subjected to solvolysis in ethanol with aqueous sulfuric acid. Unfortunately, those conditions resulted in displacement of the 4-chloro substituent with ethanol giving the 4-ethyl ether 17 in 35% yield. To circumvent this undesired

Graphical Abstract

Figure 1: Investigational non-catalytic HIV-1 Integrase inhibitors.

Scheme 1: Boehringer Ingelheim retrosynthesis of quinoline 1.

Scheme 2: Quinoline ring condensation strategies.

Scheme 3: Isatoic anhydrides from anthranilic acids with triphosgene.

Scheme 4: Substituted 2-methyl-4-hydroxyquinolines from isatoic anhydrides and ethyl acetoacetate.

Scheme 5: Mechanistic hypothesis for the cyclocondensation reaction.

Scheme 6: Quinoline synthesis with ethyl acetylpyruvate.

Scheme 7: Elaboration of the benzoic acid ethyl ester to the acetic acid residue.

Scheme 8: Umpolung addition of ethoxycarbonyl via a MAC strategy.
One hundred years of benzotropone chemistry
- Arif Dastan,
- Haydar Kilic and
- Nurullah Saracoglu
Beilstein J. Org. Chem. 2018, 14, 1120–1180, doi:10.3762/bjoc.14.98

Graphical Abstract

Scheme 1: Tropone (1), tropolone (2) and their resonance structures.

Figure 1: Natural products containing a tropone nucleus.

Figure 2: Possible isomers 11–13 of benzotropone.

Scheme 2: Synthesis of benzotropones 11 and 12.

Scheme 3: Oxidation products of benzotropylium fluoroborate (16).

Scheme 4: Oxidation of 7-bromo-5H-benzo[7]annulene (22).

Scheme 5: Synthesis of 4,5-benzotropone (11) using o-phthalaldehyde (27).

Scheme 6: Synthesis of 4,5-benzotropone (11) starting from oxobenzonorbornadiene 31.

Scheme 7: Acid-catalyzed cleavage of oxo-bridge of 34.

Scheme 8: Synthesis of 4,5-benzotropone (11) from o-xylylene dibromide (38).

Scheme 9: Synthesis of 4,5-benzotropone (11) via the carbene adduct 41.

Scheme 10: Heck coupling strategy for the synthesis of 11.

Scheme 11: Synthesis of benzofulvalenes via carbonyl group of 4,5-benzotropone (11).

Figure 3: Some cycloheptatrienylium cations.

Scheme 12: Synthesis of condensation product 63 and its subsequent oxidative cyclization products.

Figure 4: A novel series of benzo[7]annulenes prepared from 4,5-benzotropone (11).

Scheme 13: Preparation of substituted benzo[7]annulene 72 using the Mukaiyama-Michael reaction.

Figure 5: Possible benzo[7]annulenylidenes 73–75.

Scheme 14: Thermal and photochemical decomposition of 7-diazo-7H-benzo[7]annulene (76) and the trapping of int...

Scheme 15: Synthesis of benzoheptafulvalene 86.

Scheme 16: Synthesis of 7-(diphenylmethylene)-7H-benzo[7]annulene (89).

Scheme 17: Reaction of 4,5-benzotropone (11) with dimethyl diazomethane.

Scheme 18: Synthesis of dihydrobenzomethoxyazocine 103.

Scheme 19: Synthesis and reducibility of benzo-homo-2-methoxyazocines.

Scheme 20: Synthesis of 4,5-benzohomotropones 104 and 115 from 4,5-benzotropones 11 and 113.

Scheme 21: A catalytic deuterogenation of 4,5-benzotropone (11) and synthesis of 5-monosubstituted benzo[7]ann...

Scheme 22: Synthesis of methyl benzo[7]annulenes 131 and 132.

Scheme 23: Ambident reactivity of halobenzo[7]annulenylium cations 133a/b.

Scheme 24: Preparation of benzo[7]annulenylidene–iron complexes 147.

Scheme 25: Synthesis of 1-ethynylbenzotropone (150) and the etheric compound 152 from 4,5-benzotropone (11) wi...

Scheme 26: Thermal decomposition of 4,5-benzotropone (11).

Scheme 27: Reaction of 4,5-benzotropone (11) with 1,2-ethanediol and 1,2-ethanedithiol.

Scheme 28: Conversions of 1-benzosuberone (162) to 2,3-benzotropone (12).

Scheme 29: Synthesis strategies for 2,3-bezotropone (12) using 1-benzosuberones.

Scheme 30: Oxidation-based synthesis of 2,3-benzotropone (12) via 1-benzosuberone (162).

Scheme 31: Synthesis of 2,3-benzotropone (12) from α-tetralone (171) via ring-expansion.

Scheme 32: Preparation of 2,3-benzotropone (12) by using of benzotropolone 174.

Figure 6: Benzoheptafulvenes as condensation products of 2,3-benzotropone (12).

Scheme 33: Conversion of 2,3-benzotropone (12) to tosylhydrazone salt 182 and gem-dichloride 187.

Figure 7: Benzohomoazocines 191–193 and benzoazocines 194–197.

Scheme 34: From 2,3-benzotropone (12) to carbonium ions 198–201.

Scheme 35: Cycloaddition reactions of 2,3-benzotropone (12).

Scheme 36: Reaction of 2,3-benzotropone (12) with various reagents and compounds.

Figure 8: 3,4-Benzotropone (13) and its resonance structure.

Scheme 37: Synthesis of 6,7-benzobicyclo[3.2.0]hepta-3,6-dien-2-one (230).

Figure 9: Photolysis and thermolysis products of 230.

Figure 10: Benzotropolones and their tautomeric structures.

Scheme 38: Synthesis strategies of 4,5-benzotropolone (238).

Scheme 39: Synthesis protocol for 2-hydroxy-4,5-benzotropone (238) using oxazole-benzo[7]annulene 247.

Figure 11: Some quinoxaline and pyrazine derivatives 254–256 prepared from 4,5-benzotropolone (238).

Scheme 40: Nitration product of 4,5-benzotropolone (238) and its isomerization to 1-nitro-naphthoic acid (259)....

Scheme 41: Synthesis protocol for 6-hydroxy-2,3-benzotropone (239) from benzosuberone (162).

Scheme 42: Various reactions via 6-hydroxy-2,3-benzotropone (239).

Scheme 43: Photoreaction of 6-hydroxy-2,3-benzotropone (239).

Scheme 44: Synthesis of 7-hydroxy-2,3-benzotropone (241) from benzosuberone (162).

Scheme 45: Synthesis strategy for 7-hydroxy-2,3-benzotropone (241) from ketone 276.

Scheme 46: Synthesis of 7-hydroxy-2,3-benzotropone (241) from β-naphthoquinone (280).

Scheme 47: Synthesis of 7-hydroxy-2,3-benzotropone (241) from bicyclic endoperoxide 213.

Scheme 48: Synthesis of 7-hydroxy-2,3-benzotropone (241) by ring-closing metathesis.

Figure 12: Various monosubstitution products 289–291 of 7-hydroxy-2,3-benzotropone (241).

Scheme 49: Reaction of 7-hydroxy-2,3-benzotropone (241) with various reagents.

Scheme 50: Synthesis of 4-hydroxy-2,3-benzotropones 174 and 304 from diketones 300/301.

Scheme 51: Catalytic hydrogenation of diketones 300 and 174.

Scheme 52: Synthesis of halo-benzotropones from alkoxy-naphthalenes 306, 307 and 310.

Figure 13: Unexpected byproducts 313–315 during synthesis of chlorobenzotropone 309.

Figure 14: Some halobenzotropones and their cycloadducts.

Scheme 53: Multisep synthesis of 2-chlorobenzotropone 309.

Scheme 54: A multistep synthesis of 2-bromo-benzotropone 26.

Scheme 55: A multistep synthesis of bromo-2,3-benzotropones 311 and 316.

Scheme 56: Oxidation reactions of 8-bromo-5H-benzo[7]annulene (329) with some oxidants.

Scheme 57: Synthesis of 2-bromo-4,5-benzotropone (26).

Scheme 58: Synthesis of 6-chloro-2,3-benzotropone (335) using LiCl and proposed intermediate 336.

Scheme 59: Reaction of 7-bromo-2,3-benzotropone (316) with methylamine.

Scheme 60: Reactions of bromo-2,3-benzotropones 26 and 311 with dimethylamine.

Scheme 61: Reactions of bromobenzotropones 311 and 26 with NaOMe.

Scheme 62: Reactions of bromobenzotropones 26 and 312 with t-BuOK in the presence of DPIBF.

Scheme 63: Cobalt-catalyzed reductive cross-couplings of 7-bromo-2,3-benzotropone (316) with cyclic α-bromo en...

Figure 15: Cycloadduct 357 and its di-π-methane rearrangement product 358.

Scheme 64: Catalytic hydrogenation of 2-chloro-4,5-benzotropone (311).

Scheme 65: Synthesis of dibromo-benzotropones from benzotropones.

Scheme 66: Bromination/dehydrobromination of benzosuberone (162).

Scheme 67: Some transformations of isomeric dibromo-benzotropones 261A/B.

Scheme 68: Transformations of benzotropolone 239B to halobenzotropolones 369–371.

Figure 16: Bromobenzotropolones 372–376 and 290 prepared via bromination/dehydrobromination strategy.

Scheme 69: Synthesis of some halobenzotropolones 289, 377 and 378.

Figure 17: Bromo-chloro-derivatives 379–381 prepared via chlorination.

Scheme 70: Synthesis of 7-iodo-3,4-benzotropolone (382).

Scheme 71: Hydrogenation of bromobenzotropolones 369 and 370.

Scheme 72: Debromination reactions of mono- and dibromides 290 and 375.

Figure 18: Nitratation and oxidation products of some halobenzotropolenes.

Scheme 73: Azo-coupling reactions of some halobenzotropolones 294, 375 and 378.

Figure 19: Four possible isomers of dibenzotropones 396–399.

Figure 20: Resonance structures of tribenzotropone (400).

Scheme 74: Two synthetic pathways for tribenzotropone (400).

Scheme 75: Synthesis of tribenzotropone (400) from dibenzotropone 399.

Scheme 76: Synthesis of tribenzotropone (400) from 9,10-phenanthraquinone (406).

Scheme 77: Synthesis of tribenzotropone (400) from trifluoromethyl-substituted arene 411.

Figure 21: Dibenzosuberone (414).

Figure 22: Reduction products 415 and 416 of tribenzotropone (400).

Figure 23: Structures of tribenzotropone dimethyl ketal 417 and 4-phenylfluorenone (412) and proposed intermed...

Figure 24: Structures of benzylidene- and methylene-9H-tribenzo[a,c,e][7]annulenes 419 and 420 and chiral phos...

Figure 25: Structures of tetracyclic alcohol 422, p-quinone methide 423 and cation 424.

Figure 26: Structures of host molecules 425–427.

Scheme 78: Synthesis of non-helical overcrowded derivatives syn/anti-431.

Figure 27: Hexabenzooctalene 432.

Figure 28: Structures of possible eight isomers 433–440 of naphthotropone.

Scheme 79: Synthesis of naphthotropone 437 starting from 1-phenylcycloheptene (441).

Scheme 80: Synthesis of 10-hydroxy-11H-cyclohepta[a]naphthalen-11-one (448) from diester 445.

Scheme 81: Synthesis of naphthotropone 433.

Scheme 82: Synthesis of naphthotropones 433 and 434 via cycloaddition reaction.

Scheme 83: Synthesis of naphthotropone 434 starting from 452.

Figure 29: Structures of tricarbonyl(tropone)irons 458, and possible cycloadducts 459.

Scheme 84: Synthesis of naphthotropone 436.

Scheme 85: Synthesis of precursor 465 for naphthotropone 435.

Scheme 86: Generation of naphthotropone 435 from 465.

Figure 30: Structures of tropylium cations 469 and 470.

Figure 31: Structures of tropylium ions 471+.BF4−, 472+.BF4−, and 473+.BF4−.

Scheme 87: Synthesis of tropylium ions 471+.BF4− and 479+.ClO4−.

Scheme 88: Synthesis of 1- and 2-methylanthracene (481 and 482) via carbene–carbene rearrangement.

Figure 32: Trapping products 488–490.

Scheme 89: Generation and chemistry of a naphthoannelated cycloheptatrienylidene-cycloheptatetraene intermedia...

Scheme 90: Proposed intermediates and reaction pathways for adduct 498.

Scheme 91: Exited-state intramolecular proton transfer of 505.

Figure 33: Benzoditropones 506 and 507.

Scheme 92: Synthesis of benzoditropone 506e.

Scheme 93: Synthetic approaches for dibenzotropone 507 via tropone (1).

Scheme 94: Formation mechanisms of benzoditropone 507 and 516 via 515.

Scheme 95: Synthesis of benzoditropones 525 and 526 from pyromellitic dianhydride (527).

Figure 34: Possible three benzocyclobutatropones 534–536.

Scheme 96: Synthesis of benzocyclobutatropones 534 and 539.

Scheme 97: Synthesis attempts for benzocyclobutatropone 545.

Scheme 98: Generation and trapping of symmetric benzocyclobutatropone 536.

Scheme 99: Synthesis of chloro-benzocyclobutatropone 552 and proposed mechanism of fluorenone derivatives.

Scheme 100: Synthesis of tropolone analogue 559.

Scheme 101: Synthesis of tropolones 561 and 562.

Figure 35: o/p-Tropoquinone rings (563 and 564) and benzotropoquinones (565–567).

Scheme 102: Synthesis of benzotropoquinone 566.

Scheme 103: Synthesis of benzotropoquinone 567 via a Diels–Alder reaction.

Figure 36: Products 575–577 through 1,2,3-benzotropoquinone hydrate 569.

Scheme 104: Structures 578–582 prepared from tropoquinone 567.

Figure 37: Two possible structures 583 and 584 for dibenzotropoquinone, and precursor compound 585 for 583.

Scheme 105: Synthesis of saddle-shaped ketone 592 using dibenzotropoquinone 584.
New approaches to organocatalysis based on C–H and C–X bonding for electrophilic substrate activation
- Pavel Nagorny and
- Zhankui Sun
Beilstein J. Org. Chem. 2016, 12, 2834–2848, doi:10.3762/bjoc.12.283
- determined to be 3.5 × 106 M−1 (CH3CN). In addition to the aforementioned studies, recent results by Huber and Codée indicate that not only 1-chloroisochroman, but also more complex substrates such as 2,3,4,6-tetra-O-benzylglucosyl chloride could undergo halogen bond-donor-catalyzed solvolysis [86]. Further

Graphical Abstract

Figure 1: Electrophile Activation by Hydrogen Bond Donors [1-16].

Figure 2: Early examples of C–H hydrogen bonds and their recent use in supramolecular chemistry [18,19,32-34].

Scheme 1: Design of 1,2,3-triazole-based catalysts for trityl group transfer through chloride anion binding b...

Scheme 2: Examples of chiral triazole-based catalysts for anion activation designed by Mancheno and co-worker...

Scheme 3: Application of chiral triazole-based catalysts L3 and L4 for counterion activation of pyridinium, q...

Scheme 4: Ammonium salt anion binding via C–H hydrogen bonds in solid state [40-45,50,51].

Scheme 5: Early examples of ammonium salts being used for electrophilic activation of imines in aza-Diels–Ald...

Scheme 6: Ammonium salts as hydrogen bond-donor catalysts by Bibal and co-workers [53,54].

Scheme 7: Tetraalkylammonium catalyst (L6)-catalyzed dearomatization of isoquinolinium salts [50].

Scheme 8: Tetraalkylammonium catalyst L6 complexation to halogen-containing substrates [51].

Scheme 9: Tetraalkylammonium-catalyzed aza-Diels–Alder reaction by Maruoka and co-workers [52].

Scheme 10: (A) Alkylpyridinium catalysts L13-catalyzed reaction of 1-isochroman and silyl ketene acetals by Be...

Scheme 11: Mixed N–H/C–H two hydrogen bond donors L14 and L15 as organocatalysts for ROP of lactide by Bibal a...

Scheme 12: Examples of stable complexes based on halogen bonding [68,69].

Scheme 13: Interaction between (−)-sparteine hydrobromide and (S)-1,2-dibromohexafluoropropane in the cocrysta...

Scheme 14: Iodine-catalyzed reactions that are computationally proposed to proceed through halogen bond to car...

Scheme 15: Transfer hydrogenation of phenylquinolines catalyzed by haloperfluoroalkanes by Bolm and co-workers ...

Scheme 16: Halogen bond activation of benzhydryl bromides by Huber and co-workers [82].

Scheme 17: Halogen bond-donor-catalyzed addition to oxocarbenium ions by Huber and co-workers [89].

Scheme 18: Halogen bond-donor activation of α,β-unsaturated carbonyl compounds in the [2 + 4] cycloaddition re...

Scheme 19: Halogen bond donor activation of imines in the [2 + 4] cycloaddition reaction of imine and Danishef...

Scheme 20: Transfer hydrogenation catalyzed by a chiral halogen bond donor by Tan and co-workers [91].

Scheme 21: Allylation of benzylic alcohols by Takemoto and co-workers [92].

Scheme 22: NIS induced semipinacol rearrangement via C–X bond cleavage [93].






