Search results
Search for "styrene" in Full Text gives 219 result(s) in Beilstein Journal of Organic Chemistry. Showing first 200.
Regioselectivity of the SEAr-based cyclizations and SEAr-terminated annulations of 3,5-unsubstituted, 4-substituted indoles
- Jonali Das and
- Sajal Kumar Das
Beilstein J. Org. Chem. 2022, 18, 293–302, doi:10.3762/bjoc.18.33
- regioselectivity in favor of the 4,5-fused indole system. Based on their experimental and computational investigations, the researchers hypothesized that Thorpe–Ingold effect could induce dispersive interactions between the indole and styrene moieties, triggering the preferential formation of the 3,4-fused indoles

Graphical Abstract

Scheme 1: SEAr-based, CAr–C bond-forming cyclization or annulation of: (A) substituted arenes/heteroarenes an...

Scheme 2: Indole C3 regioselective intramolecular alkylation of indolyl allyl carbonates.

Scheme 3: Indole C3 regioselective Michael-type cyclization in the total synthesis of (−)-indolactam V.

Scheme 4: Synthesis of azepino[4,3,2-cd]indoles via indole C3 regioselective aza-Michael addition/cyclization...

Scheme 5: Indole C3 regioselective Pictet−Spengler reaction of 2-(1H-indol-4-yl)ethanamines.

Scheme 6: Indole C3 regioselective hydroindolation of cis-β-(α′,α′-dimethyl)-4′-methindolylstyrenes.

Scheme 7: Indole C3 regioselective cyclization leading to the formation of polycyclic azepino[5,4,3-cd]indole...

Scheme 8: Synthesis of azepino[3,4,5-cd]indoles via iridium-catalyzed asymmetric [4 + 3] cycloaddition of rac...

Scheme 9: Aldimine condensation/1,6-hydride transfer/Mannich-type cyclization cascade of indole-derived pheny...

Scheme 10: Indole C5 regioselective intramolecular FC acylation of 4-substituted indoles.

Scheme 11: Catalyst-dependent regioselectivity switching in the cyclization of ethyl 2-diazo-4-(4-indolyl)-3-o...

Scheme 12: Indole C5 regioselective cyclization of α-carbonyl sulfoxonium ylides.

Scheme 13: Indole C5 regioselective cyclization of an indole-tethered donor–acceptor cyclopropane.

Scheme 14: Indole C5 regioselective epoxide–arene cyclization.
Stepwise PEG synthesis featuring deprotection and coupling in one pot
- Logan Mikesell,
- Dhananjani N. A. M. Eriyagama,
- Yipeng Yin,
- Bao-Yuan Lu and
- Shiyue Fang
Beilstein J. Org. Chem. 2021, 17, 2976–2982, doi:10.3762/bjoc.17.207
- conditions and used TLC to monitor the progress of the 1,2-elimination (3a–j) or 1,4-elimination (3k–l) reactions. Initially, compound 3a (1 equiv) was treated with LDA (1 equiv) with catalytic amount of t-BuOK (0.1 equiv) in THF at −78 °C [29][30]. Complete consumption of 3a to give methoxide and styrene
- synthesis would be to react (PEG)4, which is commercially available and inexpensive, with styrene to give 6 [32], and tosylation of 6 to give the monomer (Scheme 3). However, the reported conditions for the synthesis of 6 without using an expensive catalyst gave low yields. We did not test the conditions
- reaction, which mainly include styrene and p-toluenesulfonic acid. As a result, when the reaction is performed at large scales, there is a high probability that the product can be purified by methods such as partition, precipitation and crystallization. The major advantage of using a base-labile protecting

Graphical Abstract

Scheme 1: A comparison of the new PEG synthesis method with a typical known PEG synthesis method.

Scheme 2: Screening base-labile protecting groups for stepwise PEG synthesis. All compounds (3a–l) underwent ...

Scheme 3: Synthesis of monomer 2.

Scheme 4: PEG synthesis using the one-pot PEG elongation approach.

Scheme 5: A proposed route for the synthesis of long and asymmetric PEGs using a base-labile protecting group....
Iron-catalyzed domino coupling reactions of π-systems
- Austin Pounder and
- William Tam
Beilstein J. Org. Chem. 2021, 17, 2848–2893, doi:10.3762/bjoc.17.196
- reported a similar approach towards the assembly of 2,2-disubstituted indolines from N-sulfonylanilines and substituted styrene derivatives [93]. In 2014, the Jiao group investigated the carbosulfonation of alkenes 60 for the synthesis of oxindoles 90 through sequential C–S/C–C-bond formation (Scheme 16
- compatible with the nucleophilic capture process. In the proposed mechanism, the α-hydrogen of the alkyl nitrile is deprotonated to form an organosilver species which undergoes SET oxidation with Ag(I) to afford the alkyl radical. Next, the α-cyanocarbon radical can add across the styrene derivative
- electronic properties of the alkenes to control the chronology of the additions. The rate of addition of the acyl radical to an electro-deficient alkene is about three times greater than that of a styrene derivative [109][110]. The electrophilic radical, adjacent to an EWG, will favor the subsequent addition

Graphical Abstract

Figure 1: Price comparison among iron and other transition metals used in catalysis.

Scheme 1: Typical modes of C–C bond formation.

Scheme 2: The components of an iron-catalyzed domino reaction.

Scheme 3: Iron-catalyzed tandem cyclization and cross-coupling reactions of iodoalkanes 1 with aryl Grignard ...

Scheme 4: Three component iron-catalyzed dicarbofunctionalization of vinyl cyclopropanes 14.

Scheme 5: Three-component iron-catalyzed dicarbofunctionalization of alkenes 21.

Scheme 6: Double carbomagnesiation of internal alkynes 31 with alkyl Grignard reagents 32.

Scheme 7: Iron-catalyzed cycloisomerization/cross-coupling of enyne derivatives 35 with alkyl Grignard reagen...

Scheme 8: Iron-catalyzed spirocyclization/cross-coupling cascade.

Scheme 9: Iron-catalyzed alkenylboration of alkenes 50.

Scheme 10: N-Alkyl–N-aryl acrylamide 60 CDC cyclization with C(sp3)–H bonds adjacent to a heteroatom.

Scheme 11: 1,2-Carboacylation of activated alkenes 60 with aldehydes 65 and alcohols 67.

Scheme 12: Iron-catalyzed dicarbonylation of activated alkenes 68 with alcohols 67.

Scheme 13: Iron-catalyzed cyanoalkylation/radical dearomatization of acrylamides 75.

Scheme 14: Synergistic photoredox/iron-catalyzed 1,2-dialkylation of alkenes 82 with common alkanes 83 and 1,3...

Scheme 15: Iron-catalyzed oxidative coupling/cyclization of phenol derivatives 86 and alkenes 87.

Scheme 16: Iron-catalyzed carbosulfonylation of activated alkenes 60.

Scheme 17: Iron-catalyzed oxidative spirocyclization of N-arylpropiolamides 91 with silanes 92 and tert-butyl ...

Scheme 18: Iron-catalyzed free radical cascade difunctionalization of unsaturated benzamides 94 with silanes 92...

Scheme 19: Iron-catalyzed cyclization of olefinic dicarbonyl compounds 97 and 100 with C(sp3)–H bonds.

Scheme 20: Radical difunctionalization of o-vinylanilides 102 with ketones and esters 103.

Scheme 21: Dehydrogenative 1,2-carboamination of alkenes 82 with alkyl nitriles 76 and amines 105.

Scheme 22: Iron-catalyzed intermolecular 1,2-difunctionalization of conjugated alkenes 107 with silanes 92 and...

Scheme 23: Four-component radical difunctionalization of chemically distinct alkenes 114/115 with aldehydes 65...

Scheme 24: Iron-catalyzed carbocarbonylation of activated alkenes 60 with carbazates 117.

Scheme 25: Iron-catalyzed radical 6-endo cyclization of dienes 119 with carbazates 117.

Scheme 26: Iron-catalyzed decarboxylative synthesis of functionalized oxindoles 130 with tert-butyl peresters ...

Scheme 27: Iron‑catalyzed decarboxylative alkylation/cyclization of cinnamamides 131/134.

Scheme 28: Iron-catalyzed carbochloromethylation of activated alkenes 60.

Scheme 29: Iron-catalyzed trifluoromethylation of dienes 142.

Scheme 30: Iron-catalyzed, silver-mediated arylalkylation of conjugated alkenes 115.

Scheme 31: Iron-catalyzed three-component carboazidation of conjugated alkenes 115 with alkanes 101/139b and t...

Scheme 32: Iron-catalyzed carboazidation of alkenes 82 and alkynes 160 with iodoalkanes 20 and trimethylsilyl ...

Scheme 33: Iron-catalyzed asymmetric carboazidation of styrene derivatives 115.

Scheme 34: Iron-catalyzed carboamination of conjugated alkenes 115 with alkyl diacyl peroxides 163 and acetoni...

Scheme 35: Iron-catalyzed carboamination using oxime esters 165 and arenes 166.

Scheme 36: Iron-catalyzed iminyl radical-triggered [5 + 2] and [5 + 1] annulation reactions with oxime esters ...

Scheme 37: Iron-catalyzed decarboxylative alkyl etherification of alkenes 108 with alcohols 67 and aliphatic a...

Scheme 38: Iron-catalyzed inter-/intramolecular alkylative cyclization of carboxylic acid and alcohol-tethered...

Scheme 39: Iron-catalyzed intermolecular trifluoromethyl-acyloxylation of styrene derivatives 115.

Scheme 40: Iron-catalyzed carboiodination of terminal alkenes and alkynes 180.

Scheme 41: Copper/iron-cocatalyzed cascade perfluoroalkylation/cyclization of 1,6-enynes 183/185.

Scheme 42: Iron-catalyzed stereoselective carbosilylation of internal alkynes 187.

Scheme 43: Synergistic photoredox/iron catalyzed difluoroalkylation–thiolation of alkenes 82.

Scheme 44: Iron-catalyzed three-component aminoazidation of alkenes 82.

Scheme 45: Iron-catalyzed intra-/intermolecular aminoazidation of alkenes 194.

Scheme 46: Stereoselective iron-catalyzed oxyazidation of enamides 196 using hypervalent iodine reagents 197.

Scheme 47: Iron-catalyzed aminooxygenation for the synthesis of unprotected amino alcohols 200.

Scheme 48: Iron-catalyzed intramolecular aminofluorination of alkenes 209.

Scheme 49: Iron-catalyzed intramolecular aminochlorination and aminobromination of alkenes 209.

Scheme 50: Iron-catalyzed intermolecular aminofluorination of alkenes 82.

Scheme 51: Iron-catalyzed aminochlorination of alkenes 82.

Scheme 52: Iron-catalyzed phosphinoylazidation of alkenes 108.

Scheme 53: Synergistic photoredox/iron-catalyzed three-component aminoselenation of trisubstituted alkenes 82.
Exfoliated black phosphorous-mediated CuAAC chemistry for organic and macromolecular synthesis under white LED and near-IR irradiation
- Azra Kocaarslan,
- Zafer Eroglu,
- Önder Metin and
- Yusuf Yagci
Beilstein J. Org. Chem. 2021, 17, 2477–2487, doi:10.3762/bjoc.17.164

- ’’-pentamethyldiethylenetriamine (PMDETA, Aldrich), sodium azide (NaN3, Panreac), copper(II) chloride (CuIICl2, Merck), black phosphorus, dimethyl sulfoxide (DMSO) was used as received. Propargyl acrylate (Sigma), styrene (Merck) were purified before by using a basic alumina column to remove the inhibitor and then stored in the

Graphical Abstract

Figure 1: Structures of azide and alkyne functional molecules and polymers used in the photoinduced CuAAC rea...

Figure 2: UV–vis spectra of CuICl, CuIICl2 and BPNs.

Figure 3: a) 1H NMR spectra of the model reaction between benzyl azide (Az-1) and phenylacetylene (Alk-3) bef...

Scheme 1: Proposed mechanism for photoinduced CuAAC reaction using exfoliated BPNs.

Figure 4: a) 1H NMR spectrum of chain end modified PCL-Anth; b) UV–vis spectra of (azidomethyl)anthracene (bl...

Scheme 2: Synthesis of PS-b-PCL block copolymer via exfoliated BPNs-mediated photoinduced CuAAC reaction.

Figure 5: a) GPC traces of PS-Az, PCL-Alk and block copolymer (Ps-b-PCL) b) 1H NMR spectrum of the block copo...

Scheme 3: Preparation of the cross-linked polymer by CuAAC reaction using multifunctional monomers, Az-3 and ...

Figure 6: a) DSC thermogram of photoinduced synthesis of nanocomposite networks (heating rate: 10 °C/min). b)...

Figure 7: (a, b) TEM images of cross-linked polymer at two different magnifications, c) HAADF-STEM image and ...
Constrained thermoresponsive polymers – new insights into fundamentals and applications
- Patricia Flemming,
- Alexander S. Münch,
- Andreas Fery and
- Petra Uhlmann
Beilstein J. Org. Chem. 2021, 17, 2123–2163, doi:10.3762/bjoc.17.138


Graphical Abstract

Figure 1: (a) Schematic representation of the phase stability of a binary mixture based on the free enthalpy ...

Figure 2: Illustration of the relationship between the type of miscibility gap and the temperature dependence...

Figure 3: Schematically pictured phase diagram of a binary mixture composed of a dissolved polymer with a LCS...

Figure 4: Schematic illustration of a thermo-induced swelling behavior of a star polymer composed of responsi...

Figure 5: Schematic illustration of self-assembly of block copolymer amphiphiles in a polar medium.

Figure 6: Schematic comparison of the size and conformation between free polymer chains (a), grafted polymer ...

Figure 7: Comparison of the possible phase diagrams of a polymer in solution with partially miscibility and t...

Figure 8: Selection of polymers exhibiting UCST behavior due to hydrogen bonding (blue) divided into homo- (a...

Figure 9: Part A shows the molecular structure of PDMAPS stars synthesized by Li et al. (left) demonstrating ...

Figure 10: Part A contains a schematic demonstration of conformational transitions of dual-thermoresponsive bl...

Figure 11: Part A pictures zwitterionic brushes grafted from silicon substrates obtaining a nonassociated, hyd...

Figure 12: Part A pictures the UCST phase transition of zwitterionic polymers grafted on the surface of mesopo...
An initiator- and catalyst-free hydrogel coating process for 3D printed medical-grade poly(ε-caprolactone)
- Jochen Löblein,
- Thomas Lorson,
- Miriam Komma,
- Tobias Kielholz,
- Maike Windbergs,
- Paul D. Dalton and
- Robert Luxenhofer
Beilstein J. Org. Chem. 2021, 17, 2095–2101, doi:10.3762/bjoc.17.136
- achieved with self-initiated photografting and photopolymerization (SIPGP, Scheme 1). SIPGP is a simple, solvent-free bulk/surface photografting first introduced by Deng et al. grafting maleic anhydride [20] and styrene [21] onto low-density poly(ethylene) films. Later, this was extended for other monomers

Graphical Abstract

Scheme 1: Schematic representation of the self-initiated photografting and photopolymerization (SIPGP) of 2-h...

Figure 1: A) Graph showing change in the static contact angle with time on a pristine PCL scaffold with a 500...

Figure 2: A) Optical photograph of an SIPGP-coated sample. B) 3D topography reconstruction of the SIPGP-coate...

Figure 3: A) SEM image of pristine, uncoated PCL MEW scaffolds with a hatch spacing of 150 µm × 200 µm and in...
Recent advances in the syntheses of anthracene derivatives
- Giovanni S. Baviera and
- Paulo M. Donate
Beilstein J. Org. Chem. 2021, 17, 2028–2050, doi:10.3762/bjoc.17.131
- OMe, NO2, or Cl groups, and epoxides (149 and 150), DBU as catalyst, CTAB as surfactant, and water as solvent. The use of cyclohexene oxide (149) provided oxa-aza-benzo[a]anthracene derivatives 151 in excellent yields (90–96%). On the other hand, the use of styrene oxide (150) provided oxa-aza

Graphical Abstract

Figure 1: Examples of anthracene derivatives and their applications.

Scheme 1: Rhodium-catalyzed oxidative coupling reactions of arylboronic acids with internal alkynes.

Scheme 2: Rhodium-catalyzed oxidative benzannulation reactions of 1-adamantoyl-1-naphthylamines with internal...

Scheme 3: Gold/bismuth-catalyzed cyclization of o-alkynyldiarylmethanes.

Scheme 4: [2 + 2 + 2] Cyclotrimerization reactions with alkynes/nitriles in the presence of nickel and cobalt...

Scheme 5: Cobalt-catalyzed [2 + 2 + 2] cyclotrimerization reactions with bis(trimethylsilyl)acetylene (23).

Scheme 6: [2 + 2 + 2] Alkyne-cyclotrimerization reactions catalyzed by a CoCl2·6H2O/Zn reagent.

Scheme 7: Pd(II)-catalyzed sp3 C–H alkenylation of diphenyl carboxylic acids with acrylates.

Scheme 8: Pd(II)-catalyzed sp3 C–H arylation with o-tolualdehydes and aryl iodides.

Scheme 9: Alkylation of arenes with aromatic aldehydes in the presence of acetyl bromide and ZnBr2/SiO2.

Scheme 10: BF3·H2O-catalyzed hydroxyalkylation of arenes with aromatic dialdehyde 44.

Scheme 11: Bi(OTf)3-promoted Friedel–Crafts alkylation of triarylmethanes and aromatic acylals and of arenes a...

Scheme 12: Reduction of anthraquinones by using Zn/pyridine or Zn/NaOH reductive methods.

Scheme 13: Two-step route to novel substituted Indenoanthracenes.

Scheme 14: Synthesis of 1,8-diarylanthracenes through Suzuki–Miyaura coupling reaction in the presence of Pd-P...

Scheme 15: Synthesis of five new substituted anthracenes by using LAH as reducing agent.

Scheme 16: One-pot procedure to synthesize substituted 9,10-dicyanoanthracenes.

Scheme 17: Reduction of bromoanthraquinones with NaBH4 in alkaline medium.

Scheme 18: In(III)-catalyzed reductive-dehydration intramolecular cycloaromatization of 2-benzylic aromatic al...

Scheme 19: Acid-catalyzed cyclization of new O-protected ortho-acetal diarylmethanols.

Scheme 20: Lewis acid-mediated regioselective cyclization of asymmetric diarylmethine dipivalates and diarylme...

Scheme 21: BF3·OEt2/CF3SO3H-mediated cyclodehydration reactions of 2-(arylmethyl)benzaldehydes and 2-(arylmeth...

Scheme 22: Synthesis of 2,3,6,7-anthracenetetracarbonitrile (90) by double Wittig reaction followed by deprote...

Scheme 23: Homo-elongation protocol for the synthesis of substituted acene diesters/dinitriles.

Scheme 24: Synthesis of two new parental BN anthracenes via borylative cyclization.

Scheme 25: Synthesis of substituted anthracenes from a bifunctional organomagnesium alkoxide.

Scheme 26: Palladium-catalyzed tandem C–H activation/bis-cyclization of propargylic carbonates.

Scheme 27: Ruthenium-catalyzed C–H arylation of acetophenone derivatives with arenediboronates.

Scheme 28: Pd-catalyzed intramolecular cyclization of (Z,Z)-p-styrylstilbene derivatives.

Scheme 29: AuCl-catalyzed double cyclization of diiodoethynylterphenyl compounds.

Scheme 30: Iodonium-induced electrophilic cyclization of terphenyl derivatives.

Scheme 31: Oxidative photocyclization of 1,3-distyrylbenzene derivatives.

Scheme 32: Oxidative cyclization of 2,3-diphenylnaphthalenes.

Scheme 33: Suzuki-Miyaura/isomerization/ring closing metathesis strategy to synthesize benz[a]anthracenes.

Scheme 34: Green synthesis of oxa-aza-benzo[a]anthracene and oxa-aza-phenanthrene derivatives.

Scheme 35: Triple benzannulation of substituted naphtalene via a 1,3,6-naphthotriyne synthetic equivalent.

Scheme 36: Zinc iodide-catalyzed Diels–Alder reactions with 1,3-dienes and aroyl propiolates followed by intra...

Scheme 37: H3PO4-promoted intramolecular cyclization of substituted benzoic acids.

Scheme 38: Palladium-catalyzed intermolecular direct acylation of aromatic aldehydes and o-iodoesters.

Scheme 39: Cycloaddition/oxidative aromatization of quinone and β-enamino esters.

Scheme 40: ʟ-Proline-catalyzed [4 + 2] cycloaddition reaction of naphthoquinones and α,β-unsaturated aldehydes....

Scheme 41: Iridium-catalyzed [2 + 2 + 2] cycloaddition of a 1,2-bis(propiolyl)benzene derivative with alkynes.

Scheme 42: Synthesis of several anthraquinone derivatives by using InCl3 and molecular iodine.

Scheme 43: Indium-catalyzed multicomponent reactions employing 2-hydroxy-1,4-naphthoquinone (186), β-naphthol (...

Scheme 44: Synthesis of substituted anthraquinones catalyzed by an AlCl3/MeSO3H system.

Scheme 45: Palladium(II)-catalyzed/visible light-mediated synthesis of anthraquinones.

Scheme 46: [4 + 2] Anionic annulation reaction for the synthesis of substituted anthraquinones.
Electron-rich triarylphosphines as nucleophilic catalysts for oxa-Michael reactions
- Susanne M. Fischer,
- Simon Renner,
- A. Daniel Boese and
- Christian Slugovc
Beilstein J. Org. Chem. 2021, 17, 1689–1697, doi:10.3762/bjoc.17.117

- from MZ-Gel SD plus, 500 A and 100 A, linear 5 µ; UV detector (SPD-20A) and RI detector (RID-20A)) using THF as eluent. Poly(styrene) standards in the range of 350 to 17800 g/mol purchased from Polymer Standard Service were used for calibration. Computational details All calculations were run with the
- –Currier repeat units; right: size exclusion chromatograms (in THF, relative to poly(styrene) standards) of poly4 prepared with 5 mol % TPP, MMTPP or TMTPP using a reaction time of 24 h and a reaction temperature of 23 °C (dashed lines) or 80 °C (full lines). Left: Oxidation stability of the phosphines

Graphical Abstract

Scheme 1: Mechanism for the phosphine-initiated oxa-Michael addition.

Figure 1: Above: Michael acceptors, Michael donors and catalysts used in this study; pKa (respectively pKa of...

Figure 2: Left: double-bond conversion of the polymerization of 4 initiated by 5 mol % TPP, MMTPP or TMTPP af...

Figure 3: Left: Oxidation stability of the phosphines. Phosphine oxide content in % as determined by 31P NMR ...
Chemical approaches to discover the full potential of peptide nucleic acids in biomedical applications
- Nikita Brodyagin,
- Martins Katkevics,
- Venubabu Kotikam,
- Christopher A. Ryan and
- Eriks Rozners
Beilstein J. Org. Chem. 2021, 17, 1641–1688, doi:10.3762/bjoc.17.116

Graphical Abstract

Figure 1: Structure of DNA and PNA.

Figure 2: PNA binding modes: (A) PNA–dsDNA 1:1 triplex; (B) PNA–DNA–PNA strand-invasion triplex; (C) the Hoog...

Figure 3: Structure of P-form PNA–DNA–PNA triplex from reference [41]. (A) view in the major groove and (B) view ...

Figure 4: Structures of backbone-modified PNA.

Figure 5: Structures of PNA having α- and γ-substituted backbones.

Figure 6: Structures of modified nucleobases in PNA to improve Hoogsteen hydrogen bonding to guanine and aden...

Figure 7: Proposed hydrogen bonding schemes for modified PNA nucleobases designed to recognize pyrimidines or...

Figure 8: Modified nucleobases to modulate Watson–Crick base pairing and chemically reactive crosslinking PNA...

Figure 9: Examples of triplets formed by Janus-wedge PNA nucleobases (blue). R1 denotes DNA, RNA, or PNA back...

Figure 10: Examples of fluorescent PNA nucleobases. R1 denotes DNA, RNA, or PNA backbones.

Figure 11: Endosomal entrapment and escape pathways of PNA and PNA conjugates.

Figure 12: (A) representative cell-penetrating peptides (CPPs), (B) conjugation designs and linker chemistries....

Figure 13: Proposed delivery mode by pHLIP-PNA conjugates (A) the transmembrane section of pHLIP interacting w...

Figure 14: Structures of modified penetratin CPP conjugates with PNA linked through either disulfide (for stud...

Figure 15: Chemical structure of C9–PNA, a stable amphipathic (cyclic-peptide)–PNA conjugate.

Figure 16: Structures of PNA conjugates with a lipophilic triphenylphosphonium cation (TPP–PNA) through (A) th...

Figure 17: Structures of (A) chloesteryl–PNA, (B) cholate–PNA and (C) cholate–PNA(cholate)3.

Figure 18: Structures of PNA–GalNAc conjugates (A) (GalNAc)2K, (B) triantennary (GalNAc)3, and (C) trivalent (...

Figure 19: Vitamin B12–PNA conjugates with different linkages.

Figure 20: Structures of (A) neomycin B, (B) PNA–neamine conjugate, and (C) PNA–neosamine conjugate.

Figure 21: PNA clamp (red) binding to target DNA containing a mixture of sequences (A) PNA binds with higher a...

Figure 22: Rolling circle amplification using PNA openers (red) to invade a dsDNA target forming a P-loop. A p...

Figure 23: Molecular beacons containing generic fluorophores (Fl) and quenchers (Q) recognizing a complementar...

Figure 24: (A) Light-up fluorophores such as thiazole orange display fluorescence enhancement upon binding to ...

Figure 25: Templated fluorogenic detection of oligonucleotides using two PNAs. (A) Templated FRET depends on h...

Figure 26: Lateral flow devices use a streptavidin labeled strip on nitrocellulose paper to anchor a capture P...
Methodologies for the synthesis of quaternary carbon centers via hydroalkylation of unactivated olefins: twenty years of advances
- Thiago S. Silva and
- Fernando Coelho
Beilstein J. Org. Chem. 2021, 17, 1565–1590, doi:10.3762/bjoc.17.112
- ., styrene derivatives [17]) that produce hundreds of tons for polymer synthesis. The goal of this review is to demonstrate the utility of olefins as starting materials in the synthesis of all-carbon quaternary centers through hydroalkylation reactions. Only the hydroalkylation of unactivated olefins as

Graphical Abstract

Figure 1: Some examples of natural products and drugs containing quaternary carbon centers.

Scheme 1: Simplified mechanism for olefin hydrofunctionalization using an electrophilic transition metal as a...

Scheme 2: Selected examples of quaternary carbon centers formed by the intramolecular hydroalkylation of β-di...

Scheme 3: Control experiments and the proposed mechanism for the Pd(II)-catalyzed intermolecular hydroalkylat...

Scheme 4: Intermolecular olefin hydroalkylation of less reactive ketones under Pd(II) catalysis using HCl as ...

Scheme 5: A) Selected examples of Pd(II)-mediated quaternary carbon center synthesis by intermolecular hydroa...

Scheme 6: Selected examples of quaternary carbon center synthesis by gold(III) catalysis. This is the first r...

Scheme 7: Selected examples of inter- (A) and intramolecular (B) olefin hydroalkylations promoted by a silver...

Scheme 8: A) Intermolecular hydroalkylation of N-alkenyl β-ketoamides under Au(I) catalysis in the synthesis ...

Scheme 9: Asymmetric pyrrolidine synthesis through intramolecular hydroalkylation of α-substituted N-alkenyl ...

Scheme 10: Proposed mechanism for the chiral gold(I) complex promotion of the intermolecular olefin hydroalkyl...

Scheme 11: Selected examples of carbon quaternary center synthesis by gold and evidence of catalytic system pa...

Scheme 12: Synthesis of a spiro compound via an aza-Michael addition/olefin hydroalkylation cascade promoted b...

Scheme 13: A selected example of quaternary carbon center synthesis using an Fe(III) salt as a catalyst for th...

Scheme 14: Intermolecular hydroalkylation catalyzed by a cationic iridium complex (Fuji (2019) [47]).

Scheme 15: Generic example of an olefin hydrofunctionalization via MHAT (Shenvi (2016) [51]).

Scheme 16: The first examples of olefin hydrofunctionalization run under neutral conditions (Mukaiyama (1989) [56]...

Scheme 17: A) Aryl olefin dimerization catalyzed by vitamin B12 and triggered by HAT. B) Control experiment to...

Scheme 18: Generic example of MHAT diolefin cycloisomerization and possible competitive pathways. Shenvi (2014...

Scheme 19: Selected examples of the MHAT-promoted cycloisomerization reaction of unactivated olefins leading t...

Scheme 20: Regioselective carbocyclizations promoted by an MHAT process (Norton (2008) [76]).

Scheme 21: Selected examples of quaternary carbon centers synthetized via intra- (A) and intermolecular (B) MH...

Scheme 22: A) Proposed mechanism for the Fe(III)/PhSiH3-promoted radical conjugate addition between olefins an...

Scheme 23: Examples of cascade reactions triggered by HAT for the construction of trans-decalin backbone uniti...

Scheme 24: A) Selected examples of the MHAT-promoted radical conjugate addition between olefins and p-quinone ...

Scheme 25: A) MHAT triggered radical conjugate addition/E1cB/lactonization (in some cases) cascade between ole...

Scheme 26: A) Spirocyclization promoted by Fe(III) hydroalkylation of unactivated olefins. B) Simplified mecha...

Scheme 27: A) Selected examples of the construction of a carbon quaternary center by the MHAT-triggered radica...

Scheme 28: Hydromethylation of unactivated olefins under iron-mediated MHAT (Baran (2015) [95]).

Scheme 29: The hydroalkylation of unactivated olefins via iron-mediated reductive coupling with hydrazones (Br...

Scheme 30: Selected examples of the Co(II)-catalyzed bicyclization of dialkenylarenes through the olefin hydro...

Scheme 31: Proposed mechanism for the bicyclization of dialkenylarenes triggered by a MHAT process (Vanderwal ...

Scheme 32: Enantioconvergent cross-coupling between olefins and tertiary halides (Fu (2018) [108]).

Scheme 33: Proposed mechanism for the Ni-catalyzed cross-coupling reaction between olefins and tertiary halide...

Scheme 34: Proposed catalytic cycles for a MHAT/Ni cross-coupling reaction between olefins and halides (Shenvi...

Scheme 35: Selected examples of the hydroalkylation of olefins by a dual catalytic Mn/Ni system (Shenvi (2019) ...

Scheme 36: A) Selected examples of quaternary carbon center synthesis by reductive atom transfer; TBC: 4-tert-...

Scheme 37: A) Selected examples of quaternary carbon centers synthetized by radical addition to unactivated ol...

Scheme 38: A) Selected examples of organophotocatalysis-mediated radical polyene cyclization via a PET process...

Scheme 39: A) Sc(OTf)3-mediated carbocyclization approach for the synthesis of vicinal quaternary carbon cente...

Scheme 40: Scope of the Lewis acid-catalyzed methallylation of electron-rich styrenes. Method A: B(C6F5)3 (5.0...

Scheme 41: The proposed mechanism for styrene methallylation (Oestreich (2019) [123]).
Cascade intramolecular Prins/Friedel–Crafts cyclization for the synthesis of 4-aryltetralin-2-ols and 5-aryltetrahydro-5H-benzo[7]annulen-7-ols
- Jie Zheng,
- Shuyu Meng and
- Quanrui Wang
Beilstein J. Org. Chem. 2021, 17, 1481–1489, doi:10.3762/bjoc.17.104
- vinylboronate afforded the o-hydroxyethyl-styrene 12a in 78% yield [22][23]. Next, Dess–Martin oxidation of the alcohol 12a was carried out, and the desired 2-(2-vinylphenyl)acetaldehyde (13a) was successfully obtained in 85% yield. Obviously, this modified method has the advantages of mild reaction conditions

Graphical Abstract

Figure 1: Parent structure of 2,4-disubstituted tetralins (1) and selected medicinally useful derivatives 2–4....

Scheme 1: Reported strategies for the synthesis of tetralin-2-ol ring systems.

Scheme 2: Designed cascade reactions to 4-substituted tetralin-2-ols.

Scheme 3: The documented synthesis of 2-(2-vinylphenyl)acetaldehyde (13a).

Scheme 4: Modified synthesis of 2-(2-vinylphenyl)acetaldehydes 13a–g and 1-vinyl-2-naphthaldehyde (13h).

Scheme 5: Lewis acid-catalyzed Prins/Friedel–Crafts reaction of 13a with veratrole.

Figure 2: The speculated stereostructures of compound cis-14aa and trans-14aa.

Scheme 6: Use of different nucleophiles for the cascade reaction with 13a. Reaction conditions: a mixture of ...

Scheme 7: Reaction of aldehydes 13b–h with veratrole or furan. Reaction conditions: a mixture of 13b–h (1.40 ...

Scheme 8: Synthesis of 5-aryltetrahydro-5H-benzo[7]annulen-7-ols 20a, b.

Scheme 9: Conversion of 2-hydroxy-4-(2-furyl)tetralin (14af) into PAT analogue 22.

Figure 3: Crystal structure of the tosylate 21. The displacement ellipsoids are drawn at the 30% probability ...
A comprehensive review of flow chemistry techniques tailored to the flavours and fragrances industries
- Guido Gambacorta,
- James S. Sharley and
- Ian R. Baxendale
Beilstein J. Org. Chem. 2021, 17, 1181–1312, doi:10.3762/bjoc.17.90

Graphical Abstract

Figure 1: Representative shares of the global F&F market (2018) segmented on their applications [1].

Figure 2: General structure of an international fragrance company [2].

Figure 3: The Michael Edwards fragrance wheel.

Figure 4: Examples of oriental (1–3), woody (4–7), fresh (8–10), and floral (11 and 12) notes.

Figure 5: A basic depiction of batch vs flow.

Scheme 1: Examples of reactions for which flow processing outperforms batch.

Scheme 2: Some industrially important aldol-based transformations.

Scheme 3: Biphasic continuous aldol reactions of acetone and various aldehydes.

Scheme 4: Aldol synthesis of 43 in flow using LiHMDS as the base.

Scheme 5: A semi-continuous synthesis of doravirine (49) involving a key aldol reaction.

Scheme 6: Enantioselective aldol reaction using 5-(pyrrolidin-2-yl)tetrazole (51) as catalyst in a microreact...

Scheme 7: Gröger's example of asymmetric aldol reaction in aqueous media.

Figure 6: Immobilised reagent column reactor types.

Scheme 8: Photoinduced thiol–ene coupling preparation of silica-supported 5-(pyrrolidin-2-yl)tetrazole 63 and...

Scheme 9: Continuous-flow approach for enantioselective aldol reactions using the supported catalyst 67.

Scheme 10: Ötvös’ employment of a solid-supported peptide aldol catalyst in flow.

Scheme 11: The use of proline tetrazole packed in a column for aldol reaction between cyclohexanone (65) and 2...

Scheme 12: Schematic diagram of an aminosilane-grafted Si-Zr-Ti/PAI-HF reactor for continuous-flow aldol and n...

Scheme 13: Continuous-flow condensation for the synthesis of the intermediate 76 to nabumetone (77) and Microi...

Scheme 14: Synthesis of ψ-Ionone (80) in continuous-flow via aldol condensation between citral (79) and aceton...

Scheme 15: Synthesis of β-methyl-ionones (83) from citral (79) in flow. The steps are separately described, an...

Scheme 16: Continuous-flow synthesis of 85 from 84 described by Gavriilidis et al.

Scheme 17: Continuous-flow scCO2 apparatus for the synthesis of 2-methylpentanal (87) and the self-condensed u...

Scheme 18: Chen’s two-step flow synthesis of coumarin (90).

Scheme 19: Pechmann condensation for the synthesis of 7-hydroxyxcoumarin (93) in flow. The setup extended to c...

Scheme 20: Synthesis of the dihydrojasmonate 35 exploiting nitro derivative proposed by Ballini et al.

Scheme 21: Silica-supported amines as heterogeneous catalyst for nitroaldol condensation in flow.

Scheme 22: Flow apparatus for the nitroaldol condensation of p-hydroxybenzaldehyde (102) to nitrostyrene 103 a...

Scheme 23: Nitroaldol reaction of 64 to 105 employing a quaternary ammonium functionalised PANF.

Scheme 24: Enantioselective nitroaldol condensation for the synthesis of 108 under flow conditions.

Scheme 25: Enatioselective synthesis of 1,2-aminoalcohol 110 via a copper-catalysed nitroaldol condensation.

Scheme 26: Examples of Knoevenagel condensations applied for fragrance components.

Scheme 27: Flow apparatus for Knoevenagel condensation described in 1989 by Venturello et al.

Scheme 28: Knoevenagel reaction using a coated multichannel membrane microreactor.

Scheme 29: Continuous-flow apparatus for Knoevenagel condensation employing sugar cane bagasse as support deve...

Scheme 30: Knoevenagel reaction for the synthesis of 131–135 in flow using an amine-functionalised silica gel. ...

Scheme 31: Continuous-flow synthesis of compound 137, a key intermediate for the synthesis of pregabalin (138)...

Scheme 32: Continuous solvent-free apparatus applied for the synthesis of compounds 140–143 using a TSE. Throu...

Scheme 33: Lewis et al. developed a spinning disc reactor for Darzens condensation of 144 and a ketone to furn...

Scheme 34: Some key industrial applications of conjugate additions in the F&F industry.

Scheme 35: Continuous-flow synthesis of 4-(2-hydroxyethyl)thiomorpholine 1,1-dioxide (156) via double conjugat...

Scheme 36: Continuous-flow system for Michael addition using CsF on alumina as the catalyst.

Scheme 37: Calcium chloride-catalysed asymmetric Michael addition using an immobilised chiral ligand.

Scheme 38: Continuous multistep synthesis for the preparation of (R)-rolipram (173). Si-NH2: primary amine-fun...

Scheme 39: Continuous-flow Michael addition using ion exchange resin Amberlyst® A26.

Scheme 40: Preparation of the heterogeneous catalyst 181 developed by Paixão et al. exploiting Ugi multicompon...

Scheme 41: Continuous-flow system developed by the Paixão’s group for the preparation of Michael asymmetric ad...

Scheme 42: Continuous-flow synthesis of nitroaldols catalysed by supported catalyst 184 developed by Wennemers...

Scheme 43: Heterogenous polystyrene-supported catalysts developed by Pericàs and co-workers.

Scheme 44: PANF-supported pyrrolidine catalyst for the conjugate addition of cyclohexanone (65) and trans-β-ni...

Scheme 45: Synthesis of (−)-paroxetine precursor 195 developed by Ötvös, Pericàs, and Kappe.

Scheme 46: Continuous-flow approach for the 5-step synthesis of (−)-oseltamivir (201) as devised by Hayashi an...

Scheme 47: Continuous-flow enzyme-catalysed Michael addition.

Scheme 48: Continuous-flow copper-catalysed 1,4 conjugate addition of Grignard reagents to enones. Reprinted w...

Scheme 49: A collection of commonly encountered hydrogenation reactions.

Figure 7: The ThalesNano H-Cube® continuous-flow hydrogenator.

Scheme 50: Chemoselective reduction of an α,β-unsaturated ketone using the H-Cube® reactor.

Scheme 51: Incorporation of Lindlar’s catalyst into the H-Cube® reactor for the reduction of an alkyne.

Scheme 52: Continuous-flow semi-hydrogenation of alkyne 208 to 209 using SACs with H-Cube® system.

Figure 8: The standard setups for tube-in-tube gas–liquid reactor units.

Scheme 53: Homogeneous hydrogenation of olefins using a tube-in-tube reactor setup.

Scheme 54: Recyclable heterogeneous flow hydrogenation system.

Scheme 55: Leadbeater’s reverse tube-in-tube hydrogenation system for olefin reductions.

Scheme 56: a) Hydrogenation using a Pd-immobilised microchannel reactor (MCR) and b) a representation of the i...

Scheme 57: Hydrogenation of alkyne 238 exploiting segmented flow in a Pd-immobilised capillary reactor.

Scheme 58: Continuous hydrogenation system for the preparation of cyrene (241) from (−)-levoglucosenone (240).

Scheme 59: Continuous hydrogenation system based on CSMs developed by Hornung et al.

Scheme 60: Chemoselective reduction of carbonyls (ketones over aldehydes) in flow.

Scheme 61: Continuous system for the semi-hydrogenation of 256 and 258, developed by Galarneau et al.

Scheme 62: Continuous synthesis of biodiesel fuel 261 from lignin-derived furfural acetone (260).

Scheme 63: Continuous synthesis of γ-valerolacetone (263) via CTH developed by Pineda et al.

Scheme 64: Continuous hydrogenation of lignin-derived biomass (products 265, 266, and 267) using a sustainable...

Scheme 65: Ru/C or Rh/C-catalysed hydrogenation of arene in flow as developed by Sajiki et al.

Scheme 66: Polysilane-immobilized Rh–Pt-catalysed hydrogenation of arenes in flow by Kobayashi et al.

Scheme 67: High-pressure in-line mixing of H2 for the asymmetric reduction of 278 at pilot scale with a 73 L p...

Figure 9: Picture of the PFR employed at Eli Lilly & Co. for the continuous hydrogenation of 278 [287]. Reprinted ...

Scheme 68: Continuous-flow asymmetric hydrogenation using Oppolzer's sultam 280 as chiral auxiliary.

Scheme 69: Some examples of industrially important oxidation reactions in the F&F industry. CFL: compact fluor...

Scheme 70: Gold-catalysed heterogeneous oxidation of alcohols in flow.

Scheme 71: Uozumi’s ARP-Pt flow oxidation protocol.

Scheme 72: High-throughput screening of aldehyde oxidation in flow using an in-line GC.

Scheme 73: Permanganate-mediated Nef oxidation of nitroalkanes in flow with the use of in-line sonication to p...

Scheme 74: Continuous-flow aerobic anti-Markovnikov Wacker oxidation.

Scheme 75: Continuous-flow oxidation of 2-benzylpyridine (312) using air as the oxidant.

Scheme 76: Continuous-flow photo-oxygenation of monoterpenes.

Scheme 77: A tubular reactor design for flow photo-oxygenation.

Scheme 78: Glucose oxidase (GOx)-mediated continuous oxidation of glucose using compressed air and the FFMR re...

Scheme 79: Schematic continuous-flow sodium hypochlorite/TEMPO oxidation of alcohols.

Scheme 80: Oxidation using immobilised TEMPO (344) was developed by McQuade et al.

Scheme 81: General protocol for the bleach/catalytic TBAB oxidation of aldehydes and alcohols.

Scheme 82: Continuous-flow PTC-assisted oxidation using hydrogen peroxide. The process was easily scaled up by...

Scheme 83: Continuous-flow epoxidation of cyclohexene (348) and in situ preparation of m-CPBA.

Scheme 84: Continuous-flow epoxidation using DMDO as oxidant.

Scheme 85: Mukayama aerobic epoxidation optimised in flow mode by the Favre-Réguillon group.

Scheme 86: Continuous-flow asymmetric epoxidation of derivatives of 359 exploiting a biomimetic iron catalyst.

Scheme 87: Continuous-flow enzymatic epoxidation of alkenes developed by Watts et al.

Scheme 88: Engineered multichannel microreactor for continuous-flow ozonolysis of 366.

Scheme 89: Continuous-flow synthesis of the vitamin D precursor 368 using multichannel microreactors. MFC: mas...

Scheme 90: Continuous ozonolysis setup used by Kappe et al. for the synthesis of various substrates employing ...

Scheme 91: Continuous-flow apparatus for ozonolysis as developed by Ley et al.

Scheme 92: Continuous-flow ozonolysis for synthesis of vanillin (2) using a film-shear flow reactor.

Scheme 93: Examples of preparative methods for ajoene (386) and allicin (388).

Scheme 94: Continuous-flow oxidation of thioanisole (389) using styrene-based polymer-supported peroxytungstat...

Scheme 95: Continuous oxidation of thiosulfinates using Oxone®-packed reactor.

Scheme 96: Continuous-flow electrochemical oxidation of thioethers.

Scheme 97: Continuous-flow oxidation of 400 to cinnamophenone (235).

Scheme 98: Continuous-flow synthesis of dehydrated material 401 via oxidation of methyl dihydrojasmonate (33).

Scheme 99: Some industrially important transformations involving Grignard reagents.

Scheme 100: Grachev et al. apparatus for continuous preparation of Grignard reagents.

Scheme 101: Example of fluidized Mg bed reactor with NMR spectrometer as on-line monitoring system.

Scheme 102: Continuous-flow synthesis of Grignard reagents and subsequent quenching reaction.

Figure 10: Membrane-based, liquid–liquid separator with integrated pressure control [52]. Adapted with permission ...

Scheme 103: Continuous-flow synthesis of 458, an intermediate to fluconazole (459).

Scheme 104: Continuous-flow synthesis of ketones starting from benzoyl chlorides.

Scheme 105: A Grignard alkylation combining CSTR and PFR technologies with in-line infrared reaction monitoring....

Scheme 106: Continuous-flow preparation of 469 from Grignard addition of methylmagnesium bromide.

Scheme 107: Continuous-flow synthesis of Grignard reagents 471.

Scheme 108: Preparation of the Grignard reagent 471 using CSTR and the continuous process for synthesis of the ...

Scheme 109: Continuous process for carboxylation of Grignard reagents in flow using tube-in-tube technology.

Scheme 110: Continuous synthesis of propargylic alcohols via ethynyl-Grignard reagent.

Scheme 111: Silica-supported catalysed enantioselective arylation of aldehydes using Grignard reagents in flow ...

Scheme 112: Acid-catalysed rearrangement of citral and dehydrolinalool derivatives.

Scheme 113: Continuous stilbene isomerisation with continuous recycling of photoredox catalyst.

Scheme 114: Continuous-flow synthesis of compound 494 as developed by Ley et al.

Scheme 115: Selected industrial applications of DA reaction.

Scheme 116: Multistep flow synthesis of the spirocyclic structure 505 via employing DA cycloaddition.

Scheme 117: Continuous-flow DA reaction developed in a plater flow reactor for the preparation of the adduct 508...

Scheme 118: Continuous-flow DA reaction using a silica-supported imidazolidinone organocatalyst.

Scheme 119: Batch vs flow for the DA reaction of (cyclohexa-1,5-dien-1-yloxy)trimethylsilane (513) with acrylon...

Scheme 120: Continuous-flow DA reaction between 510 and 515 using a shell-core droplet system.

Scheme 121: Continuous-flow synthesis of bicyclic systems from benzyne precursors.

Scheme 122: Continuous-flow synthesis of bicyclic scaffolds 527 and 528 for further development of potential ph...

Scheme 123: Continuous-flow inverse-electron hetero-DA reaction to pyridine derivatives such as 531.

Scheme 124: Comparison between batch and flow for the synthesis of pyrimidinones 532–536 via retro-DA reaction ...

Scheme 125: Continuous-flow coupled with ultrasonic system for preparation of ʟ-ascorbic acid derivatives 539 d...

Scheme 126: Two-step continuous-flow synthesis of triazole 543.

Scheme 127: Continuous-flow preparation of triazoles via CuAAC employing 546-based heterogeneous catalyst.

Scheme 128: Continuous-flow synthesis of compounds 558 through A3-coupling and 560 via AgAAC both employing the...

Scheme 129: Continuous-flow photoinduced [2 + 2] cycloaddition for the preparation of bicyclic derivatives of 5...

Scheme 130: Continuous-flow [2 + 2] and [5 + 2] cycloaddition on large scale employing a flow reactor developed...

Scheme 131: Continuous-flow preparation of the tricyclic structures 573 and 574 starting from pyrrole 570 via [...

Scheme 132: Continuous-flow [2 + 2] photocyclization of cinnamates.

Scheme 133: Continuous-flow preparation of cyclobutane 580 on a 5-plates photoreactor.

Scheme 134: Continuous-flow [2 + 2] photocycloaddition under white LED lamp using heterogeneous PCN as photocat...

Figure 11: Picture of the parallel tube flow reactor (PTFR) "The Firefly" developed by Booker-Milburn et al. a...

Scheme 135: Continuous-flow acid-catalysed [2 + 2] cycloaddition between silyl enol ethers and acrylic esters.

Scheme 136: Continuous synthesis of lactam 602 using glass column reactors.

Scheme 137: In situ generation of ketenes for the Staudinger lactam synthesis developed by Ley and Hafner.

Scheme 138: Application of [2 + 2 + 2] cycloadditions in flow employed by Ley et al.

Scheme 139: Examples of FC reactions applied in F&F industry.

Scheme 140: Continuous-flow synthesis of ibuprofen developed by McQuade et al.

Scheme 141: The FC acylation step of Jamison’s three-step ibuprofen synthesis.

Scheme 142: Synthesis of naphthalene derivative 629 via FC acylation in microreactors.

Scheme 143: Flow system for rapid screening of catalysts and reaction conditions developed by Weber et al.

Scheme 144: Continuous-flow system developed by Buorne, Muller et al. for DSD optimisation of the FC acylation ...

Scheme 145: Continuous-flow FC acylation of alkynes to yield β-chlorovinyl ketones such as 638.

Scheme 146: Continuous-flow synthesis of tonalide (619) developed by Wang et al.

Scheme 147: Continuous-flow preparation of acylated arene such as 290 employing Zr4+-β-zeolite developed by Kob...

Scheme 148: Flow system applied on an Aza-FC reaction catalysed by the thiourea catalyst 648.

Scheme 149: Continuous hydroformylation in scCO2.

Scheme 150: Two-step flow synthesis of aldehyde 655 through a sequential Heck reaction and subsequent hydroform...

Scheme 151: Single-droplet (above) and continuous (below) flow reactors developed by Abolhasani et al. for the ...

Scheme 152: Continuous hydroformylation of 1-dodecene (655) using a PFR-CSTR system developed by Sundmacher et ...

Scheme 153: Continuous-flow synthesis of the aldehyde 660 developed by Eli Lilly & Co. [32]. Adapted with permissio...

Scheme 154: Continuous asymmetric hydroformylation employing heterogenous catalst supported on carbon-based sup...

Scheme 155: Examples of acetylation in F&F industry: synthesis of bornyl (S,R,S-664) and isobornyl (S,S,S-664) ...

Scheme 156: Continuous-flow preparation of bornyl acetate (S,R,S-664) employing the oscillating flow reactor.

Scheme 157: Continuous-flow synthesis of geranyl acetate (666) from acetylation of geraniol (343) developed by ...

Scheme 158: 12-Ttungstosilicic acid-supported silica monolith-catalysed acetylation in flow.

Scheme 159: Continuous-flow preparation of cyclopentenone 676.

Scheme 160: Two-stage synthesis of coumarin (90) via acetylation of salicylaldehyde (88).

Scheme 161: Intensification process for acetylation of 5-methoxytryptamine (677) to melatonin (678) developed b...

Scheme 162: Examples of macrocyclic musky odorants both natural (679–681) and synthetic (682 and 683).

Scheme 163: Flow setup combined with microwave for the synthesis of macrocycle 686 via RCM.

Scheme 164: Continuous synthesis of 2,5-dihydro-1H-pyrroles via ring-closing metathesis.

Scheme 165: Continuous-flow metathesis of 485 developed by Leadbeater et al.

Figure 12: Comparison between RCM performed using different routes for the preparation of 696. On the left the...

Scheme 166: Continuous-flow RCM of 697 employed the solid-supported catalyst 698 developed by Grela, Kirschning...

Scheme 167: Continuous-flow RORCM of cyclooctene employing the silica-absorbed catalyst 700.

Scheme 168: Continuous-flow self-metathesis of methyl oleate (703) employing SILP catalyst 704.

Scheme 169: Flow apparatus for the RCM of 697 using a nanofiltration membrane for the recovery and reuse of the...

Scheme 170: Comparison of loadings between RCMs performed with different routes for the synthesis of 709.
Prins cyclization-mediated stereoselective synthesis of tetrahydropyrans and dihydropyrans: an inspection of twenty years
- Asha Budakoti,
- Pradip Kumar Mondal,
- Prachi Verma and
- Jagadish Khamrai
Beilstein J. Org. Chem. 2021, 17, 932–963, doi:10.3762/bjoc.17.77
- the synthesis of diol by the condensation of styrene and paraformaldehyde in the presence of a Brønsted acid [23][24]. The major breakthrough for this reaction was reported by Hanschke in 1955, when the THP ring was selectively constructed through a Prins reaction involving 3-butene-1-ol and a variety

Graphical Abstract

Scheme 1: General strategy for the synthesis of THPs.

Scheme 2: Developments towards the Prins cyclization.

Scheme 3: General stereochemical outcome of the Prins cyclization.

Scheme 4: Regioselectivity in the Prins cyclization.

Scheme 5: Mechanism of the oxonia-Cope reaction in the Prins cyclization.

Scheme 6: Cyclization of electron-deficient enantioenriched alcohol 27.

Scheme 7: Partial racemization through 2-oxonia-Cope allyl transfer.

Scheme 8: Partial racemization by reversible 2-oxonia-Cope rearrangement.

Scheme 9: Rychnovsky modification of the Prins cyclization.

Scheme 10: Synthesis of (−)-centrolobine and the C22–C26 unit of phorboxazole A.

Scheme 11: Axially selective Prins cyclization by Rychnovsky et al.

Scheme 12: Mechanism for the axially selectivity Prins cyclization.

Scheme 13: Mukaiyama aldol–Prins cyclization reaction.

Scheme 14: Application of the aldol–Prins reaction.

Scheme 15: Hart and Bennet's acid-promoted Prins cyclization.

Scheme 16: Tetrahydropyran core of polycarvernoside A as well as (−)-clavoslide A and D.

Scheme 17: Scheidt and co-workers’ route to tetrahydropyran-4-one.

Scheme 18: Mechanism for the Lewis acid-catalyzed synthesis of tetrahydropyran-4-one.

Scheme 19: Hoveyda and co-workers’ strategy for 2,6-disubstituted 4-methylenetetrahydropyran.

Scheme 20: Funk and Cossey’s ene-carbamates strategy.

Scheme 21: Yadav and Kumar’s cyclopropane strategy for THP synthesis.

Scheme 22: 2-Arylcylopropylmethanolin in centrolobine synthesis.

Scheme 23: Yadav and co-workers’ strategy for the synthesis of THP.

Scheme 24: Yadav and co-workers’ Prins–Ritter reaction sequence for 4-amidotetrahydropyran.

Scheme 25: Yadav and co-workers’ strategy to prelactones B, C, and V.

Scheme 26: Yadav and co-workers’ strategy for the synthesis of (±)-centrolobine.

Scheme 27: Loh and co-workers’ strategy for the synthesis of zampanolide and dactylolide.

Scheme 28: Loh and Chan’s strategy for THP synthesis.

Scheme 29: Prins cyclization of cyclohexanecarboxaldehyde.

Scheme 30: Prins cyclization of methyl ricinoleate (127) and benzaldehyde (88).

Scheme 31: AlCl3-catalyzed cyclization of homoallylic alcohol 129 and aldehyde 130.

Scheme 32: Martín and co-workers’ stereoselective approach for the synthesis of highly substituted tetrahydrop...

Scheme 33: Ene-IMSC strategy by Marko and Leroy for the synthesis of tetrahydropyran.

Scheme 34: Marko and Leroy’s strategy for the synthesis of tetrahydropyrans 146.

Scheme 35: Sakurai dimerization/macrolactonization reaction for the synthesis of cyanolide A.

Scheme 36: Hoye and Hu’s synthesis of (−)-dactyloide by intramolecular Sakurai cyclization.

Scheme 37: Minehan and co-workers’ strategy for the synthesis of THPs 157.

Scheme 38: Yu and co-workers’ allylic transfer strategy for the construction of tetrahydropyran 161.

Scheme 39: Reactivity enhancement in intramolecular Prins cyclization.

Scheme 40: Floreancig and co-workers’ Prins cyclization strategy to (+)-dactyloide.

Scheme 41: Panek and Huang’s DHP synthesis from crotylsilanes: a general strategy.

Scheme 42: Panek and Huang’s DHP synthesis from syn-crotylsilanes.

Scheme 43: Panek and Huang’s DHP synthesis from anti-crotylsilanes.

Scheme 44: Roush and co-workers’ [4 + 2]-annulation strategy for DHP synthesis [82].

Scheme 45: TMSOTf-promoted annulation reaction.

Scheme 46: Dobb and co-workers’ synthesis of DHP.

Scheme 47: BiBr3-promoted tandem silyl-Prins reaction by Hinkle et al.

Scheme 48: Substrate scope of Hinkle and co-workers’ strategy.

Scheme 49: Cho and co-workers’ strategy for 2,6 disubstituted 3,4-dimethylene-THP.

Scheme 50: Furman and co-workers’ THP synthesis from propargylsilane.

Scheme 51: THP synthesis from silyl enol ethers.

Scheme 52: Rychnovsky and co-workers’ strategy for THP synthesis from hydroxy-substituted silyl enol ethers.

Scheme 53: Li and co-workers’ germinal bissilyl Prins cyclization strategy to (−)-exiguolide.

Scheme 54: Xu and co-workers’ hydroiodination strategy for THP.

Scheme 55: Wang and co-workers’ strategy for tetrahydropyran synthesis.

Scheme 56: FeCl3-catalyzed synthesis of DHP from alkynylsilane alcohol.

Scheme 57: Martín, Padrón, and co-workers’ proposed mechanism of alkynylsilane Prins cyclization for the synth...

Scheme 58: Marko and co-workers’ synthesis of 2,6-anti-configured tetrahydropyran.

Scheme 59: Loh and co-workers’ strategy for 2,6-syn-tetrahydropyrans.

Scheme 60: Loh and co-workers’ strategy for anti-THP synthesis.

Scheme 61: Cha and co-workers’ strategy for trans-2,6-tetrahydropyran.

Scheme 62: Mechanism proposed by Cha et al.

Scheme 63: TiCl4-mediated cyclization to trans-THP.

Scheme 64: Feng and co-workers’ FeCl3-catalyzed Prins cyclization strategy to 4-hydroxy-substituted THP.

Scheme 65: Selectivity profile of the Prins cyclization under participation of an iron ligand.

Scheme 66: Sequential reactions involving Prins cyclization.

Scheme 67: Banerjee and co-workers’ strategy of Prins cyclization from cyclopropane carbaldehydes and propargy...

Scheme 68: Mullen and Gagné's (R)-[(tolBINAP)Pt(NC6F5)2][SbF6]2-catalyzed asymmetric Prins cyclization strateg...

Scheme 69: Yu and co-workers’ DDQ-catalyzed asymmetric Prins cyclization strategy to trisubstituted THPs.

Scheme 70: Lalli and Weghe’s chiral-Brønsted-acid- and achiral-Lewis-acid-promoted asymmetric Prins cyclizatio...

Scheme 71: List and co-workers’ iIDP Brønsted acid-promoted asymmetric Prins cyclization strategy.

Scheme 72: Zhou and co-workers’ strategy for chiral phosphoric acid (CPA)-catalyzed cascade Prins cyclization.

Scheme 73: List and co-workers’ approach for asymmetric Prins cyclization using chiral imidodiphosphoric acid ...
Synthetic reactions driven by electron-donor–acceptor (EDA) complexes
- Zhonglie Yang,
- Yutong Liu,
- Kun Cao,
- Xiaobin Zhang,
- Hezhong Jiang and
- Jiahong Li
Beilstein J. Org. Chem. 2021, 17, 771–799, doi:10.3762/bjoc.17.67
- trifluoromethylation (Scheme 25). A variety of olefins, such as ene carbamates, styrene, aliphatic olefins, vinyl ethers, and acrylates are compatible in this approach, affording corresponding β-(trifluoromethyl)alkynes with good to excellent yield. The bifunctionalization was achieved by an EDA-complex-initiated

Graphical Abstract

Scheme 1: The electron transfer process in EDA complexes.

Scheme 2: Synthesis of benzo[b]phosphorus oxide 3 initiated by an EDA complex.

Scheme 3: Mechanism of the synthesis of quinoxaline derivative 7.

Scheme 4: Synthesis of imidazole derivative 10 initiated by an EDA complex.

Scheme 5: Synthesis of sulfamoylation product 12 initiated by an EDA complex.

Scheme 6: Mechanism of the synthesis of sulfamoylation product 12.

Scheme 7: Synthesis of indole derivative 22 initiated by an EDA complex.

Scheme 8: Synthesis of perfluoroalkylated pyrimidines 26 initiated by an EDA complex.

Scheme 9: Synthesis of phenanthridine derivative 29 initiated by an EDA complex.

Scheme 10: Synthesis of cis-tetrahydroquinoline derivative 32 initiated by an EDA complex.

Scheme 11: Mechanism of the synthesis of cis-tetrahydroquinoline derivative 32.

Scheme 12: Synthesis of phenanthridine derivative 38 initiated by an EDA complex.

Scheme 13: Synthesis of spiropyrroline derivative 40 initiated by an EDA complex.

Scheme 14: Synthesis of benzothiazole derivative 43 initiated by an EDA complex.

Scheme 15: Synthesis of perfluoroalkyl-s-triazine derivative 45 initiated by an EDA complex.

Scheme 16: Synthesis of indoline derivative 47 initiated by an EDA complex.

Scheme 17: Mechanism of the synthesis of spirocyclic indoline derivative 47.

Scheme 18: Synthesis of cyclobutane product 50 initiated by an EDA complex.

Scheme 19: Mechanism of the synthesis of spirocyclic indoline derivative 50.

Scheme 20: Synthesis of 1,3-oxazolidine compound 59 initiated by an EDA complex.

Scheme 21: Synthesis of trifluoromethylated product 61 initiated by an EDA complex.

Scheme 22: Synthesis of indole alkylation product 64 initiated by an EDA complex.

Scheme 23: Synthesis of perfluoroalkylation product 67 initiated by an EDA complex.

Scheme 24: Synthesis of hydrotrifluoromethylated product 70 initiated by an EDA complex.

Scheme 25: Synthesis of β-trifluoromethylated alkyne product 71 initiated by an EDA complex.

Scheme 26: Mechanism of the synthesis of 2-phenylthiophene derivative 74.

Scheme 27: Synthesis of allylated product 80 initiated by an EDA complex.

Scheme 28: Synthesis of trifluoromethyl-substituted alkynyl product 84 initiated by an EDA complex.

Scheme 29: Synthesis of dearomatized fluoroalkylation product 86 initiated by an EDA complex.

Scheme 30: Mechanism of the synthesis of dearomatized fluoroalkylation product 86.

Scheme 31: Synthesis of C(sp3)–H allylation product 91 initiated by an EDA complex.

Scheme 32: Synthesis of perfluoroalkylation product 93 initiated by an EDA complex.

Scheme 33: Synthesis of spirocyclic indolene derivative 95 initiated by an EDA complex.

Scheme 34: Synthesis of perfluoroalkylation product 97 initiated by an EDA complex.

Scheme 35: Synthesis of alkylated indole derivative 100 initiated by an EDA complex.

Scheme 36: Mechanism of the synthesis of alkylated indole derivative 100.

Scheme 37: Synthesis of arylated oxidized indole derivative 108 initiated by an EDA complex.

Scheme 38: Synthesis of 4-ketoaldehyde derivative 111 initiated by an EDA complex.

Scheme 39: Mechanism of the synthesis of 4-ketoaldehyde derivative 111.

Scheme 40: Synthesis of perfluoroalkylated olefin 118 initiated by an EDA complex.

Scheme 41: Synthesis of alkylation product 121 initiated by an EDA complex.

Scheme 42: Synthesis of acylation product 123 initiated by an EDA complex.

Scheme 43: Mechanism of the synthesis of acylation product 123.

Scheme 44: Synthesis of trifluoromethylation product 126 initiated by an EDA complex.

Scheme 45: Synthesis of unnatural α-amino acid 129 initiated by an EDA complex.

Scheme 46: Synthesis of thioether derivative 132 initiated by an EDA complex.

Scheme 47: Synthesis of S-aryl dithiocarbamate product 135 initiated by an EDA complex.

Scheme 48: Mechanism of the synthesis of S-aryl dithiocarbamate product 135.

Scheme 49: Synthesis of thioether product 141 initiated by an EDA complex.

Scheme 50: Mechanism of the synthesis of borate product 144.

Scheme 51: Synthesis of boronation product 148 initiated by an EDA complex.

Scheme 52: Synthesis of boration product 151 initiated by an EDA complex.

Scheme 53: Synthesis of boronic acid ester derivative 154 initiated by an EDA complex.

Scheme 54: Synthesis of β-azide product 157 initiated by an EDA complex.

Scheme 55: Decarboxylation reaction initiated by an EDA complex.

Scheme 56: Synthesis of amidated product 162 initiated by an EDA complex.

Scheme 57: Synthesis of diethyl phenylphosphonate 165 initiated by an EDA complex.

Scheme 58: Mechanism of the synthesis of diethyl phenylphosphonate derivative 165.

Scheme 59: Synthesis of (Z)-2-iodovinyl phenyl ether 168 initiated by an EDA complex.

Scheme 60: Mechanism of the synthesis of (Z)-2-iodovinyl phenyl ether derivative 168.

Scheme 61: Dehalogenation reaction initiated by an EDA complex.
Valorisation of plastic waste via metal-catalysed depolymerisation
- Francesca Liguori,
- Carmen Moreno-Marrodán and
- Pierluigi Barbaro
Beilstein J. Org. Chem. 2021, 17, 589–621, doi:10.3762/bjoc.17.53

- °C provided a hydrocarbon oil in 92% yield, 71.4% of which were attributable to styrene monomer [156]. A decrease of 56 kJ mol−1 for the activation energy of PS depolymerisation was calculated in the presence of the catalysts. More recently, high-porosity montmorillonite (Mt) was used to prepare Mg
- -, Zn-, Al-, Cu- or Fe-decorated heterogeneous catalysts [157][158]. An oil yield around 89% was obtained at 450 °C over 20% Fe@Mt, composed by 51%, 10% and 6% (wt) styrene, toluene and ethylbenzene, respectively, and additional oligomers. 3.2 Polyesters 3.2.1 PET: PET is one of the most widely used

Graphical Abstract

Figure 1: Potential classification of plastic recycling processes. The area covered by the present review is ...

Figure 2: EG produced during glycolytic depolymerisation of PET using DEG + DPG as solvent and titanium(IV) n...

Scheme 1: Simplified representation of the conversion of 1,4-PBD to C16–C44 macrocycles using Ru metathesis c...

Figure 3: Main added-value monomers obtainable by catalytic depolymerisation of PET via chemolytic methods.

Scheme 2: Hydrogenolytic depolymerisation of PET by ruthenium complexes.

Scheme 3: Depolymerisation of PET via catalytic hydrosilylation by Ir(III) pincer complex.

Scheme 4: Catalytic hydrolysis (top) and methanolysis (bottom) reactions of PET.

Scheme 5: Depolymerisation of PET by glycolysis with ethylene glycol.

Figure 4: Glycolysis of PET: evolution of BHET yield over time, with and without zinc acetate catalyst (196 °...

Scheme 6: Potential activated complex for the glycolysis reaction of PET catalysed by metallated ILs and evol...

Scheme 7: One-pot, two-step process for PET repurposing via chemical recycling.

Scheme 8: Synthetic routes to PLA.

Scheme 9: Structures of the zinc molecular catalysts used for PLA-methanolysis in various works. a) See [265], b) ...

Scheme 10: Depolymerisation of PLLA by Zn–N-heterocyclic carbene complex.

Scheme 11: Salalen ligands.

Scheme 12: Catalytic hydrogenolysis of PLA.

Scheme 13: Catalytic hydrosilylation of PLA.

Scheme 14: Hydrogenative depolymerisation of PBT and PCL by molecular Ru catalysts.

Scheme 15: Glycolysis reaction of PCT by diethylene glycol.

Scheme 16: Polymerisation–depolymerisation cycle of 3,4-T6GBL.

Scheme 17: Polymerisation–depolymerisation cycle of 2,3-HDB.

Scheme 18: Hydrogenative depolymerisation of PBPAC by molecular Ru catalysts.

Scheme 19: Catalytic hydrolysis (top), alcoholysis (middle) and aminolysis (bottom) reactions of PBPAC.

Scheme 20: Hydrogenative depolymerisation of PPC (top) and PEC (bottom) by molecular Ru catalysts.

Scheme 21: Polymerisation-depolymerisation cycle of BEP.

Scheme 22: Hydrogenolysis of polyamides using soluble Ru catalysts.

Scheme 23: Catalytic depolymerisation of epoxy resin/carbon fibres composite.

Scheme 24: Depolymerisation of polyethers with metal salt catalysts and acyl chlorides.

Scheme 25: Proposed mechanism for the iron-catalysed depolymerisation reaction of polyethers. Adapted with per...
A new and efficient methodology for olefin epoxidation catalyzed by supported cobalt nanoparticles
- Lucía Rossi-Fernández,
- Viviana Dorn and
- Gabriel Radivoy
Beilstein J. Org. Chem. 2021, 17, 519–526, doi:10.3762/bjoc.17.46

- the oxidant agent. Among the reported methods that make use of peroxides as oxidants, cobalt nanoparticles supported on CNTs together with TBHP as oxidant for the epoxidation of styrene, gave good selectivity to styrene oxide but conversions were lower than 40% [41]. More recently, Hutchings et al
- suspension was stirred for 2 h (see Experimental for details). The CoNPs-based catalysts were ready for use after filtration and drying in an oven at 150 °C for 1 h. Styrene (1a) was chosen as model substrate for testing the activity and selectivity of a variety of CoNPs-based catalysts (Table 1). The
- the CuNPs supported on MgO as catalyst, but a similar result to that obtained in DMF was observed (Table 1, entry 5). The use of H2O2 as co-oxidant improved the conversion of the starting styrene (1a) only for the CoNPs/MgO catalyst, but lead to low selectivity due to the formation of benzaldehyde and

Graphical Abstract

Figure 1: TEM micrograph and size distribution graphic for CoNPs@MgO catalyst (scale bar = 20 nm).

Scheme 1: Plausible mechanistic pathway for olefin epoxidation catalyzed by CoNPs/MgO in the presence of t-Bu...
CF3-substituted carbocations: underexploited intermediates with great potential in modern synthetic chemistry
- Anthony J. Fernandes,
- Armen Panossian,
- Bastien Michelet,
- Agnès Martin-Mingot,
- Frédéric R. Leroux and
- Sébastien Thibaudeau
Beilstein J. Org. Chem. 2021, 17, 343–378, doi:10.3762/bjoc.17.32


Graphical Abstract

Figure 1: Stabilizing interaction in the CF3CH2+ carbenium ion (top) and structure of the first observable fl...

Scheme 1: Isodesmic equations accounting for the destabilizing effect of the CF3 group. ΔE in kcal⋅mol−1, cal...

Scheme 2: Stabilizing effect of fluorine atoms by resonance electron donation in carbenium ions (δ in ppm).

Scheme 3: Direct in situ NMR observation of α-(trifluoromethyl)carbenium ion or protonated alcohols. Δδ = δ19...

Scheme 4: Reported 13C NMR chemical shifts for the α-(trifluoromethyl)carbenium ion 10c (δ in ppm).

Scheme 5: Direct NMR observation of α-(trifluoromethyl)carbenium ions in situ (δ in ppm).

Scheme 6: Illustration of the ion pair solvolysis mechanism for sulfonate 13f. YOH = solvent.

Figure 2: Solvolysis rate for 13a–i and 17.

Figure 3: Structures of allyl triflates 18 and 19 and allyl brosylate 20. Bs = p-BrC6H4SO2.

Figure 4: Structure of tosylate derivatives 21.

Figure 5: a) Structure of triflate derivatives 22. b) Stereochemistry outcomes of the reaction starting from (...

Scheme 7: Solvolysis reaction of naphthalene and anthracenyl derivatives 26 and 29.

Figure 6: Structure of bisarylated derivatives 34.

Figure 7: Structure of bisarylated derivatives 36.

Scheme 8: Reactivity of 9c in the presence of a Brønsted acid.

Scheme 9: Cationic electrocyclization of 38a–c under strongly acidic conditions.

Scheme 10: Brønsted acid-catalyzed synthesis of indenes 42 and indanes 43.

Scheme 11: Reactivity of sulfurane 44 in triflic acid.

Scheme 12: Solvolysis of triflate 45f in alcoholic solvents.

Scheme 13: Synthesis of labeled 18O-52.

Scheme 14: Reactivity of sulfurane 53 in triflic acid.

Figure 8: Structure of tosylates 56 and 21f.

Scheme 15: Resonance forms in benzylic carbenium ions.

Figure 9: Structure of pyrrole derivatives 58 and 59.

Scheme 16: Resonance structure 60↔60’.

Scheme 17: Ga(OTf)3-catalyzed synthesis of 3,3’- and 3,6’-bis(indolyl)methane from trifluoromethylated 3-indol...

Scheme 18: Proposed reaction mechanism.

Scheme 19: Metal-free 1,2-phosphorylation of 3-indolylmethanols.

Scheme 20: Superacid-mediated arylation of thiophene derivatives.

Scheme 21: In situ mechanistic NMR investigations.

Scheme 22: Proposed mechanisms for the prenyltransferase-catalyzed condensation.

Scheme 23: Influence of a CF3 group on the allylic SN1- and SN2-mechanism-based reactions.

Scheme 24: Influence of the CF3 group on the condensation reaction.

Scheme 25: Solvolysis of 90 in TFE.

Scheme 26: Solvolysis of allyl triflates 94 and 97 and isomerization attempt of 96.

Scheme 27: Proposed mechanism for the formation of 95.

Scheme 28: Formation of α-(trifluoromethyl)allylcarbenium ion 100 in a superacid.

Scheme 29: Lewis acid activation of CF3-substituted allylic alcohols.

Scheme 30: Bimetallic-cluster-stabilized α-(trifluoromethyl)carbenium ions.

Scheme 31: Reactivity of cluster-stabilized α-(trifluoromethyl)carbenium ions.

Scheme 32: α-(Trifluoromethyl)propargylium ion 122↔122’ generated from silyl ether 120 in a superacid.

Scheme 33: Formation of α-(trifluoromethyl)propargylium ions from CF3-substituted propargyl alcohols.

Scheme 34: Direct NMR observation of the protonation of some trifluoromethyl ketones in situ and the correspon...

Scheme 35: Selected resonance forms in protonated fluoroketone derivatives.

Scheme 36: Acid-catalyzed Friedel–Crafts reactions of trifluoromethyl ketones 143a,b and 147a–c.

Scheme 37: Enantioselective hydroarylation of CF3-substituted ketones.

Scheme 38: Acid-catalyzed arylation of ketones 152a–c.

Scheme 39: Reactivity of 156 in a superacid.

Scheme 40: Reactivity of α-CF3-substituted heteroaromatic ketones and alcohols as well as 1,3-diketones.

Scheme 41: Reactivity of 168 with benzene in the presence of a Lewis or Brønsted acid.

Scheme 42: Acid-catalyzed three-component asymmetric reaction.

Scheme 43: Anodic oxidation of amines 178a–c and proposed mechanism.

Scheme 44: Reactivity of 179b in the presence of a strong Lewis acid.

Scheme 45: Trifluoromethylated derivatives as precursors of trifluoromethylated iminium ions.

Scheme 46: Mannich reaction with trifluoromethylated hemiaminal 189.

Scheme 47: Suitable nucleophiles reacting with 192 after Lewis acid activation.

Scheme 48: Strecker reaction involving the trifluoromethylated iminium ion 187.

Scheme 49: Reactivity of 199 toward nucleophiles.

Scheme 50: Reactivity of 204a with benzene in the presence of a Lewis acid.

Scheme 51: Reactivity of α-(trifluoromethyl)-α-chloro sulfides in the presence of strong Lewis acids.

Scheme 52: Anodic oxidation of sulfides 213a–h and Pummerer rearrangement.

Scheme 53: Mechanism for the electrochemical oxidation of the sulfide 213a.

Scheme 54: Reactivity of (trifluoromethyl)diazomethane (217a) in HSO3F.

Figure 10: a) Structure of diazoalkanes 217a–c and b) rate-limiting steps of their decomposition.

Scheme 55: Deamination reaction of racemic 221 and enantioenriched (S)-221.

Scheme 56: Deamination reaction of labeled 221-d2. Elimination products were formed in this reaction, the yiel...

Scheme 57: Deamination reaction of 225-d2. Elimination products were also formed in this reaction in undetermi...

Scheme 58: Formation of 229 from 228 via 1,2-H-shift.

Scheme 59: Deamination reaction of 230. Elimination products were formed in this reaction, the yield of which ...

Scheme 60: Deamination of several diazonium ions. Elimination products were formed in these reactions, the yie...

Scheme 61: Solvolysis reaction mechanism of alkyl tosylates.

Scheme 62: Solvolysis outcome for the tosylates 248 and 249 in HSO3FSbF5.

Figure 11: Solvolysis rate of 248, 249, 252, and 253 in 91% H2SO4.

Scheme 63: Illustration of the reaction pathways. TsCl, pyridine, −5 °C (A); 98% H2SO4, 30 °C (B); 98% H2SO4, ...

Scheme 64: Proposed solvolysis mechanism for the aliphatic tosylate 248.

Scheme 65: Solvolysis of the derivatives 259 and 260.

Scheme 66: Solvolysis of triflate 261. SOH = solvent.

Scheme 67: Intramolecular Friedel–Crafts alkylations upon the solvolysis of triflates 264 and 267.

Scheme 68: α-CF3-enhanced γ-silyl elimination of cyclobutyltosylates 270a,b.

Scheme 69: γ-Silyl elimination in the synthesis of a large variety of CF3-substituted cyclopropanes. Pf = pent...

Scheme 70: Synthetic pathways to 281. aNMR yields.

Scheme 71: The cyclopropyl-substituted homoallylcyclobutylcarbenium ion manifold.

Scheme 72: Reactivity of CF3-substituted cyclopropylcarbinyl derivatives 287a–c. LG = leaving group.

Scheme 73: Reactivity of CF3-substituted cyclopropylcarbinyl derivatives 291a–c.

Scheme 74: Superacid-promoted dimerization or TFP.

Scheme 75: Reactivity of TFP in a superacid.

Scheme 76: gem-Difluorination of α-fluoroalkyl styrenes via the formation of a “hidden” α-RF-substituted carbe...

Scheme 77: Solvolysis of CF3-substituted pentyne 307.

Scheme 78: Photochemical rearrangement of 313.

Figure 12: Structure of 2-norbornylcarbenium ion 318 and argued model for the stabilization of this cation.

Figure 13: Structures and solvolysis rate (TFE, 25 °C) of the sulfonates 319–321. Mos = p-MeOC6H4SO2.

Scheme 79: Mechanism for the solvolysis of 323. SOH = solvent.

Scheme 80: Products formed by the hydrolysis of 328.

Scheme 81: Proposed carbenium ion intermediates in an equilibrium during the solvolysis of tosylates 328, 333,...
The preparation and properties of 1,1-difluorocyclopropane derivatives
- Kymbat S. Adekenova,
- Peter B. Wyatt and
- Sergazy M. Adekenov
Beilstein J. Org. Chem. 2021, 17, 245–272, doi:10.3762/bjoc.17.25
- alkenes, electron-rich styrenes, and styrenes with electron-withdrawing substituents in the structure [40]. The yields of the gem-difluorocyclopropanes from the styrene derivatives were almost all excellent. On the other hand, simple alkenes gave lower yields. The reaction of various functionalized

Graphical Abstract

Scheme 1: Synthesis of 1,1-difluoro-2,3-dimethylcyclopropane (2).

Scheme 2: Cyclopropanation via dehydrohalogenation of chlorodifluoromethane.

Scheme 3: Difluorocyclopropanation of methylstyrene 7 using dibromodifluoromethane and zinc.

Scheme 4: Synthesis of difluorocyclopropanes from the reaction of dibromodifluoromethane and triphenylphosphi...

Scheme 5: Generation of difluorocarbene in a catalytic two-phase system and its addition to tetramethylethyle...

Scheme 6: The reaction of methylstyrene 7 with chlorodifluoromethane (11) in the presence of a tetraarylarson...

Scheme 7: Pyrolysis of sodium chlorodifluoroacetate (12) in refluxing diglyme in the presence of alkene 13.

Scheme 8: Synthesis of boron-substituted gem-difluorocyclopropanes 16.

Scheme 9: Addition of sodium bromodifluoroacetate (17) to alkenes.

Scheme 10: Addition of sodium bromodifluoroacetate (17) to silyloxy-substituted cyclopropanes 20.

Scheme 11: Synthesis of difluorinated nucleosides.

Scheme 12: Addition of butyl acrylate (26) to difluorocarbene generated from TFDA (25).

Scheme 13: Addition of difluorocarbene to propargyl esters 27 and conversion of the difluorocyclopropenes 28 t...

Scheme 14: The generation of difluorocyclopropanes using MDFA 30.

Scheme 15: gem-Difluorocyclopropanation of styrene (32) using difluorocarbene generated from TMSCF3 (31) under...

Scheme 16: Synthesis of a gem-difluorocyclopropane derivative using HFPO (41) as a source of difluorocarbene.

Scheme 17: Cyclopropanation of (Z)-2-butene in the presence of difluorodiazirine (44).

Scheme 18: The cyclopropanation of 1-octene (46) using Seyferth's reagent (45) as a source of difluorocarbene.

Scheme 19: Alternative approaches for the difluorocarbene synthesis from trimethyl(trifluoromethyl)tin (48).

Scheme 20: Difluorocyclopropanation of cyclohexene (49).

Scheme 21: Synthesis of difluorocyclopropane derivative 53 using bis(trifluoromethyl)cadmium (51) as the diflu...

Scheme 22: Addition of difluorocarbene generated from tris(trifluoromethyl)bismuth (54).

Scheme 23: Addition of a stable (trifluoromethyl)zinc reagent to styrenes.

Scheme 24: The preparation of 2,2-difluorocyclopropanecarboxylic acids of type 58.

Scheme 25: Difluorocyclopropanation via Michael cyclization.

Scheme 26: Difluorocyclopropanation using N-acylimidazolidinone 60.

Scheme 27: Difluorocyclopropanation through the cyclization of phenylacetonitrile (61) and 1,2-dibromo-1,1-dif...

Scheme 28: gem-Difluoroolefins 64 for the synthesis of functionalized cyclopropanes 65.

Scheme 29: Preparation of aminocyclopropanes 70.

Scheme 30: Synthesis of fluorinated methylenecyclopropane 74 via selenoxide elimination.

Scheme 31: Reductive dehalogenation of (1R,3R)-75.

Scheme 32: Synthesis of chiral monoacetates by lipase catalysis.

Scheme 33: Transformation of (±)-trans-81 using Rhodococcus sp. AJ270.

Scheme 34: Transformation of (±)-trans-83 using Rhodococcus sp. AJ270.

Scheme 35: Hydrogenation of difluorocyclopropenes through enantioselective hydrocupration.

Scheme 36: Enantioselective transfer hydrogenation of difluorocyclopropenes with a Ru-based catalyst.

Scheme 37: The thermal transformation of trans-1,2-dichloro-3,3-difluorocyclopropane (84).

Scheme 38: cis–trans-Epimerization of 1,1-difluoro-2,3-dimethylcyclopropane.

Scheme 39: 2,2-Difluorotrimethylene diradical intermediate.

Scheme 40: Ring opening of stereoisomers 88 and 89.

Scheme 41: [1,3]-Rearrangement of alkenylcyclopropanes 90–92.

Scheme 42: Thermolytic rearrangement of 2,2-difluoro-1-vinylcyclopropane (90).

Scheme 43: Thermal rearrangement for ethyl 3-(2,2-difluoro)-3-phenylcyclopropyl)acrylates 93 and 95.

Scheme 44: Possible pathways of the ring opening of 1,1-difluoro-2-vinylcyclopropane.

Scheme 45: Equilibrium between 1,1-difluoro-2-methylenecyclopropane (96) and (difluoromethylene)cyclopropane 97...

Scheme 46: Ring opening of substituted 1,1-difluoro-2,2-dimethyl-3-methylenecyclopropane 98.

Scheme 47: 1,1-Difluorospiropentane rearrangement.

Scheme 48: Acetolysis of (2,2-difluorocyclopropyl)methyl tosylate (104) and (1,1-difluoro-2-methylcyclopropyl)...

Scheme 49: Ring opening of gem-difluorocyclopropyl ketones 106 and 108 by thiolate nucleophiles.

Scheme 50: Hydrolysis of gem-difluorocyclopropyl acetals 110.

Scheme 51: Ring-opening reaction of 2,2-difluorocyclopropyl ketones 113 in the presence of ionic liquid as a s...

Scheme 52: Ring opening of gem-difluorocyclopropyl ketones 113a by MgI2-initiated reaction with diarylimines 1...

Scheme 53: Ring-opening reaction of gem-difluorocyclopropylstannanes 117.

Scheme 54: Preparation of 1-fluorovinyl vinyl ketone 123 and the synthesis of 2-fluorocyclopentenone 124. TBAT...

Scheme 55: Iodine atom-transfer ring opening of 1,1-difluoro-2-(1-iodoalkyl)cyclopropanes 125a–c.

Scheme 56: Ring opening of bromomethyl gem-difluorocyclopropanes 130 and formation of gem-difluoromethylene-co...

Scheme 57: Ring-opening aerobic oxidation reaction of gem-difluorocyclopropanes 132.

Scheme 58: Dibrominative ring-opening functionalization of gem-difluorocyclopropanes 134.

Scheme 59: The selective formation of (E,E)- and (E,Z)-fluorodienals 136 and 137 from difluorocyclopropyl acet...

Scheme 60: Proposed mechanism for the reaction of difluoro(methylene)cyclopropane 139 with Br2.

Scheme 61: Thermal rearrangement of F2MCP 139 and iodine by CuI catalysis.

Scheme 62: Synthesis of 2-fluoropyrroles 142.

Scheme 63: Ring opening of gem-difluorocyclopropyl ketones 143 mediated by BX3.

Scheme 64: Lewis acid-promoted ring-opening reaction of 2,2-difluorocyclopropanecarbonyl chloride (148).

Scheme 65: Ring-opening reaction of the gem-difluorocyclopropyl ketone 106 by methanolic KOH.

Scheme 66: Hydrogenolysis of 1,1-difluoro-3-methyl-2-phenylcyclopropane (151).

Scheme 67: Synthesis of monofluoroalkenes 157.

Scheme 68: The stereoselective Ag-catalyzed defluorinative ring-opening diarylation of 1-trimethylsiloxy-2,2-d...

Scheme 69: Synthesis of 2-fluorinated allylic compounds 162.

Scheme 70: Pd-catalyzed cross-coupling reactions of gem-difluorinated cyclopropanes 161.

Scheme 71: The (Z)-selective Pd-catalyzed ring-opening sulfonylation of 2-(2,2-difluorocyclopropyl)naphthalene...

Figure 1: Structures of zosuquidar hydrochloride and PF-06700841.

Scheme 72: Synthesis of methylene-gem-difluorocyclopropane analogs of nucleosides.

Figure 2: Anthracene-difluorocyclopropane hybrid derivatives.

Figure 3: Further examples of difluorcyclopropanes in modern drug discovery.
Au(III) complexes with tetradentate-cyclam-based ligands
- Ann Christin Reiersølmoen,
- Thomas N. Solvi and
- Anne Fiksdahl
Beilstein J. Org. Chem. 2021, 17, 186–192, doi:10.3762/bjoc.17.18
- cyclopropanation of styrene with propargyl ester [49][50][51][52] (Table 1) were selected as test reactions. These reactions have previously been studied with different gold(I) and gold(III) catalysts and a variety of substrates, thus providing a solid background for comparison. A large difference in the catalytic
- diamides 1a,b and tetraamides 2a,b. Au(III) coordination conditions for ligands 5a,b and 6a,b. Coordination of 5b was unsuccessful. The catalytic activity of Au(III) complexes evaluated in a) alkyne carboalkoxylation and b) cyclopropanation of styrene with propargyl ester. Supporting Information

Graphical Abstract

Scheme 1: Synthetic protocols for the preparation of potential ligands 1–4.

Scheme 2: Reduction of diamides 1a,b and tetraamides 2a,b.

Scheme 3: Au(III) coordination conditions for ligands 5a,b and 6a,b. Coordination of 5b was unsuccessful.

Figure 1: 1H NMR study of the formation of complex 6a-Au(III) by AuCl3 coordination to ligand 6a.
Amine–borane complex-initiated SF5Cl radical addition on alkenes and alkynes
- Audrey Gilbert,
- Pauline Langowski,
- Marine Delgado,
- Laurent Chabaud,
- Mathieu Pucheault and
- Jean-François Paquin
Beilstein J. Org. Chem. 2020, 16, 3069–3077, doi:10.3762/bjoc.16.256
- styrene [48][49][50]. We envisioned that it could be possible to replace the Et3B in Dolbier’s protocol by a stable amine–borane complex that could perform the radical initiation of SF5Cl on its addition on alkenes. This would address the drawbacks associated with the use of Et3B as the radical initiator
- reactions on styrene, the desired addition product 2c was only observed with a low NMR yield of 8% with the Et3B-mediated reaction, while the DICAB protocol led to a 15% isolated yield of the 2:1 addition product 2d, with no sign of the desired compound 2c. Furthermore, as expected, a low yield of 15% of

Graphical Abstract

Scheme 1: Dolbier’s protocol for the SF5Cl radical addition on alkenes.

Scheme 2: Proposed mechanism for the amine–borane complex-initiated radical addition of SF5Cl on alkenes.

Figure 1: Structures and acronyms of the amine-borane complexes investigated.

Scheme 3: Scope of the Et3B and the DICAB-initiated SF5Cl additions on alkenes. Unless noted otherwise, isola...

Scheme 4: Scope of the Et3B and the DICAB-initiated SF5Cl additions on alkynes. Unless noted otherwise, isola...
Photosensitized direct C–H fluorination and trifluoromethylation in organic synthesis
- Shahboz Yakubov and
- Joshua P. Barham
Beilstein J. Org. Chem. 2020, 16, 2151–2192, doi:10.3762/bjoc.16.183

Graphical Abstract

Figure 1: Fluorine-containing drugs.

Figure 2: Fluorinated agrochemicals.

Scheme 1: Selectivity of fluorination reactions.

Scheme 2: Different mechanisms of photocatalytic activation. Sub = substrate.

Figure 3: Jablonski diagram showing visible-light-induced energy transfer pathways: a) absorption, b) IC, c) ...

Figure 4: Schematic illustration of TTET.

Figure 5: Organic triplet PSCats.

Figure 6: Additional organic triplet PSCats.

Figure 7: A) Further organic triplet PSCats and B) transition metal triplet PSCats.

Figure 8: Different fluorination reagents grouped by generation.

Scheme 3: Synthesis of Selectfluor®.

Scheme 4: General mechanism of PS TTET C(sp3)–H fluorination.

Scheme 5: Selective benzylic mono- and difluorination using 9-fluorenone and xanthone PSCats, respectively.

Scheme 6: Chen’s photosensitized monofluorination: reaction scope.

Scheme 7: Chen’s photosensitized benzylic difluorination reaction scope.

Scheme 8: Photosensitized monofluorination of ethylbenzene on a gram scale.

Scheme 9: Substrate scope of Tan’s AQN-photosensitized C(sp3)–H fluorination.

Scheme 10: AQN-photosensitized C–H fluorination reaction on a gram scale.

Scheme 11: Reaction mechanism of the AQN-assisted fluorination.

Figure 9: 3D structures of the singlet ground and triplet excited states of Selectfluor®.

Scheme 12: Associated transitions for the activation of acetophenone by violet light.

Scheme 13: Ethylbenzene C–H fluorination with various PSCats and conditions.

Scheme 14: Effect of different PSCats on the C(sp3)–H fluorination of cyclohexane (39).

Scheme 15: Reaction scope of Chen’s acetophenone-photosensitized C(sp3)–H fluorination reaction.

Figure 10: a) Site-selectivity of Chen’s acetophenone-photosensitized C–H fluorination reaction [201]. b) Site-sele...

Scheme 16: Formation of the AQN–Selectfluor® exciplex Int1.

Scheme 17: Generation of the C3 2° pentane radical and the Selectfluor® N-radical cation from the exciplex.

Scheme 18: Hydrogen atom abstraction by the Selectfluor® N-radical cation from pentane to give the C3 2° penta...

Scheme 19: Fluorine atom transfer from Selectfluor® to the C3 2° pentane radical to yield 3-fluoropentane and ...

Scheme 20: Barrierless fluorine atom transfer from Int1 to the C3 2° pentane radical to yield 3-fluoropentane,...

Scheme 21: Ketone-directed C(sp3)–H fluorination.

Scheme 22: Ketone-directed fluorination through a 5- and a 6-membered transition state, respectively.

Scheme 23: Effect of different PSCats on the photosensitized C(sp3)–H fluorination of 47.

Scheme 24: Substrate scope of benzil-photoassisted C(sp3)–H fluorinations.

Scheme 25: A) Benzil-photoassisted enone-directed C(sp3)–H fluorination. B) Classification of the reaction mod...

Scheme 26: A) Xanthone-photoassisted ketal-directed C(sp3)–H fluorination. B) Substrate scope. C) C–H fluorina...

Scheme 27: Rationale for the selective HAT at the C2 C–H bond of galactose acetonide.

Scheme 28: Photosensitized C(sp3)–H benzylic fluorination of a peptide using different PSCats.

Scheme 29: Peptide scope of 5-benzosuberenone-photoassisted C(sp3)–H fluorinations.

Scheme 30: Continuous flow PS TTET monofluorination of 72.

Scheme 31: Photosensitized C–H fluorination of N-butylphthalimide as a PSX.

Scheme 32: Substrate scope and limitations of the PSX C(sp3)–H monofluorination.

Scheme 33: Substrate crossover monofluorination experiment.

Scheme 34: PS TTET mechanism proposed by Hamashima and co-workers.

Scheme 35: Photosensitized TFM of 78 to afford α-trifluoromethylated ketone 80.

Scheme 36: Substrate scope for photosensitized styrene TFM to give α-trifluoromethylated ketones.

Scheme 37: Control reactions for photosensitized TFM of styrenes.

Scheme 38: Reaction mechanism for photosensitized TFM of styrenes to afford α-trifluoromethylated ketones.

Scheme 39: Reaction conditions for TFMs to yield the cis- and the trans-product, respectively.

Scheme 40: Substrate scope of trifluoromethylated (E)-styrenes.

Scheme 41: Strategies toward trifluoromethylated (Z)-styrenes.

Scheme 42: Substrate scope of trifluoromethylated (Z)-styrenes.

Scheme 43: Reaction mechanism for photosensitized TFM of styrenes to afford E- or Z-products.
Reactions of 3-aryl-1-(trifluoromethyl)prop-2-yn-1-iminium salts with 1,3-dienes and styrenes
- Thomas Schneider,
- Michael Keim,
- Bianca Seitz and
- Gerhard Maas
Beilstein J. Org. Chem. 2020, 16, 2064–2072, doi:10.3762/bjoc.16.173
- ] cycloaddition reactions [45]. Thus, while styrene and maleic anhydride react only at elevated temperatures, with the more electrophilic (methoxycarbonyl)maleic anhydride two 2:1 adducts are already formed at room temperature: one by two consecutive Diels–Alder reactions, the other one by a Diels–Alder/ene
- reaction of propyn-1-iminium salt 1a with styrene in acetonitrile was considered first and was monitored by 19F NMR spectroscopy. Whereas no reaction appeared to occur at room temperature, a slow transformation into two fluorine-containing products was observed at 70 °C, which after neutralization and work
- reaction mechanism. Thus, the reaction of 1a with α-methylstyrene or 1,1-diphenylethene proceeded at a faster rate than with styrene and yielded 3-methyl- and 3-phenyl-2-vinylindenes 12b and 12c (structure confirmed by an XRD analysis, see Figure 2) in high yields. Benzo[a]fluorenes were found to a minor

Graphical Abstract

Scheme 1: Diels–Alder reaction of propyn-1-iminium salt 1a compared with the reported [29] reaction of 4-phenyl-1...

Scheme 2: Sequential Diels–Alder/intramolecular SE(Ar) reaction of propyn-1-iminium triflates 1a,b. Condition...

Scheme 3: Diels–Alder reaction of 1a and anthracene followed by an intramolecular SE(Ar) reaction.

Figure 1: Solid-state molecular structure of 11 (ORTEP plot).

Scheme 4: Reactions of propyn-1-iminium salt 1a with styrenes.

Figure 2: Solid-state molecular structure of 12c (ORTEP plot).

Figure 3: Solid-state molecular structure of 12d (ORTEP plot). Both the R and the S enantiomer are present in...

Scheme 5: A mechanistic proposal for the reaction of alkyne 1a with styrenes.

Scheme 6: Reaction of alkyne 1a with 1,2-dihydronaphthalene.

Scheme 7: Synthesis and solid-state molecular structure (ORTEP plot) of pentafulvene 19; selected bond distan...

Scheme 8: Proposed mechanistic pathway leading to fulvene 19.
One-pot synthesis of oxazolidinones and five-membered cyclic carbonates from epoxides and chlorosulfonyl isocyanate: theoretical evidence for an asynchronous concerted pathway
- Esra Demir,
- Ozlem Sari,
- Yasin Çetinkaya,
- Ufuk Atmaca,
- Safiye Sağ Erdem and
- Murat Çelik
Beilstein J. Org. Chem. 2020, 16, 1805–1819, doi:10.3762/bjoc.16.148
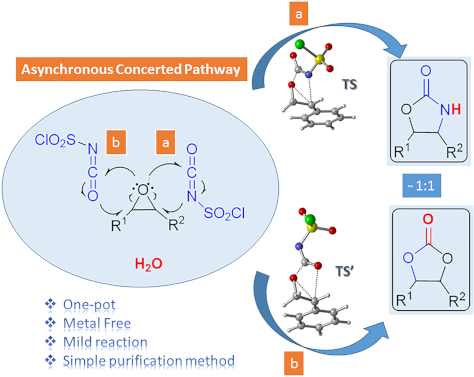
- ), respectively (Table 2). These results show that the relative configuration is preserved. 4-Phenyl-1,3-dioxolan-2-one (8f) and 4-phenyloxazolidin-2-one (9f) were obtained from the reaction of CSI with styrene oxide (7f) showing the regioselective nature of the reaction. In addition, we report the synthesis of

Graphical Abstract

Scheme 1: Oxazolidinone (1), five-membered cyclic carbonate (2) and some important compounds containing an ox...

Scheme 2: Proposed mechanisms by Keshava Murthy and Dhar [41] and De Meijere and co-workers [42].

Figure 1: Possible pathways for the formation of oxazolidinone intermediates 10 and 11. Optimized transition ...

Figure 2: Potential energy profile related to the formation of oxazolidinone intermediates 10 and 11 at the P...

Figure 3: IRC calculated for the formation of (a) 10 and (b) 11 at M06-2X/6-31+G(d,p) level. I-1, I-15, I-35, ...

Figure 4: Optimized geometries for the stationary points for the formation of 10 at PCM(DCM)/M06-2X/6-31+G(d,...

Scheme 3: Proposed mechanisms for the formation of oxazolidinone 9f.

Figure 5: Potential energy profiles for paths 1a (blue), 1b (red), 2 (green) and relative Gibbs free energies...

Figure 6: Optimized geometries for the stationary points of path 1b at PCM(DCM)/M06-2X/6-31+G(d,p)//M06-2X/6-...

Scheme 4: Proposed mechanism for the formation of five-membered cyclic carbonate 8f.

Figure 7: Potential energy profile and relative Gibbs free energies (kcal/mol) in DCM related to the formatio...

Figure 8: Optimized geometries for the stationary points of step 1 for the formation of 16 at PCM(DCM)/M06-2X...

Figure 9: Optimized geometries for the stationary points of step 2 for the formation of 17 at PCM(DCM)/M06-2X...

Figure 10: Optimized geometries for the stationary points of step 3 for the formation of PC8 at PCM(DCM)/M06-2...
Heterogeneous photocatalysis in flow chemical reactors
- Christopher G. Thomson,
- Ai-Lan Lee and
- Filipe Vilela
Beilstein J. Org. Chem. 2020, 16, 1495–1549, doi:10.3762/bjoc.16.125

- the efficiency of synthesising and purifying immobilised photosensitisers [63]. A BODIPY photosensitiser was immobilised to an aldehyde-functionalised Merrifield resin (poly(styrene-co-divinylbenzene-co-4-formylstyrene)) in continuous flow and concurrently in an analogous batch procedure. The coupling

Graphical Abstract

Figure 1: A) Bar chart of the publications per year for the topics “Photocatalysis” (49,662 instances) and “P...

Figure 2: A) Professor Giacomo Ciamician and Dr. Paolo Silber on their roof laboratory at the University of B...

Scheme 1: PRC trifluoromethylation of N-methylpyrrole (1) using hazardous gaseous CF3I safely in a flow react...

Figure 3: A) Unit cells of the three most common crystal structures of TiO2: rutile, brookite, and anatase. R...

Figure 4: Illustration of the key semiconductor photocatalysis events: 1) A photon with a frequency exceeding...

Figure 5: Photocatalytic splitting of water by oxygen vacancies on a TiO2(110) surface. Reprinted with permis...

Figure 6: Proposed adsorption modes of A) benzene, B) chlorobenzene, C) toluene, D) phenol, E) anisole, and F...

Figure 7: Structures of the sulfonate-containing organic dyes RB5 (3) and MX-5B (4) and the adsorption isothe...

Figure 8: Idealised triclinic unit cell of a g-C3N4 type polymer, displaying possible hopping transport scena...

Figure 9: Idealised structure of a perfect g-C3N4 sheet. The central unit highlighted in red represents one t...

Figure 10: Timeline of the key processes of charge transport following the photoexcitation of g-C3N4, leading ...

Scheme 2: Photocatalytic bifunctionalisation of heteroarenes using mpg-C3N4, with the selected examples 5 and ...

Figure 11: A) Structure of four linear conjugated polymer photocatalysts for hydrogen evolution, displaying th...

Figure 12: Graphical representation of the common methods used to immobilise molecular photocatalysts (PC) ont...

Figure 13: Wireless light emitter-supported TiO2 (TiO2@WLE) HPCat spheres powered by resonant inductive coupli...

Figure 14: Graphical representation of zinc–perylene diimide (Zn-PDI) supramolecular assembly photocatalysis v...

Scheme 3: Upconversion of NIR photons to the UV frequency by NaYF4:Yb,Tm nanocrystals sequentially coated wit...

Figure 15: Types of reactors employed in heterogeneous photocatalysis in flow. A) Fixed bed reactors and the s...

Figure 16: Electrochemical potential of common semiconductor, transition metal, and organic dye-based photocat...

Scheme 4: Possible mechanisms of an immobilised molecular photoredox catalyst by oxidative or reductive quenc...

Scheme 5: Scheme of the CMB-C3N4 photocatalytic decarboxylative fluorination of aryloxyacetic acids, with the...

Scheme 6: Scheme of the g-C3N4 photocatalytic desilylative coupling reaction in flow and proposed mechanism [208].

Scheme 7: Proposed mechanism of the radical cyclisation of unsaturated alkyl 2-bromo-1,3-dicarbonyl compounds...

Scheme 8: N-alkylation of benzylamine and schematic of the TiO2-coated microfluidic device [213].

Scheme 9: Proposed mechanism of the Pt@TiO2 photocatalytic deaminitive cyclisation of ʟ-lysine (23) to ʟ-pipe...

Scheme 10: A) Proposed mechanism for the photocatalytic oxidation of phenylboronic acid (24). B) Photos and SE...

Scheme 11: Proposed mechanism for the DA-CMP3 photocatalytic aza-Henry reaction performed in a continuous flow...

Scheme 12: Proposed mechanism for the formation of the cyclic product 32 by TiO2-NC HPCats in a slurry flow re...

Scheme 13: Reaction scheme for the photocatalytic synthesis of homo and hetero disulfides in flow and scope of...

Scheme 14: Reaction scheme for the MoOx/TiO2 HPCat oxidation of cyclohexane (34) to benzene. The graph shows t...

Scheme 15: Proposed mechanism of the TiO2 HPC heteroarene C–H functionalisation via aryl radicals generated fr...

Scheme 16: Scheme of the oxidative coupling of benzylamines with the HOTT-HATN HPCat and selected examples of ...

Scheme 17: Photocatalysis oxidation of benzyl alcohol (40) to benzaldehyde (41) in a microflow reactor coated ...

Figure 17: Mechanisms of Dexter and Forster energy transfer.

Scheme 18: Continuous flow process for the isomerisation of alkenes with an ionic liquid-immobilised photocata...

Scheme 19: Singlet oxygen synthetic step in the total synthesis of canataxpropellane [265].

Scheme 20: Scheme and proposed mechanism of the singlet oxygen photosensitisation by CMP_X HPCats, with the st...

Scheme 21: Structures of CMP HPCat materials applied by Vilela and co-workers for the singlet oxygen photosens...

Scheme 22: Polyvinylchloride resin-supported TDCPP photosensitisers applied for singlet oxygen photosensitisat...

Scheme 23: Structure of the ionically immobilised TPP photosensitiser on amberlyst-15 ion exchange resins (TPP...

Scheme 24: Photosensitised singlet oxygen oxidation of citronellol (46) in scCO2, with automatic phase separat...

Scheme 25: Schematic of PS-Est-BDP-Cl2 being applied for singlet oxygen photosensitisation in flow. A) Pseudo-...

Scheme 26: Reaction scheme of the singlet oxygen oxidation of furoic acid (54) using a 3D-printed microfluidic...

Figure 18: A) Photocatalytic bactericidal mechanism by ROS oxidative cleavage of membrane lipids (R = H, amino...

Figure 19: A) Suggested mechanisms for the aqueous pollutant degradation by TiO2 in a slurry flow reactor [284-287]. B)...

Figure 20: Schematic of the flow system used for the degradation of aqueous oxytetracycline (56) solutions [215]. M...

Scheme 27: Degradation of a salicylic acid (57) solution by a coupled solar photoelectro-Fenton (SPEF) process...

Figure 21: A) Schematic flow diagram using the TiO2-coated NETmix microfluidic device for an efficient mass tr...
An overview on disulfide-catalyzed and -cocatalyzed photoreactions
- Yeersen Patehebieke
Beilstein J. Org. Chem. 2020, 16, 1418–1435, doi:10.3762/bjoc.16.118

- . Huang and co-workers proposed a polar radical crossover cycloaddition mechanism for this Diels–Alder cycloaddition (Scheme 6). The electron transfer from the electron-rich styrene 14 to the activated acridinium photocatalyst 15 oxidizes the styrene 14 to form the styrene radical 16 and the acridine
- radical 17 (Mes–Acr–Ph•). The subsequent reaction of the formed styrene radical 16 with another styrene 18 gives the radical species 19 and the reoxidation of the acridine radical 17 by a thiyl radical, which is generated by the homolysis of diphenyl disulfide, regenerating the photocatalyst. In a
- hydroxylation product 36 and frees the disulfide for the next cycle. The generation of the disulfide–olefin complex 37 from styrene and disulfide initiates the hydroxymethylation. The following 3O2 trapping by the intermediate 38, which is generated by the addition of a thiyl radical to the styrene under

Graphical Abstract

Scheme 1: [3 + 2] cyclization catalyzed by diaryl disulfide.

Scheme 2: [3 + 2] cycloaddition catalyzed by disulfide.

Scheme 3: Disulfide-bridged peptide-catalyzed enantioselective cycloaddition.

Scheme 4: Disulfide-catalyzed [3 + 2] methylenecyclopentane annulations.

Scheme 5: Disulfide as a HAT cocatalyst in the [4 + 2] cycloaddition reaction.

Scheme 6: Proposed mechanism of the [4 + 2] cycloaddition reaction using disulfide as a HAT cocatalyst.

Scheme 7: Disulfide-catalyzed ring expansion of vinyl spiro epoxides.

Scheme 8: Disulfide-catalyzed aerobic oxidation of diarylacetylene.

Scheme 9: Disulfide-catalyzed aerobic photooxidative cleavage of olefins.

Scheme 10: Disulfide-catalyzed aerobic oxidation of 1,3-dicarbonyl compounds.

Scheme 11: Proposed mechanism of the disulfide-catalyzed aerobic oxidation of 1,3-dicarbonyl compounds.

Scheme 12: Disulfide-catalyzed oxidation of allyl alcohols.

Scheme 13: Disulfide-catalyzed diboration of alkynes.

Scheme 14: Dehalogenative radical cyclization catalyzed by disulfide.

Scheme 15: Hydrodifluoroacetamidation of alkenes catalyzed by disulfide.

Scheme 16: Plausible mechanism of the hydrodifluoroacetamidation of alkenes catalyzed by disulfide.

Scheme 17: Disulfide-cocatalyzed anti-Markovnikov olefin hydration reactions.

Scheme 18: Disulfide-catalyzed decarboxylation of carboxylic acids.

Scheme 19: Proposed mechanism of the disulfide-catalyzed decarboxylation of carboxylic acids.

Scheme 20: Disulfide-catalyzed decarboxylation of carboxylic acids.

Scheme 21: Disulfide-catalyzed conversion of maleate esters to fumarates and 5H-furanones.

Scheme 22: Disulfide-catalyzed isomerization of difluorotriethylsilylethylene.

Scheme 23: Disulfide-catalyzed isomerization of allyl alcohols to carbonyl compounds.

Scheme 24: Proposed mechanism for the disulfide-catalyzed isomerization of allyl alcohols to carbonyl compound...

Scheme 25: Diphenyl disulfide-catalyzed enantioselective synthesis of ophirin B.

Scheme 26: Disulfide-catalyzed isomerization in the total synthesis of (+)-hitachimycin.

Scheme 27: Disulfide-catalyzed isomerization in the synthesis of (−)-gloeosporone.






